Introduction
Bone metabolism is strictly coordinated by two
functional cells, osteoclasts and osteoblasts (1). The former cells are responsible for
bone resorption and the latter cells are for bone formation
(1). Bone tissue in the skeleton
is continuously regenerated and renewed to maintain the quality and
quantity through the bone remodeling process (2). The remodeling process begins with
osteoclastic bone resorption, followed by osteoblastic bone
formation (3). The imbalance of
bone remodeling causes metabolic bone diseases such as osteoporosis
and the increased risk of age-related bone fracture.
Currently, it is well recognized that osteoblasts
play a crucial role in regulating bone resorption via the
expression of receptor activator of nuclear factor-κB (RANK) ligand
(RANKL), which responds to a variety of bone resorptive agents
(1–3). Osteoprotegerin (OPG), which is
synthesized in osteoblasts and secreted, belongs to the tumor
necrosis factor receptor family as well as RANK on osteoclasts
(4). OPG binds to RANKL as a decoy
receptor, and prevents RANKL from binding to RANK, resulting in the
suppression of bone resorption via inhibiting osteoclastogenesis
(4). OPG-knock out mice reportedly
suffer from severe osteoporosis (5). Therefore, it is currently recognized
that the RANK/RANKL/OPG axis plays a central regulatory system in
osteoclast functions (6). It has
been shown that bone morphogenetic protein (BMP)-2 stimulates OPG
production in human osteoblastic cell line (7). BMPs, multifunctional cytokines,
belong to the transforming growth factor-β (TGF-β) superfamily
(8). Regarding the intracellular
signaling of BMPs, it is firmly established that the effects of
BMPs are exerted mainly through the Smad-dependent pathway
(8). In addition, accumulating
evidence indicates that the Smad-independent pathway mediates
numerous effects of BMP (9). We
have recently shown that BMP-4 stimulates the synthesis of OPG at
least in part through the activation of p70 S6 kinase in
osteoblast-like MC3T3-E1 cells (10). However, the exact mechanism behind
the BMP-induced OPG synthesis in osteoblasts has not yet been
clarified.
Heat shock proteins (HSPs) are induced in response
to various environmental stress such as heat (11). HSPs play an essential role as
molecular chaperones in protein folding and the prevention of
aggregation. Among them, HSP90 (also known as HSPC) is abundantly
expressed in a variety type of unstressed cells and represents 1–2%
of total cellular proteins, which increases to 4–6% under the
stress conditions (12). Since
client proteins of HSP90 are involved in a variety of oncogenic
signaling pathways, HSP90 inhibition has emerged as one of the
strategies for anticancer chemotherapeutics, and HSP90 inhibitors
including 17-allylamino-17demethoxy-geldanamycin (17-AAG),
17-dimethylamino-ethylamino-17-demethoxy-geldanamycin (17-DMAG) and
geldanamycin, are developed (13–18).
With regard to HSP90 inhibitor-effects on bone metabolism, 17-AAG
reportedly amplifies osteoclast formation and potentiates
osteolytic bone metastasis in bone metastasis of breast cancer
cells (19). In addition, it has
been shown that geldanamycin induces autophagy and apoptosis of
osteosarcoma cells (20). However,
the exact roles of HSP90 in osteoblast functions remains to be
elucidated.
In the present study, we investigated whether HSP90
is involved in the BMP-4-induced OPG synthesis in osteoblast-like
MC3T3-E1 cells using HSP90 inhibitors. We herein demonstrate that
HSP90 inhibitors suppress the BMP-4-stimulated OPG synthesis
through downregulating p70 S6 kinase in osteoblasts.
Materials and methods
Materials
17-AAG and 17-DMAG were purchased from
Calbiochem-Novabiochem Co. (La Jolla, CA, USA). Geldanamycin was
obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). BMP-4 and
mouse OPG enzyme-linked immunosorbent assay (ELISA) kits were
purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Phospho-specific Smad1/5 antibodies, phospho-specific p70 S6 kinase
antibodies and p70 S6 kinase antibodies were obtained from Cell
Signaling Technology, Inc. (Beverly, MA, USA).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). An ECL Western blotting detection system was obtained from GE
Healthcare Life Sciences (Chalfont, UK). Other materials and
chemicals were obtained from commercial sources. 17-AAG, 17-DMAG
and geldanamycin were dissolved in dimethyl sulfoxide. The maximum
concentration of dimethyl sulfoxide was 0.1%, which did not affect
the assay for OPG, real-time RT-PCR or western blot analysis.
Cell culture
Cloned osteoblast-like MC3T3-E1 cells that have been
derived from newborn mouse calvaria (21) were maintained as previously
described (22). Briefly, the
cells were cultured in α-minimum essential medium (α-MEM)
containing 10% fetal bovine serum (FBS) at 37°C in a humidified
atmosphere of 5% CO2/95% air. The cells were seeded into
35-mm diameter dishes (5×104 cells/dish) or 90-mm
diameter dishes (2×105 cells/dish) in α-MEM containing
10% FBS. After 5 days, the medium was exchanged for α-MEM
containing 0.3% FBS. The cells were used for experiments after 48
h.
Measurement of OPG
The cultured cells were stimulated by 30 ng/ml of
BMP-4 or vehicle in 1 ml of α-MEM containing 0.3% FBS for 48 h.
When indicated, the cells were pretreated with various doses of
17-AAG, 17-DMAG or geldanamycin for 60 min. The conditioned medium
was collected at the end of incubation, and the OPG concentration
was then measured using the OPG ELISA kit according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The cultured cells were pretreated with 0.3 µM of
geldanamycin or vehicle for 60 min, and then stimulated by 30 ng/ml
of BMP-4 or vehicle in 1 ml of α-MEM containing 0.3% FBS for 6 h.
Total RNA was isolated and reverse transcribed into complementary
DNA using TRIzol Reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Heysham, Lancashire, UK) and Omniscript Reverse Transcriptase
kit (Qiagen Inc., Valencia, CA, USA), respectively. RT-qPCR was
performed in capillaries using a Light Cycler system with the Light
Cycler Fast Start DNA Master SYBR-Green I (Roche Diagnostics,
Basel, Switzerland). Sense and antisense primers for mouse OPG mRNA
or GAPDH mRNA were purchased from Takara Bio, Inc. (Tokyo, Japan;
primer set ID, MA026526). The amplified products were determined by
melting curve analysis and agarose electrophoresis. The OPG mRNA
levels were normalized to those of GAPDH mRNA.
Western blot analysis
The cultured cells were pretreated with various
doses of 17-AAG or 17-DMAG for 60 min, and then stimulated by 30
ng/ml of BMP-4 or vehicle in 1 ml α-MEM containing 0.3% FBS for the
indicated periods. The cells were washed twice with
phosphate-buffered saline, and then lysed, homogenized and
sonicated in a lysis buffer containing 62.5 mM Tris/HCl, pH 6.8, 2%
sodium dodecyl sulfate (SDS), 50 mM dithiothreitol and 10%
glycerol. SDS-polyacrylamide gel electrophoresis (PAGE) was
performed by the method of Laemmli (23) in 10% polyacrylamide gels. The
protein was fractionated and transferred onto an Immun-Blot PVDF
membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
membranes were blocked with 5% fat-free dry milk in Tris-buffered
saline-Tween (TBS-T; 20 mM Tris-HCl, pH 7.6, 137 mM NaCl, 0.1%
Tween-20) for 1 h before incubation with primary antibodies. A
western blot analysis was performed as described previously
(24) using antibodies against
phospho-specific Smad1/5 antibodies, GAPDH, phospho-specific p70 S6
kinase antibodies or p70 S6 kinase as primary antibodies at a
dilution of 1:1,000 in 5% milk in TBS-T overnight at 4°C.
Peroxidase-labeled antibodies raised in goat against rabbit IgG
(KPL, Inc., Gaithersburg, MD, USA) were used as secondary
antibodies at a dilution of 1:1,000 in 5% milk in TBS-T for 1 h at
room temperature. The peroxidase activity on the PVDF sheet was
visualized on X-ray film by means of the ECL Western blotting
detection system.
Densitometric analysis
A densitometric analysis of the western blots was
performed using a scanner and image analysis software program
(Image J, version 1.48; National Institutes of Health, Bethesda,
MD, USA). The phosphorylated protein levels were calculated as
follows: The background-subtracted signal intensity of each
phosphorylation signal was respectively normalized to the total
protein signal and plotted as the fold increase in comparison to
that of the control cells treated without stimulation.
Statistical analysis
The data were analyzed by ANOVA followed by
Bonferroni method for multiple comparisons between pairs, and
P<0.05 was considered to be statistically significant. All data
are presented as the mean ± SEM of triplicate determinations from
three independent cell preparations.
Results
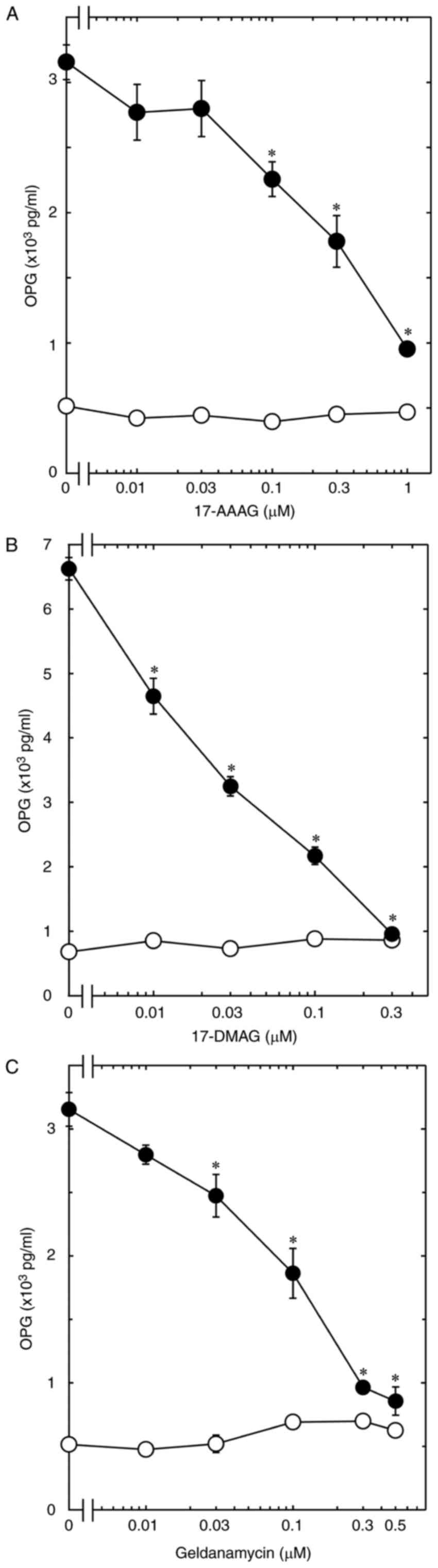
Effects of 17-AAG, 17-DMAG or
geldanamycin on the BMP-4-stimulated OPG release in MC3T3-E1
cells
In order to investigate the involvement of HSP90 in
the BMP-4-induced synthesis of OPG in osteoblast-like MC3T3-E1
cells, we first examined the effects of 17-AAG (13), 17-DMAG (15) and geldanamycin (14), as HSP90 inhibitors, on the
BMP-4-stimulated release of OPG. 17-AAG, which alone had little
effect on the release, significantly reduced the BMP-4-stimulated
OPG release in a dose-dependent manner over the range 0.01 and 1 µM
(Fig. 1A). The maximum effect of
17-AAG was observed at 1 µM, which caused an approximately 80%
decrease in the BMP-4-effect. In addition, 17-DMAG and geldanamycin
as well as 17-AAG markedly suppressed the OPG release (Fig. 1B and C). The maximum effects of
17-DMAG and geldanamycin were observed at 0.3 and 0.5 µM,
respectively, which caused almost complete suppression in the
BMP-4-effect.
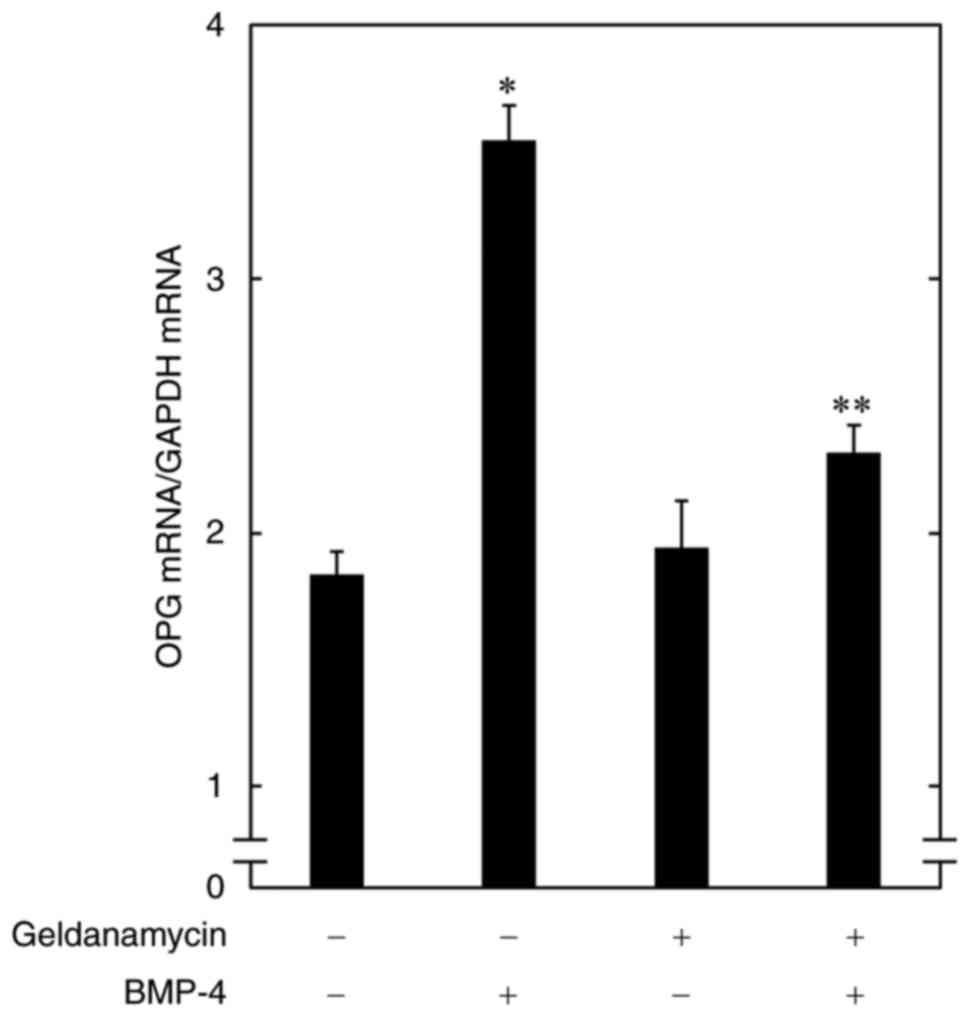
Effect of geldanamycin on the
BMP-4-induced expression levels of OPG mRNA in MC3T3-E1 cells
To clarify whether the inhibition by HSP90
inhibitors of the BMP-4-induced OPG release is mediated through
transcriptional events, we examined the effect of geldanamycin on
the OPG mRNA expression induced by BMP-4 in osteoblast-like
MC3T3-E1 cells. Geldanamycin, which by itself had little effect on
the basal levels, significantly suppressed the BMP-4-induced
expression levels of OPG mRNA (Fig.
2).
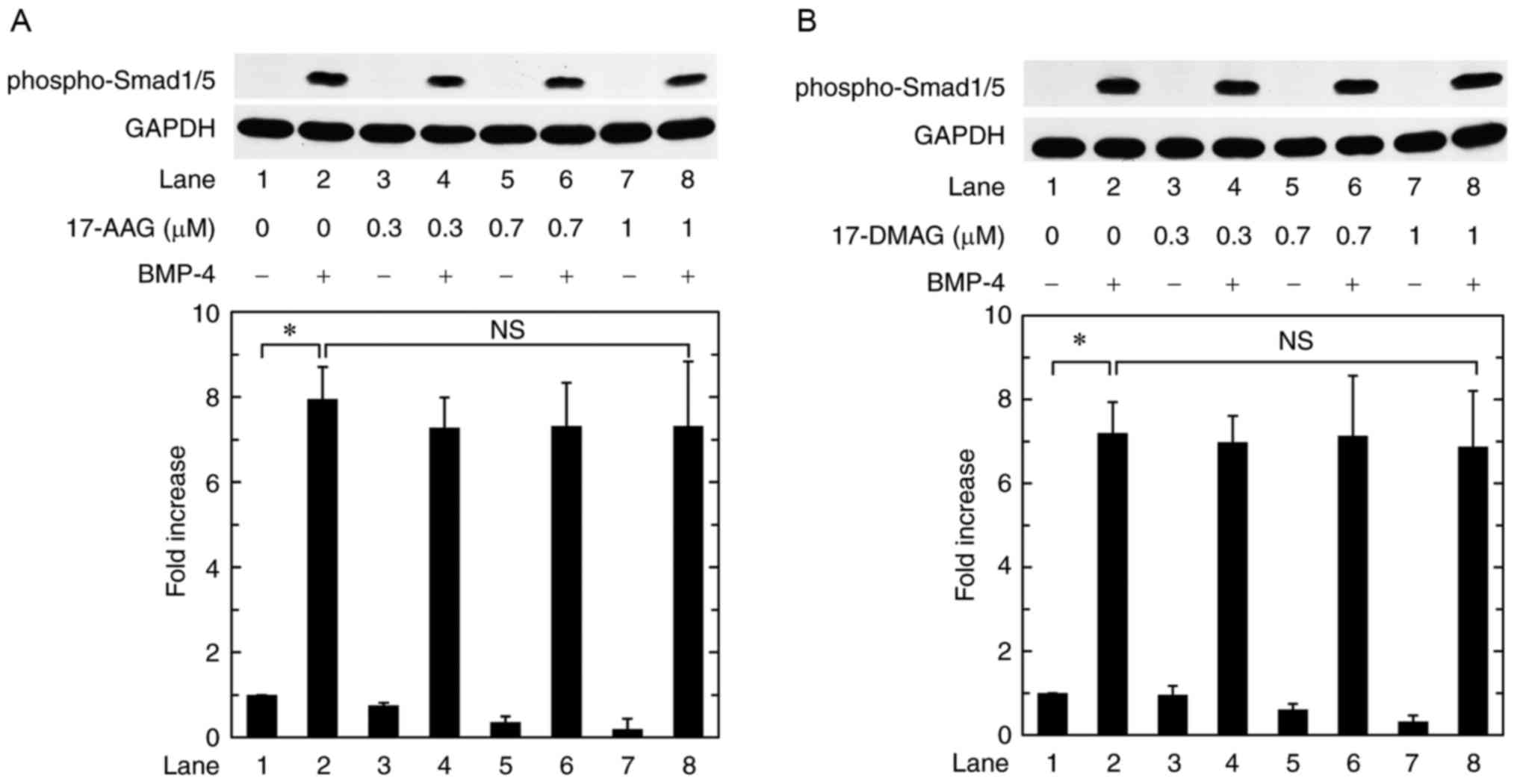
Effects of 17-AAG or 17-DMAG on the
BMP-4-induced phosphorylation of Smad1/5 in MC3T3-E1 cells
Regarding the intracellular signaling of BMPs, the
Smad protein family such as Smad1, Smad5 and Smad8 plays an
important role (8). Therefore, we
examined the effect of 17-AAG or 17-DMAG on the BMP-4-induced
phosphorylation of Smad1/5 in osteoblast-like MC3T3-E1 cells.
However, neither 17-AAG nor 17-DMAG affected the BMP-4-induced
phosphorylation of Smad1/5 up to 1 µM (Fig. 3A and B).
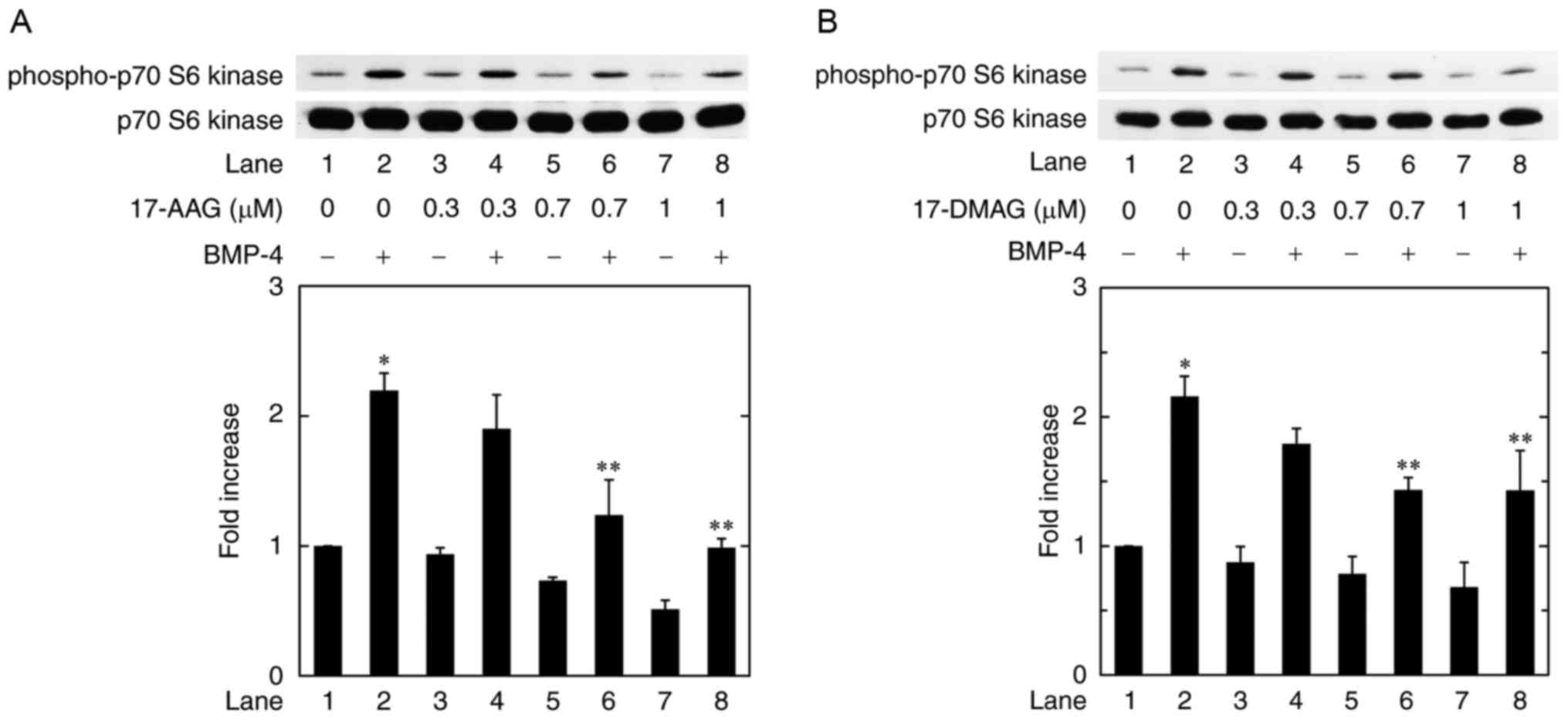
Effects of 17-AAG or 17-DMAG on the
BMP-4-induced phosphorylation of p70 S6 kinase in MC3T3-E1
cells
It is currently recognized that not only the
Smad-dependent pathway but also the Smad-independent pathways
mediate the effects of BMPs (9).
We have recently demonstrated that p70 S6 kinase functions as a
positive regulator in the BMP-4-stimulated synthesis of OPG in
osteoblast-like MC3T3-E1 cells (10). In order to investigate whether the
activation of p70 S6 kinase is implicated in the HSP90
inhibitor-effect on the BMP-4-induced OPG synthesis in MC3T3-E1
cells, we examined the effect of 17-AAG on the BMP-4-induced
phosphorylation of p70 S6 kinase. 17-AAG at 0.7 and 1.0 µM
significantly attenuated the BMP-4-induced phosphorylation of p70
S6 kinase dose dependently over the range 0.3 and 1 µM (Fig. 4A). In addition, the phosphorylation
of p70 S6 kinase was remarkably inhibited by 17-DMAG (Fig. 4B).
Discussion
In the present study, we demonstrated that HSP90
inhibitors including 17-AAG (13),
17-DMAG (15) and geldanamycin
(14) significantly attenuated the
BMP-4-stimulated release of OPG in osteoblast-like MC3T3-E1 cells.
In addition, the expression levels of OPG mRNA induced by BMP-4
were markedly suppressed by geldanamycin. Therefore, our findings
suggest that the suppression by HSP90 inhibitors of the
BMP-4-stimulated synthesis of OPG is exerted at a point upstream of
transcriptional levels in MC3T3-E1 cells. This is probably the
first report showing the attenuation by HSP90 inhibitors of
BMP-stimulated OPG synthesis in osteoblasts as far as we know.
Thus, we next investigated the exact mechanism behind the
suppression by HSP90 inhibitors of the BMP-4-stimulated OPG
synthesis in osteoblast-like MC3T3-E1 cells.
Regarding the intracellular signaling in the TGF-β
superfamily including BMPs, it is firmly established that Smad
proteins act as central mediators (8). Among the Smad proteins, BMPs employ
the activation of 1, 5 and Smad8 as receptor-activated Smads
(8). Thus, in order to investigate
whether the activation of these Smads is implicated in the
inhibitory effects of HSP90 inhibitors on the BMP-4-stimulated OPG
synthesis in osteoblast-like MC3T3-E1 cells, we examined the
effects of 17-AAG or 17-DMAG on the BMP-4-induced phosphorylation
of Smad1/5. However, we found that 17-AAG and 17-DMAG failed to
affect the BMP-4-induced phosphorylation of Smad1/5. Based on these
findings, it seems unlikely that the suppression by HSP90
inhibitors of the OPG synthesis stimulated by BMP-4 is mediated
through the Smad-dependent signaling pathway. On the other hand,
accumulating evidence indicates that the TGF-β superfamily exerts
their effects on a variety of biological functions via the
Smad-independent signaling pathways in addition to the
Smad-dependent pathway (9). In our
recent study (10), we have shown
that BMP-4 stimulates OPG synthesis at least in part via p70 S6
kinase activation in osteoblast-like MC3T3-E1 cells. Thus, to
clarify whether HSP90 inhibitors affect the BMP-4-induced
activation of p70 S6 kinase in MC3T3-E1 cells, we examined the
effects of 17-AAG or 17-DMAG on the BMP-4-induced phosphorylation
of p70 S6 kinase. We showed here that the phosphorylation levels of
p70 S6 kinase induced by BMP-4 were remarkably reduced by both
17-AAG and 17-DMAG. Taking our findings into account, it is most
likely that HSP90 inhibitors suppress the BMP-4-stimulated OPG
synthesis via attenuating p70 S6 kinase in osteoblast-like MC3T3-E1
cells.
HSP90 is a ubiquitous molecular chaperone which is
involved in the folding and stabilization of a variety of proteins
(25,26). It is currently recognized that
HSP90 plays important roles in cell homeostasis including the
regulation of glucocorticoid receptors (25,26).
We have found that the expression levels of HSP90 protein are quite
high in osteoblast-like MC3T3-E1 cells (27). HSP90 inhibitors, including 17-AAG,
17-DMAG and geldanamycin, are developed as anticancer agents since
numerous client proteins of HSP90 are involved in the progression
of cancer (26). On the other
hand, OPG, which has been identified as an osteoclastogenesis
inhibitory factor, functions as a negative regulator of
RANKL-mediated osteoclastic bone resorption (1). In physiological bone remodeling, bone
resorption is the primary step, and bone formation is subsequently
developed (1,3). To maintain the quality and quantity
of bone, proper remodeling cooperated by osteoclasts and
osteoblasts is required to remove old fragile skeleton and
regenerate new bone. Our present findings, demonstrating that HSP90
inhibitors reduced the BMP-4-stimulated OPG synthesis in
osteoblast-like MC3T3-E1 cells, make us to speculate that HSP90
could act as a positive regulator in the OPG synthesis in
osteoblasts. Taking our present results into account as a whole, it
is possible that the upregulation of HSP90 activity in
BMP-4-stimulated OPG synthesis in osteoblasts leads bone metabolism
toward the increase of bone formation due to the attenuation of
osteoclastic bone resorption. Therefore, our present findings might
provide a novel insight for HSP90 as a pivotal modulator of bone
remodeling, which possesses a potentiality of therapeutic strategy
for the remedy of metabolic bone diseases including osteoporosis.
HSP90 inhibitors are generally recognized as anticancer agents
(13–18), however, 17-AAG reportedly
potentiates osteolytic bone metastasis of breast cancer cells
(19). On the other hand, BMP is a
potent osteoinductive cytokine (8). Based on our present findings, it is
possible that HSP90 inhibitors upregulate RANKL-RANK-mediating bone
resorption through the reduction of OPG synthesis by BMP-4, leading
to the potentiation of osteolysis consistent with the previous
report. Thus, it seems necessary to pay attention to the
possibility of bone resorption enhanced by HSP90 inhibitors. In
addition, we used only one cell line, osteoblast-like MC3T3-E1
cells in the present study. Therefore, our findings about HSP90
inhibitor-effects on MC3T3-E1 cells should be confirmed in other
types of osteoblasts including primary cultured cells. Further
investigations would be required to clarify the details underlying
the roles of HSP90 in bone metabolism.
In conclusion, our results strongly suggest that
HSP90 inhibitors suppress the BMP-4-stimulated OPG synthesis in
osteoblasts, and that their inhibitory effects are exerted through
downregulating p70 S6 kinase.
Acknowledgements
We are very grateful to Mrs. Yumiko Kurokawa for her
skillful technical assistance. This study was supported in part by
a Grant-in-Aid for Scientific Research (26462289, 15K10487) from
the Ministry of Education, a Grant-in-Aid for Scientific Research
(H25-Aging-General-004) from the Ministry of Health, Labour and
Welfare, and the Research Funding for Longevity Sciences (25–4,
26–12) from National Center for Geriatrics
and Gerontology (NCGG), Japan.
References
|
1
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zuo C, Huang Y, Bajis R, Sahih M, Li YP,
Dai K and Zhang X: Osteoblastgenesis regulation signals in bone
remodeling. Osteoporos Int. 23:1653–1663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hadjidakis DJ and Androulakis II: Bone
remodeling. Ann N Y Acad Sci. 1092:385–396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mizuno A, Amizuka N, Irie K, Murakami A,
Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, et
al: Severe osteoporosis in mice lacking osteoclastogenesis
inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun.
247:610–615. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tat S Kwan, Padrines M, Théoleyre S,
Heymann D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: Interrelations
in bone resorption pathophysiology. Cytokine Growth Factor Rev.
15:49–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hofbauer LC, Dunstan CR, Spelsberg TC,
Riggs BL and Khosla S: Osteoprotegerin production by human
osteoblast lineage cells is stimulated by vitamin D, bone
morphogenetic protein-2 and cytokines. Biochem Biophys Res Commun.
250:776–781. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyazono K, Kamiya Y and Morikawa M: Bone
morphogenetic protein receptors and signal transduction. J Biochem.
147:35–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moustakas A and Heldin CH: Non-Smad
TGF-beta signals. J Cell Sci. 118:3573–3584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujita K, Otsuka T, Yamamoto N, Kainuma S,
Ohguchi R, Kawabata T, Sakai G, Kuroyanagi G, Matsushima-Nishiwaki
R, Kozawa O and Tokuda H: (−)-Epigallocatechin gallate but not
chlorogenic acid upregulates osteoprotegerin synthesis through
regulation of bone morphogenetic protein-4 in osteoblasts. Exp Ther
Med. 14:417–423. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mymrikov EV, Seit-Nebi AS and Gusev NB:
Large potentials of small heat shock proteins. Physiol Rev.
91:1123–1159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiosis G: Targeting chaperones in
transformed systems - a focus on Hsp90 and cancer. Expert Opin Ther
Targets. 10:37–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schulte TW and Neckers LM: The
benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds
to HSP90 and shares important biologic activities with
geldanamycin. Cancer Chemother Pharmacol. 42:273–279. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ochel HJ, Eichhorn K and Gademann G:
Geldanamycin: The prototype of a class of antitumor drugs targeting
the heat shock protein 90 family of molecular chaperones. Cell
Stress Chaperones. 6:105–112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jez JM, Chen JC, Rastelli G, Stroud RM and
Santi DV: Crystal structure and molecular modeling of 17-DMAG in
complex with human Hsp90. Chem Biol. 10:361–368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamal A, Thao L, Sensintaffar J, Zhang L,
Boehm MF, Fritz LC and Burrows FJ: A high-affinity conformation of
Hsp90 confers tumor selectivity on Hsp90 inhibitors. Nature.
425:407–410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Whitesell L and Lindquist SL: HSP90 and
the chaperoning of cancer. Nat Rev Cancer. 5:761–772. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu W and Neckers L: Targeting the
molecular chaperone heat shock protein 90 provides a multifaceted
effect on diverse cell signaling pathways of cancer cells. Clin
Cancer Res. 13:1625–1629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Price JT, Quinn JM, Sims NA, Vieusseux J,
Waldeck K, Docherty SE, Myers D, Nakamura A, Waltham MC, Gillespie
MT and Thompson EW: The heat shock protein 90 inhibitor,
17-allylamino-17-demethoxygeldanamycin, enhances osteoclast
formation and potentiates bone metastasis of a human breast cancer
cell line. Cancer Res. 65:4929–4938. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mori M, Hitora T, Nakamura O, Yamagami Y,
Horie R, Nishimura H and Yamamoto T: Hsp90 inhibitor induces
autophagy and apoptosis in osteosarcoma cells. Int J Oncol.
46:47–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kozawa O, Tokuda H, Miwa M, Kotoyori J and
Oiso Y: Cross-talk regulation between cyclic AMP production and
phosphoinositide hydrolysis induced by prostaglandin E2 in
osteoblast-like cells. Exp Cell Res. 198:130–134. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and alpha B-crystallin by cyclic AMP in C6 rat glioma cells.
J Neurochem. 66:946–950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prodromou C: Mechanisms of Hsp90
regulation. Biochem J. 473:2439–2452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Verma S, Goyal S, Jamal S, Singh A and
Grover A: Hsp90: Friends, clients and natural foes. Biochimie.
127:227–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kozawa O, Niwa M, Hatakeyama D, Tokuda H,
Oiso Y, Matsuno H, Kato K and Uematsu T: Specific induction of heat
shock protein 27 by glucocorticoid in osteoblasts. J Cell Biochem.
86:357–364. 2002. View Article : Google Scholar : PubMed/NCBI
|