Introduction
Chronic hepatitis B virus (HBV) infection is an
extremely serious health problem worldwide with a number of ensuing
complications including chronic hepatitis, liver cirrhosis and
hepatocellular carcinoma (1,2).
Statistical data indicate that ~400 million people are chronically
infected with HBV (3). It has been
reported that cellular immune responses serve an important role in
preventing the deterioration of HBV infection (4). HBV-specific cytotoxic T lymphocytes
(CTLs) are known to serve a critical role in cellular immune
responses; efficient estimation of their numbers significantly
improves the study of the viral clearance and disease pathogenesis
(5–7). For the identification of HBV-specific
CTLs, the human leukocyte antigen (HLA)-A*020-restricted p-HBV core
antigen (HBcAg18–27) epitope C18–27 is a
perfect candidate. First, HLA-A2 is the most prevalent HLA allele
globally so this epitope may be used for targeting virus-specific
CTLs in almost half the population (8). Second, the therapeutic
C18–27 peptide has been detected in the majority of
HLA-A2.1 patients with HBV (9,10).
Third, it has been reported that the presence of valine at the
C-terminal of C18–27 peptide is associated with severe
liver inflammation in Chinese patients with chronic HBV infection
(11). Together, these reasons
demonstrate that the characterization of C18–27-specific
T cells with high sensitivity is vital for the development of
immunotherapeutic approaches to diseases caused by HBV.
A wide range of assays have been developed to
measure T-cell immune responses (12) and among them peptide-major
histocompatibility complex (pMHC) tetramers are the most common and
standard methods (13). The
fluorescence-labeled pMHC tetramer binds to a specific T-cell
receptor (TCR) on the surface of a T cell using the nominal peptide
imitating the corresponding antigenic epitope. This enables the
detection of T cells by flow cytometry ex vivo (14). The pMHC tetramer technique is a
powerful method to study T-cells, allowing direct and rapid
visualization and quantification (15). It should be noted that the pMHC
tetramers technique has its own drawbacks and remains under
improvement. Phycoerythrin (PE) is the most commonly used organic
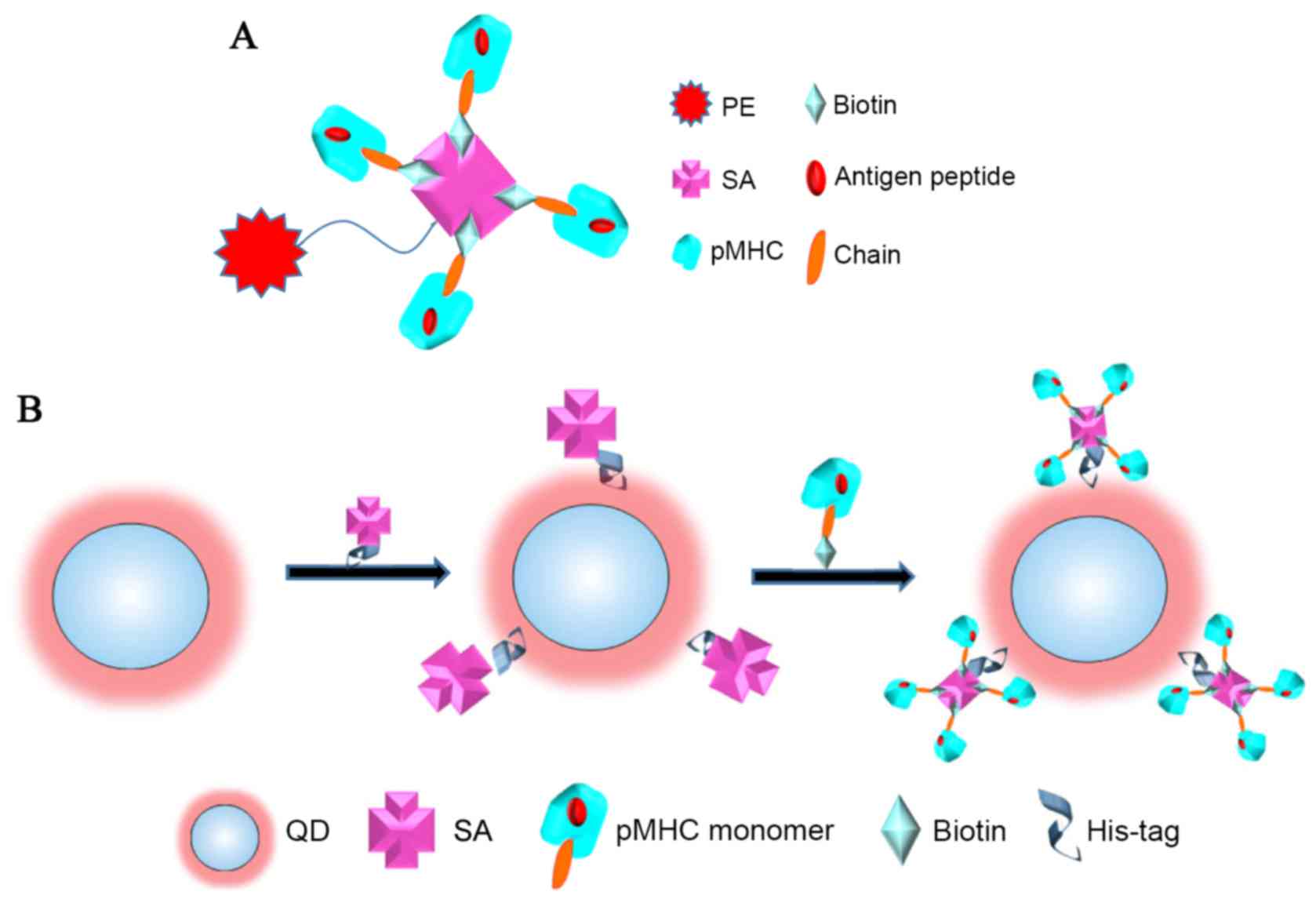
fluorochrome for labeling tetramers (16). Its size is ~0.5–1 nm (17) meaning that normally just one avidin
can be attached to a single PE organic fluorophore. The PE/pMHC
tetramers usually contain four biotinylated pMHC monomers resulting
in an avidin molecule has just four biotin-binding sites (18; as
illustrated in Fig. 1A). Thus,
they often fail to characterize CTLs where the interaction between
pMHC and TCR is relatively weak.
The new emerging inorganic quantum dot-based
multimers exhibit promising applications in cellular labeling and
possess numerous advantages over conventional tetramers.
Semiconductor nanocrystal QDs are spherical particles with
diameters typically ranging between 2–20 nm, and consist of a
semiconductor core (including CdSe and CdTe) alone or coated with a
shell. The shell of the QD (e.g., ZnS) enhances the quantum yield
and protects the core from oxidation (19). QD bioconjugates have been found to
be 2,600-fold more resistant to photobleaching than PE (20). The narrow emission and broad
excitation spectra from different QDs can be excited by a single
light source (21). In addition, a
single QD can be labeled with several proteins simultaneously. It
has been reported that ~15–20 maltose binding proteins can be
stably coated on each 6 nm diameter QD (22). Thus, a number of streptavidin
molecules may be attached to one QD nanoparticle, leading to the
QD/pMHC multimers carrying more pMHC monomers compared with
standard pMHC tetramers (as illustrated in Fig. 1B). These QD/pMHC multimers offer a
unique architecture for increasing the affinity of the TCR-pMHC
interaction and may perform higher detection frequencies than
PE/pMHC tetramers.
To date, a number of authors have made the use of
QD/pMHC multimers for immunophenotyping (23–25).
However, to the best of our knowledge, QD/pMHC multimers have not
been used for the identification of the C18–27 epitope
which serves an important role in the HBV infection. Thus, the
present study used the QD/pMHC multimers technique to detect the
ex vivo expansion of C18–27-specific T cells by
flow cytometry with a single 488-nm excitation light source. The
aim of the current study was to examine whether QD/pMHC multimers
can be used to stain C18–27-specific T cells and if the
technique were preferable to standard pMHC tetramers.
Materials and methods
Materials
The CdTe/CdS/ZnS core/shell/shell QDs were purchased
from Beida Jubang Science & Technology Co. Ltd. (Beijing,
China) and the streptavidin (SA) from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Corning Lymphocyte Serum-free medium was used
for T cell culture and purchased from Zhao Sheng Co., Ltd.
(Beijing, China). The transporters associated with antigen
processing-deficient HLA-A2-positive T2 cell line was obtained from
the Guangxi Key Laboratory of Biological Targeting Diagnosis and
Therapy Research (Guangxi, China). The K562 cell line (cat no.
CCL-243) was purchased from Xiangf Biotechnology Co., Ltd.
(Shanghai, Beijing). Peptides SLYNTVATL (SL9) and FLPSDFFPSV
(C18–27) were synthesized by the Chinese Peptide Company
(Hangzhou, China). Biotinylated pMHC monomers were from Beijing
QuantoBio Biotechnology Co., Ltd. (Beijing, China). All chemicals
used were of the highest purity available.
Synthesis of water soluble QD/pMHC
multimers and PE/pMHC tetramers
QDs with emission maximum of 635 nm were used in the
present study. QD-SA conjugates were prepared via metal-affinity
interactions between a polyhistidine (His)-tag and Zn on the
surface of the QDs. The His-tag SA (HSA) was prepared as previously
described (24). Then, 10 µl of 25
µM HSA was incubated with 10 µl of 5 µM QDs in DMSO for 2 h at room
temperature to yield QD-SA. The bioconjugates were purified by
membrane filtration and washed with DMSO three times. Then, 10 µl
QD-SA was added to 50 µl biotinylated pMHC monomers solution (0.1
mM) and stirred in the dark for 10 min; this step was repeated
several times and the multimers obtained were purified using a 100
kDa membrane filter. PE/pMHC tetramers were obtained by a similar
procedure but by adding PE-SA to biotinylated pMHC monomers
solution.
Characterization of QDs and QD
bioconjugates
The variety of QDs used in the present study was
designated CdTe/CdS/ZnS core/shell/shell QDs. The surface
properties of the QDs were characterized by transmission electron
microscopy (TEM; H7650; JEOL, Ltd., Tokyo, Japan) and
high-resolution TEM (JEM-2100F; JEOL, Ltd.). QD-bioconjugates were
characterized by TEM and dynamic light scattering (DLS; Malvern
Zetasizer; Malvern Instruments, Ltd., Malvern, UK). A fluorescence
spectrophotometer (F-7000; Hitachi, Ltd., Tokyo, Japan) was used to
characterize the fluorescence properties of the nanocrystals.
Ex vivo expansion of
HBcAg18-27-specific T-cells
Peripheral blood mononuclear cells were isolated
from 100 ml whole heparinized blood of healthy HLA-A*0201
volunteers by Ficoll/Hypaque density gradient centrifugation with
120 × g at 25°C for 20 min (Sigma-Aldrich; Merck KGaA). HLA class I
typing and A2 subtyping was performed by sequence-specific primer
polymerase chain reaction (PCR). T2 cells in serum-free
HEPES-buffered RPMI 1640 were pulsed with 40 µg/ml pHBV
C18–27 (T2/C18–27) at 37°C for 4 h and
irradiated by 200 Gy as stimulating cells. Effector cells were
designated into two groups. One group was peripheral blood
lymphocyte cells (PBLs), obtained by adherence to plastic for 1 h
in complete RPMI-1640 medium (containing 2 mM L-glutamine, 100 U/ml
each of penicillin and streptomycin, 1 mM sodium pyruvate, 1X MEM
nonessential amino acids and 10% autologous serum). The other group
was CD8+ T cells immunobead-purified from PBLs. The
effector cells were cocultured with stimulating cells at a ratio of
10:1 at 37°C under 5% CO2 and 20 IU/ml human
interleukin-2 and 10 ng/ml human interleukin-7 were added into the
coculture medium every 3 days. Lymphocytes were re-stimulated with
C18–27-pulsed irradiated T2 cells each week.
The study was approved by the Medical Ethics
Committee of Guangxi Medical University (Nanning, China). Written
informed consent was obtained from all subjects prior to enrolment
in the present study.
Analysis of T2-MHC-I binding
affinity
T2/C18–27 cells were incubated at 37°C
for 4 h in a 5% CO2 atmosphere and irradiated at 200 Gy.
Expression of MHC-I on T2/C18–27 and T2 cells subjected
to the same procedures was determined by staining with fluorescein
isothiocyanate (FITC)-conjugated anti-HLA-A2 monoclonal antibody
(mAb) BB7.2. The fluorescence index (FI) was calculated as follows:
FI=(mean FITC fluorescence with peptide C18–27-mean FITC
fluorescence without peptide)/(mean FITC fluorescence without
peptide). Peptides with FI>1 were considered as high-affinity
epitopes.
Interferon (IFN)-γ detection
assay
To ensure that the T cells were activated, secretion
of IFN-γ by the C18–27-specific T cell cultures was
evaluated by ELISA. OptEIA sets (BD Pharmingen, San Diego, CA, USA)
were used to measure the concentrations of IFN-γ in supernatants of
CTLs restimulated for 40 h with peptide-pulsed T2 cells. T2 were
pulsed with peptides at concentrations of 40 µg/ml and used at the
T cell: T2 cell ratio of 1:10. The IFN-γ in supernatants of
unstimulated T and T2 cells served as negative control.
Peptide-specific CTL cytotoxicity
assay
Cytotoxicity of C18–27-specific CTLs was
tested by a lactate dehydrogenase (LDH) release assay. The effector
cells were C18–27-specific T-cells from ex vivo
culture. Target cells were T2 pulsed with FLPSDFFPSV
(T2/C18–27) and were cocultured at effector/target
ratios of 10:1, 20:1 or 50:1 at 37°C under 5% CO2 for 4
h. K562 and T2 cells pulsed with SLYNTVATL (T2/SL9) were negative
control groups. Each assay was performed in triplicate. Percent
specific lysis=[(experimental release-spontaneous release)/(maximum
release-spontaneous release)] × 100.
Characterization of peptide-specific
T-cells by flow cytometry
In order to avoid the interference of non-specific
cells, the effector T cells were divided into two groups:
Peripheral PBLs and purified CD8+ T cells. Following two
weeks in vitro culture, PBL and CD8+ T cells were
collected and simultaneously labeled with FITC-conjugated anti CD8
mAb and PE/tetramers or QD/multimers. Four-color flow cytometry
analysis was performed on a Coulter Epics XL cytometer with a
single 488-nm excitation light source (Beckman Coulter, Inc., Brea,
CA, USA). The flow cytometry data were analyzed in real time using
Expo32 ADC software version 1.1C (Beckman Coulter).
Quantum dot cytotoxicity assay
For the cytotoxicity experiment, the harvested PBLs
were loaded into 96-well microtiter plates and incubated with
quantum dot/pMHC multimers (concentrations ranging between 0 and 5
µM) for 24 h at 37°C. Each well contained 5,000 cells and samples
were performed in triplicate. Then, 10 µl of the Cell Counting
Kit-8 (CCK-8; Sigma-Aldrich; Merck KGaA) solution was added to each
well and the cells were incubated for another 2 h at 37°C with 5%
CO2. The absorbance of samples was measured at 450 nm by
UV-vis with a Multiscan GO (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The number of cells was proportional to the
optical density (OD) value and non-treated group was expressed as
100%.
Statistical analysis
Data from the experimental and control groups were
analysed using one-way analysis of variance (ANOVA) followed by
post hoc least significant differences, Student-Neuman-Keuls or
Bonferroni tests for multiple comparisons. Data from the CCK-8
experiment were analysed using two-way ANOVA. P<0.05 was
considered to indicate a statistically significant difference. Data
are reported as the mean ± standard deviation. Statistical
comparisons were performed using GraphPad Prism software version
6.0 (GraphPad Software,. Inc., La Jolla, CA, USA).
Results
Characterization of QDs and QD
bioconjugates
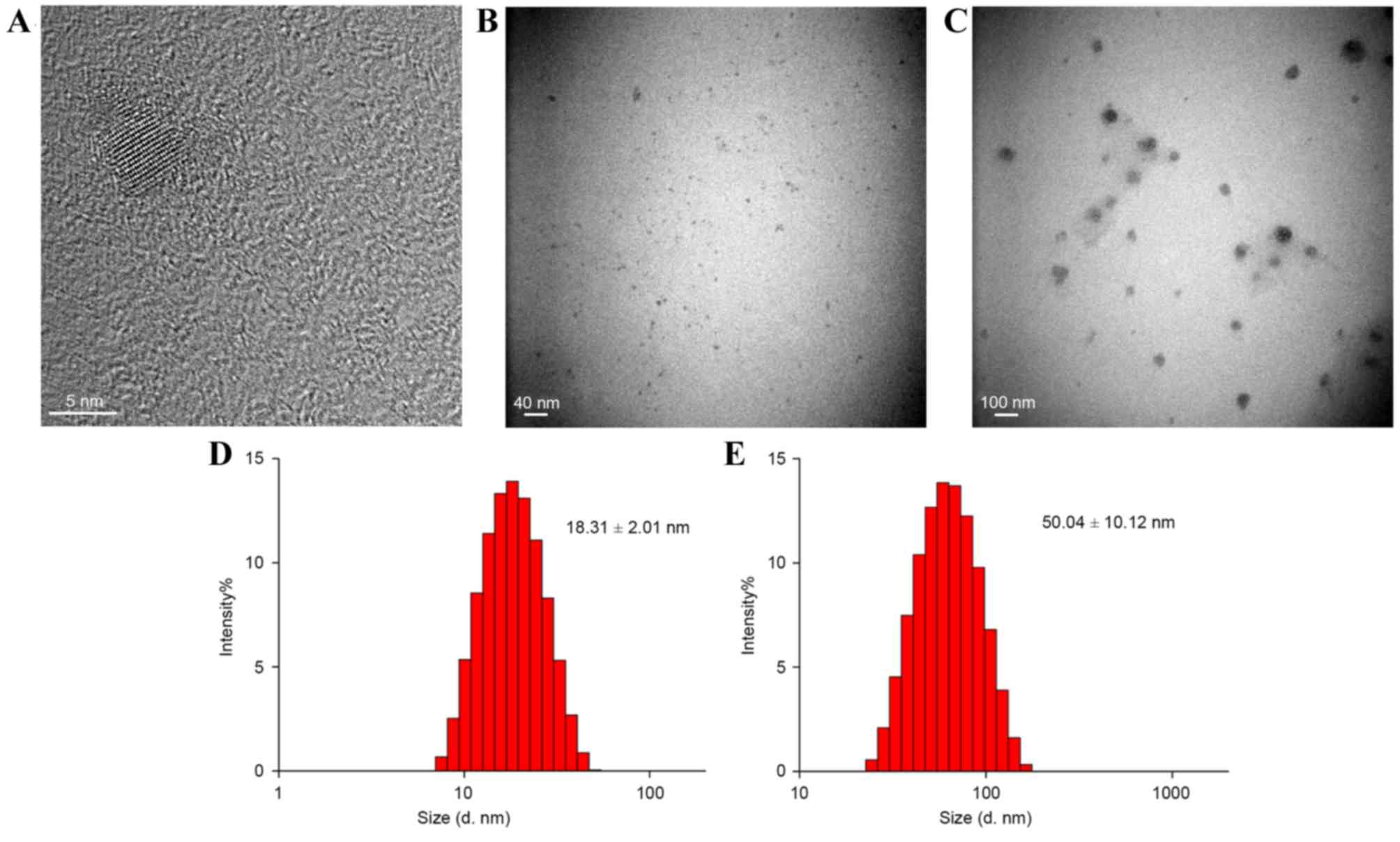
TEM and DLS were used for the characterization of
QDs, QD-SA and QD/pMHC multimers (Fig.
2). Results demonstrated that the diameter of QDs, QD-SA and
QD/pMHC multimers were ~6.0, 18.31±2.01 and 50.04±10.12 nm,
respectively (Fig. 2). The
polydispersity index of all was <0.5. The fluorescence emission
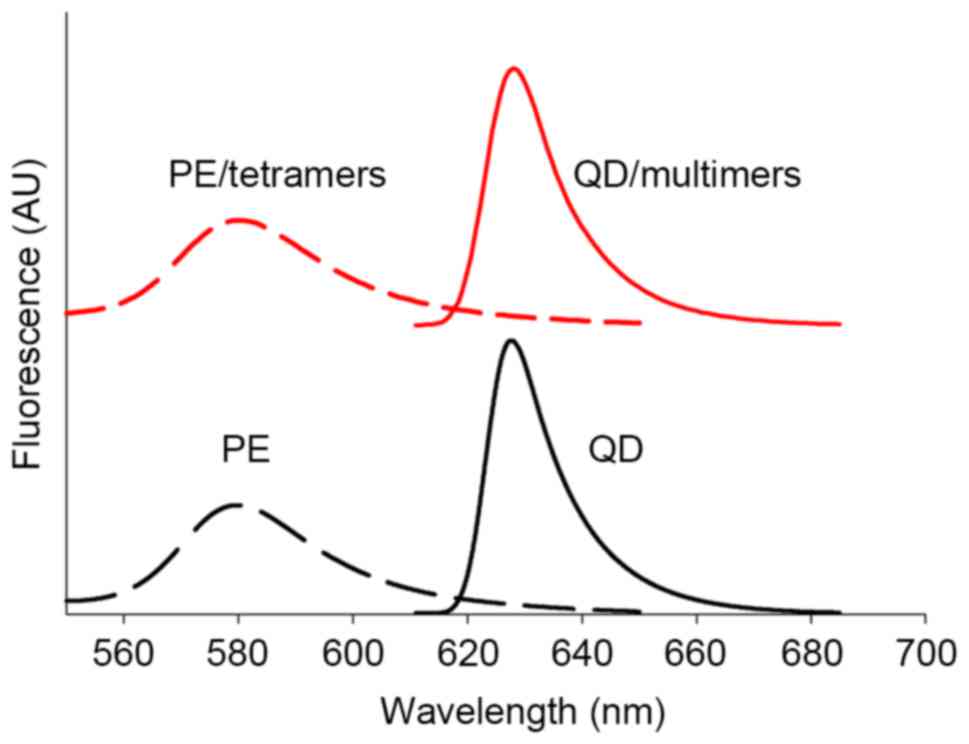
curve of original QD, PE and multimers (Fig. 3) showed that the fluorescence
properties of both QD and PE had not been influenced by the surface
modification process. These results indicated that the QDs and QD
bioconjugate nanoparticles were well-dispersed and that the
polymers or protein coated on QDs did not change the surface
properties of the CdTe/CdS/ZnS nanocrystals.
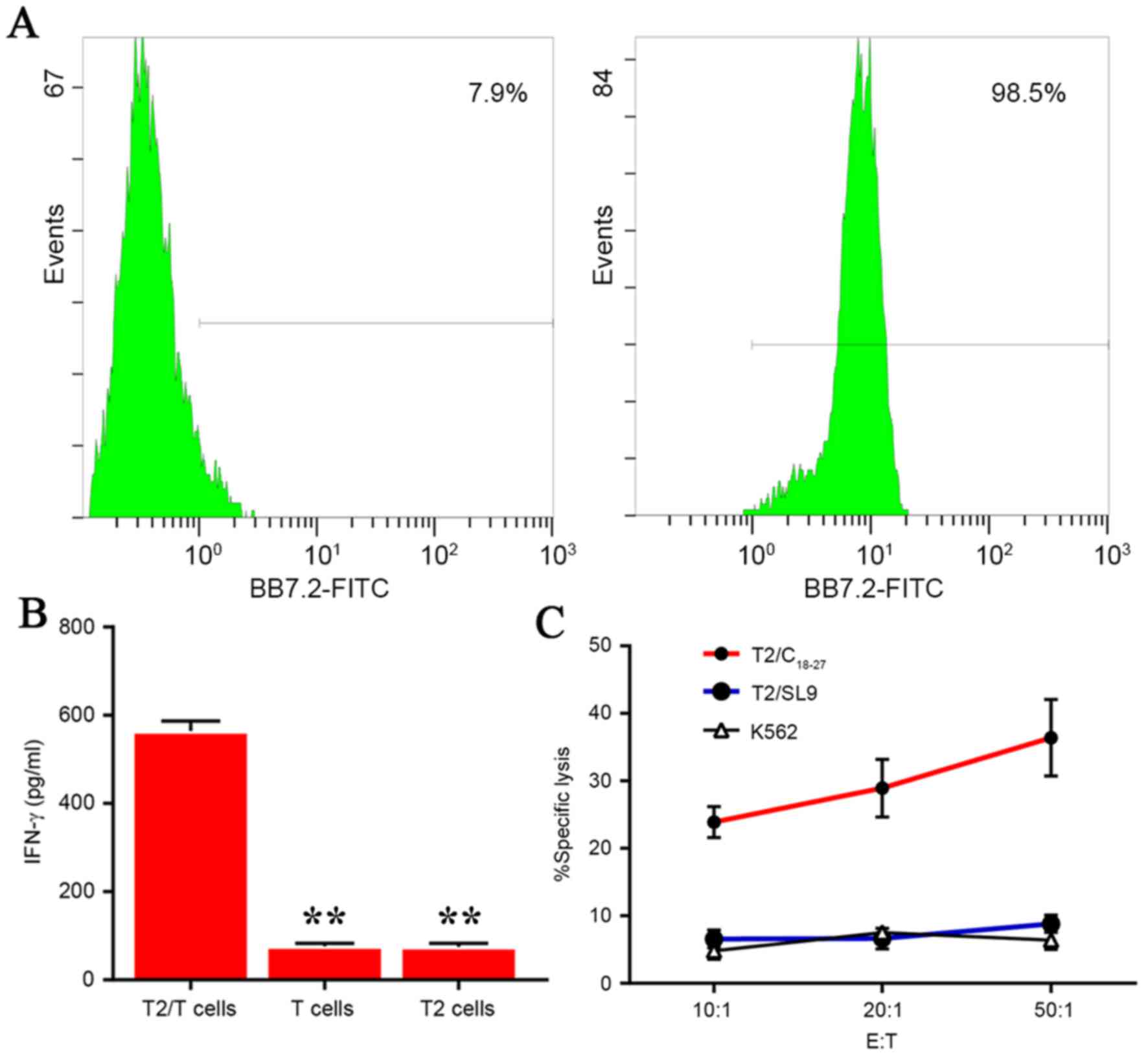
Identification of stable HLA-1
expression in T2 pulsed with antigen peptide
To ensure that MHC-I molecules expressed stably on
T2 cells, expression of HLA-A0201 on the surface of T2 cell
membranes was examined by staining with mAb FITC-BB7.2. As
presented in Fig. 4A, a flow
cytometry assay demonstrated that T2/C18–27 cells
expressed HLA-A0201 with markedly higher frequencies (98.5%)
compared with T2 cells treated in similar conditions but without
pulsing with antigen peptide, FI>1 (7.9%).
IFN-γ responses of peptide-specific
T-cells from in vitro expansion
In order to confirm that the T cells had been
activated by the selected functional epitope peptide
C18–27, the secretion of IFN-γ in supernatants of CTLs
was tested by ELISA. As demonstrated in Fig. 4B, the IFN-γ secretion from
C18–27-specific CTLs increased markedly following ex
vivo coculture with T2/C18–27 for three weeks. In
contrast, the T cells or T2 cells alone released significantly less
IFN-γ compared with the T2/T cell experimental group (P<0.01;
Fig. 4B).
Cytotoxicity of peptide-specific
CTLs
The cytotoxicity of the cultured CTLs was
investigated by LDH release assay. The results demonstrated that
cultured specific CTLs lysed the T2/C18–27 cells while
the negative control groups (K562 and T2/SL9 cells) did not induce
a CTL response and spontaneous release was below 10% of the maximum
release (P<0.01; Fig. 4C).
These results indicated that C18–27 CTLs had been
induced successfully ex vivo.
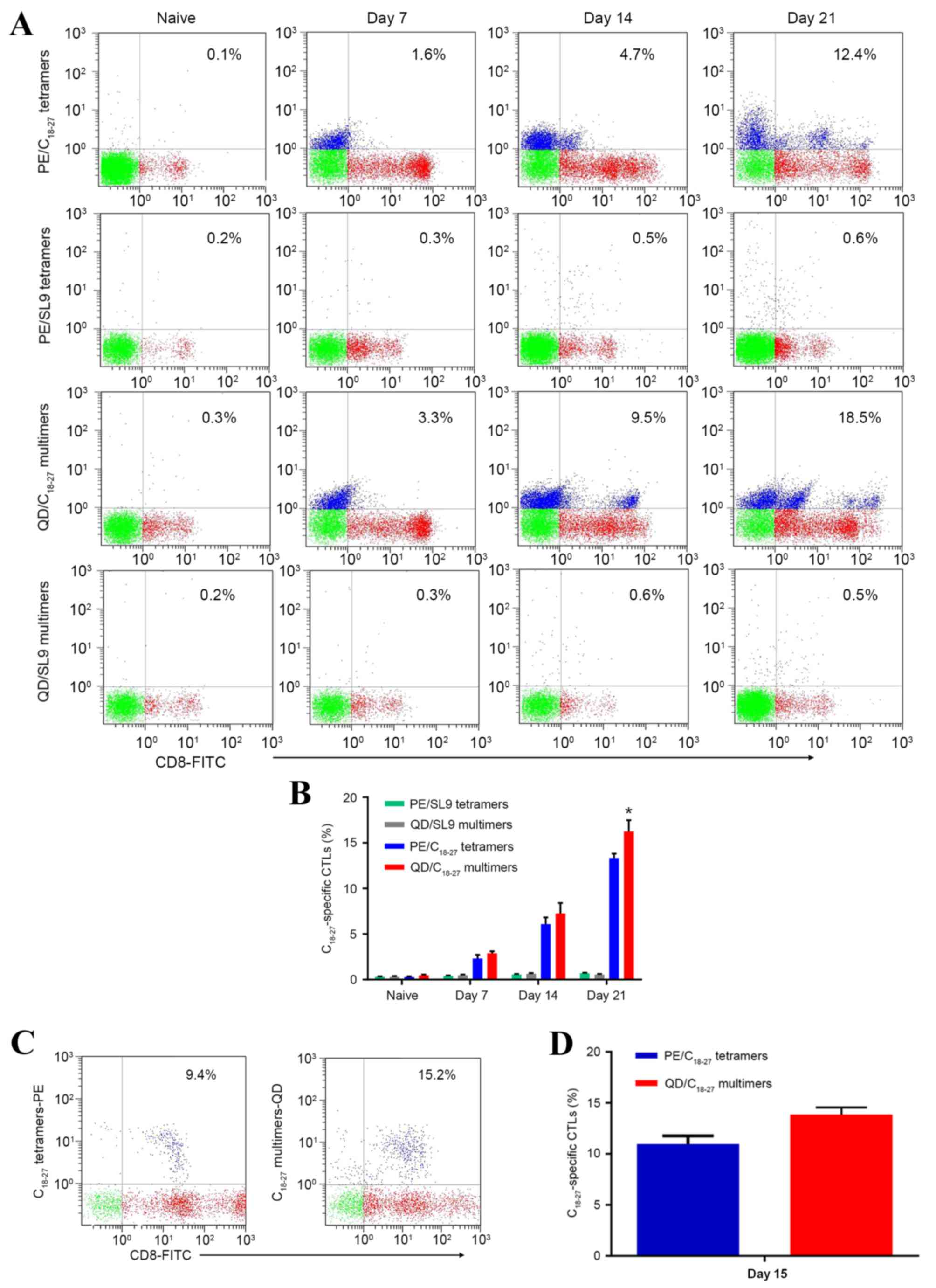
Comparison of QD/pMHC multimers and
PE/pMHC tetramers for the detection of peptide-specific T
cells
To compare the staining of
C18–27-specific T cells with PE/pMHC tetramers and
QD/pMHC multimers, SL9 multimers or tetramers were designed as
negative control materials. It is notable in Fig. 5 that QD/pMHC multimers detected
higher numbers of C18–27-specific CTLs than PE/pMHC
tetramers. The frequencies in negative control groups were
significantly lower.
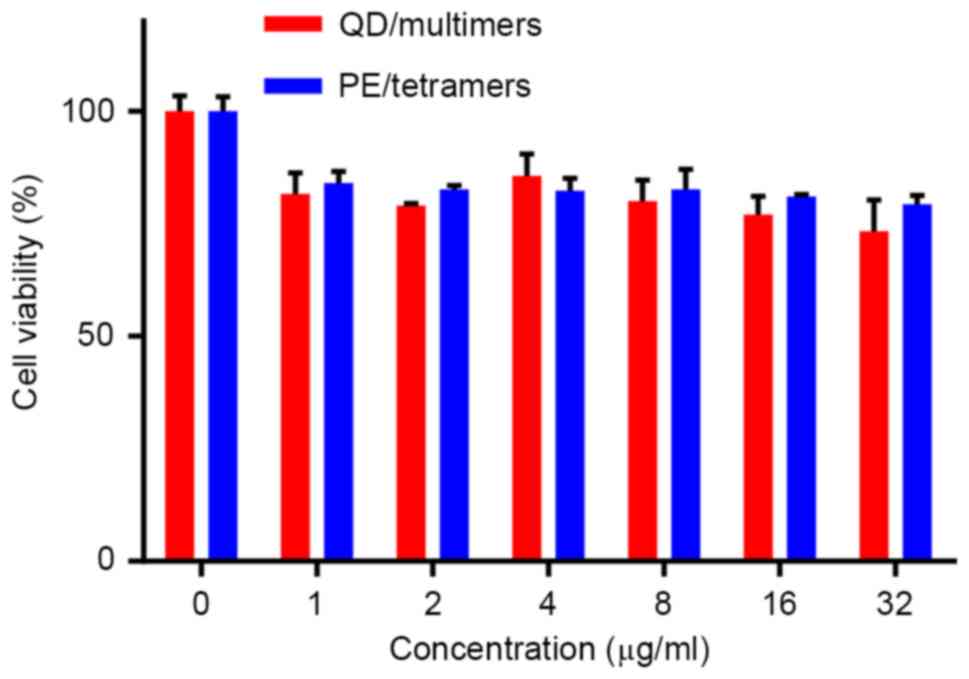
Cytotoxicity assessment of pMHCI-QD
multimers
To evaluate whether QD toxicity was harmful to T
cells, a CCK-8 kit was used. Cytotoxicity data demonstrated that
the percentage of cell death upon incubation of T cells with
QD/multimers was low (Fig. 6).
However, no significant difference in cytotoxicity between
QD/multimers and PE/tetramers was detected. It also demonstrated
that QDs with shells or coatings minimized Cd2+ release
from the particle surface and that the CdTe/CdS/ZnS
core/shell/shell QDs can be applied in T cell experiments.
Discussion
For the biological synthesis of QD/pMHC multimers,
it is important to choose an appropriate assay for solubilization
and stabilization of QD-bioconjugates in solutions (26). There are two main approaches to the
surface modification of QDs: Covalent linkage and noncovalent
binding (27–29). The covalent linkage assay usually
results in QD-protein dispersions with a large aggregation and low
quantum yield. The present study bound streptavidin to QDs via
noncovalent metal-affinity interactions. Studies (28,30,31)
have shown that in His-tag motifs to Zn atoms of QDs, the
interaction (Zn2+-His) is stronger than the majority of
antibody bindings, so streptavidin was labeled with a His-tag to
form high-affinity complexes with QDs. Then QD/pMHC multimers were
obtained by conjugating monomeric pMHCI biotinylated-proteins to
QD-SA. From the TEM images it can be observed that the QD/pMHC
multimers nanoparticles are larger than QDs, so it is supposed that
one single QD/pMHC multimer may contain several QDs and numerous
pMHC monomers (Fig. 2C), although
unforeseen aggregation did not appear. The resulting pellets
demonstrated suitable stabilization (Fig. 2E) and the photoluminescence spectra
did not demonstrate any noticeable difference in the peaks
(Fig. 3), indicating that the
surface modification did not induce any significant change in the
structures of the QDs. One possible reason may be that the exposed
charged groups and hydrophobic chains per polymer molecule in the
QD-coating bioconjugates had changed little during the QD surface
modification process (17).
QD toxicity has always been a great concern in
biomedical studies (32). The
cytotoxicity of QDs has been demonstrated to occur from released
Cd2+ ions and can be greatly reduced by using QDs with
shells or coatings (33). Cell
viability is a commonly used assay to evaluate the toxicity of
nanomaterials. In this study, CCK-8, which was considered to
possess a improved sensitivity compared with the well-known MTT
assay, was used to evaluate QD toxicity (34). The quantity of the formazan dye is
directly proportional to the occurrence of living cells in this
method. The relative viability of cells was obtained from the OD
value at 450 nm wavelength. The CdTe/CdS/ZnS core/shell/shell QDs
used in the present study did not show significant cytotoxicity
(Fig. 6), suggesting that the
CdS/ZnS coating of QDs greatly reduced the toxicity within the
biological system.
For the coculture of T2/C18–27 and T
cells, purified CD8+ T cells were developed as one group
of effector T cells in order to eliminate non-specificity influence
of other cells in PBLs. To determine whether HLA-A*0201 expressed
steadily on the surface of T2/C18–27 cells, the
T2/C18–27 cells were stained with FITC-BB7.2 mAb. As
demonstrated in Fig. 4A the
peptide C18–27 bound to T2 with strong affinity. The
secretion level of IFN-γ analyzed by ELISA assay demonstrated that
C18–27 was markedly efficient at activating
C18–27-specific CTL responses (Fig. 4B). The cytotoxicity of
peptide-specific CTLs detected by LDH release assay demonstrated
that C18–27-specific CTLs were induced successfully and
the resulting CTLs had the ability to kill target cells (Fig. 4C). The experiments were designed to
ensure that there was a sufficient number of functional
C18–27-specific T cells for parallel comparative
analyses of T cell staining with PE/pMHC tetramers and QD/pMHC
multimers.
During the expansion of C18–27-specific
CTL, PE/pMHC tetramers and QD/pMHC multimers were used to detect
the frequencies of C18–27 specific CTLs. Fig. 5A and B demonstrate that
QD/C18–27 multimers detected a higher number of T cells
compared with PE/SL9 tetramers each week during ex vivo
expansion. However, PE/SL9 tetramers exhibited a more stable
detection result compared with QD/C18–27 multimers. When
the effector T cells became purified CD8+ T cells, the
performance of the QD/C18–27 multimers was similar to
PE/SL9 tetramers following ex vivo expansion for 10 days
(data not shown). However, over 15 days the average CTL frequencies
determined by QD/C18–27 multimers were markedly higher
than PE/SL9 tetramers (Fig. 5C and
D). The result may indicate that QD/C18–27 multimers
exhibit higher detection efficiency compared with PE/SL9 tetramers
when the frequency of C18–27-specific CTL is relatively
high. In brief, QD/pMHC multimers detected a higher number of T
cells than PE/pMHC tetramers in the majority of cases and the
QD/pMHC multimers technique can improve detection sensitivity.
Since Altman et al (18) first constructed MHC-tetramers for
tagging T cells in 1996, MHC-tetramers technique have rapidly
become the gold standard for T-cell analysis of known antigen
specificity (35). The fast-moving
development of QD-bioconjugates is stimulating interfaces with
nanotechnology as well. The present study compared QD/pMHC
multimers and PE/pMHC tetramers for staining
C18–27-specific CTLs expanded ex vivo with a
single 488 nm argon laser for the first time and the results
indicated that QD/pMHC multimer performed better in the analysis of
C18–27 specific CTLs, which may improve the
understanding of T cell immune response induced by HBV. It may also
serve an important role in the development of more effective
vaccines.
Acknowledgements
The present study was supported by the National
Natural Scientific Foundation of China (grant nos. 81430055,
81172139, 81172138 and 81372452), the International Cooperation
Project of the Ministry of Science and Technology of China (grant
no. 2015DFA31320), the Project for Innovative Research Team in
Guangxi Natural Science Foundation (grant no. 2015GXNSFFA139001)
and the Project of Science and Technology of Guangxi (grant nos.
14125008-2-12 and 1599005-2-10).
References
|
1
|
Chisari FV and Ferrari C: Hepatitis B
virus immunopathogenesis. Annu Rev Immunol. 13:29–60. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beasley RP, Hwang LY, Lin CC and Chien CS:
Hepatocellular carcinoma and hepatitis B virus. A prospective study
of 22 707 men in Taiwan. Lancet. 2:1129–1133. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noordeen F: Hepatitis B virus infection:
An insight into infection outcomes and recent treatment options.
Virusdisease. 26:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guidotti LG and Chisari FV: Immunobiology
and pathogenesis of viral hepatitis. Annu Rev Pathol. 1:23–61.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wherry EJ and Ahmed R: Memory CD8 T-cell
differentiation during viral infection. J Virol. 78:5535–5545.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Virgin HW, Wherry EJ and Ahmed R:
Redefining chronic viral infection. Cell. 138:30–50. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Belyakov IM and Ahlers JD: What role does
the route of immunization play in the generation of protective
immunity against mucosal pathogens? J Immunol. 183:6883–6892. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Varela-Rohena A, Molloy PE, Dunn SM, Li Y,
Suhoski MM, Carroll RG, Milicic A, Mahon T, Sutton DH, Laugel B, et
al: Control of HIV-1 immune escape by CD8 T cells expressing
enhanced T-cell receptor. Nat Med. 14:1390–1395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bertoletti A and Gehring AJ: The immune
response during hepatitis B virus infection. J Gen Virol.
87:1439–1449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Engler OB, Dai WJ, Sette A, Hunziker IP,
Reichen J, Pichler WJ and Cerny A: Peptide vaccines against
hepatitis B virus: From animal model to human studies. Mol Immunol.
38:457–465. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Chen KY and Ren EC: Structural
insights into the binding of hepatitis B virus core peptide to
HLA-A2 alleles: Towards designing better vaccines. Eur J Immunol.
41:2097–2106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hobeika AC, Morse MA, Osada T, Ghanayem M,
Niedzwiecki D, Barrier R, Lyerly HK and Clay TM: Enumerating
antigen-specific T-cell responses in peripheral blood: A comparison
of peptide MHC Tetramer, ELISpot, and intracellular cytokine
analysis. J Immunother. 28:63–72. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dolton G, Lissina A, Skowera A, Ladell K,
Tungatt K, Jones E, Kronenberg-Versteeg D, Akpovwa H, Pentier JM,
Holland CJ, et al: Comparison of peptide-major histocompatibility
complex tetramers and dextramers for the identification of
antigen-specific T cells. Clin Exp Immunol. 177:47–63. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meidenbauer N, Hoffmann TK and Donnenberg
AD: Direct visualization of antigen-specific T cells using
peptide-MHC-class I tetrameric complexes. Methods. 31:160–171.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sims S, Willberg C and Klenerman P:
MHC-peptide tetramers for the analysis of antigen-specific T cells.
Expert Rev Vaccines. 9:765–774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reguzova AY, Karpenko LI, Mechetina LV and
Belyakov IM: Peptide-MHC multimer-based monitoring of CD8 T-cells
in HIV-1 infection and AIDS vaccine development. Expert Rev
Vaccines. 14:69–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bilan R, Fleury F, Nabiev I and Sukhanova
A: Quantum dot surface chemistry and functionalization for cell
targeting and imaging. Bioconjug Chem. 26:609–624. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altman JD, Moss PA, Goulder PJ, Barouch
DH, McHeyzer-Williams MG, Bell JI, McMichael AJ and Davis MM:
Phenotypic analysis of antigen-specific T lymphocytes. Science.
274:94–96. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hines MA and Guyot-Sionnest P: Synthesis
and characterization of strongly luminescing ZnS-capped CdSe
nanocrystals. J Phys Chem. 100:468–471. 1996. View Article : Google Scholar
|
|
20
|
Sukhanova A, Devy J, Venteo L, Kaplan H,
Artemyev M, Oleinikov V, Klinov D, Pluot M, Cohen JH and Nabiev I:
Biocompatible fluorescent nanocrystals for immunolabeling of
membrane proteins and cells. Anal Biochem. 324:60–67. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Samir TM, Mansour MM, Kazmierczak SC and
Azzazy HM: Quantum dots: Heralding a brighter future for clinical
diagnostics. Nanomedicine (Lond). 7:1755–1769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Medintz IL, Uyeda HT, Goldman ER and
Mattoussi H: Quantum dot bioconjugates for imaging, labelling and
sensing. Nat Mater. 4:435–446. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chattopadhyay PK, Price DA, Harper TF,
Betts MR, Yu J, Gostick E, Perfetto SP, Goepfert P, Koup RA, De
Rosa SC, et al: Quantum dot semiconductor nanocrystals for
immunophenotyping by polychromatic flow cytometry. Nat Med.
12:972–977. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andersen RS, Kvistborg P, Frøsig TM,
Pedersen NW, Lyngaa R, Bakker AH, Shu CJ, Straten Pt, Schumacher TN
and Hadrup SR: Parallel detection of antigen-specific T cell
responses by combinatorial encoding of MHC multimers. Nat Protoc.
7:891–902. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Howarth M, Chinnapen DJ, Gerrow K,
Dorrestein PC, Grandy MR, Kelleher NL, El-Husseini A and Ting AY: A
monovalent streptavidin with a single femtomolar biotin binding
site. Nat Methods. 3:267–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu C, Liu J, Zhang P, Li J, Ji H, Yang X
and Wang K: A recognition-before-labeling strategy for sensitive
detection of lung cancer cells with a quantum dot-aptamer complex.
Analyst. 140:6100–6107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pinaud F, King D, Moore HP and Weiss S:
Bioactivation and cell targeting of semiconductor CdSe/ZnS
nanocrystals with phytochelatin-related peptides. J Am Chem Soc.
126:6115–6123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hainfeld JF, Liu W, Halsey CM, Freimuth P
and Powell RD: Ni-NTA-gold clusters target His-tagged proteins. J
Struct Biol. 127:185–198. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akerman ME, Chan WC, Laakkonen P, Bhatia
SN and Ruoslahti E: Nanocrystal targeting in vivo. Proc Natl Acad
Sci USA. 99:pp. 12617–12621. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goldman ER, Medintz IL, Whitley JL,
Hayhurst A, Clapp AR, Uyeda HT, Deschamps JR, Lassman ME and
Mattoussi H: A hybrid quantum dot-antibody fragment fluorescence
resonance energy transfer-based TNT sensor. J Am Chem Soc.
127:6744–6751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Medintz IL, Berti L, Pons T, Grimes AF,
English DS, Alessandrini A, Facci P and Mattoussi H: A reactive
peptidic linker for self-assembling hybrid quantum dot-DNA
bioconjugates. Nano Lett. 7:1741–1748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hardman R: A toxicologic review of quantum
dots: Toxicity depends on physicochemical and environmental
factors. Environ Health Perspect. 114:165–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ryman-Rasmussen JP, Riviere JE and
Monteiro-Riviere NA: Surface coatings determine cytotoxicity and
irritation potential of quantum dot nanoparticles in epidermal
keratinocytes. J Invest Dermatol. 127:143–153. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miyamoto T, Min W and Lillehoj HS:
Lymphocyte proliferation response during Eimeria tenella infection
assessed by a new, reliable, nonradioactive colorimetric assay.
Avian Dis. 46:10–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hadrup SR and Schumacher TN: MHC-based
detection of antigen-specific CD8+ T cell responses. Cancer Immunol
Immunother. 59:1425–1433. 2010. View Article : Google Scholar : PubMed/NCBI
|