Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are
typically used to treat inflammatory states and injury.
Non-selective NSAIDs inhibit cyclooxygenase-1 (COX-1) mediated
production of prostaglandins and are known to cause vascular and
gastrointestinal toxicity, and a variety of nephrotoxic syndromes
(1). Due to these adverse effects,
there is a requirement for novel alternative treatments for
inflammatory diseases. Meanwhile, there has been an increase in the
use of herbal dietary supplements. The rise in the popularity of
natural products may be attributed to dissatisfaction with
conventional medicines (2–5).
The function of nitric oxide (NO) in the
inflammatory reaction is controversial. During inflammation and
sepsis, increased production of diverse mediators, including
endotoxins, pro-inflammatory cytokines and eicosanoids, induces
inducible nitric oxide synthase (iNOS) activity. Increased mucosal
NO production and NOS-2 activity have been associated with active
inflammatory bowel disease or pathogenesis of chronic disease in
patients (6–8) and in an experimental animal model of
induced or inherent inflammation (9). In addition, prostaglandin
E2 (PGE2) is a primary inflammatory mediator
and upregulates vascular permeability increasing factor, which may
lead to edema, pain and fever during inflammatory disease (10). Members of the COX family are
accountable for the production of prostaglandins, including
thromboxanes and prostacyclin (11,12).
COX exists as two isoforms: Inducible COX-2 and constitutive COX-1.
COX-2 levels are reduced under healthy physiological conditions,
but is transiently and rapidly induced by pro-inflammatory
mediators, activating the biosynthesis of prostaglandin and
inflammatory responses (13).
Inhibition of COX-2 may provide relief from the symptoms of
inflammation and pain (14).
Consequently, a previous study aimed to investigate selective COX-2
inhibitors (15). Fraxinellone has
been suggested to have mild but significant inhibitory effects on
the stimulation of PGE2 by LPS, and Kim et al
(16) suggested reductions in
PGE2 may be due to the transcriptional suppression of
COX-2.
D. obtusifolius is a plant genus that belongs
to the family Dipterocarpaceae, which consists of ~75 species
distributed in tropical regions (17). This family of plants is known to
contain sesquiterpenes, triterpenes, flavonoids and resveratrol
oligomers, and exhibits diverse biological anticancer, anti-human
immunodeficiency virus (18),
antibacterial and antioxidant activities (19). D. obtusifolius is a tree
with a typical height of 10–15 m that grows in cleared forests in
low altitude regions, and is widely distributed across Southeast
Asian countries. Traditionally, the resin of this plant has been
used to relieve abdominal discomfort in Cambodia, Laos and Vietnam
(20). To the best of our
knowledge, there have been no reports on the anti-inflammatory
effects of D. obtusifolius methanolic extract (DOME).
Therefore, the aim of the present study was to investigate the
anti-inflammatory effects of DOME using LPS-stimulated RAW264.7
cells. NO, cytokines and PGE2 levels were assessed
following treatment with DOME in LPS-stimulated RAW264.7 cells. To
elucidate the protective underlying mechanisms of DOME, the
expression of mRNA and proteins associated with inflammatory
responses were examined.
Materials and methods
Preparation of DOME from D.
obtusifolius leaves
D. obtusifolius was collected from the Tbaeng
region of Cambodia in 2009. The extract was obtained from 86 g
leaves with MeOH, an extract efficiency of 9.22%. A voucher
specimen (KRIB 0025367) was deposited in the herbarium of the Korea
Research Institute of Bioscience and Biotechnology (Daejeon,
Republic of Korea). The D. obtusifolius leaves were obtained
from the International Biological Material Research Center
(Daejeon, Republic of Korea).
Cell culture
The RAW264.7 macrophage cell line was provided by
the American Type Culture Collection (Manassas, VA, USA). Cells
were maintained in a 95% air, 5% CO2 atmosphere in a
37°C incubator in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (Hyclone; GE Healthcare, Logan,
UT, USA) and 1% penicillin-streptomycin-glutamine (Invitrogen;
Thermo Fisher Scientific, Inc.). RAW264.7 cells were passage weekly
at 70–80% confluence. Following pre-incubation for 4 h, 0–30 µg/ml
DOME was added to cells. In another set of cultures, the cells were
co-incubated with 10 µM p42/44 inhibitor, PD98059, 10 µM p38
inhibitor, SB203580 and 10 µM c-Jun N-terminal kinase (JNK)
inhibitor, SP600125.
Cell viability assay
Cell viability was determined by an assay based on
the mitochondrial-dependent reduction of MTT (Amresco, LLC, Solon,
OH, USA) to formazan with 100 µl DMSO per well. Based on the
results of cell viability, nontoxic concentrations of DOME were
used with RAW264.7 cells in the present study. The optical density
of formazan was measured using a microplate reader (Benchmark;
Bio-Rad Laboratories, Hercules, CA, USA) at a wavelength of 570 nm.
The optical density of formazan generated by untreated cells was
used to determine the 100% viability.
NO assay
The NO level in cell culture supernatants from
centrifugation at 100 × g for 5 min at room temperature was
determined using the Griess test. RAW264.7 cells were plated at a
density of 5×104 cells/well in 96-well plates and
subsequently incubated with or without 0.5 µg/ml LPS in the absence
or presence of various concentrations of DOME for 24 h. Nitrite in
the culture supernatants was mixed with an equal volume of Griess
reagent [1% sulfanilamide and 0.1% N-(1-naphthyl)ethylenediamine
dihydrochloride in 5% phosphoric acid]. The absorbance was measured
at a wavelength of 540 nm using a microplate reader and a series of
known concentrations of NaNO2 was used as a
standard.
PGE2 assay
The quantity of PGE2 in the supernatant
was determined using a PGE2 ELISA kit (cat. no. 514010;
Cayman Chemical Company, Ann Arbor, MI, USA) according to the
manufacturer's protocol. A total of 50 µl diluted standard:sample
2:1 was pipetted into a 96-well plate pre-coated with goat
polyclonal anti-mouse IgG-HRP antibody (sc-2005; Santa Cruz
Biotechnology, Inc.). Aliquots of a PGE2 monoclonal
antibody and PGE2 acetylcholine esterase (AChE)
conjugate were added to each well and allowed to incubate at room
temperature for 18 h. The wells were washed six times with buffer
containing 0.05% Tween-20, followed by the addition of 200 µl
Ellman's reagent containing acetylthiocholine and
5,5′-dithio-bis-(2-nitrobenzoic acid). PGE2
concentrations were determined by measuring the absorbance at a
wavelength of 405 nm using a microplate reader (Benchmark; Bio-Rad
Laboratories, Inc.).
Cytokine assays
The levels of interleukin (IL)-1β and tumor necrosis
factor (TNF)-α were determined using commercial ELISA kits for
IL-1β (cat. no. 559603) and TNF-α (cat. no. 558534) purchased from
BD Biosciences, Inc. (San Jose, CA, USA) according to the
manufacturer's protocols. The concentrations of mediators were
determined by measuring the absorbance at a wavelength of 450 nm
using a microplate reader (Benchmark; Bio-Rad Laboratories,
Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed for the detection of the mRNA
expression of iNOS, COX-2, IL-1β, TNF-α and β-actin. Following 0.5
µg/ml LPS stimulation of RAW264.7 cells for 6 h, total RNA was
isolated using TRIzol™ reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
RT reactions were performed using a kit for producing cDNA
(QuantiTect Reverse Transcription; 205313; Qiagen GmbH, Hilden,
Germany). PCR was carried out using specific forward and reverse
primers and SYBR® FAST Master Mix (KAPA, KR0389; Bioneer
Corporation, Daejeon, Korea) according to the manufacturer's
protocol. The following conditions were used for each PCR reaction:
94°C for 5 min (1 cycle); 94°C for 20 sec, 5°C for 30 sec and 72°C
for 45 sec (30 cycles); and a final extension phase at 72°C for 10
min. Primer sequences iNOS, COX-2, IL-1β, TNF-α and β-actin are
presented in Table I. β-actin
expression served as an internal housekeeping gene control. The
reaction products were separated by electrophoresis on a 1.5%
agarose gel, stained with ethidium bromide and visualized using a
UV Transilluminator imaging system. Gels were imaged using an
C-4000 Zoom camera (Olympus America, Inc., Center Valley, PA,
USA).
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward | Reverse |
|---|
| iNOS |
CAAGAGTTTGACCAGAGGACC |
TGGAACCACTCGTACTTGGGA |
| COX-2 |
GAATGCTTTGGTCTGGTGCCTG |
GTCTGCTGGTTTGGAATAGTTGC |
| IL-1β |
GTGTCTTTCCCGTGGACCTT |
TCGTTGCTTGGTTCTCCTTG |
| TNF-α |
CATCTTCTCAAAATTCGAGTGACAA |
TGGGAGTAGACAAGGTACAACCC |
| β-actin |
TGTTTGAGACCTTCAACACC |
CGCTCATTGCCGATAGTGAT |
Western blot analysis
Following 0.5 µg/ml LPS stimulation for 30 min,
5×105 cells/ml cells were harvested. Cells were washed
twice with ice-cold PBS, scraped off with a rubber policeman and
centrifuged at 1,000 × g for 5 min at 4°C. Cell pellets were
resuspended in an appropriate volume of Protein Extraction Solution
(NP-40; catalog no. EBA-1049; ELPIS-Biotech, Inc., Daejeon, Korea),
and incubated for 10 min at room temperature and subsequently for
20 min on ice. Lysates were subsequently centrifuged at 30,000 × g
for 10 min at 4°C and collected for further analysis. The
supernatant was stored at −87°C until use. Protein concentrations
of samples were determined by Bicinchoninic Acid assay (Thermo
Fisher Scientific, Inc.) using samples equilibrated to 2 mg/ml with
Protein Extraction Solution. A total of 20 µg protein was separated
by 10% SDS-PAGE and subsequently transferred onto a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA). Each
membrane was incubated for 1 h with 5% skimmed milk in TBS with
Tween-20 buffer (0.1 M Tris-HCl, pH 7.4; 0.9% NaCl; 0.1% Tween-20)
to block non-specific binding, followed by 4°C overnight incubation
with the following primary antibodies: Anti-iNOS (ADL-905-431;
1:1,000; Enzo Life Sciences, Farmingdale, NY, USA), anti-COX-2
(sc-1747; 1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), anti-β-actin (4967S; 1:2,000; Cell Signaling Technology,
Inc., Danvers, MA, USA), anti-extracellular signal regulated kinase
(ERK)2 (sc-154), anti-p38 mitogen activated protein kinase (MAPK)
(sc-7149), anti-JNK1/3 (sc-474), anti-nuclear factor (NF)-κB p65
(sc-372), anti-proliferating cell nuclear antigen (PCNA) (sc-56)
(all 1:1,000; Santa Cruz Biotechnology, Inc.), anti-phosphorylated
(p)-p38 MAPK (MA 022; 1:1,000), anti-p-JNK1/2 (both 1:1,000; Enzo
Life Sciences) and anti-p-ERK (4370; 1:1,000; Cell Signaling
Technology, Inc.). Each protein was detected using an Enhanced
Chemiluminescence detection system according to the ImageQuant
LAS4000 (GE Healthcare Life Sciences, Chalfont, UK).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Statistical significance between two groups was
determined using the Student's t-test. Data with values of
P<0.05 were considered to indicate a statistically significant
difference.
Results
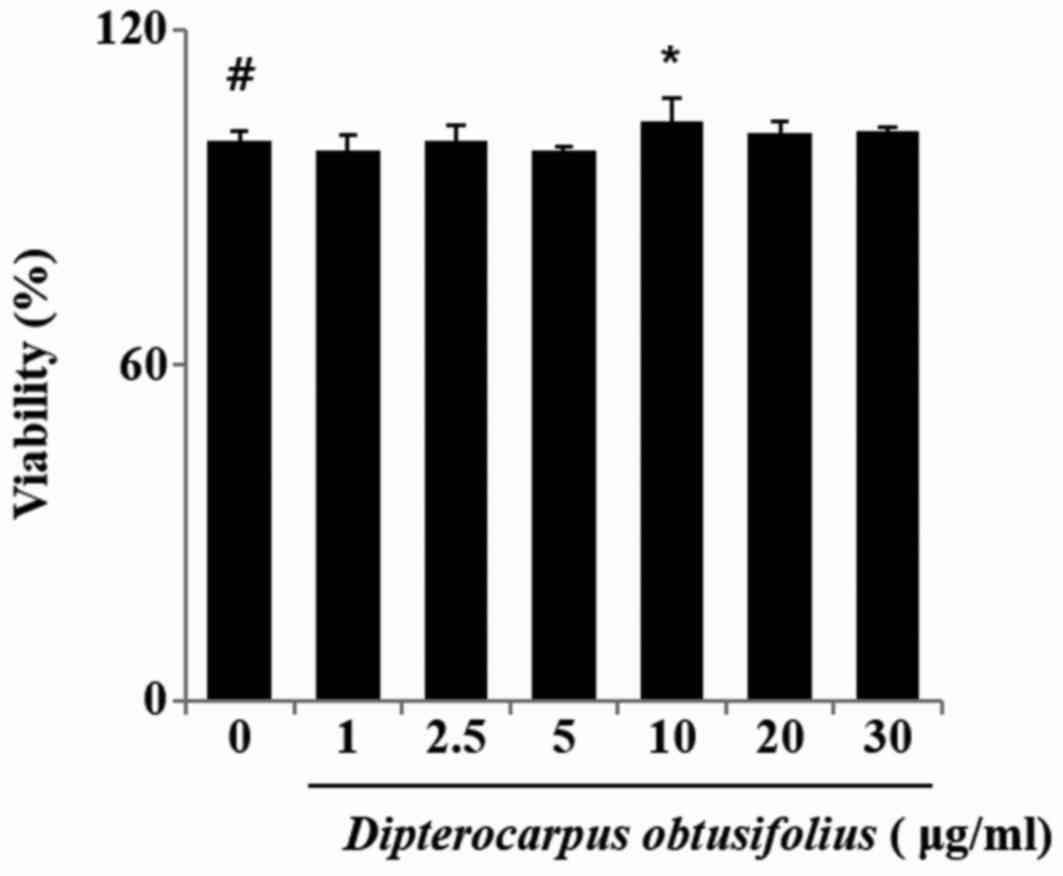
Evaluation of cytotoxicity of DOME on
RAW264.7 cells
To determine if DOME affects cell viability,
RAW264.7 cells were incubated for 24 h with the extract at a wide
range of concentrations (0–30 µg/ml), and cell viability was
evaluated by MTT assay. A statistically significant decrease in
cell survival was detected at concentrations higher than 30 µg/ml
(Fig. 1).
DOME inhibits NO production by
suppressing iNOS expression in LPS-stimulated RAW264.7 macrophage
cells
Nitric oxide serves a central role in the
inflammatory response. Unstimulated RAW264.7 cells secreted basal
levels of NO, whereas LPS stimulation induced an increase in NO
production. DOME treatment was demonstrated to inhibit NO
production in LPS-induced RAW264.7 macrophages in a dose-dependent
manner (Fig. 2A). To further
evaluate the effects of DOME on expression of iNOS, RT-qPCR and
western blot analyses were performed 6 and 18 h following LPS
stimulation. Untreated cells and those pre-incubated with DOME
expressed detectable levels of iNOS mRNA and protein, which were
elevated upon LPS addition (data not shown). Notably, DOME reduced
iNOS mRNA (Fig. 2B) and protein
(Fig. 2C) expression levels,
indicating that DOME inhibits LPS-induced NO production by
inhibiting iNOS.
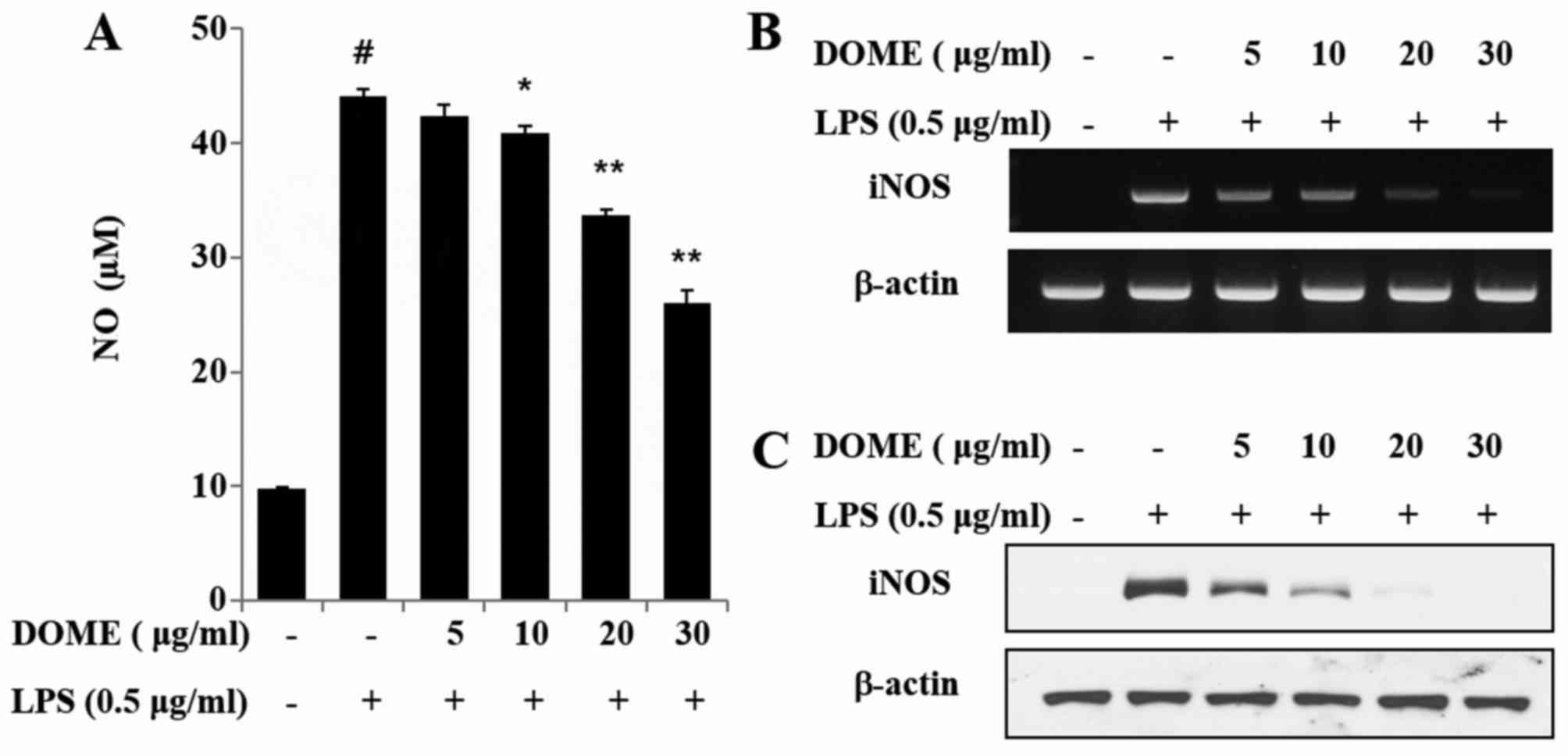 | Figure 2.DOME inhibits LPS-induced NO
production and iNOS expression in RAW264.7 cells. (A) RAW264.7
cells were pretreated with 5, 10, 20 or 30 µg/ml DOME for 1 h and
subsequently stimulated with 0.5 µg/ml LPS. After 24 h, NO
concentrations were measured using the Griess reaction. Data are
presented as the mean ± standard error (n=3). #P<0.05
vs. normal control group, *P<0.05 and **P<0.01 vs. cells
treated with LPS alone. RAW264.7 cells were pretreated with DOME
for 1 h and stimulated with LPS for 6 h. (B) mRNA and (C) protein
expression levels of iNOS, as assessed by reverse
transcription-quantitative polymerase chain reaction (n=3) and
western blot analysis (n=4), respectively. β-actin served as an
internal control. DOME, Dipterocarpus obtusifolius
methanolic extract; LPS, lipopolysaccharide; iNOS, inducible nitric
oxide synthase; NO, nitric oxide. |
DOME inhibits PGE2
production by suppressing COX-2 expression in LPS-stimulated
RAW264.7 cells
To assess whether DOME extract exerted an inhibitory
effect on PGE2 production, RAW264.7 cells were
pretreated with the extract for 1 h and stimulated with LPS.
Incubation of macrophages with LPS alone for 24 h significantly
increased the secretion of the pro-inflammatory mediator
PGE2, as measured by ELISA (P<0.05). However,
pretreatment with DOME in macrophages dose-dependently decreased
the secretion of PGE2 compared with treatment with LPS alone
(Fig. 3A; P<0.05). To further
clarify the effects of DOME on COX-2 mRNA and protein expression
levels, RT-qPCR and western blot analyses were performed 6 and 18 h
after LPS stimulation. Cells treated with DOME alone expressed
detectable levels of COX-2 mRNA and protein, which were further
increased following LPS stimulation (data not shown). DOME markedly
attenuated mRNA (Fig. 3B) and
protein (Fig. 3C) expression
levels of LPS-induced COX-2 in RAW264.7 cells. Therefore, DOME may
inhibit PGE2 production via suppression of COX-2
expression in LPS-stimulated RAW264.7 cells.
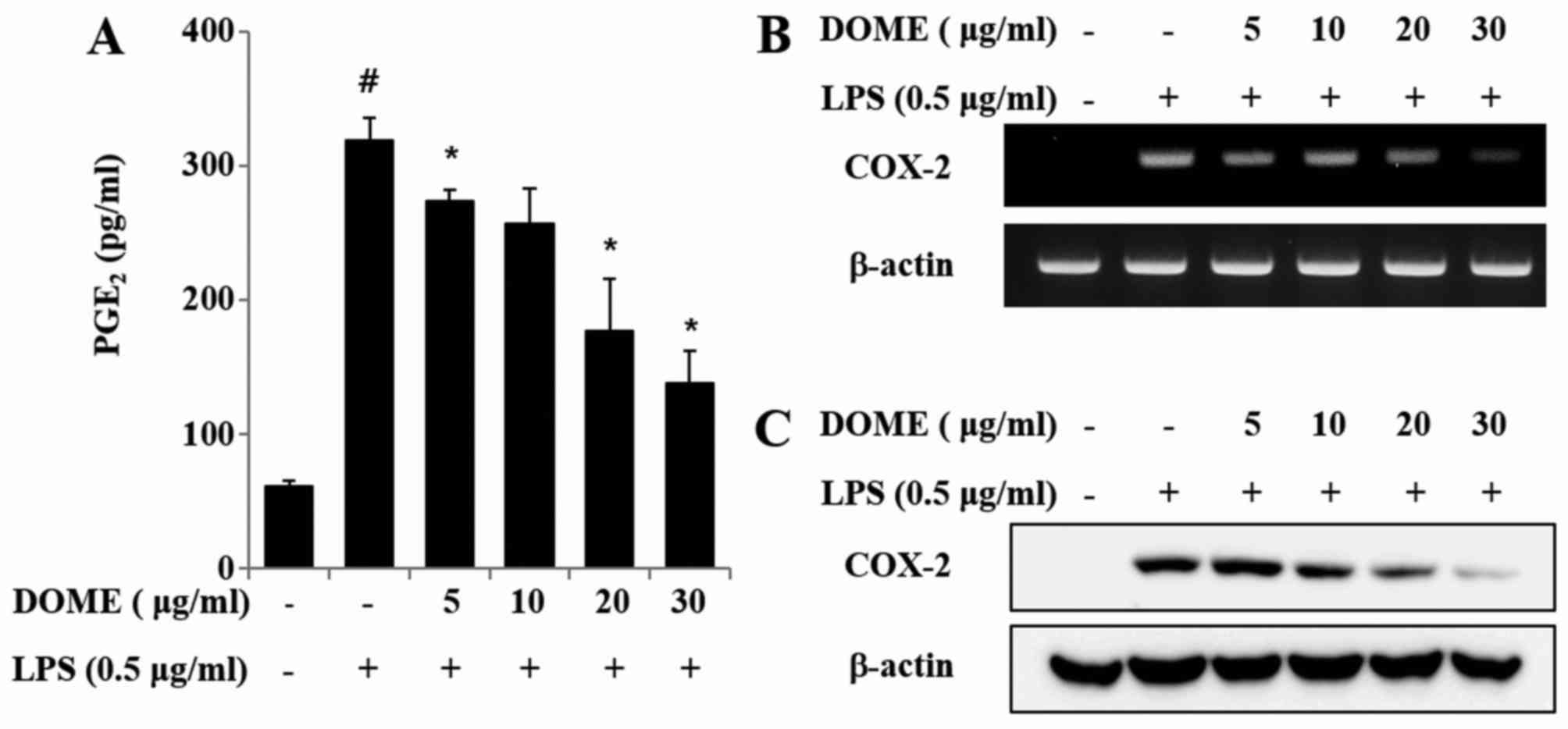 | Figure 3.DOME inhibits LPS-induced
PGE2 production and COX-2 expression in RAW264.7 cells.
(A) RAW264.7 cells were pretreated with 5, 10, 20 or 30 µg/ml DOME
for 1 h and subsequently stimulated with 0.5 µg/ml LPS. After 24 h,
PGE2 concentrations were measured using by ELISA. Data
are presented as the mean ± standard error (n=3).
#P<0.05 vs. normal control group, *P<0.05 vs.
cells treated with LPS alone. RAW264.7 cells were pretreated with
DOME for 1 h and stimulated with LPS for 6 h. (B) mRNA and (C)
protein expression levels of COX-2, as assessed by reverse
transcription-quantitative polymerase chain reaction (n=3) and
western blot analysis (n=4), respectively. β-actin served as an
internal control. DOME, Dipterocarpus obtusifolius
methanolic extract; LPS, lipopolysaccharide; PGE2,
prostaglandin E2; COX-2, cyclooxygenase-2. |
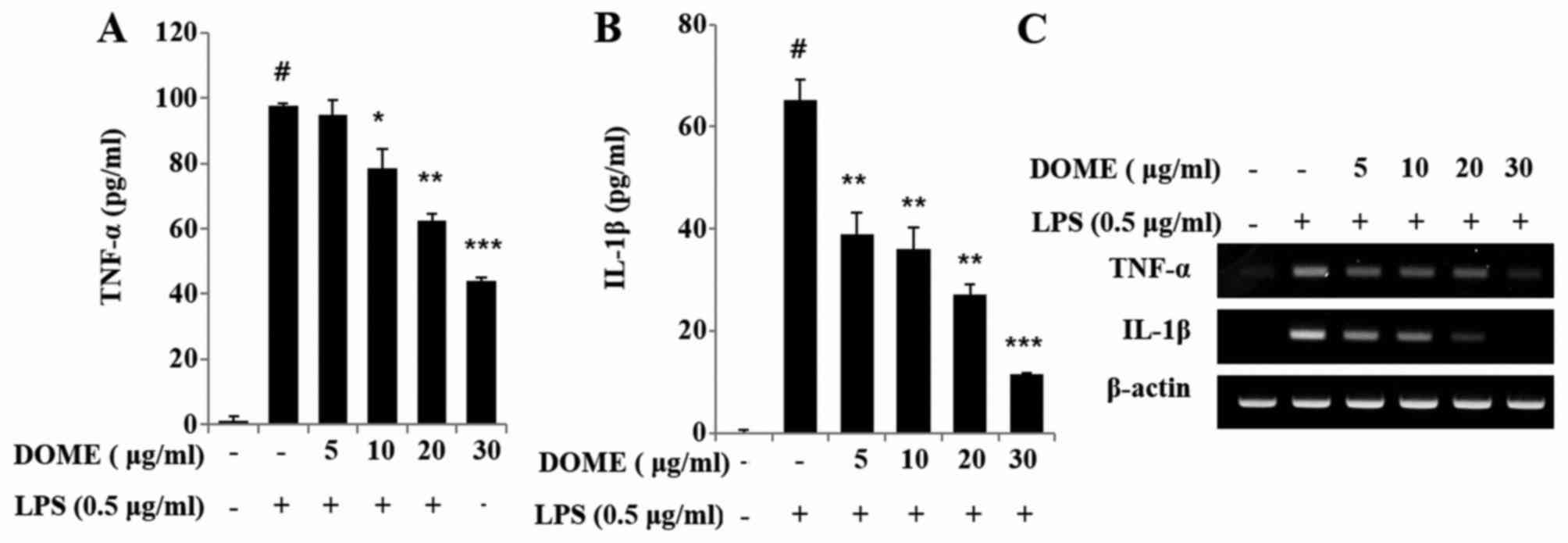
DOME reduces the release of
pro-inflammatory cytokines in LPS-stimulated RAW264.7 cells
Secretion of pro-inflammatory mediators is a
critical factor in inflammatory processes. LPS stimulation induced
an increase in TNF-α (Fig. 4A) and
IL-1β (Fig. 4B) levels in RAW264.7
cells. However, DOME significantly reduced the release of these
LPS-stimulated mediators in a dose-dependent manner. Similarly,
mRNA expression levels of TNF-α and IL-1β were increased in
LPS-stimulated RAW264.7 cells, whereas DOME suppressed this effect
(Fig. 4C).
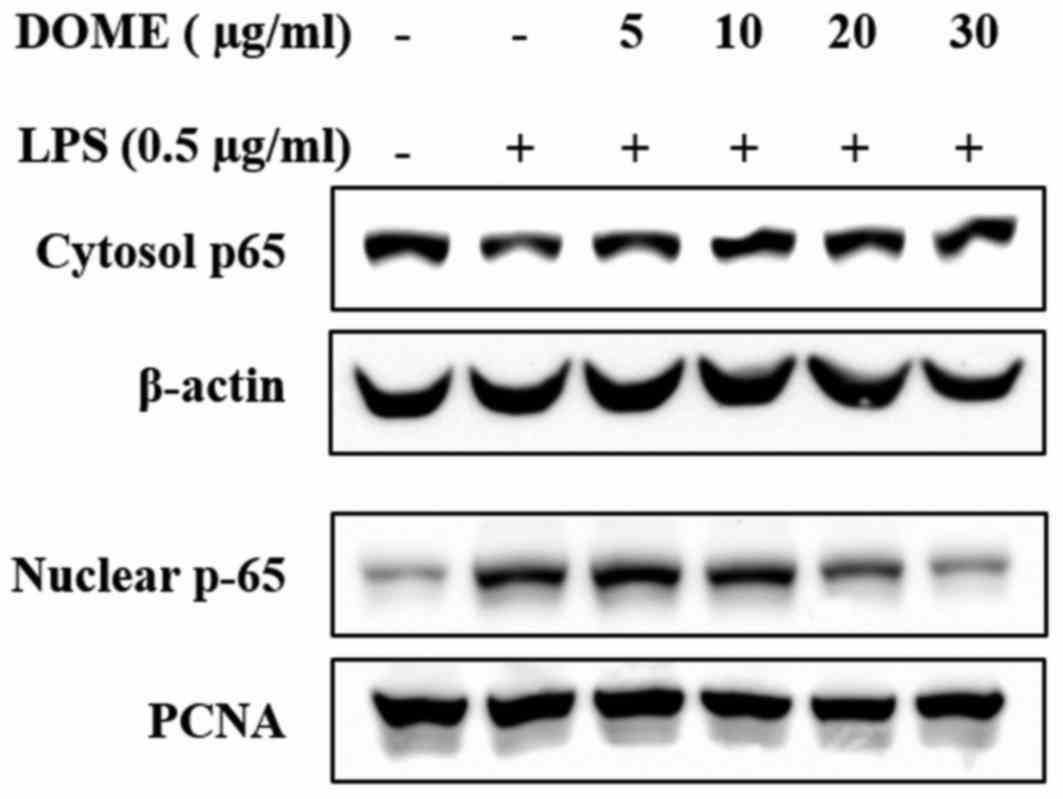
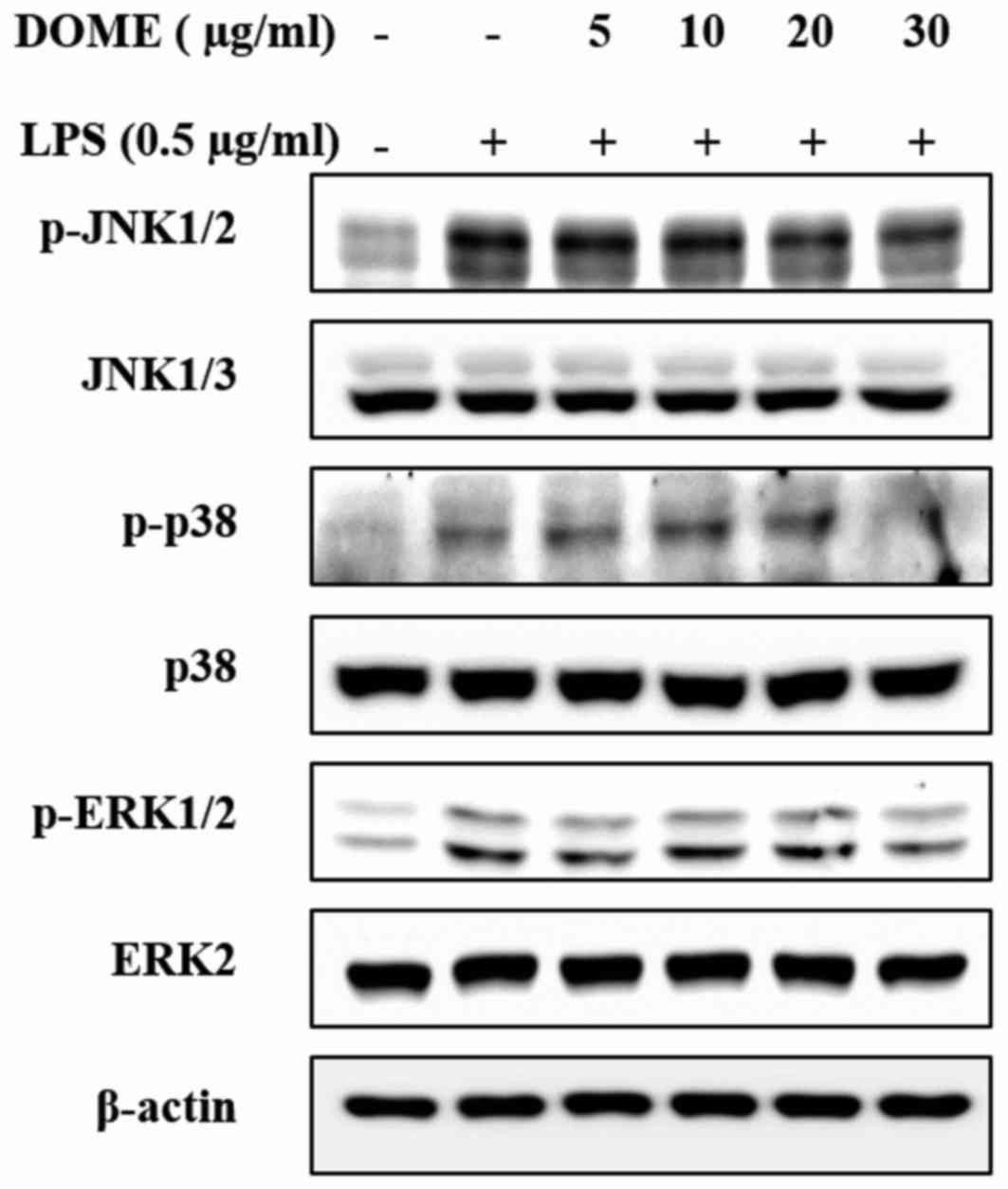
DOME inhibits the phosphorylation of
MAPKs and activation of NF-κB in LPS-stimulated RAW264.7 cells
DOME-treated RAW264.7 cells exhibited a reduction in
the translocation of NF-κB into the nucleus (Fig. 5). The MAPKs may be activated by
LPS, a key stimulator of the inflammation response, in macrophages
as well as many cell types (20).
As presented in Fig. 6, DOME
markedly decreased the expression levels of p-p38, p-JNK and p-ERK
in LPS-induced RAW264.7 cells compared with untreated cells. These
data suggested that DOME suppresses the inflammatory response via
partial regulation of MAPK signaling. To assess the role of MAPKs
in LPS-induced inflammatory mediator production, the effects of
selective MAPK inhibitors on LPS-induced NO production were
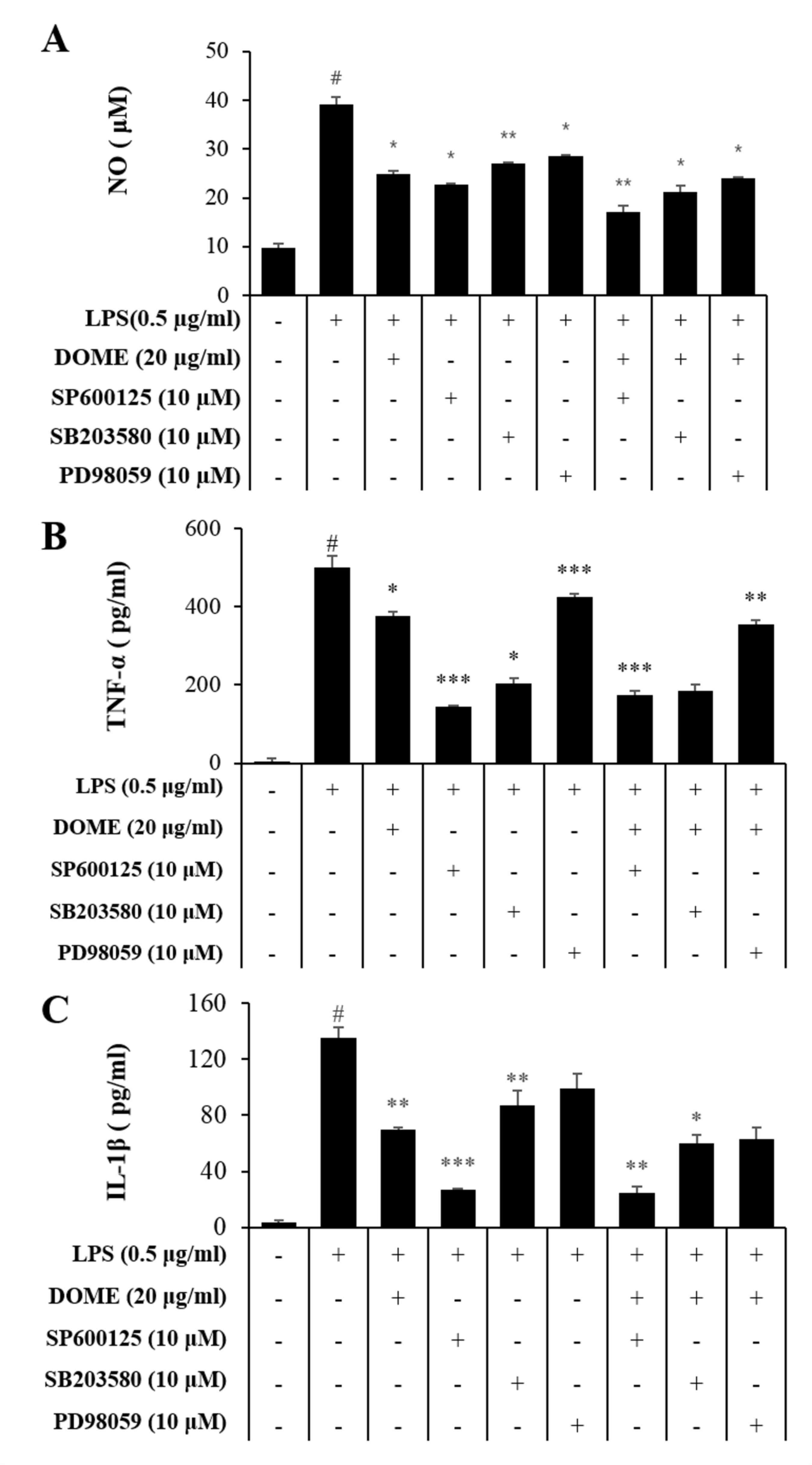
examined. Western blot analysis revealed that SP600125 (Fig. 7A), SB203580 (Fig. 7B) and PD98059 (Fig. 7C), markedly inhibited JNK, p38 and
ERK phosphorylation, respectively. Furthermore, these inhibitors
were demonstrated to markedly inhibited LPS-mediated NO (Fig. 8A), TNF-α (Fig. 8B) and IL-1β (Fig. 8C) production. These results
suggested that DOME-mediated inactivation of ERK, JNK and p38 MAPK
may be, at least in part, responsible for the suppression of NO,
IL-1β and TNF-α in RAW264.7 cells. In addition, LPS stimulation
induced an increase in the activation of NF-κB, whereas treatment
with DOME inhibited this effect. The activation is consistent with
the results of western blotting for NF-κB. Furthermore, LPS
stimulation may cause translocation of NF-κB into nucleus.
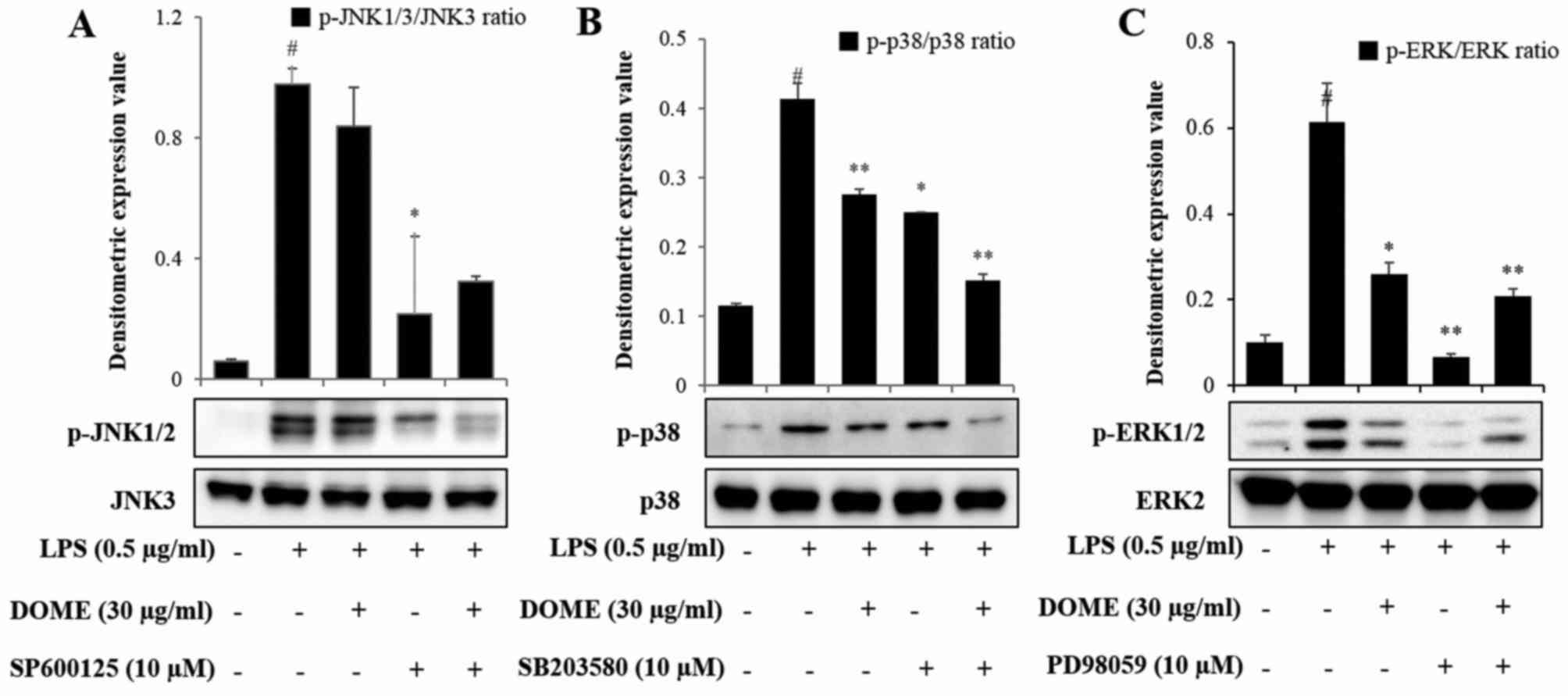 | Figure 7.Effects of mitogen activated protein
kinase inhibitors on JNK, p-38 and ERK phosphorylation. RAW264.7
cells were pretreated with 30 µg/ml DOME, 10 µM SB203580, 10 µM
PD98059 or 10 µM SP600125 for 1 h followed by the incubation with
0.5 µg/ml LPS for 1 h. (A) p-JNK, (B) p-p38 and (C) p-ERK protein
expression levels were measured by western blot analysis. Data are
presented as the mean ± standard error. #P<0.01,
*P<0.05 and **P<0.01 vs. the normal control group. DOME,
Dipterocarpus obtusifolius methanolic extract; LPS,
lipopolysaccharide; p, phosphorylated; JNK, c-Jun N-terminal
kinase; ERK, extracellular signal regulated kinase. |
Discussion
Inflammation is a host response against diverse
injuries. Macrophage cells regulate inflammatory mediators,
including NO, PGE2, IL-1β, and TNF-α (21). A previous study has indicated that
certain inducible enzymes, including iNOS and COX-2, cytokines
(IL-1β and TNF-α), and their response products are involved in
chronic inflammatory sickness (22). iNOS is involved with inflammation,
and its reaction product involved in a wide range of conditions;
for example, psoriasis and atopic inflammation. COX-2, an inducible
isoform of COX, is upregulated in inflammation (23,24).
Therefore, to assess the biological activity of DOME and to
estimate its effects against allergic inflammation, the present
study examined the anti-inflammatory effect of DOME in LPS-induced
RAW264.7 macrophage cells. DOME inhibited the production of NO and
PGE2 in LPS-induced RAW264.7 cells. Therefore, DOME may
mediate iNOS and COX-2 activity. Numerous pro-inflammatory
cytokines, including TNF-α and IL-1β, additionally contribute to
inflammatory reactions; therefore, inhibition of their activity is
a key factor to estimate the effectiveness of anti-inflammatory
medicines. In the present study, DOME considerably suppressed the
production of the pro-inflammatory mediators TNF-α, and IL-1β in
LPS-stimulated RAW264.7 cells. Therefore, DOME may have an
anti-inflammatory function (25,26).
NF-κB regulates the expression of genes encoding
pro-inflammatory inducible enzymes, including COX-2, iNOS and other
inflammatory-associated proteins (8,27,28).
In its inactive form, NF-κB forms a complex with IκB kinase. Upon
stimulation, this composite is broken down and NF-κB is activated,
translocating into the nucleus and controlling transcription of a
large number of inflammatory genes (29). As expression of these
pro-inflammatory mediators is known to be regulated by NF-κB
(30,31), the results of the present study
indicated that transcriptional inhibition of these pro-inflammatory
mediators by DOME are due to blockage of the NF-κB signaling
pathway. In addition, it was demonstrated that translocation of
activated NF-κB p65 to the nucleus is significantly inhibited by
DOME. These data indicated that DOME may inhibit NF-κB activation
by suppressing translocation of the p65 subunit of NF-κB from the
cytosol to the nucleus in LPS-induced RAW264.7 cells. DOME, an
inhibitor of MAPKs, markedly suppressed the nuclear translocation
of NF-κB p65 by LPS in RAW264.7 cells, suggesting that the MAPK
signaling pathway may serve a central role in upregulation of
NF-κB.
MAPKs are a family of serine/threonine protein
kinases responsible for the majority of cellular responses to
external stress signals and cytokines, and are important for
regulation of the production of inflammation mediators (32,33).
The MAPK superfamily have been extensively studied due to their
consistent activation by pro-inflammatory cytokines and to their
involvement in nuclear signaling. This superfamily involves JNKs,
ERKs, and p38 MAPKs (additionally known as cytokine suppressive
binding protein). ERKs are activated by growth factors and
mitogenic stimuli, whereas p38 and JNK are regulated by
stress-inducing signals and pro-inflammatory cytokines (30,34).
Therefore, this signaling pathway may be a potential therapeutic
target for the treatment of inflammatory diseases (35). In the present study, rapid
phosphorylation of JNK, ERK1/2 and p38 MAPK induced by LPS
stimulation in RAW264.7 macrophage cells were suppressed by DOME in
a dose-dependent manner, suggesting that DOME may inhibit the MAPK
signaling cascade. Treatment with DOME, the p38 MAPK inhibitor
SB203580, the JNK inhibitor SP600125 and the ERK inhibitor PD98059
blocked LPS-activated phosphorylation of MAPKs to baseline values.
Furthermore, these inhibitors reduced JNK, ERK and p38
phosphorylation by ~50%.
In conclusion, treatment with DOME synergistically
inhibited inflammatory mediators in LPS-induced RAW264.7 cells,
which may be attributed to down regulation of iNOS and COX-2. These
effects were considered to be associated with suppression of MAPK
signaling pathway molecules, resulting in inhibition of NF-κB
activation and its nuclear translocation. Therefore, these results
implicate DOME as a potential novel therapeutic approach for the
treatment of inflammatory diseases.
Acknowledgements
The present study was supported by the Bio and
Medical Technology Development Program of the National Research
Foundation (NRF) and funded by the Korean government (MSIT) (grant
nos. NRF-2016K1A1A8A01939075 and PRM0191611), and the Korea
Research Institute of Bioscience and Biotechnology Research
Initiative Program of the Republic of Korea (grant no.
KGM1221713).
References
|
1
|
Vanhoutte PM: COX-1 and vascular disease.
Clin Pharmacol Ther. 86:212–215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akatsu T, Santo RM, Nakayasu K and Kanai
A: Oriental herbal medicine induced epithelial keratopathy. Br J
Ophthalmol. 84:9342000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suk K: Regulation of neuroinflammation by
herbal medicine and its implications for neurodegenerative
diseases. A focus on traditional medicines and flavonoids.
Neurosignals. 14:23–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abu-Irmaileh BE and Afifi FU: Herbal
medicine in Jordan with special emphasis on commonly used herbs. J
Ethnopharmacol. 89:193–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmidt M, Christiansen CF, Mehnert F,
Rothman KJ and Sørensen HT: Non-steroidal anti-inflammatory drug
use and risk of atrial fibrillation or flutter: Population based
case-control study. BMJ. 343:d34502011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dijkstra G, Moshage H, van Dullemen HM, de
Jager-Krikken A, Tiebosch AT, Kleibeuker JH, Jansen PL and van Goor
H: Expression of nitric oxide synthases and formation of
nitrotyrosine and reactive oxygen species in inflammatory bowel
disease. J Pathol. 186:416–421. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guslandi M: Nitric oxide and inflammatory
bowel diseases. Eur J Clin Invest. 28:904–907. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kole L, Giri B, Manna SK, Pal B and Ghosh
S: Biochanin-A, an isoflavon, showed anti-proliferative and
anti-inflammatory activities through the inhibition of iNOS
expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB
nuclear translocation. Eur J Pharmacol. 653:8–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miller FR, Guay ME, Bauer T and Tucker HM:
Long-term flap tracheostomy in a pediatric animal model. Arch
Otolaryngol Head Neck Surg. 121:743–748. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cosme R, Lublin D, Takafuji V, Lynch K and
Roche JK: Prostanoids in human colonic mucosa: Effects of
inflammation on PGE(2) receptor expression. Hum Immunol.
61:684–696. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schneider A, Zhang Y, Zhang M, Lu WJ, Rao
R, Fan X, Redha R, Davis L, Breyer RM, Harris R, et al:
Membrane-associated PGE synthase-1 (mPGES-1) is coexpressed with
both COX-1 and COX-2 in the kidney. Kidney Int. 65:1205–1213. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Katagiri H, Ito Y, Ishii K, Hayashi I,
Suematsu M, Yamashina S, Murata T, Narumiya S, Kakita A and Majima
M: Role of thromboxane derived from COX-1 and −2 in hepatic
microcirculatory dysfunction during endotoxemia in mice.
Hepatology. 39:139–150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peskar BM: Role of cyclooxygenase isoforms
in gastric mucosal defence. J Physiol Paris. 95:3–9. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bertinaria M, Shaikh MA, Buccellati C,
Cena C, Rolando B, Lazzarato L, Fruttero R, Gasco A, Hoxha M, Capra
V, et al: Designing multitarget anti-inflammatory agents: Chemical
modulation of the lumiracoxib structure toward dual thromboxane
antagonists-COX-2 inhibitors. Chem Med Chem. 7:1647–1660. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mashita Y, Taniguchi M, Yokota A, Tanaka A
and Takeuchi K: Oral but not parenteral aspirin upregulates COX-2
expression in rat stomachs. A relationship between COX-2 expression
and PG deficiency. Digestion. 73:124–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JH, Park YM, Shin JS, Park SJ, Choi
JH, Jung HJ, Park HJ and Lee KT: Fraxinellone inhibits
lipopolysaccharide-induced inducible nitric oxide synthase and
cyclooxygenase-2 expression by negatively regulating nuclear
factor-kappa B in RAW 264.7 macrophages cells. Biol Pharm Bull.
32:1062–1068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muhtadi, Hakim EH, Juliawaty LD, Syah YM,
Achmad SA, Latip J and Ghisalberti EL: Cytotoxic resveratrol
oligomers from the tree bark of Dipterocarpus hasseltii.
Fitoterapia. 77:550–555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ukiya M, Kikuchi T, Tokuda H, Tabata K,
Kimura Y, Arai T, Ezaki Y, Oseto O, Suzuki T and Akihisa T:
Antitumor-promoting effects and cytotoxic activities of dammar
resin triterpenoids and their derivatives. Chem Biodivers.
7:1871–1884. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khiev P, Kwon OK, Song HH, Oh SR, Ahn KS,
Lee HK and Chin YW: Cytotoxic terpenes from the stems of
Dipterocarpus obtusifolius collected in Cambodia. Chem Pharm Bull
(Tokyo). 60:955–961. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park JW, Lee IC, Shin NR, Jeon CM, Kwon
OK, Ko JW, Kim JC, Oh SR, Shin IS and Ahn KS: Copper oxide
nanoparticles aggravate airway inflammation and mucus production in
asthmatic mice via MAPK signaling. Nanotoxicology. 10:445–452.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Munhoz CD, García-Bueno B, Madrigal JL,
Lepsch LB, Scavone C and Leza JC: Stress-induced neuroinflammation:
Mechanisms and new pharmacological targets. Braz J Med Biol Res.
41:1037–1046. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park JW, Kwon OK, Yuniato P, Marwoto B,
Lee J, Oh SR, Kim JH and Ahn KS: Amelioration of an LPS-induced
inflammatory response using a methanolic extract of Lagerstroemia
ovalifolia to suppress the activation of NF-κB in RAW264.7
macrophages. Int J Mol Med. 38:482–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bruch-Gerharz D, Fehsel K, Suschek C,
Michel G, Ruzicka T and Kolb-Bachofen V: A proinflammatory activity
of interleukin 8 in human skin: Expression of the inducible nitric
oxide synthase in psoriatic lesions and cultured keratinocytes. J
Exp Med. 184:2007–2012. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park JW, Kwon OK, Jang HY, Jeong H, Oh SR,
Lee HK, Han SB and Ahn KS: A leaf methanolic extract of Wercklea
insignis attenuates the lipopolysaccharide-induced inflammatory
response by blocking the NF-κB signaling pathway in RAW 264.7
macrophages. Inflammation. 35:321–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang S, Shen XY, Huang HQ, Xu SW, Yu Y,
Zhou CH, Chen SR, Le K, Wang YH and Liu PQ: Cryptotanshinone
suppressed inflammatory cytokines secretion in RAW264.7 macrophages
through inhibition of the NF-κB and MAPK signaling pathways.
Inflammation. 34:111–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kwon OK, Ahn KS, Park JW, Jang HY, Joung
H, Lee HK and Oh SR: Ethanol extract of Elaeocarpus petiolatus
inhibits lipopolysaccharide-induced inflammation in macrophage
cells. Inflammation. 35:535–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH,
Kim DS, Park JS and Cho HJ: Spinal NF-κB activation induces COX-2
upregulation and contributes to inflammatory pain hypersensitivity.
Eur J Neurosci. 19:3375–3381. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cascinu S, Scartozzi M, Carbonari G,
Pierantoni C, Verdecchia L, Mariani C, Squadroni M, Antognoli S,
Silva RR, Giampieri R and Berardi R: COX-2 and NF-KB overexpression
is common in pancreatic cancer but does not predict for COX-2
inhibitors activity in combination with gemcitabine and
oxaliplatin. Am J Clin Oncol. 30:526–530. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan LL, Liu XH, Gong QH, Wu D and Zhu YZ:
Hydrogen sulfide attenuated tumor necrosis factor-α-induced
inflammatory signaling and dysfunction in vascular endothelial
cells. PLoS One. 6:e197662011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shin IS, Shin NR, Park JW, Jeon CM, Hong
JM, Kwon OK, Kim JS, Lee IC, Kim JC, Oh SR and Ahn KS: Melatonin
attenuates neutrophil inflammation and mucus secretion in cigarette
smoke-induced chronic obstructive pulmonary diseases via the
suppression of Erk-Sp1 signaling. J Pineal Res. 58:50–60. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee SU, Ahn KS, Sung MH, Park JW, Ryu HW,
Lee HJ, Hong ST and Oh SR: Indacaterol inhibits tumor cell
invasiveness and MMP-9 expression by suppressing IKK/NF-kB
activation. Mol Cells. 37:585–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shin IS, Park JW, Shin NR, Jeon CM, Kwon
OK, Kim JS, Kim JC, Oh SR and Ahn KS: Melatonin reduces airway
inflammation in ovalbumin-induced asthma. Immunobiology.
219:901–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park JW, Kwon OK, Kim JH, Oh SR, Kim JH,
Paik JH, Marwoto B, Widjhati R, Juniarti F, Irawan D and Ahn KS:
Rhododendron album Blume inhibits iNOS and COX-2 expression in
LPS-stimulated RAW264.7 cells through the downregulation of NF-κB
signaling. Int J Mol Med. 35:987–994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shin IS, Park JW, Shin NR, Jeon CM, Kwon
OK, Lee MY, Kim HS, Kim JC, Oh SR and Ahn KS: Melatonin inhibits
MUC5AC production via suppression of MAPK signaling in human airway
epithelial cells. J Pineal Res. 56:398–407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao W, Bao C, Padalko E and Lowenstein CJ:
Acetylation of mitogen-activated protein kinase phosphatase-1
inhibits Toll-like receptor signaling. J Exp Med. 205:1491–1503.
2008. View Article : Google Scholar : PubMed/NCBI
|