Introduction
Neuroblastoma originates from the neural crest of
the sympathetic nervous system (1), and is the most common solid tumor in
children in terms of incidence and also mortality, with 800 new
cases diagnosed each year in the United States (2). Almost half of patients with
neuroblastoma are children younger than two years old, with a
median age of 17 months at diagnosis. Neuroblastoma accounts for
8–10% of all pediatric malignant tumors and 15% of
cancer-associated death in children (3–5).
Patients with neuroblastoma are usually treated with a combination
of chemotherapy, surgery and radiation (6). However, the majority of patients will
develop certain complications, including infertility, hearing loss
and cardiac dysfunction, following these procedures (7). In 2015, the molecular-targeted drug
dinutuximab, in combination with granulocyte macrophage
colony-stimulating factor, interleukin-2 and isotretinoin, was
approved as treatment for high-risk neuroblastoma, however, various
serious adverse reactions, which include infusion reactions and
neuropathy, have been reported to be associated with this treatment
regimen (8). Therefore, it is of
high importance to identify and develop treatments for
neuroblastoma that exhibit increased potency and reduced side
effects.
Honokiol
[2-(4-hydroxy-3-prop-2-enyl-phenyl)-4-prop-2-enylphenol] is a
soluble, nontoxic, natural small-molecule polyphenol from the
magnolia plant. It has been reported to inhibit thegrowth of
various types of cancer cells (9),
including oral squamous cell carcinoma (10), glioblastoma multiforme (11), human prostate cancer (12) and melanoma cells (13). A previous report also demonstrated
that honokiol kills neuroblastoma cells (14), indicating that honokiol may be a
promising agent for neuroblastoma treatment. However, the molecular
pharmacology of honokiol in the suppression of cancer cell growth
and proliferation is largely unknown.
The present study treated the Neuro-2a mouse
neuroblastoma cell line with honokiol and demonstrated that
honokiol inhibited the growth of Neuro-2a cells in a time- and
dose-dependent manner. Further mechanistic investigation revealed
that honokiol triggered receptor-interacting protein kinase 3
(RIP3)-mediated loss of cell viability in neuroblastoma cells via
the generation of reactive oxygen species (ROS).
Materials and methods
Reagents
Honokiol and MTT were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Necrosulfonamide (Nec) was from
Abcam (Cambridge, MA, USA). N-acetyl-L-cysteine (NAC)
and Lipofectamine 2000 were obtained from Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). RIP3 small interfering (si)RNA
(5′-CCAGAGACCUCAACUUUCA-3′) and control siRNA
(5′-UUCUCCGAACGUGUCACGUTT-3′) were purchased from Qiagen, Inc.
(Valencia, CA, USA). Rabbit anti-RIP3 (#95702) and β-actin
antibodies (#8457) were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA).
Cell culture and treatments
Neuro-2a mouse neuroblastoma cells were obtained
from the American Type Culture Collection (Manassas, VA, USA).
Cells were grown in Dulbecco's modified Eagle's medium (Thermo
Fisher Scientific, Inc.) supplemented with 10% (v/v)
heat-inactivated fetal bovine serum (Thermo Fisher Scientific,
Inc.), 2 mM L-glutamine, 100 IU/ml penicillin and 100 µg/ml
streptomycin in an incubator at 37°C with 5% CO2.
Transfection of 100 nM siRNA with Lipofectamine 2000 was performed
according to the manufacturer's protocol. Neuro-2a cells were
treated with 60 µM honokiol alone, or in combination with 100 nM
RIP3 siRNA for 24 h or with 1 µM RIP3 specific inhibitor Nec for 48
h. For the ROS scavenger assay, Neuro-2a cells were treated with
vehicle, 60 µM honokiol or a combination of 1 µM NAC and 60
µM honokiol for 48 h. Control cells were treated with DMSO instead
of honokiol throughout the study.
MTT assay
The cytotoxicity of honokiol in Neuro-2a cells was
determined using an MTT colorimetric assay. Briefly, Neuro-2a cells
were seeded in 200 µl at a concentration of 1×104
cells/well in 96-well tissue culture plates and incubated at 37°C
with 5% CO2. The next day, cells were treated with 0,
30, 60 or 120 µM honokiol for 24, 48 and 72 h. Following
incubation, 20 µl MTT reagent (5 mg/ml) was added to each well and
incubated for another 3 h. MTT solution was removed and
dimethylsulfoxide was added to each well to dissolve the blue
formazan product. The absorbance at 550 nm was recorded. The
relative inhibition rate of the cells was calculated according to
the following equation: Relative inhibition rate of cells = (1 -
absorbance value of sample) / (absorbance value of control) × 100.
Data were calculated from three independent experiments.
Western blot analysis
Total proteins were extracted from 2×106
cultured cells using radioimmunoprecipitation assay lysis buffer
(1% Nonidet P-40, 0.5% sodium deoxycholate and 0.1% SDS in 1X
phosphate buffer solution) containing protease inhibitors (2 µg/ml
aprotinin, 2 µg/ml leupeptin and 1 mM phenylmethylsulfonyl
fluoride) for 30 min on ice. Following centrifugation at 12,000 × g
for 15 min at 4°C, the supernatant was resuspended in buffer
containing 1% SDS and 1% dithiothreitol, and heated at 100°C for 5
min. The protein concentration was quantified using a bicinchoninic
acid protein assay, and an equal amount of protein (20 µg) was
electrophoresed by 10% SDS-PAGE and transferred onto nitrocellulose
membranes. Following blocking with 5% non-fat dry milk in
TBS-Tween-20 (TBS-T; 10 mM Tris-HCl, pH 8.0, 100 mM NaCl and 0.05%
Tween-20) at room temperature for 1 h, the membrane was incubated
at 4°C overnight with rabbit anti-RIP3 (1:1,000; #95702) or
anti-β-actin (1:1,000; #8457) primary antibodies (both from Cell
Signaling Technology, Inc.). Following incubation, the membrane was
washed twice with TBS-T for 15 min and incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (Abcam,
ab6721, 1:5,000) at room temperature for 1 h. After washing with
TBS-T, the immunoreactivities were visualized by an ECL plus
chemiluminescence kit (Beyotime Institute of Biotechnology, Haimen,
China) according to manufacturer's protocol (Pierce; Thermo Fisher
Scientific, Inc.). The relative optical density of bands were
analyzed using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA).
Measurement of intracellular ROS
Levels of ROS were measured using the ROS assay kit
from Beyotime Institute of Biotechnology, according to the
manufacturer's protocol. Briefly, following treatment,
1×106 Neuro-2a cells were incubated with 10 µM
non-fluorescent probe 2′,7′-dichlorofluorescin diacetate (DCFH-DA)
for 20 min at room temperature. DCFH-DA is able to penetrate the
cellular membrane and be hydrolyzed to dichlorofluorescin (DCFH)
carboxylate anion. DCFH is subsequently oxidized by ROS, which
results in the formation of fluorescent dichlorofluorescein (DCF).
DCF fluorescence was detected using fluorescence spectroscopy with
maximum excitation and emission of 495 and 529 nm,
respectively.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Data are presented as the mean ± standard error of the mean.
One-way analysis of variance was used to compare differences among
three or more groups, which was followed by Bonferroni post-hoc
testing for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Honokiol inhibits the proliferation of
Neuro-2a mouse neuroblastoma cells in a time- and dose-dependent
manner
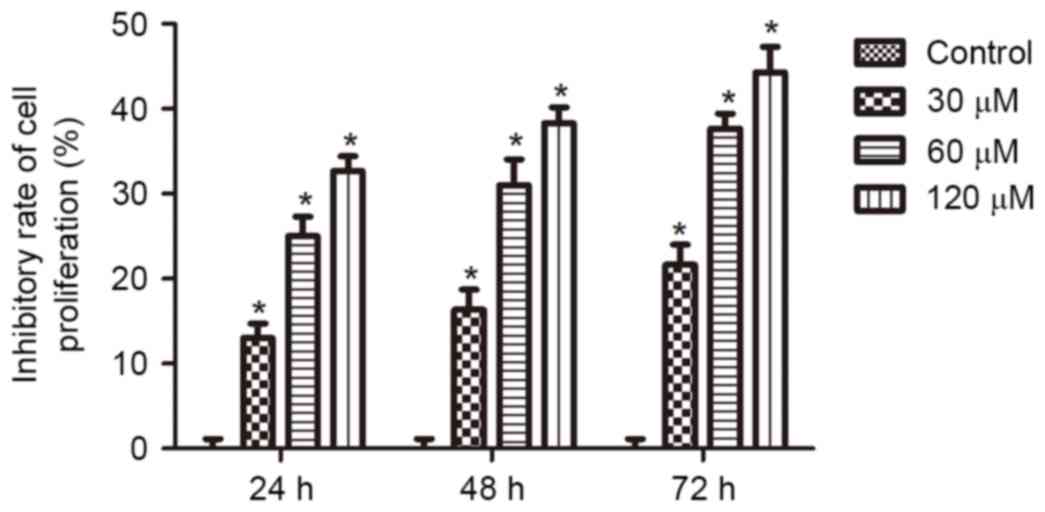
To assess the effects of honokiol on the growth of
neuroblastoma cells, Neuro-2a cells were treated with 0, 30, 60 or
120 µM honokiol for 24, 48 and 72 h. Cell viability was assessed by
MTT assay. As demonstrated in Fig.
1, following treatment for 24 h, 30, 60 and 120 µM honokiol
resulted in inhibition of cell proliferation by 12, 25 and 30%.
Furthermore, increased inhibitory effects were observed when the
treatment time was extended to 48 and 72 h. These results
demonstrate that honokiol inhibited the growth of Neuro-2a cells in
a time- and dose-dependent manner.
Honokiol promotes the expression of
RIP3 in Neuro-2a neuroblastoma cells
Necrosis is the process of cell death caused by
various external factors, including toxins, infections and trauma,
or induced by specific genes in a regulated manner (15). RIP3 has an essential role in the
necrosis pathway (16) and a
recent study reported that stress induced RIP3 upregulation in
neuroblastoma cells (17). To
investigate the mechanism and potential role of RIP3 in the
suppression of neuroblastoma cell proliferation by honokiol, the
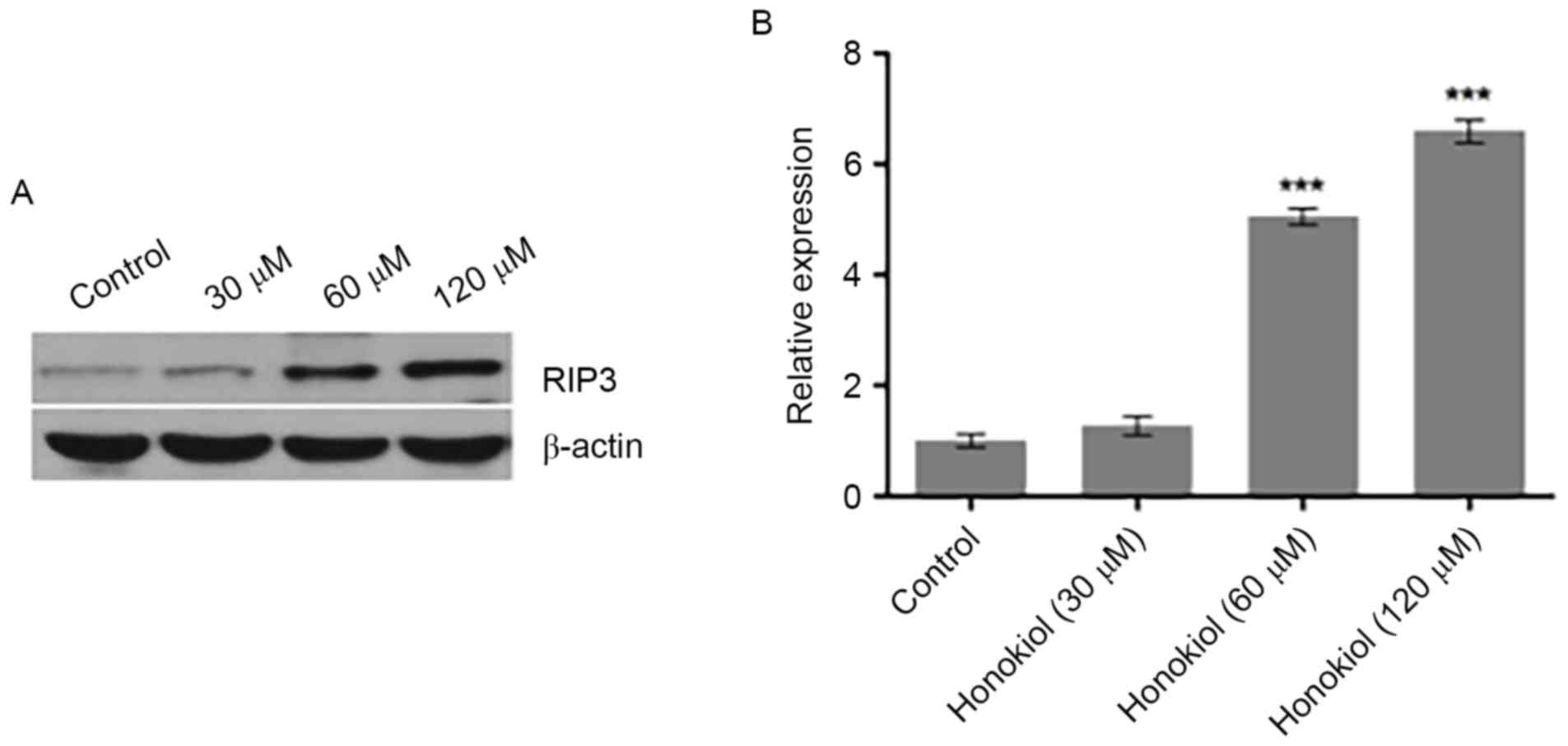
protein expression of RIP3 in Neuro-2a cells was investigated using
western blot analysis following treatment of cells with various
concentrations (0, 30, 60 and 120 µM) of honokiol for 48 h.
Compared with the untreated control, 30 µM marginally increased
RIP3 protein expression, while 60 and 120 µM honokiol significantly
increased the protein expression of RIP3 (Fig. 2). These results indicate that
honokiol promoted the expression of RIP3 in Neuro-2a neuroblastoma
cells.
Silencing RIP3 by siRNA or
pharmacological inhibition of RIP3 prevents honokiol-induced loss
of cell viability in Neuro-2a neuroblastoma cells
The observed induction of RIP3 indicates that RIP3
may have an important role in honokiol-mediated necrosis of
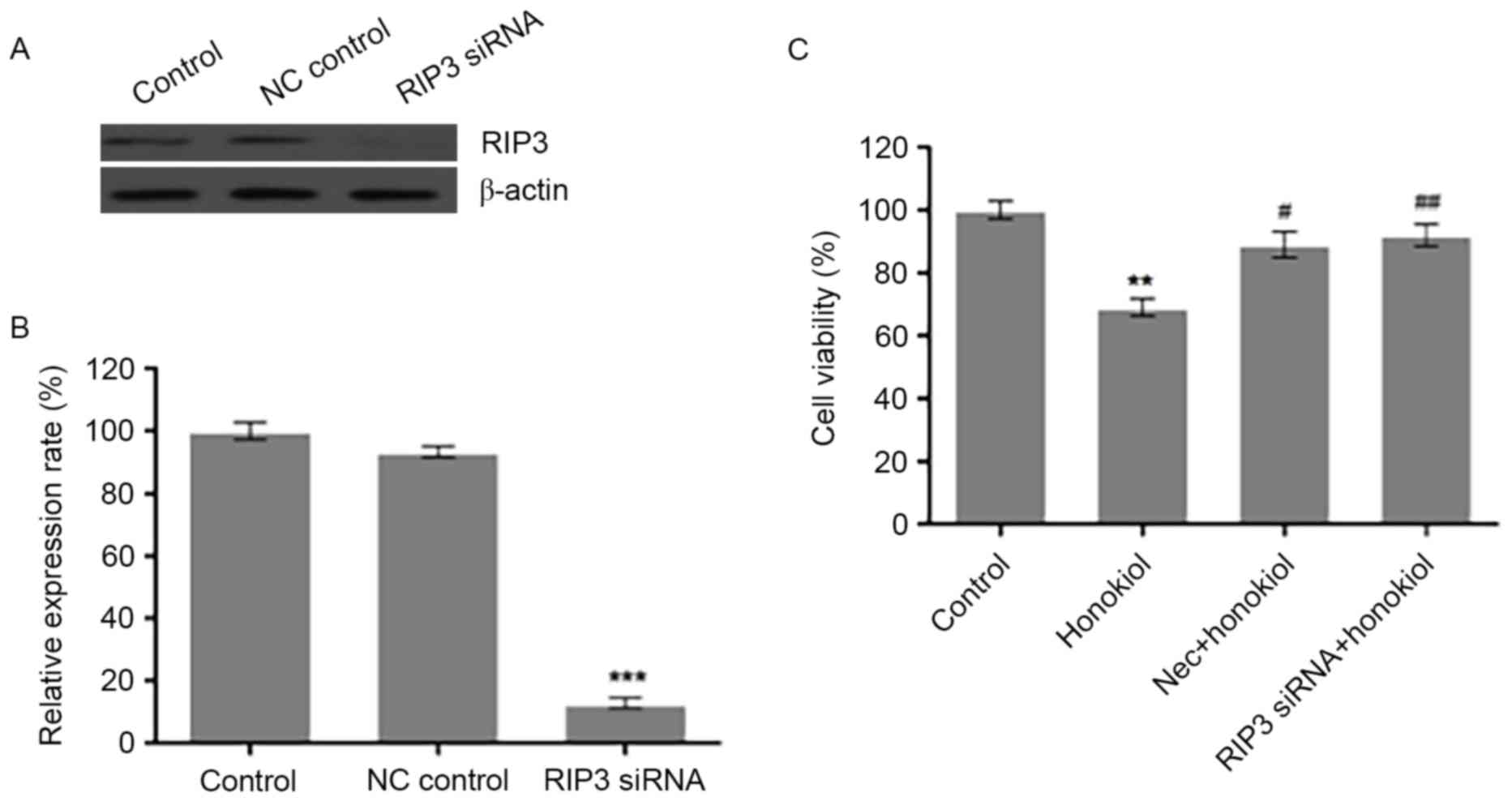
Neuro-2a cells. To test this hypothesis, the present study knocked
down RIP3 in Neuro-2a cells by siRNA. Compared with scrambled
control siRNA (NC control), transfection of 100 nM RIP3 siRNA
reduced the protein expression of RIP3 in Neuro-2a cells (Fig. 3A). Analysis of the relative optical
density of RIP3 bands demonstrated that RIP3 siRNA significantly
decreased the protein level of RIP3 (Fig. 3B), compared with the control.
Neuro-2a cells were also treated with 60 µM honokiol alone, or in
combination with RIP3 siRNA transfection for 24 h or treatment with
1 µM RIP3 specific inhibitor Nec for 48 h (Fig. 3C). Cell viability was assessed by
MTT assay. Compared with the control group, 60 µM honokiol
significantly reduced the cell viability of Neuro-2a cells, which
was significantly reversed by Nec and RIP3 siRNA treatments
(Fig. 3C). These results indicate
that induction of RIP3 contributed to the observed
honokiol-mediated loss of cell viability in Neuro-2a cells.
Honokiol inhibits Neuro-2a
neuroblastoma cell growth via ROS-mediated upregulation of
RIP3
To investigate whether honokiol inhibits Neuro-2a
neuroblastoma cell growth via ROS-mediated upregulation of RIP3,
Neuro-2a cells were treated with vehicle, honokiol (30, 60 and 120
µM) or a combination of ROS scavenger NAC (1 µM) and
honokiol (60 µM) for 48 h. The intracellular ROS levels were
measured using DCFH-DA. Compared with the vehicle control, 60 and
120 µM honokiol significantly increased the intracellular ROS
levels, while NAC treatment significantly prevented the induction
of ROS by honokiol (Fig. 4A). In
addition, Neuro-2a cells were treated with vehicle, 60 µM honokiol
or a combination of 1 µM NAC and 60 µM honokiol for 48 h,
and the protein expression of RIP3 was determined by western blot
analysis. As demonstrated in Fig.
4B, the 60 µM honokiol-induced increase of RIP3 protein
expression was reversed to a certain extent by NAC. Analysis of the
relative optical density of RIP3 bands demonstrated that 60 µM
honokiol significantly increased the protein expression of RIP3,
compared with the control group, which was significantly prevented
by NAC (Fig. 4C). These results
indicate that honokiol may suppress Neuro-2a neuroblastoma cell
growth, at least partially, through ROS-mediated upregulation of
RIP3.
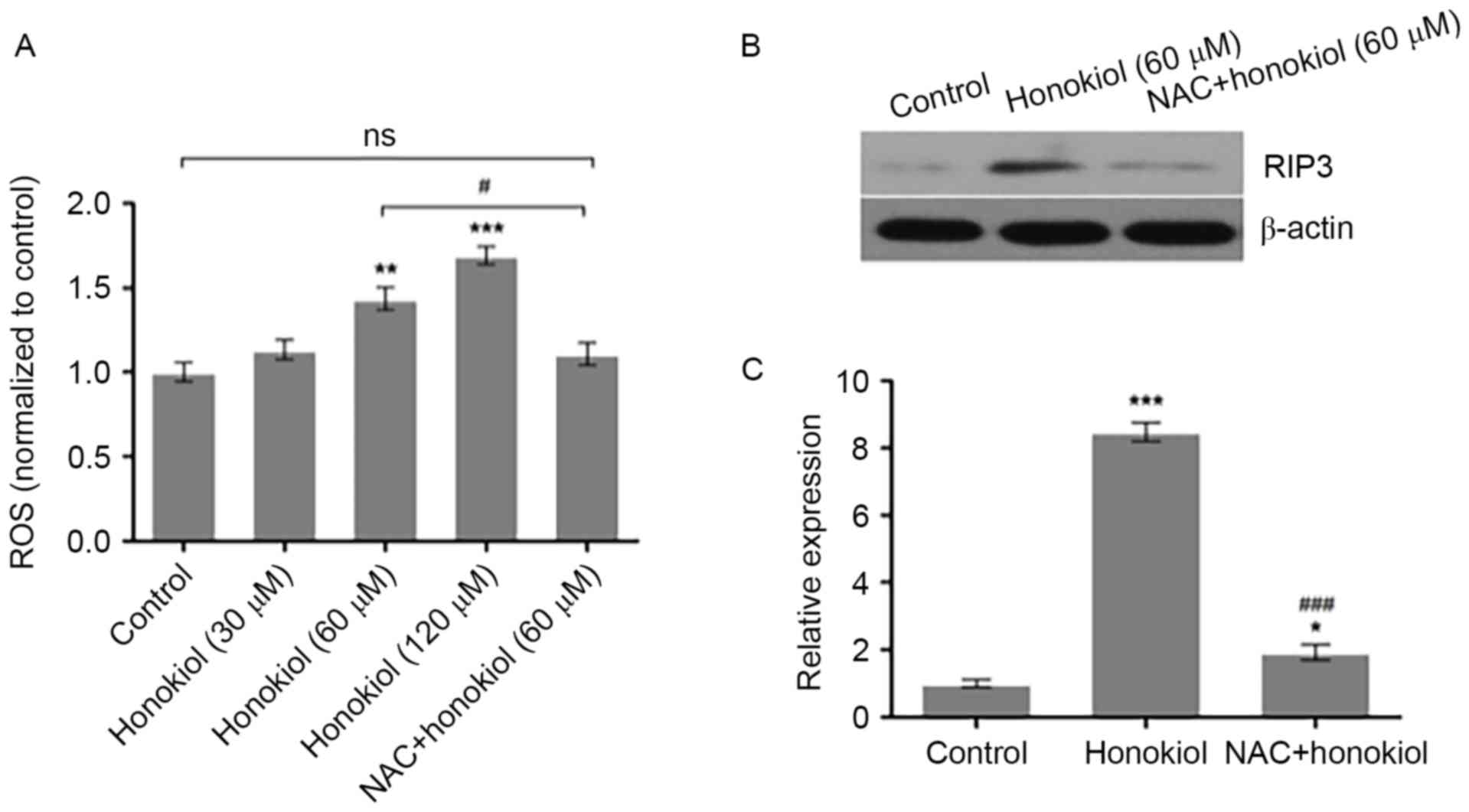 | Figure 4.ROS scavenger NAC prevents the
honokiol-induced increase of RIP3 in Neuro-2a neuroblastoma cells.
(A) Neuro-2a cells were treated with vehicle, 30, 60 and 120 µM
honokiol, or a combination of ROS scavenger NAC (1 µM) and
honokiol (60 µM) for 48 h. The intracellular ROS levels were
measured using 2′, 7′-dichlorofluorescin diacetate. (B) Neuro-2a
cells were treated with vehicle, 60 µM honokiol or a combination of
1 µM NAC and 60 µM honokiol for 48 h, and the protein
expression of RIP3 was determined by western blot analysis with
β-actin as loading control. (C) Relative optical density of RIP3
bands to β-actin bands was analyzed using Image-Pro Plus 6.0
software. *P<0.05, **P<0.01 and ***P<0.001 vs. control
group; #P<0.05 and ###P<0.001 vs. 60 µM
honokiol group. ROS, reactive oxygen species; NAC,
N-acetyl-L-cysteine; RIP3, receptor-interacting protein
kinase 3; ns, not significant. |
Discussion
Honokiol has been demonstrated to inhibit the growth
of various types of cancer cells (9–14).
However, the molecular mechanism of honokiol in the suppression of
cancer cell growth and proliferation is largely unknown. The
present study demonstrated that honokiol inhibited the growth of
Neuro-2a neuroblastoma cells in a time- and dose-dependent manner,
which was accompanied by upregulation of RIP3. Furthermore,
silencing RIP3 by siRNA or pharmacological inhibition of RIP3
reversed honokiol-induced loss of cell viability in Neuro-2a cells.
Importantly, honokiol significantly increased the intracellular ROS
levels and the expression of RIP3, while the ROS scavenger
NAC significantly prevented the induction of ROS and RIP3 by
honokiol. These results indicate that honokiol may trigger
RIP3-mediated loss of cell viability in neuroblastoma cells via the
generation of ROS.
Honokiol has been demonstrated to inhibit the growth
of human glioma via multiple mechanisms. Honokiol was reported to
suppress human glioma growth via induction of apoptosis and cell
cycle arrest in tumor cells by activating a p53/cyclin D1/cyclin
dependent kinase (CDK) 6/CDK4/E2F transcription factor 1-dependent
pathway (18). Notably, honokiol
was also reported to induce autophagy in neuroblastoma cells via
the phosphatidylinositol 3-kinase/Akt/mechanistic target of
rapamycin and endoplasmic reticulum stress/ROS/extracellular
signal-regulated kinase 1/2 signaling pathways, and the suppression
of cell migration (19). In
addition, necrosis is the process of cell death that is caused by
certain external factors, which include toxins, infections and
trauma, or induced by specific genes in a regulated manner
(15). The results of the present
study indicated that honokiol induced the expression of RIP3, which
is upregulated by stress in neuroblastoma cells and has an
essential role in necrosis (16–17).
Furthermore, silencing RIP3 by siRNA or pharmacological inhibition
of RIP3 reversed honokiol-induced loss of cell viability in
Neuro-2a cells. The current study has identified an additional
mechanism by which honokiol induces loss of cell viability in
neuroblastoma cells, indicating that honokiol may be a potential
candidate drug for treating brain tumors such as neuroblastoma.
It was reported that honokiol inhibited the growth
of malignant glioma via ROS (20).
Furthermore, it has been reported that ROS inhibited RIP
protein-induced necroptosis of MiaPaCa-2 and BxPC-3 cells (21). These advances led us to hypothesize
that honokiol may suppress neuroblastoma cell growth via
ROS-mediated upregulation of RIP3. Indeed, the present study
revealed that honokiol significantly increased the intracellular
ROS levels and the protein expression of RIP3, while the ROS
scavenger NAC significantly prevented the induction of ROS and
RIP3 expression by honokiol. These results indicate that honokiol
may stimulate the expression of RIP3, at least partially, through
the production of ROS in neuroblastoma cells.
In conclusion, the present study demonstrated that
honokiol inhibited cell proliferation, promoted the production of
ROS and stimulated the protein expression of RIP3 in Neuro-2a mouse
neuroblastoma cells. The results of the current study indicate that
honokiol may suppress neuroblastoma cell growth via ROS-mediated
upregulation of RIP3, providing the basis for further development
of honokiol for the treatment of neuroblastoma.
Acknowledgements
This work was supported by the National Natural
Science Fund of China (grant no. 81502187).
References
|
1
|
Hoehner JC, Gestblom C, Hedborg F,
Sandstedt B, Olsen L and Påhlman S: A developmental model of
neuroblastoma: Differentiating stroma-poor tumors' progress along
an extra-adrenal chromaffin lineage. Lab Invest. 75:659–675.
1996.PubMed/NCBI
|
|
2
|
Spix C, Pastore G, Sankila R, Stiller CA
and Steliarova-Foucher E: Neuroblastoma incidence and survival in
European children (1978–1997): Report from the automated childhood
cancer information system project. Eur J Cancer. 42:2081–2091.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Izbicki T, Mazur J and Izbicka E:
Epidemiology and etiology of neuroblastoma: An overview. Anticancer
Res. 23:755–760. 2003.PubMed/NCBI
|
|
4
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Bernardi B, Mosseri V, Rubie H, Castel
V, Foot A, Ladenstein R, Laureys G, Beck-Popovic M, de Lacerda AF,
Pearson AD, et al: Treatment of localised resectable neuroblastoma.
Results of the LNESG1 study by the SIOP Europe Neuroblastoma Group.
Br J Cancer. 99:1027–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zage PE, Kletzel M, Murray K, Marcus R,
Castleberry R, Zhang Y, London WB and Kretschmar C; Children's
Oncology Group, : Outcomes of the POG 9340/9341/9342 trials for
children with high-risk neuroblastoma: A report from the children's
oncology group. Pediatr Blood Cancer. 51:747–753. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoy SM: Dinutuximab: A review in high-risk
neuroblastoma. Target Oncol. 11:247–253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bai X, Cerimele F, Ushio-Fukai M, Waqas M,
Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K, et
al: Honokiol, a small molecular weight natural product, inhibits
angiogenesis in vitro and tumor growth in vivo. J Biol Chem.
278:35501–35507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen XR, Lu R, Dan HX, Liao G, Zhou M, Li
XY and Ji N: Honokiol: A promising small molecular weight natural
agent for the growth inhibition of oral squamous cell carcinoma
cells. Int J Oral Sci. 3:34–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Duan X, Yang G, Zhang X, Deng L,
Zheng H, Deng C, Wen J, Wang N, Peng C, et al: Honokiol crosses BBB
and BCSFB, and inhibits brain tumor growth in rat 9L intracerebral
gliosarcoma model and human U251 xenograft glioma model. PLoS One.
6:e184902011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shigemura K, Arbiser JL, Sun SY, Zayzafoon
M, Johnstone PA, Fujisawa M, Gotoh A, Weksler B, Zhau HE and Chung
LW: Honokiol, a natural plant product, inhibits the bone metastatic
growth of human prostate cancer cells. Cancer. 109:1279–1289. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prasad R, Kappes JC and Katiyar SK:
Inhibition of NADPH oxidase 1 activity and blocking the binding of
cytosolic and membrane-bound proteins by honokiol inhibit migratory
potential of melanoma cells. Oncotarget. 7:7899–7912. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin JW, Chen JT, Hong CY, Lin YL, Wang KT,
Yao CJ, Lai GM and Chen RM: Honokiol traverses the blood-brain
barrier and induces apoptosis of neuroblastoma cells via an
intrinsic bax-mitochondrion-cytochrome c-caspase protease pathway.
Neuro Oncol. 14:302–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Proskuryakov SY, Konoplyannikov AG and
Gabai VL: Necrosis: A specific form of programmed cell death? Exp
Cell Res. 283:1–16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moriwaki K and Chan FK: RIP3: A molecular
switch for necrosis and inflammation. Genes Dev. 27:1640–1649.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai R, Xue W, Liu S, Petersen RB, Huang K
and Zheng L: Overexpression of glyceraldehyde 3-phosphate
dehydrogenase prevents neurovascular degeneration after retinal
injury. FASEB J. 29:2749–2758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin CJ, Chang YA, Lin YL, Liu SH, Chang CK
and Chen RM: Preclinical effects of honokiol on treating
glioblastoma multiforme via G1 phase arrest and cell apoptosis.
Phytomedicine. 23:517–527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin CJ, Chen TL, Tseng YY, Wu GJ, Hsieh
MH, Lin YW and Chen RM: Honokiol induces autophagic cell death in
malignant glioma through reactive oxygen species-mediated
regulation of the p53/PI3K/Akt/mTOR signaling pathway. Toxicol Appl
Pharmacol. 304:59–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hahm ER, Sakao K and Singh SV: Honokiol
activates reactive oxygen species-mediated cytoprotective autophagy
in human prostate cancer cells. Prostate. 74:1209–1221. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang M, Harashima N, Moritani T, Huang W
and Harada M: The roles of ROS and caspases in TRAIL-induced
apoptosis and necroptosis in human pancreatic cancer cells. PLoS
One. 10:e01273862015. View Article : Google Scholar : PubMed/NCBI
|