Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune
disease characterized by chronic inflammation of the synovium and
hyperplasia of synovial fibroblasts, eroding adjacent cartilage and
bone, thus causing subsequent joint destruction (1). The normal synovium forms a thin
membrane of one to three cell layers at the edges of joints and
provides lubrication and nutrients for cartilage. In RA, however,
the synovial lining thickens and transforms into an inflammatory
tissue known as the pannus (2).
The major cells in the thickened lining and resultant pannus are
activated RA fibroblast-like synoviocytes (RASFs). Notably, these
cells exhibit tumor cell-like features, including increased
proliferation, prolonged survival, resistance to apoptosis and
invasiveness of adjacent tissues. Thus, RASFs are described as
‘tumor-like transformed’ cells (3,4).
Degradation of articular cartilage is one of the
early features of RA and is mediated by increased activity of
proteolytic systems (2). In
particular, RASFs exhibit increased production of the
matrix-degrading enzyme family of matrix metalloproteinases (MMPs)
(3,5–7).
MMPs can digest substrates, including fibrillar collagen I and II
(8,9) and aggrecan, which predominantly
exists in cartilage (10). MMPs
are inactive precursors that must be processed by proprotein
convertases, which cleave at single basic or paired basic residues
of the proproteins to produce biologically active proteins
(11). Proprotein convertase
subtilisin/kexin type 6 (PCSK6) is a proprotein convertase that
functions in proteolysis of various precursor proteins and
regulation of protein maturation. Our previous study demonstrated
that knockdown of PCSK6 can influence both expression and activity
of MMP-2 and MMP-9 (12). These
results revealed that PCSK6 contributes to hyperplasia of synovial
lining cells, but the pathways involved remained unknown. Our
previous study also revealed that the expression of PCSK6 is
significantly higher in RASFs than in those of osteoarthritis, and
silencing PCSK6 could inhibit the proliferation, migration and
invasion of RASFs (12). Besides
functioning in cells, PCSK6 can be secreted outside cells. However,
the role and mechanism of secreted PCSK6 on RASFs have not been
investigated. The aim of the present study was to investigate the
involvement and functional role of PCSK6 in hyperplasia of RASFs,
and the potential cell signaling pathways involved in this
process.
Materials and methods
Synovial fibroblast isolation and
treatment
Synovial tissues were collected during knee joint
replacement surgeries from 7 patients with RA (5 females, 2 males;
mean age=68.1 years). All patients met the American College of
Rheumatology Classification Criteria (13). Participants provided written
informed consent to participate in the study and to allow genetic
analysis of biological samples. The Ethical Committee of Shandong
Academy of Medicinal Sciences (Jinan, China) approved this study.
The experiment was in compliance with the Helsinki Declaration.
RASFs were isolated from synovial tissues after
collagenase digestion as previously described (12). The purity of the RASFs was
determined by flow cytometry analysis using a FACSAria equipped
with FACSDiva 2.0 (BD Biosciences, Franklin Lakes, NJ USA). Fourth
generation RASFs were collected by centrifugation at 800 × g for 1
min at 4°C and stained with anti-cluster of differentiation CD68
[fluorescein isothiocyanate (FITC)-conjugated; 1:20 dilution;
11-0689-41, eBioscience; Thermo Fisher Scientific, Inc., Waltham,
MA, USA] and anti-fibroblast marker ER-TR7 [Phycoerythrin
(PE)-conjugated; 1:50 dilution; sc-73355, Santa Cruz Biotechnology,
Inc. Dallas, TX, USA] primary antibodies on ice for 45 min.
Synovial fibroblasts were cultured in Dulbecco's
modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.)
supplemented with 10% heat-inactivated fetal bovine serum (FBS).
Cells from passages three to eight were used in all experiments.
Before treatment, cells were pre-incubated in DMEM without FBS
(basal medium) for 4 h and transferred to fresh basal medium. Cells
were stimulated with recombinant human PCSK6 (rhPCSK6) from wheat
germ (cat. no. H00005046-Q01; Abonova, Taipei, Taiwan) at a
concentration of 150 ng/ml for 24 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed as described previously
(13). Relative mRNA levels were
measured using the cycle threshold (2−ΔΔCq) method
(14). Three independent
experiments were completed and each reaction was performed in
triplicate.
Western blotting
Western blotting was performed using whole cell
lysates as previously described (12), using the following antibodies:
Anti-PACE4, (1:2,000; cat. no. ab39877; Abcam, Cambridge, MA, USA),
anti-human β-actin (1:1,000; cat. no. 4970s; Cell Signaling
Technology, Inc., Danvers, MA, USA) anti-MMP9 (1:1,000; cat. no.
ab137867; Abcam), anti-extracellular signal-regulated kinase
(ERK)1/2 (1:2,000; cat. no. 8727s, Cell Signaling Technology,
Inc.), anti-phosphorylated (p)-ERK1/2 (1:2,000; cat. no. 4370s,
Cell Signaling Technology, Inc.), anti-phosphorylated-signal
transducers and activators of transcription 3 (STAT3) (1:1,000;
cat. no. 9131s; Cell Signaling Technology, Inc.); anti-STAT3
(1:1,000; cat. no. 4904p; Cell Signaling Technology, Inc.),
anti-p-nuclear factor (NF)-κB p65 subunit (1:1,000; cat. no. 3033s;
Cell Signaling Technology, Inc.) and anti-NF-κB p65 subunit
(1:1,000; cat. no. 8242s; Cell Signaling Technology, Inc.).
Antibodies were incubated with membranes at 4°C overnight. After
three washes, membranes were incubated with an anti-rabbit
horseradish peroxidase-conjugated secondary antibody (1:1,000;
A0208; Beyotime Institute of Biotechnology, Beijing, China).
Immunoreactivity was detected using an enhanced chemiluminescence
detection system (GE Healthcare, Chicago, IL, USA). β-actin served
as an internal loading control.
MTT assay
RASFs (2×104 cells/well) were seeded into
96-well culture plates and cultured at 37°C to 80% confluence.
Cultures were treated with 150 ng/ml rhPCSK6. After incubation for
24, 48 or 72 h, 20 µl 5 mg/ml MTT (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) in PBS were added to each
well, and cultures were incubated for 4 h at 37°C. MTT solution was
removed and 150 µl dimethyl sulfoxide was added to extract
MTT-formazan products at room temperature for 10 min. Absorbance
was measured in triplicate at 490 nm using a spectrophotometer.
Cell cycle analysis
Cells were plated in 6-well culture plates at
1.0×106 cells/well and treated with rhPCSK6 as described
above. After removal of culture medium, cells were harvested at 24
h by trypsinization, washed twice with ice-cold PBS and fixed
overnight with cold 70% ethanol. Prior to analysis, fixed cells
were rinsed, resuspended in PBS, and stained for 30 min at 37°C
with 1 ml 0.05 mg/ml propidium iodide solution (Beijing Dingguo
Changsheng Biotechnology Co., Ltd., Beijing, China) containing 10
µg/ml RNase. Cells were detected with a Coulter Epics XL flow
cytometer (Beckman Coulter, Inc., Brea, CA, USA) and DNA content
was analyzed with Beckman Coulter EXPO32 software (Beckman Coulter,
Inc.).
Apoptosis assay
For apoptosis assays, RASFs were serum-deprived in
the presence or absence of rhPCSK6 (150 ng/ml) for 24 h and
detected using an Annexin V-FITC apoptosis detection kit (BD
Biosciences, San Jose, CA, USA) according to manufacturer's
protocol. Cells were detected with a Coulter Epics XL flow
cytometer (Beckman Coulter, Inc.) and DNA content analyzed with
Beckman Coulter EXPO32 software.
Invasion/migration assays
RASF invasion was measured using the Cyto Select
24-well cell invasion assay kit (basement membrane, colorimetric
format; Cell Biolabs, Inc., San Diego, CA, USA) according to
manufacturer's protocol. Invasive cells on the bottom of the
membrane were stained and analyzed using light microscopy to count
total cells in five random fields of each membrane at ×20
magnification. The results are expressed as invasive cells per
field.
Cell migration was detected using a scratch
migration assay as previously described (12). To eliminate the effect of cell
proliferation, RASFs were treated with 5 mg/ml mitomycin C (cat.
no. M0503; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 4 h.
Cell monolayers were scratched before stimulation and then
incubated with 150 ng/ml rhPCSK6 for 24 h. Cells that migrated to
wound areas were counted under a microscope at 20× magnification
and expressed as a percentage of controls.
Measuring interleukin (IL)-1α, IL-1β,
IL-6, IL-17 and tumor necrosis factor (TNF)-α levels with an
enzyme-linked immunosorbent assay (ELISA)
RASFs were treated with 150 ng/ml rhPCSK6 for 24 h,
and the conditioned culture medium was collected and centrifuged at
1,200 × g for 5 min at 4°C. The supernatant (100 µl) was added to a
96-well microplate, and incubated overnight at 4°C. Following
gentle washing with PBS + 1% Tween-20, blocking was performed using
1% bovine serum albumin + 5% sucrose for 1 h at 37°C. Following
three PBS washes, antibodies diluted 1:1,000 against IL-1α (cat.
no. ab92396), IL-1β (cat. no. ab2105), IL-17 (cat. no. ab79056),
TNF-α (cat. no. ab667 or IL-6 (cat. no. ab6672) (all from Abcam)
were applied to the plate for overnight incubation at 4°C. The
following day, the plate was washed and blocked again as
aforementioned, and incubated with anti-rabbit IgG horseradish
peroxidase antibody (1:2,000; cat. no. A0208, Beyotime Institute of
Biotechnology) for 3 h at 37°C. Staining was developed using a TMB
kit (cat. no. PR1200, Beijing Solarbio Science & Technology
Co., Ltd.). The absorbance at 450 nm was measured using a plate
reader. Cell medium plus transfection reagent served as a
control.
Pathway inhibitor analysis
To verify the induced pathways, specific inhibitors
of the ERK1/2, STAT3 and NF-κB signaling pathways were used.
Briefly, 1 µmol/l NF-κB activation inhibitor pyrrolidine
dithiocarbamate (PDTC; cat. no. ab141406; Abcam), the STAT
inhibitor stattic (cat. no. ab120952 Abcam), or the ERK-1/2
inhibitor PD98059 (cat. no. ab120234, Abcam) was added to cells in
the presence of 150 ng/ml rhPCSK6 for 2 h. RASF cells were then
analyzed for changes in proliferation, migration, invasion,
secretion of inflammatory cytokines, cell cycle and apoptosis.
Statistical analysis
All results were confirmed in at least three
independent experiments. One-way analysis of variance was used for
multiple comparison between different groups, followed by a
Bonferroni post hoc test. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
rPCSK6 stimulates RASF cell
proliferation, migration and invasion in vitro
As PCSK6 has been reported to be significantly
associated with RA, and as it is a secretory protein, the present
study stimulated RASFs with rhPCSK6 to assess its effects on the
biological activity of RASFs. MTT, wound healing and Transwell
assays were performed. To determine the effective concentration, 1,
10, 50, 100 and 150 ng/ml rPCSK6 was used. The MTT results
demonstrated that 50, 100 and 150 ng/ml rhPCSK6 could induce the
proliferation (only data of 150 ng/ml is presented, P<0.05 vs.
control; Fig. 1A) of RASFs without
cell death. As 150 ng/ml of rhPCSK6 has the most marked effect, it
was used for subsequent experiments. In addition, the wound healing
and Transwell results demonstrated that rhPCSK6 could promote the
migration and invasion of RASFs (P<0.05; Fig. 1B and C, respectively).
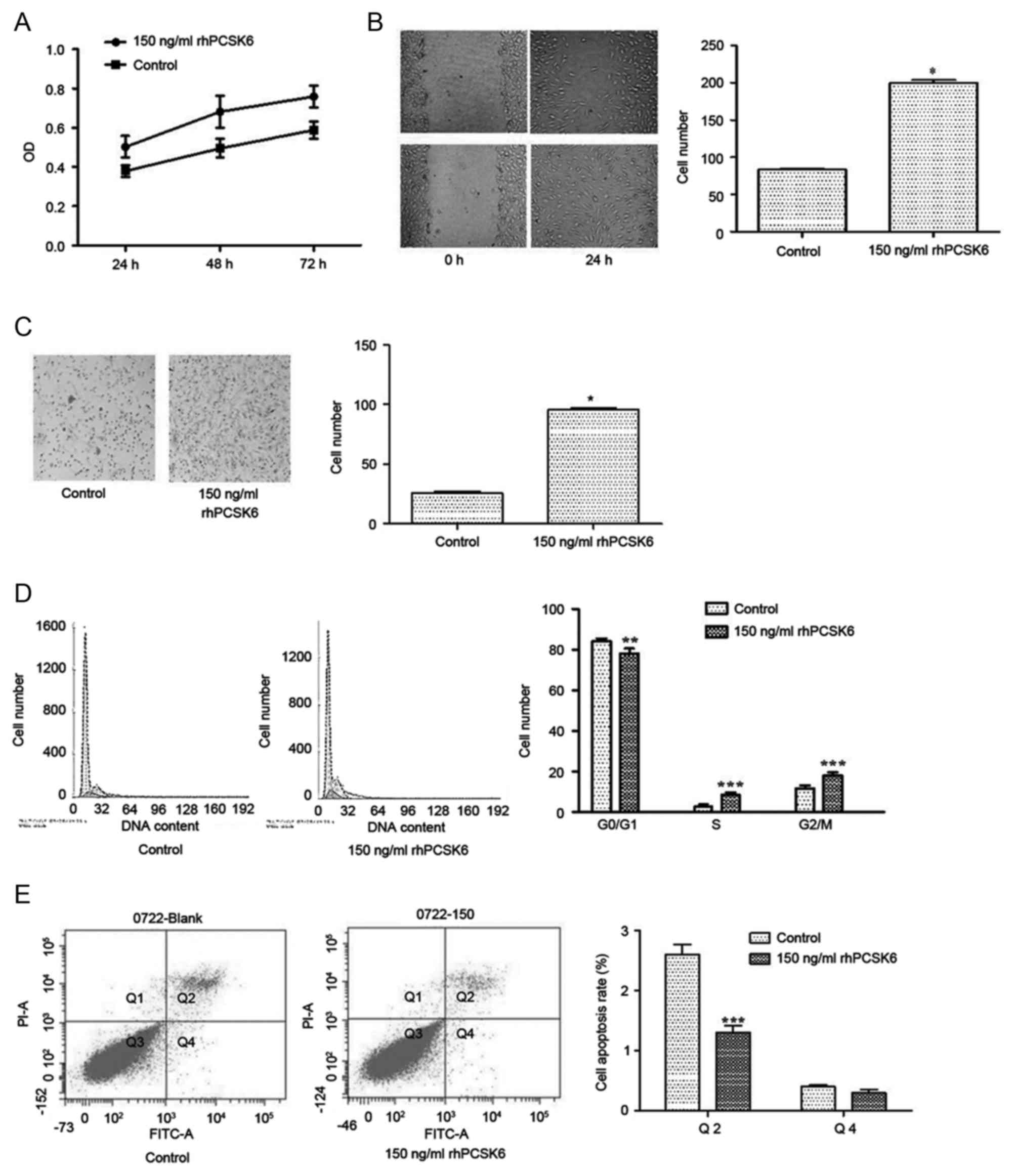 | Figure 1.Effect of rhPCSK6 stimulation on RASF
proliferation, migration, invasion, cell cycle and apoptosis. (A)
RASFs were stimulated with rPCSK6 for 24, 48 or 72 h and cell
proliferation was measured with MTT assays. (B) Non-proliferating
RASF cultures were scratched and cell migration to the scratched
area was measured with wound healing assays. (C) The mean number of
RASFs that migrated through Transwell filters, a measure of
invasive ability. (D) Effects of rhPCSK6 on RASF cell cycle
distribution were measured with flow cytometry on propidium
iodide-stained cells. (E) Annexin V-FITC staining measured
apoptotic RASF cells after treatment with rhPCSK6. Data are
expressed as the mean ± standard error of three independent
experiments conducted in triplicate. *P<0.05, **P<0.01 and
***P<0.001 vs. Control. rhPCSK6, recombinant human proprotein
convertase subtilisin/kexin type 6; RASFs, rheumatoid arthritis
fibroblast-like synoviocytes; PI, propidium iodide; FITC,
fluorescein isothiocyanate; OD, optical density. |
To further analyze the effects of rhPCSK6 on the
cell proliferation, flow cytometry was used and the results
revealed that the ratio of G0/G1 phase cells
was significantly lower in RASFs stimulated with rhPCSK6 after 24 h
(Fig. 1D), indicating that PCSK6
could disturb the cell cycle in RASFs. However, proliferation
changes could also result from reduced cell apoptosis; therefore,
whether PCSK6 could protect from apoptosis was investigated by
adding exogenous rhPCSK6 to the cultures of starved RASFs. Annexin
V-FITC and propidium iodide staining revealed that exogenous
rhPCSK6 significantly decreased the number of apoptotic cells
induced by serum deprivation (Fig.
1E).
rhPCSK6 promotes the production of
pro-inflammatory factors in RASFs
Pro-inflammatory cytokines serve prominent roles in
RA. As reported, RASFs can secrete IL-1α, IL-1β, IL-6, IL-17 and
TNF-α, and are closely associated with synovitis and joint
destruction (15). Levels of these
three pro-inflammatory cytokines were detected in the supernatants
of RASFs following stimulation with 150 ng/ml rhPCSK6 or its
vehicle controls by ELISA. Significantly, rhPCSK6 stimulation
increased the secretion of IL-1α, IL-1β and IL-6 (P<0.05,
Fig. 2A) from RASF cells, but not
the secretion of IL-17 and TNF-α (data not shown).
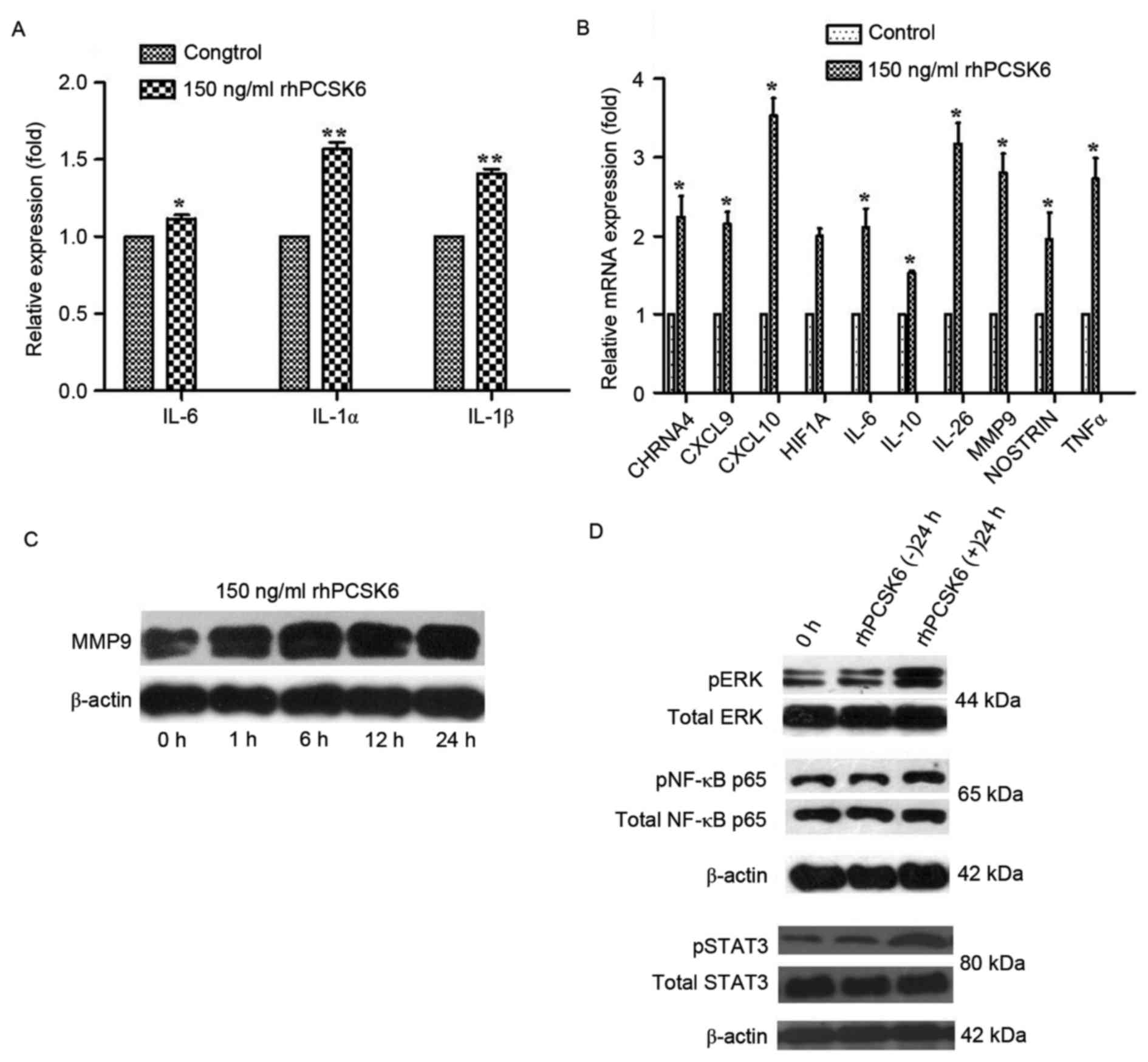 | Figure 2.Effect of rhPCSK6 stimulation on the
cytokine secretion, gene expression, and pathway activation of
RASFs. (A) ELISA was applied to detect the secretion of various
cytokines (IL-1α, IL-1β and IL-6) in the supernatants of RASFs. (B)
Reverse transcription-quantitative polymerase chain reaction
measurements of RASF cell expression of genes associated with
rheumatoid arthritis. Western blot analysis of (C) MMP-9 and (D)
ERK1/21/2, STAT3 and NF-κB protein expression in RASF cell lysates.
Data are expressed as the mean ± standard error of three
independent experiments conducted in triplicate. *P<0.05,
**P<0.01 vs. Control. rhPCSK6, recombinant human proprotein
convertase subtilisin/kexin type 6; RASFs, rheumatoid arthritis
fibroblast-like synoviocytes; IL, interleukin; HIF1A,
hypoxia-inducible factor 1A; CXCL, C-X-C chemokine motif; MMP-9,
matrix metalloproteinase 9; NOSTRIN, nitric oxide synthase traffic
inducer; CHRNA4, neuronal acetylcholine receptor subunit alpha-4;
ERK, extracellular signal-regulated kinase; NF-κB, nuclear
factor-κB; TNF-α, tumor necrosis factor-α. |
To gain further insight into the role of PCSK6 in RA
pathology, expression of genes associated with cell proliferation,
angiogenesis, hypoxia and invasion were assayed using qPCR. It was
demonstrated the expression of various genes involved in
angiogenesis (MMP-9 and nitric oxide synthase traffic
inducer) hypoxia (hypoxia inducible factor-IA),
proliferation (CHRNA4), and inflammation [C-X-C chemokine
motif (CXCL9), CXCL10, TNF-α, IL-6,
IL-10 and IL-26] were all upregulated after rhPCSK6
stimulation (Fig. 2B).
RASFs express MMP-2 and MMP-9. MMP-2 is
constitutively expressed by most cell types, including synovial
fibroblasts, while basal levels of MMP-9 are undetectable in most
cell types except for malignant or inflammatory cells, such as
RASFs (7). The present study only
tested the effect of rhPCSK6 on MMP-9, because MMP-2 expression in
RASFs is so high that no change can be detected after rhPCSK6
stimulation. Western blot analysis demonstrated that stimulation
with rhPCSK6 increased the protein expression levels of MMP-9
(Fig. 2C).
rhPCSK6 induces activation of the
ERK1/2, STAT3 and NF-κB signaling pathways
To investigate the pathway by which PCSK6 directly
or indirectly regulates cell proliferation, migration, invasion,
and secretion of proinflammatory cytokines, the present study
examined ERK1/2, STAT3 and NF-κB activity in RASFs. Phosphorylation
of ERK1/2 catalytically activates the protein, and ERK1/2 isoforms
serve a major role in cell proliferation signaling (16). Western blot analysis demonstrated
that rhPCSK6 increases levels of phosphorylated forms of ERK1/2 in
RASFs (Fig. 2D).
In vivo and in vitro studies support
the role of STAT3 in contributing to the chronicity of inflammatory
arthritis (17). Therefore, the
present study investigated STAT3 activation by PCSK6 and
demonstrated that treatment of RASFs with rhPCSK6 increased levels
of p-STAT3 (Fig. 2D). In addition,
NF-κB is a critical activator of inflammatory processes (18). rhPCSK6 also induced the
phosphorylated forms of NF-κB in RASFs (Fig. 2D).
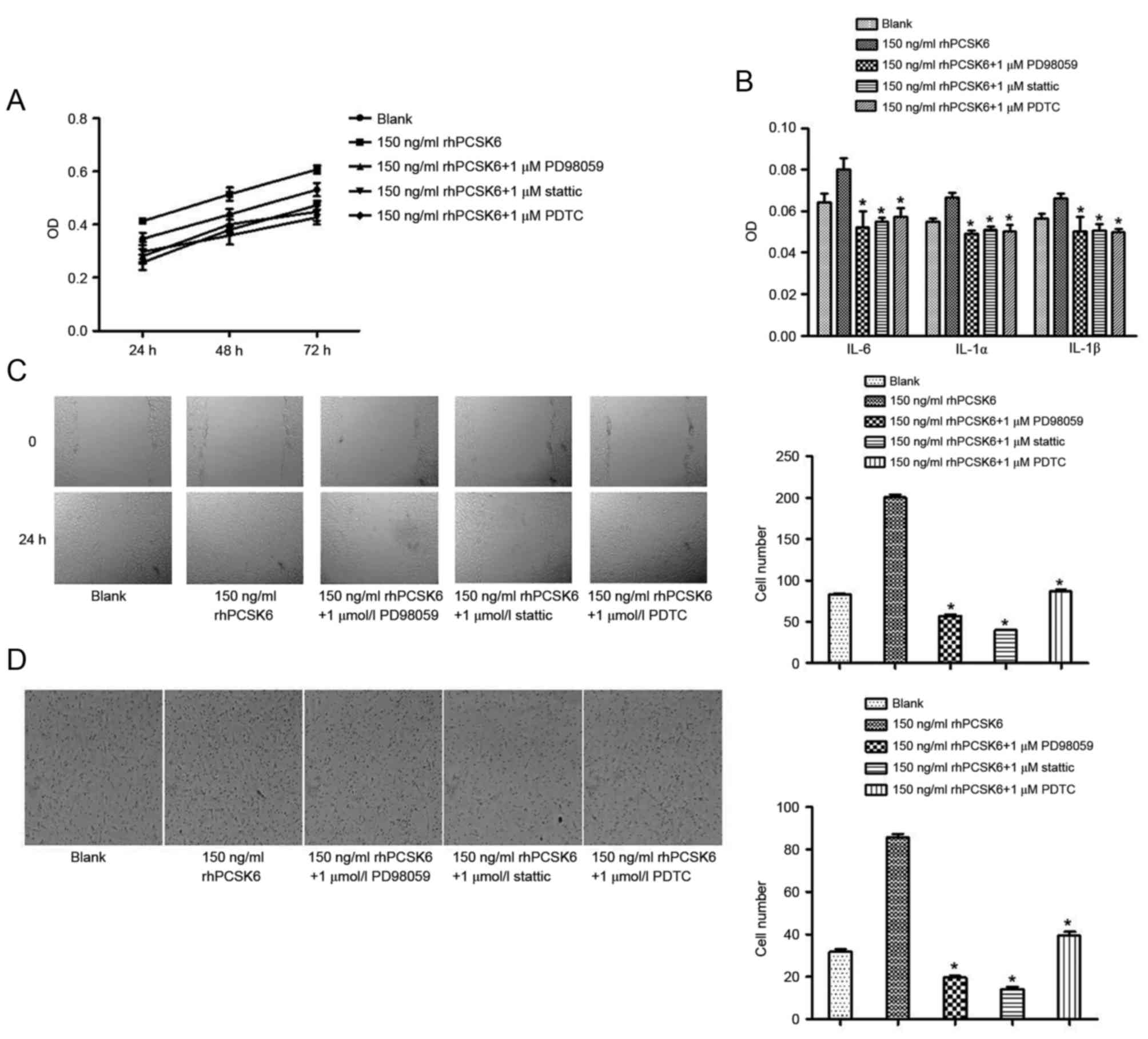
To confirm the signaling pathways responsible for
PCSK6 stimulation of RASFs, specific pathway inhibitors were used,
including the NF-κB activation inhibitor PDTC, the ERK1/2 inhibitor
PD98059 and the STAT3 inhibitor stattic. These chemical inhibitors
exhibit no cytotoxicity at the concentrations used in these
experiments (19).
rhPCSK6-stimulated proliferation, migration, invasion and secretion
of cytokines were markedly decreased in the presence of PDTC,
PD98059 and stattic. Therefore, rhPCSK6 can stimulate RASF
bioactivity via the NF-κB, ERK1/2 and STAT3 signaling pathways
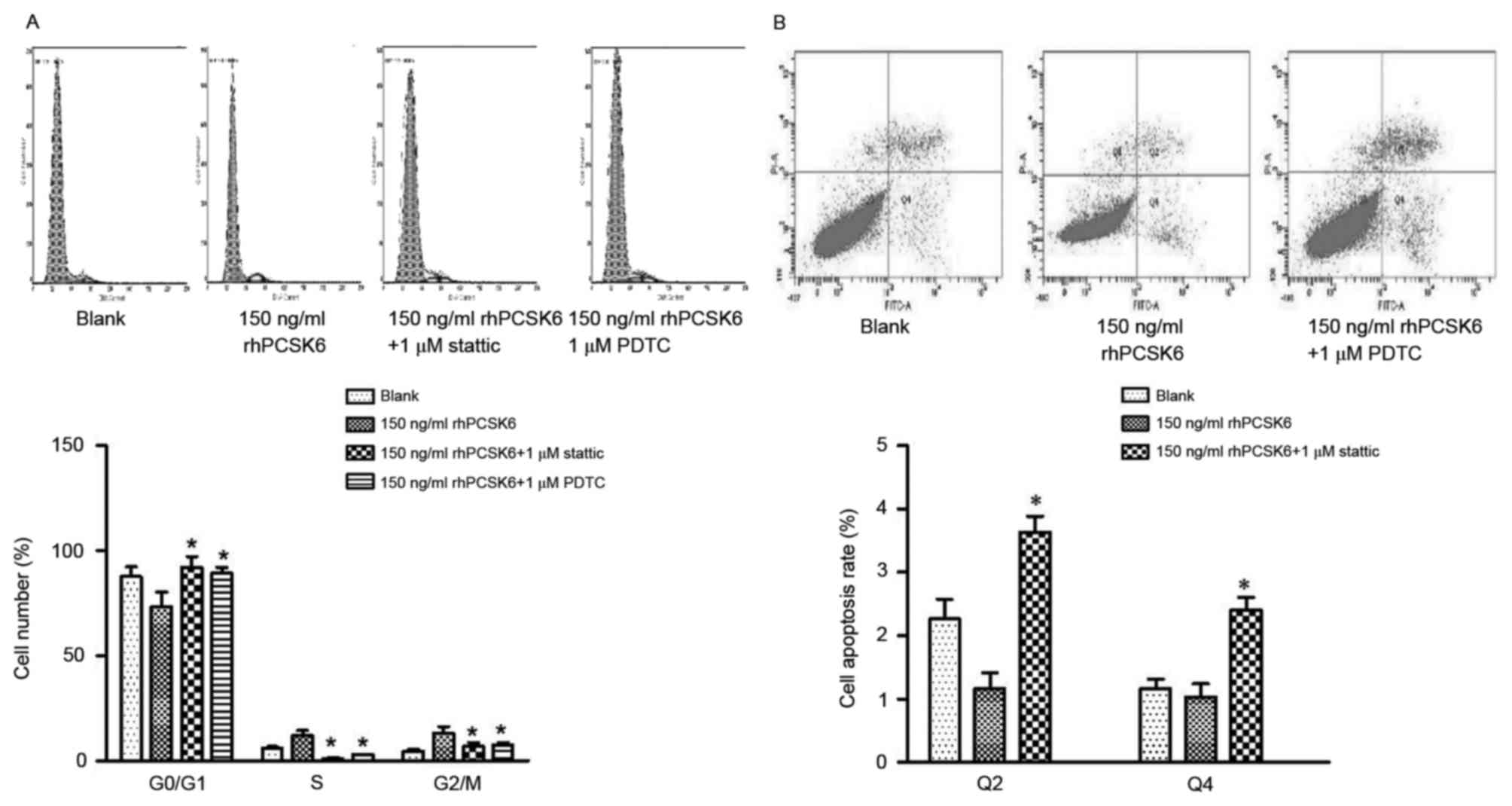
(Fig. 3). Furthermore, the effect
of rhPCSK6 on cell cycle and apoptosis was assessed. The result
revealed that PDTC and stattic can recover
G0/G1 arrest of RASFs, respectively,
indicating that PCSK6 can affect the cell cycle of RASFs via NF-κB
and STAT3 activation. In RASF apoptosis assays, PDTC was
demonstrated to recover reduced cell apoptosis induced by rhPCSK6,
which highlights the role of NF-κB activation in PCSK6 stimulation
on RASFs (Fig. 4).
Discussion
PCSK6 is a neuroendocrine-specific mammalian
subtilisin-related endoprotease (20) that exhibits a C-terminal
cysteine-rich region (21) and
functions in the secretory pathway. Our previous study demonstrated
RASFs, but not osteoarthritis synovial fibroblasts, produce high
levels of PCSK6 (12). As a
secretory protein, the present study aimed to investigate the role
of exogenous PCSK6 on RASF. rhPCSK6 was used in this study. The
host is wheat germ, which eliminated the issue of
lipopolysaccharide contamination. The present study demonstrated
that PCSK6 may serve important roles in cell proliferation,
migration, invasion and angiogenesis, because exogenous PCSK6
enhanced RASF survival, inflammation and invasion capacity
(22). These findings suggested
that PCSK6 may be an important therapeutic target in RA.
Furthermore, RA is characterized as a chronic
inflammatory disease, in which pro-inflammatory cytokines including
IL-1α, IL-1β and IL-6 contribute to synovitis and joint
destruction. Our previous results demonstrated that silencing PCSK6
in RASFs could regulate the production of these parameters
(12); the present study also
revealed that rhPCSK6 treatment exerts the same effects on RASFs,
suggesting that PCSK6 exacerbates inflammation by regulating
expression or secretion of cytokines.
RASFs are a dominant cell type in RA synovium and
mediate persistent inflammation as well as cartilage and bone
destruction (23). In the present
study, MMP-9 expression in RASFs increased after stimulation with
rhPCSK6, consistent with a previous report that PCSK6 could process
immature MMP-9 to its mature form (11). Xue et al (24) reported that endogenous MMP-9
contributes to prolonged survival and invasive and inflammatory
properties of RASFs. While MMP-9 is able to degrade the
extracellular matrix, it is also implicated in the development of
cartilage and bone erosion in RA (25). A previous study by Perisic et
al (26) demonstrated that
PCSK6 mRNA expression levels are positively correlated with
typical markers of inflammation and apoptosis, including IL-1β and
TNF-α, and proteins involved in matrix degradation, such as MMP-9,
in vascular disease. These findings confirm the results of the
present study and further validate the pro-metastasis and
angiogenesis functions of PCSK6 in RA.
In RA, increased RASF proliferation and/or decreased
RASF apoptosis contributes to synovial hyperplasia. Hyperplasia of
synovial fibroblasts contributes to RA pathogenesis and is capable
of eroding adjacent cartilage and bone, causing subsequent joint
destruction. In this study, rhPCSK6 stimulation of RASFs
significantly promoted cell proliferation, migration and invasion,
suggesting that PCSK6 may serve an important role in hyperplasia
and in the erosion capacity of synovial fibroblasts. Further
analysis revealed that PCSK6 can reduce G0/G1
cell cycle arrest and protect RASFs from apoptosis, demonstrating
that rhPCSK6 promotes RASF cell proliferation. In our previous
study (12), increased expression
of the cyclin-dependent kinase inhibitor p27KIP was
observed in PCSK6-silenced RASFs, consistent with a previous
finding in prostate cancer (27).
A lack of mitogenic activity is associated with higher cell
quiescence states characterized by the increased expression of
p27KIP (28). In
addition, previous studies have demonstrated that mitogen-activated
protein kinase (MAPK) p42 and p44 (ERK1/21/2) are required for
fibroblast proliferation to pass the G1 restriction
point and enter S-phase (29).
Overall, the results of the present study indicated that PCSK6
promotes the proliferation of RASFs by disturbing cell cycle arrest
via targeting p27KIP or the activity of the MAPK
signaling pathway. This study also demonstrated that
rhPCSK6-mediated activation of RASFs is likely via the NF-κB, STAT3
and ERK1/2 signaling pathways, as rhPCSK6 treatment induced the
phosphorylation levels of these pathways. Furthermore, specific
inhibitors of these pathways significantly prevented RASF
proliferation by influencing both cell cycle arrest and apoptosis.
Consistently, previous data (12)
has demonstrated that the ERK1/2 isoforms serve a major role in
cell proliferation signaling, while STAT3 contributes to the
chronicity of inflammatory arthritis and NF-κB is a critical
activator of inflammatory processes. In the present study,
rhPCSK6-stimulated proliferation, invasion, migration and secretion
of proinflammatory cytokines were inhibited in the presence of
NF-κB, STAT3 and ERK1/2 inhibitors, confirming a role of these
pathways in mediating the effects of PCSK6 on RASF cells.
In conclusion, the present study revealed that after
secretion into the joint, PCSK6 could exacerbate the progression of
RA through its effects on RASFs. Furthermore, the NF-κB, STAT3 and
ERK1/2 signaling pathways may mediate the pro-inflammatory role of
PCSK6 in RA. Therefore, PCSK6 may be a potential therapeutic target
for RA.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (NSFC) (81102275), the Natural Science
Foundation of Shandong Province (ZR2015LY029), the Key Research
Project of Shandong Province (2017GSF218082) and the Innovation
Project of Shandong Medical Academy.
References
|
1
|
Sokka T: Work disability in early
rheumatoid arthritis. Clin Exp Rheumatol. 21(5 Suppl 31): S71–S74.
2003.PubMed/NCBI
|
|
2
|
Noss EH and Brenner MB: The role and
therapeutic implications of fibroblast-like synoviocytes in
inflammation and cartilage erosion in rheumatoid arthritis. Immunol
Rev. 223:252–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tchetverikov I, Lard LR, De Groot J,
Verzijl N, TeKoppele JM, Breedveld FC, Huizinga TW and Hanemaaijer
R: Matrix metalloproteinases-3, −8, −9 as markers of disease
activity and joint damage progression in early rheumatoid
arthritis. Ann Rheum Dis. 62:1094–1099. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tolboom TC, Van der Helm-van Mil AH,
Nelissen RG, Breedveld FC, Toes RE and Huizinga TW: Invasiveness of
fibroblast-like synoviocytes is an individual patient
characteristic associated with the rate of joint destruction in
patients with rheumatoid arthritis. Arthritis Rheum. 52:1999–2002.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cunnane G, Fitzgerald O, Hummel KM,
Youssef PP, Gay RE, Gay S and Bresnihan B: Synovialtissue protease
gene expression and joint erosions in early rheumatoid arthritis.
Arthritis Rheum. 44:1744–1753. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Konttinen YT, Ainola M, Valleala H, Ma J,
Ida H, Mandelin J, Kinne RW, Santavirta S, Sorsa T, López-Otín C
and Takagi M: Analysis of 16 different matrix metalloproteinases
(MMP-1 to MMP-20) in the synovial membrane: Different profiles in
trauma and rheumatoid arthritis. Ann Rheum Dis. 58:691–697. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xue M, March L, Sambrook PN and Jackson
CJ: Differential regulation of matrix metalloproteinase 2 and
matrix metalloproteinase 9 by activated protein C: Relevance to
inflammation in rheumatoid arthritis. Arthritis Rheum.
56:2864–2874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van den Steen PE, Grillet B and Opdenakker
G: Gelatinase B participates in collagen II degradation and
releases glycosylated remnant epitopes in rheumatoid arthritis. Adv
Exp Med Biol. 564:45–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Charni-Ben Tabassi N, Desmarais S,
Bay-Jensen AC, Delaissé JM, Percival MD and Garnero P: The type II
collagen fragments Helix-II and CTX-II reveal different enzymatic
pathways of human cartilage collagen degradation. Osteoarthritis
Cartilage. 16:1183–1191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mahloogi H, Bassi DE and Klein-Szanto AJ:
Malignant conversion of non-tumorigenic murine skin keratinocytes
overexpressing PACE4. Carcinogenesis. 23:565–572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang F, Wang L, Jiang H, Chang X and Pan
J: Inhibition of PCSK6 may play a protective role in the
development of rheumatoid arthritis. J Rheumatol. 42:161–169. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hochberg MC, Chang RW, Dwosh I, Lindsey S,
Pincus T and Wolfe F: The American college of rheumatology 1991
revised criteria for the classification of global functional status
in rheumatoid arthritis. Arthritis Rheum. 35:498–502. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: Key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Posada J and Cooper JA: Requirements for
phosphorylation of MAP kinase during meiosis in Xenopus oocytes.
Science. 255:212–215. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Walker JG and Smith MD: The Jak-STAT
pathway in rheumatoid arthritis. J Rheumatol. 32:1650–1653.
2005.PubMed/NCBI
|
|
18
|
Roman-Blas JA and Jimenez SA: NF-κB as a
potential therapeutic target in osteoarthritis and rheumatoid
arthritis. Osteoarthritis Cartilage. 14:839–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Q, Wu J, Cao Q, Xiao L, Wang L, He
D, Ouyang G, Lin J, Shen B, Shi Y, et al: A critical role of Cyr61
in interleukin-17-dependent proliferation of fibroblast-like
synoviocytes in rheumatoid arthritis. Arthritis Rheum.
60:3602–3612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mains RE, Berard CA, Denault JB, Zhou A,
Johnson RC and Leduc R: PACE4: A subtilisin-like endoprotease with
unique properties. Biochem J. 321:587–593. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seidah NG, Chrétien M and Day R: The
family of subtilisin/kexin like pro-protein and pro-hormone
convertases: Divergent or shared functions. Biochimie. 76:197–209.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsuji A, Hashimoto E, Ikoma T, Taniguchi
T, Mori K, Nagahama M and Matsuda Y: Inactivation of proprotein
convertase, PACE4, by alpha1-antitrypsin Portland (alpha1-PDX), a
blocker of proteolytic activation of bone morphogenetic protein
during embryogenesis: Evidence that PACE4 is able to form an
SDS-stable acyl intermediate with alpha1-PDX. J Biochem.
126:591–603. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huber LC, Distler O, Tarner I, Gay RE, Gay
S and Pap T: Synovial fibroblasts: Key players in rheumatoid
arthritis. Rheumatology (Oxford). 45:669–675. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xue M, McKelvey K, Shen K, Minhas N, March
L, Park SY and Jackson CJ: Endogenous MMP-9 and not MMP-2 promotes
rheumatoid synovial fibroblast survival, inflammation and cartilage
degradation. Rheumatology (Oxford). 53:2270–2279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brenner M and Gulko PS: The arthritis
severity locus Cia5a regulates the expression of inflammatory
mediators including Syk pathway genes and proteases in
pristane-induced arthritis. BMC Genomics. 13:7102012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perisic L, Hedin E, Razuvaev A, Lengquist
M, Osterholm C, Folkersen L, Gillgren P, Paulsson-Berne G, Ponten
F, Odeberg J and Hedin U: Profiling of atherosclerotic lesions by
gene and tissue microarrays reveals PCSK6 as a novel protease in
unstable carotid atherosclerosis. Arterioscler Thromb Vasc Biol.
33:2432–2443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
D'Anjou F, Routhier S, Perreault JP, Latil
A, Bonnel D, Fournier I, Salzet M and Day R: Molecular validation
of PACE4 as a target in prostate cancer. Transl Oncol. 4:157–172.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chu IM, Hengst L and Slingerland JM: The
Cdk inhibitor p27 in human cancer: Prognostic potential and
relevance to anticancer therapy. Nat Rev Cancer. 8:253–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pagès G, Lenormand P, L'Allemain G,
Chambard JC, Meloche S and Pouysségur J: Mitogen-activated protein
kinases p42mapk and p44mapk are required for fibroblast
proliferation. Proc Natl Acad Sci USA. 90:pp. 8319–8323. 1993;
View Article : Google Scholar : PubMed/NCBI
|