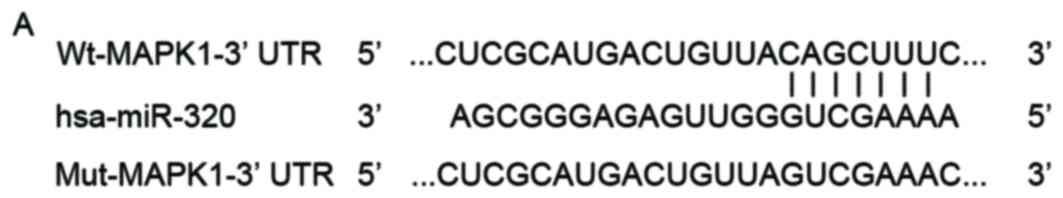Introduction
Ovarian cancer is the second most common cancer
worldwide, and the most fatal gynaecological malignancy of all
gynecological cancers, with over 238,700 newly diagnosed cases and
151,900 fatalities per year (1,2).
Epithelial ovarian cancer (EOC) accounts for ~90% of all ovarian
cancer cases, and consists of five subtypes, including high-grade
serous carcinoma (70%), low-grade serous carcinoma (<5%),
mucinous carcinoma (3%), endometrioid carcinoma (10%) and
clear-cell carcinoma (10%) (3).
Despite progress in the traditional treatments for EOC, the overall
survival rate for patients with this malignancy remains
dissatisfactory over the past 50 years (4). Furthermore, an increased number of
patients are presenting with EOC with local or distant metastasis
at the time of diagnosis, due to an absence of early diagnostic
biomarkers, and this results in poor prognosis and short survival
time (5). Therefore, further
investigations are necessary in order to elucidate the underlying
molecular mechanisms of EOC occurrence and progression, and
identify novel efficient targets for diagnosis, therapy and
prognosis of this disease.
MicroRNAs (miRNAs) represent a large family of
non-coding, single stranded, endogenous and short RNA molecules
with 18–25 nucleotides (6). miRNAs
regulate gene expression by base-pairing with the 3′ untranslated
regions (3′UTRs) of their target genes, resulting in translational
suppression or mRNA degradation, and ultimately controlling the
protein expression of target genes (7). It has previously been demonstrated
that miRNAs are important in various biological processes,
including cell proliferation, cell cycle, differentiation and
metastasis (8–10). miRNAs have been reported to be
downregulated or upregulated in a variety of human malignancies
(11–13). Furthermore, previous studies
demonstrated that deregulated miRNAs are involved in the formation
and progression of the majority of human cancers, including EOC
(14), bladder (15), gastric (16), glioma (17) and breast cancers (18). These abnormally expressed miRNAs
may function as oncogenes or tumor suppressor genes depending on
the roles of their target genes and tumor types (19). These findings suggest that miRNAs
may be useful in the diagnosis and prognosis of human cancers, and
may additionally act as therapeutic targets for their
treatment.
Abnormal expression of miR-320 has been reported in
multiple types of cancer, including breast (20,21),
gastric (22), colorectal
(23), glioma (24,25)
and bladder cancers (26).
However, the role of miR-320 in EOC remains to be elucidated. The
present study aimed to investigate the expression pattern and
regulatory role of miR-320 in EOC, and its associated underlying
mechanism.
Materials and methods
Ethical approval and human tissue
The present study was approved by the Ethics
Committee of Shengli Oilfield Central Hospital (Dongying, China).
In addition, written informed consent was obtained from all
patients. A total of 56 EOC tumor tissues and their paired adjacent
normal ovarian epithelium tissues were obtained from the Department
of Gynaecology and Obstetrics, Shengli Oilfield Central Hospital,
between 2012 and 2015. None of these EOC patients (n=56; female;
age, 39–72 years) were treated with other treatments prior to
surgery. Tissues specimens were immediately snap-frozen in liquid
nitrogen and stored at −70°C in a freezer.
Cell lines
A total of 4 EOC cell lines (CAOV3, OVCAR3, SKOV3,
ES-2) and the human normal ovarian epithelial cell line NOEC, were
purchased from American Type Culture Collection (Manassas, VA,
USA). EOC cells were cultured in RPMI-1640 medium supplemented with
10% fetal bovine serum and 1% penicillin/streptomycin, all obtained
from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). NOEC
cells were grown in Ham's F-12 medium (Gibco; Thermo Fisher
Scientific Inc.) with 10% FBS and 1% penicillin/streptomycin. All
cells were maintained in a humidified environment at 37°C with 5%
CO2.
Cell transfection
miR-320 mimics, the corresponding negative miRNA
mimics controls (miR-NC), small interfering (si)RNA targeting
mitogen activated protein kinase (MAPK; si-MAPK1) and its negative
control scrambled siRNA (si-NC) were synthesized by Shanghai
GenePharma Co., Ltd (Shanghai, China). The miR-320 mimics sequence
was 5′-AAAAGCUGGGUUGAGAGGGGCG-3′ and the miR-NC sequence was
5′-UUCUCCGAACGUGUCACGUTT-3′. The si-MAPK1 sequence was
5′-AGUUCGAGUAUACUUCAAGUU-3′ and the si-NC sequence was
5′-UUCUCCGAACGUGUCACGUTT-3′. Cells were seeded into 6-well plates
at a density of 50–60% confluence in FBS-free RPMI-1640 medium for
1 day prior to transfection. Cells were transfected with miR-320
mimics (100 pmol), miR-NC (100 pmol), si-MAPK1 (100 pmol) or si-NC
(100 pmol) by using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Cell culture medium was replaced with
RPMI-1640 medium containing 10% FBS at 8 h post-transfection.
Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) was performed to detect miR-320 and MAPK1 mRNA expression
at 48 h post-transfection. Western blotting analysis was applied to
detect MAPK1 protein expression at 72 h post-transfection. The Cell
Counting kit 8 (CCK8) and cell invasion assays were performed at 24
h and 48 h following transfection, respectively.
RNA extraction and RT-qPCR
Total RNA was isolated from tissues and cells using
a TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. For quantification
of miR-320, reverse transcription was conducted with TaqMan
MicroRNA Reverse Transcription Kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and followed by qPCR with TaqMan Human
MicroRNA assay kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The cycling conditions were as follows: 50°C for 2 min, 95°C
for 10 min, followed by 40 cycles of denaturation at 95°C for 15
sec and annealing/extension at 60°C for 1 min. For MAPK1 mRNA
expression, M-MLV Reverse Transcription system (Promega
Corporation, Madison, WI, USA) was used to synthesize cDNA. The
detection of MAPK1 mRNA expression was conducted using SYBR Premix
Ex Taq (Takara Biotechnology Co., Ltd, Dalian, China). The
thermocycling conditions were as follows: 5 min at 95°C, followed
by 40 cycles of 95°C for 30 sec and 65°C for 45 sec. U6 small
nuclear RNA and β-actin were used as internal standard references
for miR-320 and MAPK1, respectively. The primers sequences were
designed as follows: miR-320, 5′-ACACTCCAGCTGGGAAAAGCTGGGTTGAGA-3′
(forward) and 5′-TGGTGTCGTGGAGTCG-3′ (reverse); U6,
5′-CTCGCTTCGGCAGCACA-3′ (forward) and 5′-AACGCTTCACGAATTTGCGT-3′
(reverse); MAPK1, 5′-TGGATTCCCTGGTTCTCTCTAAAG-3′ (forward) and
5′-GGGTCTGTTTTCCGAGGATGA-3′ (reverse); and β-actin,
5′-CCTGGCACCCAGCACAAT-3′ (forward) and 5′-GCTGATCCACATCTGCTGGAA-3′
(reverse). Relative expression was quantified by the
2−ΔΔCq method (27).
CCK8 assay
Cell proliferation was evaluated by using a CCK8
assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan). A
total of 24 h following transfection, transfected cells were seeded
in 96-well plates (3,000 cells/well). Cells were then incubated in
a humidified environment at 37°C with 5% CO2 for 4
consecutive days. At every 24 h, a CCK8 assay was performed
according to the manufacturer's protocol. Briefly, 10 µl CCK8
reagent was added into each well. Following incubation at 37°C for
an additional 4 h, absorbance at a wavelength of 450 nm was
measured using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Each assay was performed in triplicate.
Cell invasion assay
Transwell chambers (8-mm pore size; Costar; Corning
Incorporated, Corning, NY, USA) precoated with Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA) were used to conduct the cell
invasion assay. A total of 500 µl RPMI-1640 medium containing 20%
FBS was added into the lower chamber, and 5×104
transfected cells in 200 µl FBS-free culture medium were plated in
the upper chamber. Following incubation for 48 h at 37°C, cells
that remained in the upper surface of the membrane were removed by
cotton swabs. The invaded cells were fixed with 4% paraformaldehyde
at room temperature for 10 min and stained with 0.1% crystal violet
at room temperature for 10 min. Following washing, five randomly
selected visual fields per membrane were photographed and counted
under an inverted fluorescence microscope (magnification, ×200;
CKX41; Olympus Corporation, Tokyo, Japan).
miR-320 target prediction
The computational methods TargetScan (www.targetscan.org) and PicTar (www.pictar.mdc-berlin.de/) were used to predict the
potential targets of miR-320.
Luciferase assay
Cells were seeded in 24-well plates at a density of
40–50% confluence. Following incubation overnight,
Lipofectamine® 2000 was employed to co-transfect cells
with miR-320 mimics or miR-NC, and psiCHECK wild-type MAPK1 3′UTR
luciferase plasmid (psiCHECK-Wt-MAPK1-3′UTR; Shanghai GenePharma
Co., Ltd) or psiCHECK mutant MAPK1 3′UTR luciferase plasmid
(psiCHECK-Mut-MAPK1-3′UTR; Shanghai GenePharma Co., Ltd). At 48 h
following transfection, the cells were harvested and subjected to
luciferase assay by using the Dual-Luciferase® Reporter
Assay system (Promega Corporation). Firefly luciferase activity was
normalized to Renilla luciferase activity.
Western blotting
Total protein was extracted from transfected cells
at 72 h post-transfection with ice-cold radioimmunoprecipitation
assay lysis buffer containing proteinase inhibitor (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). Concentrations of total protein
were detected using a bicinchoninic assay kit (Pierce; Thermo
Fisher Scientific, Inc.). Equal amounts protein (20 µg) were
resolved using SDS-PAGE on a 10% gel. Subsequently, proteins were
transferred to polyvinylidene difluoride membranes and then blocked
with 5% non-fat milk in Tris-buffered saline containing 0.1%
Tween-20 (TBST) at room temperature for 1 h. The membranes were
incubated with primary antibodies at 4°C overnight, followed by
washing with TBST three times and incubated with a goat anti-mouse
horseradish peroxidase-conjugated secondary antibody (1:5,000
dilution; catalog no. sc-2005; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) at room temperature for 2 h. Finally, protein
bands were visualized using an enhanced chemiluminescence solution
(Bio-Rad Laboratories, Inc.) and analyzed using Quantity One
software, version 4.62 (Bio-Rad Laboratories, Inc.). Primary
antibodies used in the present study included mouse anti-human
MAPK1 monoclonal antibody (1:1,000 dilution; catalog no. sc-81459;
Santa Cruz Biotechnology, Inc.) and mouse anti-human β-actin
monoclonal antibody (1:1,000 dilution; catalog no. sc-47778; Santa
Cruz Biotechnology, Inc.). β-actin was used as a loading
control.
Statistical analysis
Data are expressed as the mean ± standard deviation.
All statistical analyses were performed with Student's t-tests or
one-way analysis of variance using SPSS software, version 18.0
(SPSS, Inc., Chicago, IL, USA). The correlation between miR-320 and
MAPK1 mRNA expression was analyzed with Spearman's correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-320 expression is downregulated in
EOC tissues and cell lines
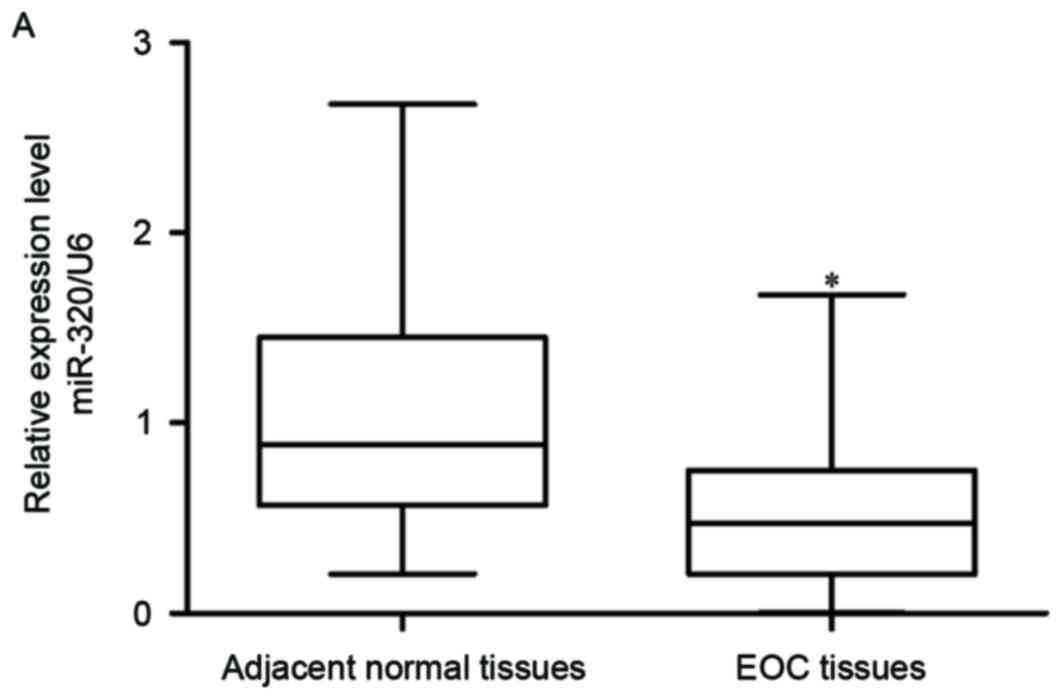
To assess miR-320 expression levels, RT-qPCR was
performed in 56 EOC tumor tissues and matched adjacent normal
ovarian epithelium tissues. The data indicated that expression
levels of miR-320 were decreased in EOC tissues compared with in
matched adjacent normal ovarian epithelium tissues (Fig. 1A; P<0.05). Following this,
miR-320 expression was quantified in a panel of EOC cell lines in
addition to the human normal ovarian epithelial cell line NOEC.
Compared with NOEC, miR-320 expression was significantly
downregulated in all four tested EOC cell lines (Fig. 1B).
Furthermore, correlation between miR-320 and the
clinicopathological variables of patients with EOC was
investigated. As presented in Table
I, miR-320 expression was strongly correlated with FIGO stage
(P=0.013) and lymph node metastasis (P=0.001). However, no
correlation was observed with other clinicopathological
characteristics, including age, differentiation and tumor size (all
P>0.05). These results suggested that miR-320 may be important
in EOC formation and progression.
 | Table I.Correlation of miRNA-320 with
clinical characteristics in patients with epithelial ovarian
cancer. |
Table I.
Correlation of miRNA-320 with
clinical characteristics in patients with epithelial ovarian
cancer.
|
|
| miR-320
expression |
|
|---|
|
|
|
|
|
|---|
| Features | No. of
patients | Low | High | P-value |
|---|
| Age (years) |
|
|
| 0.269 |
|
<60 | 29 | 14 | 15 |
|
|
≥60 | 27 | 17 | 10 |
|
| FIGO stage |
|
|
| 0.013 |
|
I–II | 30 | 12 | 18 |
|
|
III–IV | 26 | 19 | 7 |
|
|
Differentiation |
|
|
| 0.420 |
|
1/2 | 28 | 17 | 11 |
|
| 3 | 28 | 14 | 14 |
|
| Tumor size
(cm) |
|
|
| 0.243 |
|
<5 | 25 | 16 | 9 |
|
| ≥5 | 31 | 15 | 16 |
|
| Lymph node
metastasis |
|
|
| 0.001 |
|
Negative | 27 | 9 | 18 |
|
|
Positive | 29 | 22 | 7 |
|
Overexpression of miR-320 suppresses
cell proliferation and invasion in EOC
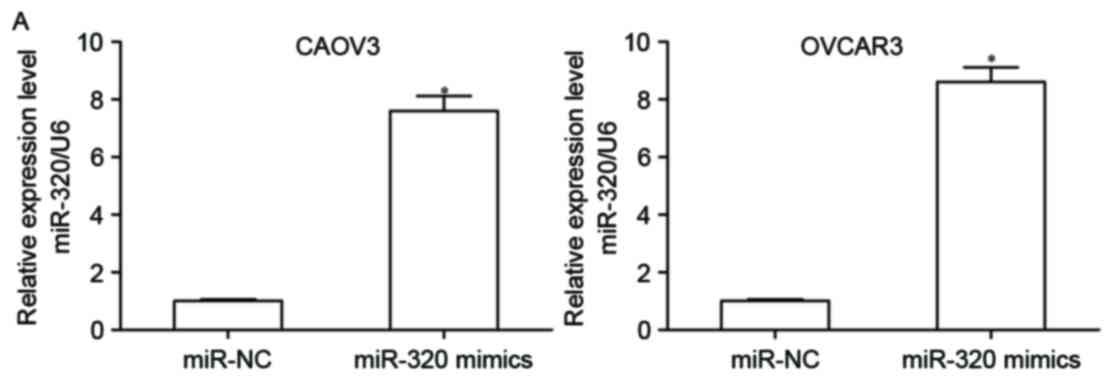
To explore the biological role of miR-320 in EOC, a
gain-of-function analysis was conducted. CAOV3 and OVCAR3 cells
were transfected with miR-320 mimics or miR-NC. A total of 48 h
following transfection, RT-qPCR was performed to detect miR-320
expression and it was observed that miR-320 expression levels in
CAOV3 and OVCAR3 cells were significantly increased following
transfection with miR-320 mimics (Fig.
2A; P<0.05). To determine if miR-320 contributes to EOC
progression, CCK8 and cell invasion assays were performed in CAOV3
and OVCAR3 cells following transfection with miR-320 mimics or
miR-NC. CCK8 assay demonstrated that upregulation of miR-320
decreased CAOV3 and OVCAR3 cell proliferation (Fig. 2B; P<0.05). Similarly,
overexpression of miR-320 resulted in a significant reduction of
cell invasion capacity of the CAOV3 and OVCAR3 cells (Fig. 2C; P<0.05). These findings
suggested that miR-320 may suppress EOC cell growth and
metastasis.
MAPK1 is a direct target of miR-320 in
EOC
To explore the mechanisms underlying the tumor
suppressive role of miR-320 in EOC, Targetscan and PicTar were used
to predict the potential targets of miR-320. As presented in
Fig. 3A, the seed sequence of
miR-320 was complementary to the 3′UTR of MAPK1. MAPK1 is
overexpressed in EOC tissues and cell lines (28,29),
and contributes to the tumorigenesis and tumor development in EOC
(28), which led to the hypothesis
that MAPK1 may be a direct target of miR-320 in EOC. To determine
whether MAPK1 is a direct target gene of miR-320, a luciferase
reporter assay was performed in CAOV3 and OVCAR3 cells
co-transfected with miR-320 mimics or miR-NC, and luciferase
reporter vector containing the wild type or mutant 3′UTR of MAPK1.
The results demonstrated that transfection of miR-320 resulted in a
significant inhibition of luciferase activities by
psiCHECK-Wt-MAPK1-3′UTR (Fig. 3B;
P<0.05). However, these repressive effects of miR-320 on
luciferase activities were reversed following transfection with
psiCHECK-Mut-MAPK1-3′UTR. The present study then sought to
investigate whether ectopic expression of miR-320 regulated
endogenous MAPK1 expression. RT-qPCR and western blotting verified
that upregulation of miR-320 suppressed MAPK1 expression in CAOV3
and OVCAR3 cells at the mRNA (Fig.
3C; P<0.05) and protein (Fig.
3D; P<0.05) levels. Collectively, these results suggested
that miR-320 decreased MAPK1 expression by targeting specific sites
within the 3′UTR of MAPK1.
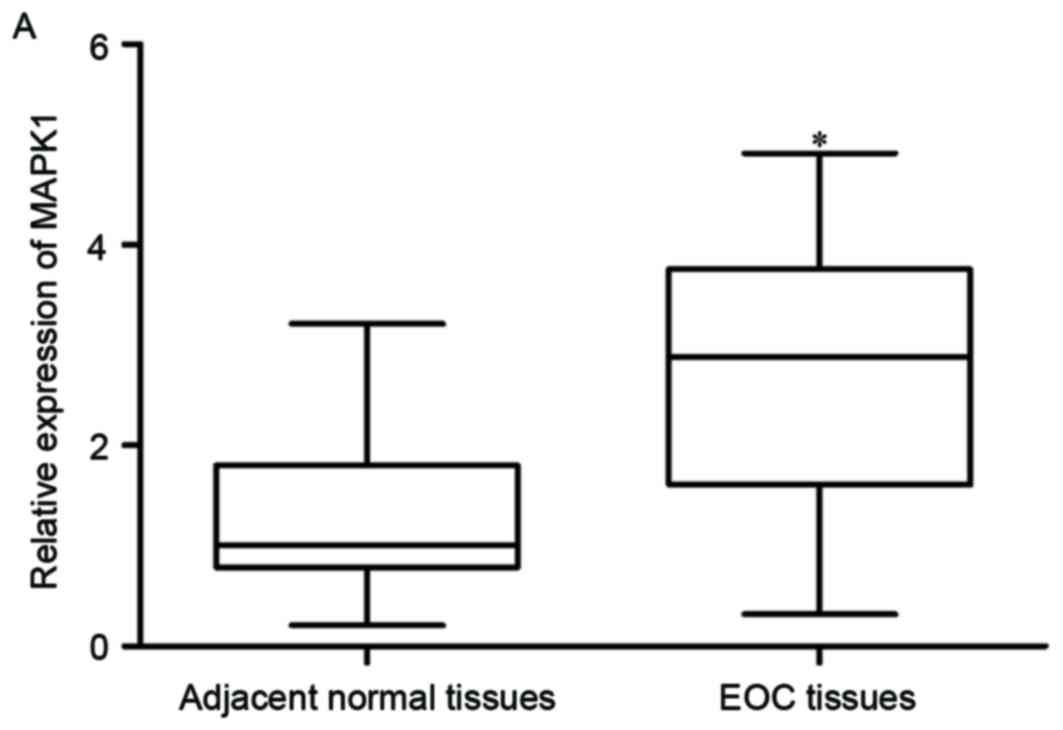
MAPK1 is upregulated in EOC tissues
and negatively correlated with miR-320 expression
MAPK1 was identified as a direct target gene of
miR-320 in EOC; therefore, MAPK1 expression in EOC tissues and
matched adjacent normal ovarian epithelium tissues was measured. As
expected, MAPK1 mRNA was significantly increased in EOC tissues,
compared with normal ovarian epithelium tissues (Fig. 4A; P<0.05). Furthermore,
Spearman's correlation analysis indicated an inverse correlation
between MAPK1 mRNA and miR-320 expression (Fig. 4B; r=-0.4078; P=0.0018).
miR-320 inhibits cell proliferation
and invasion of EOC by downregulation of MAPK1
To evaluate the role of MAPK1 in EOC, a
loss-of-function assay was performed. CAOV3 and OVCAR3 cells were
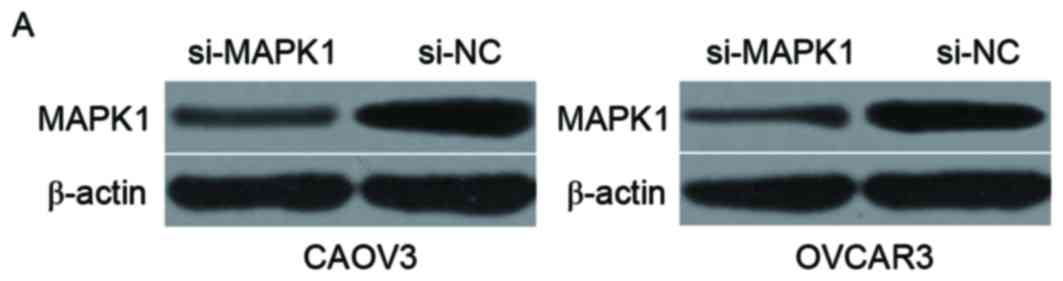
injected with si-MAPK1 or si-NC. Western blotting verified that
MAPK1 protein was downregulated in CAOV3 and OVCAR3 cells following
transfection with si-MAPK1 (Fig.
5A; P<0.05). CCK8 assay demonstrated that downregulation of
MAPK1 suppressed proliferation in CAOV3 and OVCAR3 cells, which was
consistent with miR-320 overexpression (Fig. 5B; P<0.05). Furthermore,
consistent with miR-320 mimics, cell invasive abilities were
decreased in si-MAPK1-transfected CAOV3 and OVCAR3 cell lines
(Fig. 5C; P<0.05). These
results demonstrated that miR-320 inhibits cell proliferation and
invasion of EOC by negative regulation of MAPK1.
Discussion
Previous studies have suggested that miRNAs are
important in tumorigenesis and tumor development, and thus may
prove as novel targets for the treatment and prognosis of various
cancers (30,31). Abnormal expression of miR-320 has
been reported in various types of cancers, including breast
(20,21), gastric (22), colorectal (23), glioma (24,25),
bladder (26) and cervical cancers
(32). In addition, expression
levels of miR-320 have been demonstrated to be correlated with
clinicopathological variables of multiple human cancers. For
example, in non-small cell lung cancer, miR-320 is correlated with
TNM classification and metastasis (33). In breast cancer, a significant
correlation has been observed between low miR-320 expression level
and tumor size, clinical stage, lymph node metastasis and distant
metastasis (34). The present
study measured miR-320 expression in EOC tissues and cell lines.
Data from RT-qPCR demonstrated that miR-320 was significantly
downregulated in EOC tissues and cell lines. Low miR-320 expression
was significantly correlated with FIGO stage and lymph node
metastasis of EOC patients. These findings suggested that miR-320
deregulation is a common event in human cancer, and may be
important in tumorigenesis and tumor development.
Previous studies have demonstrated that miR-320
regulates the formation and progression of human cancer.
Introduction of miR-320 inhibits cell proliferation in osteosarcoma
(35), colorectal adenoma
(36), non-small cell lung cancer
(33), cervical cancer (32), glioma (25), multiple myeloma (37) and breast cancer (21). Additionally, upregulation of
miR-320 results in a significant decrease in the motility of breast
cancer (20,21), salivary adenoid cystic carcinoma
(38), nasopharyngeal carcinoma
(39), glioma (24) and non-small cell lung cancer
(33). It has previously been
demonstrated that miR-320 promotes Fluorouracil resistance in
pancreatic cancer (40), enhances
the chemosensitivity of tamoxifen-resistant breast cancer cells to
tamoxifen (41), improves the
chemosensitivity and radiosensitivity of colon cancer (42), and represses tube formation of
vascular endothelial cells in oral cancer (43). In the present study, the CCK8 assay
revealed that miR-320 inhibited cell growth in EOC cells. The cell
invasion assay indicated that restoration of expression of miR-320
decreased invasion activity in EOC cells. Collectively, these
experiments indicated that miR-320 may act as a tumor suppressor in
human cancers, and may be used as a novel molecular therapeutic
target for anti-tumor treatments.
The present study then aimed to investigate the
molecular mechanism by which miR-320 acts as a tumor suppressor in
EOC. Previous studies identified numerous targets of miR-320
including E2F transcription factor 1 (35), cyclin dependent kinase 6 (36), MCI1 (32), PBX Homeobox 3 (37), RAB11A (21) and metadherin (20). To explore the targets of miR-320,
Targetscan and PicTar were used. MAPK1 was predicated to be a
potential target of miR-320. A luciferase reporter assay was then
performed to verify that miR-320 directly targeted the 3′UTR of
MAPK1. Subsequently, it was demonstrated that miR-320 negatively
regulated MAPK1 expression at the mRNA and protein level in EOC
cells. Furthermore, MAPK1 expression was upregulated in EOC tissues
and negatively correlated with miR-320 expression. Additionally,
consistent with miR-320 overexpression, cell proliferation and
invasion were decreased in si-MAPK1-transfected EOC cells. These
results verified MAPK1 as a novel direct target of miR-320 in
EOC.
The MAPK signaling cascades are composed of
membrane-to-nucleus signaling modules which are important in
multiple physiological processes (44). MAPK1, additionally termed,
extracellular regulated kinase 2, has been reported to be
abnormally expressed in various human cancers, including cervical
(45), myeloma (46), sacral chordoma (47), non-small cell lung cancer (48) and gastric cancer (49). Rahman et al (29) reported that MAPK1 is highly
expressed in ovarian cancer tissues and cell lines (28,29).
Functional assays have demonstrated that MAPK1 underexpression
suppresses growth and metastasis in ovarian cancer SKOV3 cells
(28). In accordance with previous
studies, the results of the present study demonstrated that MAPK1
was significantly upregulated in EOC tissues, and the
downregulation of MAPK1 repressed the proliferation and invasive
ability of EOC cells. MAPK1 may be investigated as a useful
therapeutic target for the treatment of patients with EOC.
In conclusion, the results of the present study
demonstrated that miR-320 was decreased in EOC tissues and cell
lines. Low miR-320 expression was significantly correlated with
FIGO stage and lymph node metastasis of EOC patients. Furthermore,
ectopic expression of miR-320 inhibited EOC cell proliferation and
invasion through directly targeting MAPK1. These findings may
provide a novel insight into the potential carcinogenic and
progressive mechanisms in EOC, and may be used in the development
of novel treatment strategies for patients with this malignancy.
Further investigations are required to explore whether the
potential of miR-320 may be fully realised in EOC.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prat J: Ovarian carcinomas: Five distinct
diseases with different origins, genetic alterations, and
clinicopathological features. Virchows Arch. 460:237–249. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Kim S and Kim IM: Regulation of
metastasis by microRNAs in ovarian cancer. Front Oncol. 4:1432014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tung CS, Wong KK and Mok SC: Biomarker
discovery in ovarian cancer. Womens Health (Lond). 4:27–40. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vasudevan S: Posttranscriptional
upregulation by microRNAs. Wiley Interdiscip Rev RNA. 3:311–330.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seashols-Williams SJ, Budd W, Clark GC, Wu
Q, Daniel R, Dragoescu E and Zehner ZE: miR-9 acts as an oncomiR in
prostate cancer through multiple pathways that drive tumour
progression and metastasis. PLoS One. 11:e01596012016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sulaiman SA, Ab Mutalib NS and Jamal R:
miR-200c regulation of metastases in ovarian cancer: Potential role
in epithelial and mesenchymal transition. Front Pharmacol.
7:2712016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao L, Zhou H, Li XP, Chen J, Fang C, Mao
CX, Cui JJ, Zhang W, Zhou HH, Yin JY and Liu ZQ: MicroRNA-138 acts
as a tumor suppressor in non small cell lung cancer via targeting
YAP1. Oncotarget. 7:40038–40046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Das DK, Naidoo M, Ilboudo A, Park JY, Ali
T, Krampis K, Robinson BD, Osborne JR and Ogunwobi OO: miR-1207-3p
regulates the androgen receptor in prostate cancer via
FNDC1/fibronectin. Exp Cell Res. 348:190–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gopalan V, Islam F, Pillai S, Tang JC,
Tong DK, Law S, Chan KW and Lam AK: Overexpression of microRNA-1288
in oesophageal squamous cell carcinoma. Exp Cell Res. 348:146–154.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Dou Y and Sheng M: Inhibition of
microRNA-383 has tumor suppressive effect in human epithelial
ovarian cancer through the action on caspase-2 gene. Biomed
Pharmacother. 83:1286–1294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao J, Lin HY, Zhu YY, Zhu YP and Chen
LW: MiR-126 regulates proliferation and invasion in the bladder
cancer BLS cell line by targeting the PIK3R2-mediated PI3K/Akt
signaling pathway. Onco Targets Ther. 9:5181–5193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu D, Niu X, Pan H, Zhou Y, Zhang Z, Qu P
and Zhou J: Tumor-suppressing effects of microRNA-429 in human
renal cell carcinoma via the downregulation of Sp1. Oncol Lett.
12:2906–2911. 2016.PubMed/NCBI
|
|
17
|
Zhou Y, Liu Y, Hu C and Jiang Y:
MicroRNA-16 inhibits the proliferation, migration and invasion of
glioma cells by targeting Sal-like protein 4. Int J Mol Med.
38:1768–1776. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan Y, Jiao G, Wang C, Yang J and Yang W:
MicroRNA-421 inhibits breast cancer metastasis by targeting
metastasis associated 1. Biomed Pharmacother. 83:1398–1406. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu J, Wang JG, Zhang L, Yang HP, Wang L,
Ding D, Chen Q, Yang WL, Ren KH, Zhou D, et al: MicroRNA-320a
inhibits breast cancer metastasis by targeting metadherin.
Oncotarget. 7:38612–38625. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang B, Yang Z, Wang H, Cao Z, Zhao Y,
Gong C, Ma L, Wang X, Hu X and Chen S: MicroRNA-320a inhibits
proliferation and invasion of breast cancer cells by targeting
RAB11A. Am J Cancer Res. 5:2719–2729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Zeng J, Pan J, Geng X, Li L, Wu J,
Song P, Wang Y, Liu J and Wang L: MiR-320a inhibits gastric
carcinoma by targeting activity in the FoxM1-P27KIP1 axis.
Oncotarget. 7:29275–29286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao H, Dong T, Zhou H, Wang L, Huang A,
Feng B, Quan Y, Jin R, Zhang W, Sun J, et al: miR-320a suppresses
colorectal cancer progression by targeting Rac1. Carcinogenesis.
35:886–895. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo T, Feng Y, Liu Q, Yang X, Jiang T,
Chen Y and Zhang Q: MicroRNA-320a suppresses in GBM patients and
modulates glioma cell functions by targeting IGF-1R. Tumour Biol.
35:11269–11275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun JY, Xiao WZ, Wang F, Wang YQ, Zhu YH,
Wu YF, Miao ZL and Lin YC: MicroRNA-320 inhibits cell proliferation
in glioma by targeting E2F1. Mol Med Rep. 12:2355–2359. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shang C, Zhang H, Guo Y, Hong Y, Liu Y and
Xue Y: MiR-320a down-regulation mediates bladder carcinoma invasion
by targeting ITGB3. Mol Biol Rep. 41:2521–2527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yiwei T, Hua H, Hui G, Mao M and Xiang L:
HOTAIR interacting with MAPK1 regulates ovarian cancer skov3 cell
proliferation, migration, and invasion. Med Sci Monit.
21:1856–1863. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rahman MT, Nakayama K, Rahman M, Katagiri
H, Katagiri A, Ishibashi T, Ishikawa M, Sato E, Iida K, Nakayama N,
et al: KRAS and MAPK1 gene amplification in type II ovarian
carcinomas. Int J Mol Sci. 14:13748–13762. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu WK, Lee CW, Cho CH, Fan D, Wu K, Yu J
and Sung JJ: MicroRNA dysregulation in gastric cancer: A new player
enters the game. Oncogene. 29:5761–5771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miao J, Wu S, Peng Z, Tania M and Zhang C:
MicroRNAs in osteosarcoma: Diagnostic and therapeutic aspects.
Tumour Biol. 34:2093–2098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang T, Zou P, Wang T, Xiang J, Cheng J,
Chen D and Zhou J: Down-regulation of miR-320 associated with
cancer progression and cell apoptosis via targeting Mcl-1 in
cervical cancer. Tumour Biol. 37:8931–8940. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lei T, Zhu Y, Jiang C, Wang Y, Fu J, Fan Z
and Qin H: MicroRNA-320 was downregulated in non-small cell lung
cancer and inhibited cell proliferation, migration and invasion by
targeting fatty acid synthase. Mol Med Rep. 14:1255–1262. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang H, Yu J, Wang L, Ding D, Zhang L, Chu
C, Chen Q, Xu Z, Zou Q and Liu X: miR-320a is an independent
prognostic biomarker for invasive breast cancer. Oncol Lett.
8:1043–1050. 2014.PubMed/NCBI
|
|
35
|
Wu H, Li W, Zhang M, Zhu S, Zhang D and
Wang X: Inhibitory roles of miR-320 in osteosarcoma via regulating
E2F1. J Cancer Res Ther. 12:68–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tadano T, Kakuta Y, Hamada S, Shimodaira
Y, Kuroha M, Kawakami Y, Kimura T, Shiga H, Endo K, Masamune A, et
al: MicroRNA-320 family is downregulated in colorectal adenoma and
affects tumor proliferation by targeting CDK6. World J Gastrointest
Oncol. 8:532–542. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu Y, Wu D, Wang J, Li Y, Chai X and Kang
Q: miR-320a regulates cell proliferation and apoptosis in multiple
myeloma by targeting pre-B-cell leukemia transcription factor 3.
Biochem Biophys Res Commun. 473:1315–1320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun L, Liu B, Lin Z, Yao Y, Chen Y, Li Y,
Chen J, Yu D, Tang Z, Wang B, et al: MiR-320a acts as a prognostic
factor and Inhibits metastasis of salivary adenoid cystic carcinoma
by targeting ITGB3. Mol Cancer. 14:962015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qi X, Li J, Zhou C, Lv C and Tian M:
MicroRNA-320a inhibits cell proliferation, migration and invasion
by targeting BMI-1 in nasopharyngeal carcinoma. FEBS Lett.
588:3732–3738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang W, Zhao L, Wei X, Wang L, Liu S, Yang
Y, Wang F, Sun G, Zhang J, Ma Y, et al: MicroRNA-320a promotes 5-FU
resistance in human pancreatic cancer cells. Sci Rep. 6:276412016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu M, Ding K, Zhang G, Yin M, Yao G, Tian
H, Lian J, Liu L, Liang M, Zhu T and Sun F: MicroRNA-320a
sensitizes tamoxifen-resistant breast cancer cells to tamoxifen by
targeting ARPP-19 and ERRγ. Sci Rep. 5:87352015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wan LY, Deng J, Xiang XJ, Zhang L, Yu F,
Chen J, Sun Z, Feng M and Xiong JP: miR-320 enhances the
sensitivity of human colon cancer cells to chemoradiotherapy in
vitro by targeting FOXM1. Biochem Biophys Res Commun. 457:125–132.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu YY, Chen YL, Jao YC, Hsieh IS, Chang KC
and Hong TM: miR-320 regulates tumor angiogenesis driven by
vascular endothelial cells in oral cancer by silencing neuropilin
1. Angiogenesis. 17:247–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995.PubMed/NCBI
|
|
45
|
Li XW, Tuergan M and Abulizi G: Expression
of MAPK1 in cervical cancer and effect of MAPK1 gene silencing on
epithelial-mesenchymal transition, invasion and metastasis. Asian
Pac J Trop Med. 8:937–943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tsubaki M, Takeda T, Ogawa N, Sakamoto K,
Shimaoka H, Fujita A, Itoh T, Imano M, Ishizaka T, Satou T and
Nishida S: Overexpression of survivin via activation of ERK1/2,
Akt, and NF-κB plays a central role in vincristine resistance in
multiple myeloma cells. Leuk Res. 39:445–452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang K, Chen H, Zhang B, Sun J, Lu J,
Chen K and Yang H: Overexpression of Raf-1 and ERK1/2 in sacral
chordoma and association with tumor recurrence. Int J Clin Exp
Pathol. 8:608–614. 2015.PubMed/NCBI
|
|
48
|
You B, Yang YL, Xu Z, Dai Y, Liu S, Mao
JH, Tetsu O, Li H, Jablons DM and You L: Inhibition of ERK1/2
down-regulates the Hippo/YAP signaling pathway in human NSCLC
cells. Oncotarget. 6:4357–4368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fei B and Wu H: MiR-378 inhibits
progression of human gastric cancer MGC-803 cells by targeting
MAPK1 in vitro. Oncol Res. 20:557–564. 2012. View Article : Google Scholar : PubMed/NCBI
|