Introduction
The safe and efficient delivery of therapeutic genes
across the plasma membrane of the cell is crucial for effective
gene therapy. Although viral vectors may be used for gene
transfection, their application is limited due to potential side
effects. By contrast, ultrasound techniques may have great
potential for non-viral gene delivery in vitro and in
vivo in the absence of severe adverse effects. However, due to
cytoplasmic and nuclear membrane barriers, transfection efficiency
is relatively low (1). Numerous
methods have been assessed to address this issue and increase
transfection efficiency.
Controversy surrounds the development of an
efficient method to deliver DNA or drugs into cells. One of these
methods is ultrasound-targeted microbubble destruction (UTMD)
(2–4). Compared with other direct DNA
delivery methods, including microinjection and electroporation,
UTMD may be simpler to implement (5,6).
Sonication (ultrasound), which may target gene delivery to a
specific area, alters the transient permeability of plasma
membranes to facilitate intake (5). In the present study, UTMD technology
was used to facilitate the entry of plasmid (p)DNA into the
cytoplasm without causing severe cell damage.
Due to the nuclear membrane barrier, delivery of
genes to the nucleus is challenging. Certain studies have
demonstrated that pDNA microinjection results in <3% nuclear
import and low expression (7–9). It
is therefore important to overcome this obstacle to increase
nuclear import during gene transfer. A previous study used numerous
nuclear localization signals (NLS) to promote nuclear gene import;
however, efficiency remained unsatisfactory (10).
The present study examined gene transfection by UTMD
using 293T cells with a microbubble contrast agent. A specific NLS
was bound by peptide nucleic acid (PNA) to pDNA. The
antibody-targeted microbubbles (AT-MCB) recognized and bound to the
antigen on the cell membrane. Subsequently, the DNA with NLS was
guided to bring the pDNA into the cell, and this may have improved
nuclear intake and transfection efficiency. AT-MCB may improve
cellular uptake of pDNA, and PNA-binding NLS may promote nuclear
import of pDNA. Therefore, combining these two methods may
significantly enhance transfection efficiency.
Materials and methods
Antibody-targeted microbubble
preparation
Electrostatic adsorption has been reported to be an
efficient method of constructing AT-MCB (11). Normal saline (5 ml) was poured into
a small bottle with SonoVue freeze-dried powder (25 mg; Bracco
Suisse S.A., Geneva, Switzerland) and shaken well for 10 sec to
form a microbubble suspension. The diameters of the microbubbles
were 0.8–10 µm, with the majority being 2–5 µm. A monoclonal
anti-SV40Tag antibody (ab82118; Abcam, Cambridge, MA, USA)
conjugated to fluorescein isothiocyanate was dissolved 1:50 in PBS.
The microbubble suspension was mixed with the diluted antibody at a
ratio of 2:1, and the pH of the reaction liquid was adjusted to pH
4.0–5.0 prior to incubation at 4°C for 2 h, during which the
mixture was shaken several times. The clear liquid at the bottom of
the container (containing the unbound antibodies) was subsequently
removed and washed with a buffer. Following 10–15 min, the liquid
separated into layers, and the bottom clear liquid (containing the
unbound antibodies) was again removed. Finally, PBS was added to
suspend the floating foam to target the microbubble suspension for
an anti-SV40Tag antibody.
Targeted microbubble
identification
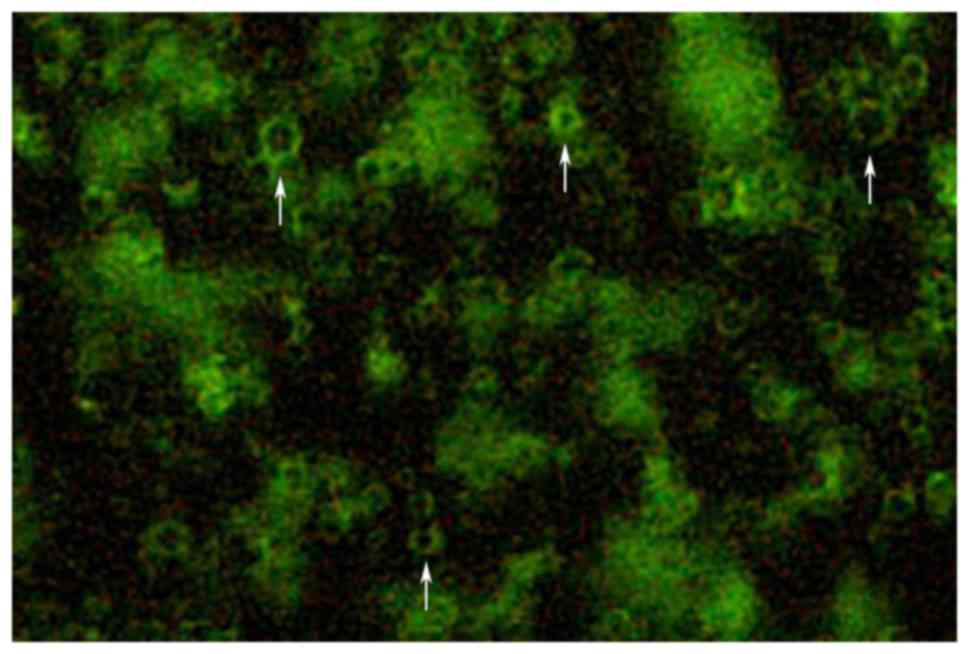
The prepared targeted microbubble suspension was
observed under a fluorescence microscope to determine the
combination of antibodies and microbubbles, as well as the status
and distribution of the targeted microbubbles (Fig. 1).
Enhanced green fluorescent protein
(EGFP)-N3 plasmid construction
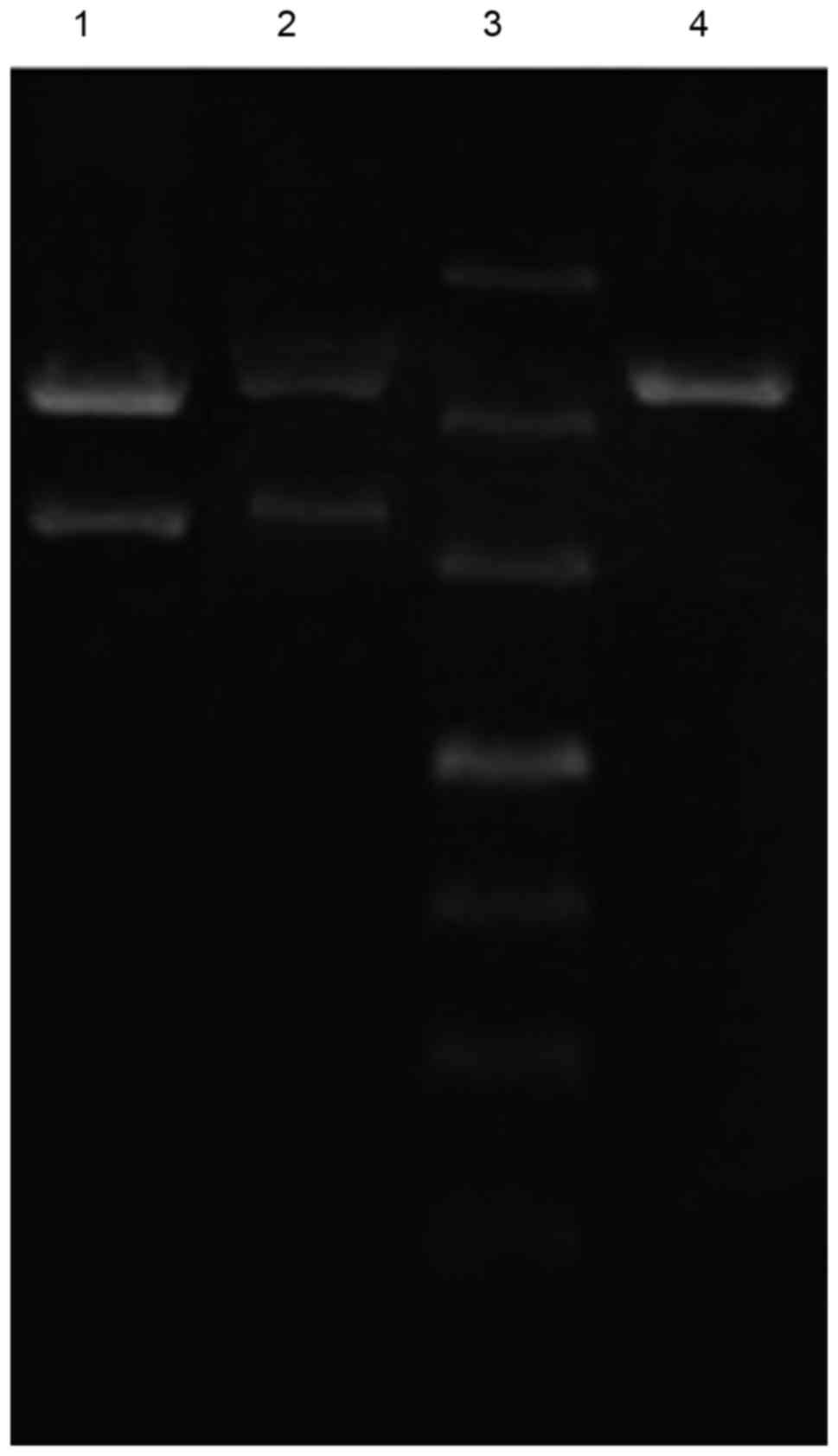
To hybridize EGFP-N3 and PNA, EGFP-N3 was added to
the sample at a proportion of EGFP-N3: biotin-PNA=3:100 in a
graduated 200-µl Eppendorf tube to cause the plasmid EGFP-N3 to
completely hybridize with the biotin PNA, which produced a
sepharose gel. Subsequently, a 1-µl sample was taken from the
Eppendorf tube (60 V at constant voltage for 6 h). The band was
observed under a blue light transilluminator. A specific NLS
sequence (PKKKRKV) was designed to embed into biotin PNA. The
redundant PKKKRKV-PNA was removed by liquid chromatography to
measure the optical density (OD) using an ultraviolet
spectrophotometer. The purified sample was 450 µl at 8.2 µg/ml,
with an OD260/280 of 1.72. The biotinylated PNA/DNA compound was
mixed with a streptavidin SV40T antigen NLS and incubated for 20
min at 4°C; subsequently, centrifugal washing at 500 × g for 30 sec
was performed twice to remove the unbound NLS and construct the
NLS-PNA/EGFP-N3 karyotheca-targeted gene (Fig. 2).
Cell culture
The 293T cells were obtained from the China Center
for Type Culture Collection (Wuhan, China), and cultured in
Dulbecco's modified Eagle's medium (DMEM; ScienCell Research
Laboratories, Carlsbad, CA, USA) containing 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
cells were cultured in 10-cm culture dishes at 37°C with 5%
CO2. Adherent cells were removed by trypsinization and
seeded at a density of 2×105 cells per well (in 2 ml DMEM) in
6-well plates (Corning Incorporated, Corning, NY, USA) 24 h prior
to transfection.
UTMD-mediated transfection of EGFP-N3
plasmid
The present study was divided into three groups, and
each group had three subgroups. Group 1: control, CMCB + DNA,
AT-MCB + DNA; Group 2: control, CMCB + DNA, CMCB + NLS-PNA-DNA;
Group 3: AT-MCB + DNA, CMCB + NLS-PNA-DNA, AT-MCB + NLS-PNA-DNA.
The control group received no treatment and the negative group was
CMCB + DNA, which were treated with ordinary microbubbles (CMCB)
and pDNA. 293T cells were transfected with CMCB and NLS-PNA-DNA,
AT-MCB and pDNA, AT-MCB and NLS-PNA-DNA in group CMCB +
NLS-PNA-DNA, AT-MCB + DNA, AT-MCB + NLS-PNA-DNA respectively.
Transfection was performed when the cells reached
65–75% confluence. The culture medium was replaced with 2 ml
Opti-mimimal essential medium (Gibco; Thermo Fisher Scientific,
Inc.) per well. Plasmid (10 µg) was added to each well along with
microbubbles prior to transfection. Ultrasound was subsequently
used to irradiate the cell, plasmid and microbubble suspension
(UGT2007; Ultrasonic Research Institute of Chongqing Medical
University, Chongqing, China). The parameters were set to different
acoustic intensities and exposure times, and the transducer was
sterilized prior to immersion in the cell suspension.
It has been previously demonstrated that gene
transfection efficiency in vitro may be affected by
alterations in ultrasound parameters, including duration and
intensity (12,13). Transfections were performed using
the optimal ultrasound exposure parameters, which were determined
by our previous study: 1×107 microbubbles/ml and 1.5
W/cm2 for 45 sec with a 20% duty cycle (14). The various parameters were assessed
by cell viability and pDNA uptake.
Cell viability
The 293T cells were seeded in 96-well plates
(5×103 cells/well in 100 µl DMEM. The viability of the
cells was measured using a Cell Counting kit-8 (CCK-8; Dojindo
Laboratories, Kumamoto, Japan). Following incubation at 37°C for 24
h, 10 µl CCK-8 was added per well and cells were incubated for 2 h
at 37°C. The absorbance was measured at a wavelength of 450 nm
using an automatic microplate reader (Lambda 1050; PerkinElmer,
Inc., Waltham, MA, USA). The percentage cell viability was
calculated as follows: Cell viability (%) = (ODsample /
ODcontrol) × 100.
Cellular uptake of pDNA
EGFP-N3 was used to transfect the 293T cells in the
presence or absence of UTMD. Following 48 h of incubation at 37°C
in the incubator, the 293T cells were observed under an inverted
fluorescence microscope to determine the presence of the EGFP-N3
plasmid (15). The transfected
cells were allowed to incubate at 37°C for a further 6 h after
which they were removed by trypsinization and washed in PBS. The
percentage of cells that had endocytosed EGFP-N3 was determined
using flow cytometry (Beckman Coulter, Inc., Brea, CA, USA) and
calculated as follows: EGFP-N3 uptake (%) = (EGFP-N3-positive cell
population / total cell population) × 100 using FlowJo version
7.6.1 (FlowJo LLC, Ashland, OR, USA).
Nuclear uptake of pDNA
The transfected cells in different groups were fixed
with 4% paraformaldehyde for 15 min and rinsed with PBS three
times. The nuclear stain DAPI (Beyotime Biotechnology Inc.,
Nantong, China) was added for 20 min to determine the cellular
localization of fluorescence plasmids under an inverted
fluorescence microscope (Olympus Corporation, Tokyo, Japan). ImageJ
software version 1.46 (National Institutes of Health, Bethesda, MD,
USA) was used to quantify the fluorescence intensity (FL) of the
cell (FLcell) and the nucleus (FLnucleus).
Nuclear uptake (%) = (FLnucleus / FLcell) ×
100%.
Reverse transcription-polymerase chain
reaction (RT-PCR)
To evaluate the relative mRNA expression levels of
EGFP-N3, total mRNA was extracted from 293T cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). A total of 2
µg RNA of each sample was reverse-transcribed into cDNA using an
RT-for-PCR kit (Takara Bio, Inc., Otsu, Japan). RT-PCR was
performed using a Light Cycler 480 instrument with software version
1.5 (Roche Diagnostics GmbH, Mannheim, Germany). The amplification
was performed using a Light Cycler 480 SYBR Green Master Mix (Roche
Diagnostics GmbH). The program was performed as follows: 10 sec
complete denaturation at 95°C and followed by 30 cycles of
exponential amplification. Each cycle consisted of a 30 sec
denaturation step at 95°C, a 20 sec annealing step at 60°C, and a
20 sec incubation at 72°C for extension. The primers were as
follows: Forward, 5′-TTTATGGTGAGCAAGGGCGAG-3′ and reverse,
5′-TTTTGGTGCAGATGAACTTCAG-3′ for EGFP-N3; and forward,
5′-TCAAGAAGGTGGTGAAGCAGG-3′ and reverse, 5′-TCAAAGGTGGAGGAGTGGGT-3′
for GAPDH. The results were normalized to GAPDH expression
(16). Each experiment was
replicated three times.
Western blot analysis
EGFP-N3 protein expression levels were determined by
western blot analysis. Total proteins were extracted from the 293T
cells with RIPA lysis buffer product. The lysate was incubated on
ice for 30 min and then centrifuged at 16,000 × g at 4°C for 30 min
in a centrifuge (HI650; Hunan Xiangyi Laboratory Instrument
Development Co., Ltd., Hunan, China). The supernatant containing
proteins was collected and 20 µg protein from each sample were
subjected to 10% sodium dodecyl sulfate-polyacrylamide gel and
electrophoresis. Following electrophoresis, the proteins were
transferred to polyvinylidene fluoride (PVDF) membranes. The
membranes were incubated in TBST solution containing 5% non-fat
milk to block the nonspecific reaction. The membranes were
incubated with an anti-EGFP antibody (ab6556; 1:1,000; Abcam) and
anti-GAPDH antibody (ab9485; 1:1,000; Abcam) at 4°C overnight.
Then, the membranes were washed with TBST and incubated with
horseradish peroxidase (HRP)-conjugated secondary antibodies
(ab6721; 1:10,000; Abcam) for 1 h at room temperature. Following
which, the membranes were washed with TBST and incubated in
developing solution (NCI5079; Thermo Fisher Scientific, Inc.).
Finally, the membranes were exposed to X-rays to detect the
expression bands. The density of the respective bands was
quantitated by BandScan software version 5.1 (Glyko; BioMarin
Pharmaceutical, Inc., Novato, CA, USA). The result of protein
expression were normalized to the protein expression of GAPDH.
Statistical analysis
Statistical analyses were performed using SPSS
software version 18.0 (SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard deviation. Groups were compared
using Student-Newman-Keuls q-test or independent-samples t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cytoplasmic uptake of pDNA is
increased by AT-MCB
Transfections were performed using the optimal
ultrasound exposure parameters, which were determined by our
previous study (14):
1×107 microbubbles/ml and 1.5 W/cm2 for 45
sec with a 20% duty cycle. After 24 h, flow cytometry was used to
analyze the cytoplasmic uptake of pDNA. The percentage of
fluorescent 293T cells following exposure to optimal ultrasound
parameters in the presence of AT-MCB was 45%, which was greater
compared with ordinary microbubbles (CMCB; 9%; Fig. 3). The cell viability was >85%
under the optimal exposure conditions. These results suggested that
the optimal parameters of ultrasound exposure combined with AT-MCB
may be an effective and safe method of gene delivery. The
differences between all three groups and the blank control group
were statistically significant (P<0.01), as were the differences
between the three groups and the negative control group. Cells were
observed under a fluorescence microscope, and those in the AT-MCB +
NLS-PNA-DNA group exhibited the greatest fluorescence. AT-MCB + DNA
and CMCB + NLS-PNA-DNA had the greatest quantity of
fluorescence-labeled cells in each of the subgroups (Fig. 4).
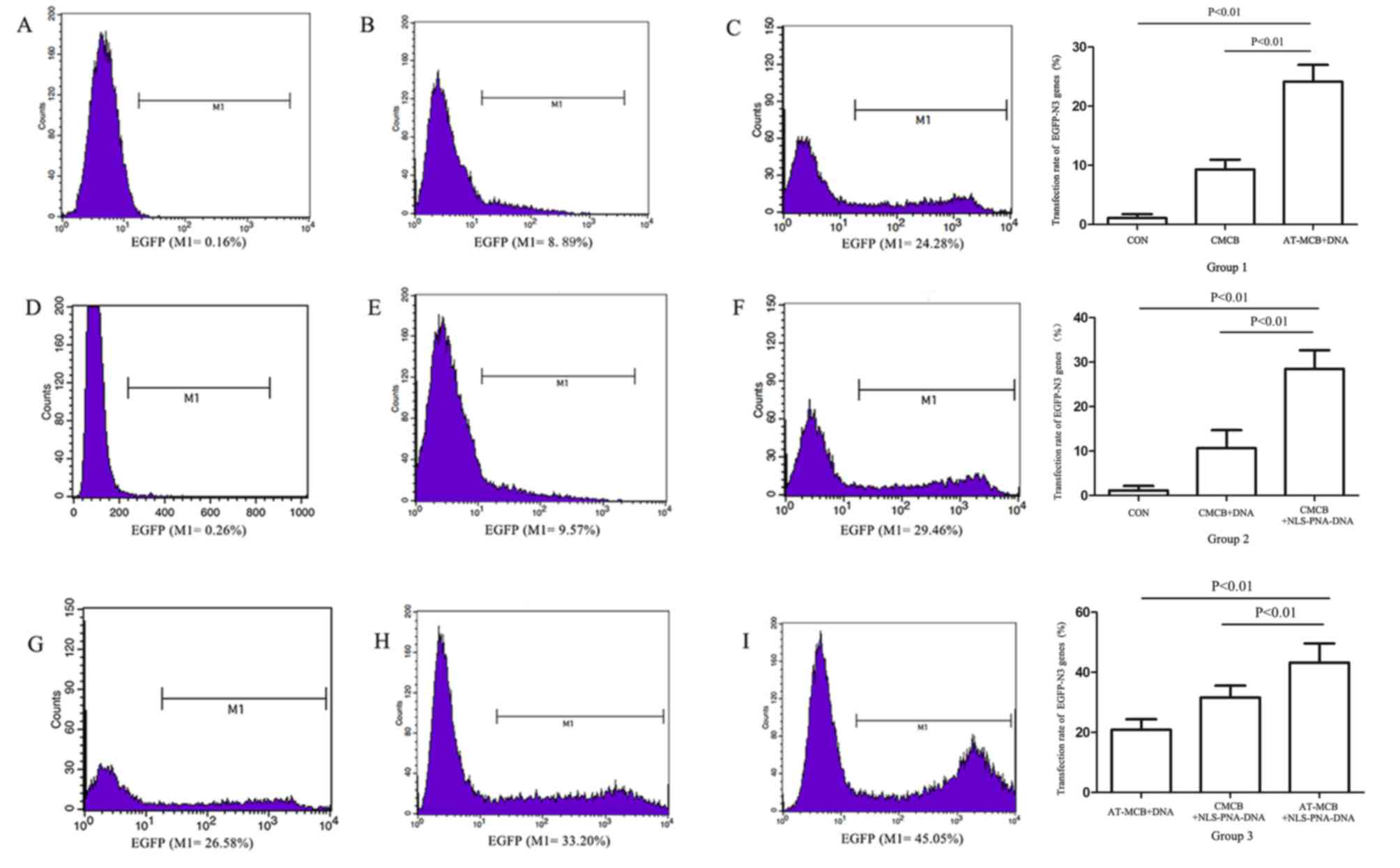 | Figure 3.Flow cytometric analysis and a graph
of the results following gene transfection in the three groups
(group 1, 2 and 3). Histograms reveal the expression of EGFP by
cells from: (A) Group 1, control; (B) group 1, CMCB + DNA; (C)
group 1, AT-MCB + DNA; (D) group 2, control; (E) group 2, CMCB +
DNA; (F) group 2, CMCB + NLS-PNA-DNA; (G) group 3, AT-MCB + DNA;
(H) group 3, CMCB + NLS-PNA-DNA; and (I) group 3, AT-MCB +
NLS-PNA-DNA. EGFP, enhanced green fluorescent protein; CMCB,
ordinary microbubbles; AT-MCB, antibody targeted microbubbles; NLS,
nuclear localization signal; PNA, peptide nucleic acid. |
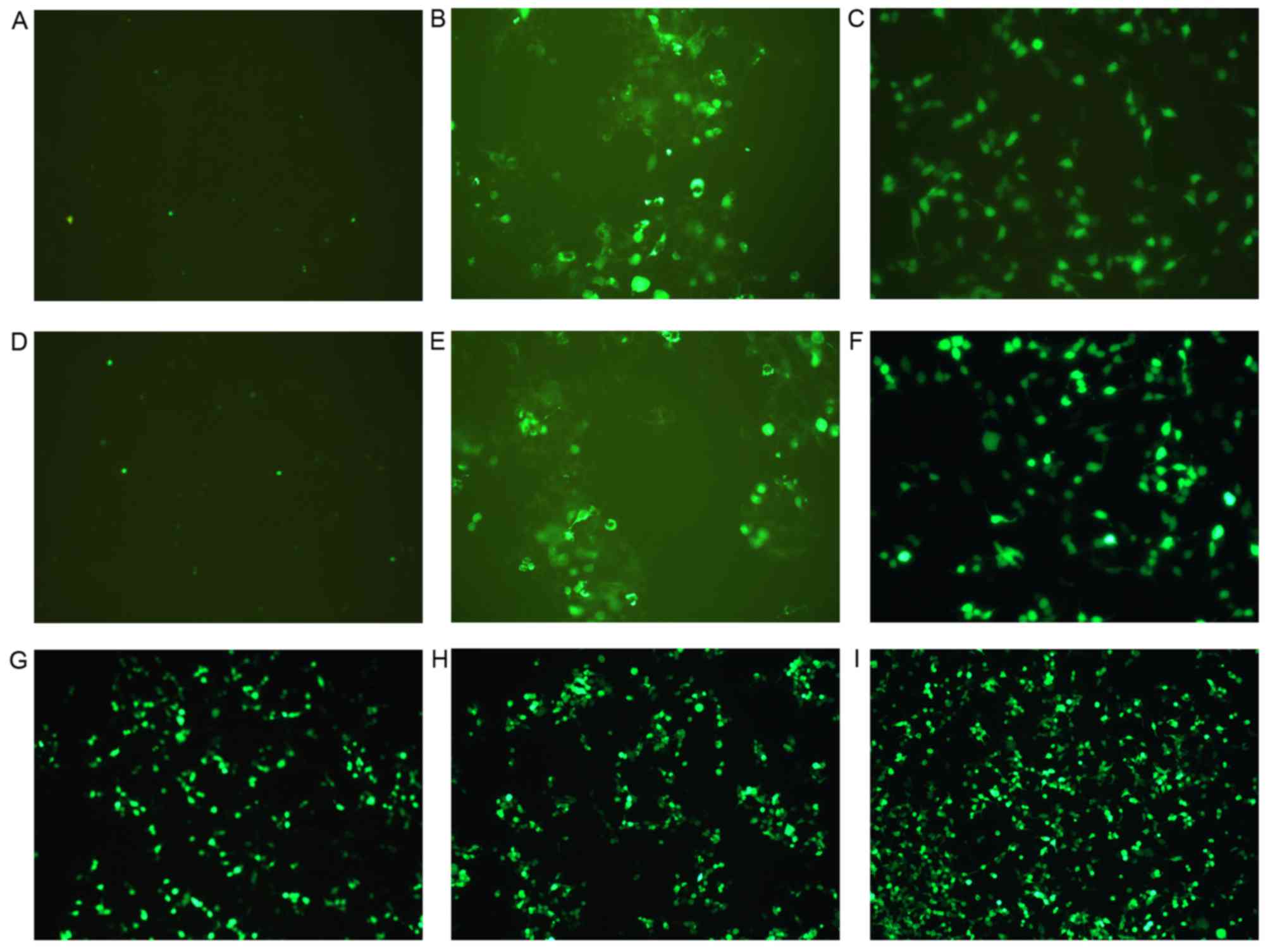 | Figure 4.Fluorescence of cells following gene
transfection in the three groups (group 1, 2 and 3). Images reveal
the expression of enhanced green fluorescent protein by cells from:
(A) Group 1, control; (B) group 1, CMCB + DNA; (C) group 1, AT-MCB
+ DNA; (D) group 2, control; (E) group 2, CMCB + DNA; (F) group 2,
CMCB + NLS-PNA-DNA; (G) group 3, AT-MCB + DNA; (H) group 3, CMCB +
NLS-PNA-DNA; and (I) group 3, AT-MCB + NLS-PNA-DNA. EGFP, enhanced
green fluorescent protein; CMCB, ordinary microbubbles; AT-MCB,
antibody targeted microbubbles; NLS, nuclear localization signal;
PNA, peptide nucleic acid (magnification, ×60). |
Cellular and nuclear uptake of pDNA is
increased by AT-MCB combined with PNA-binding NLS
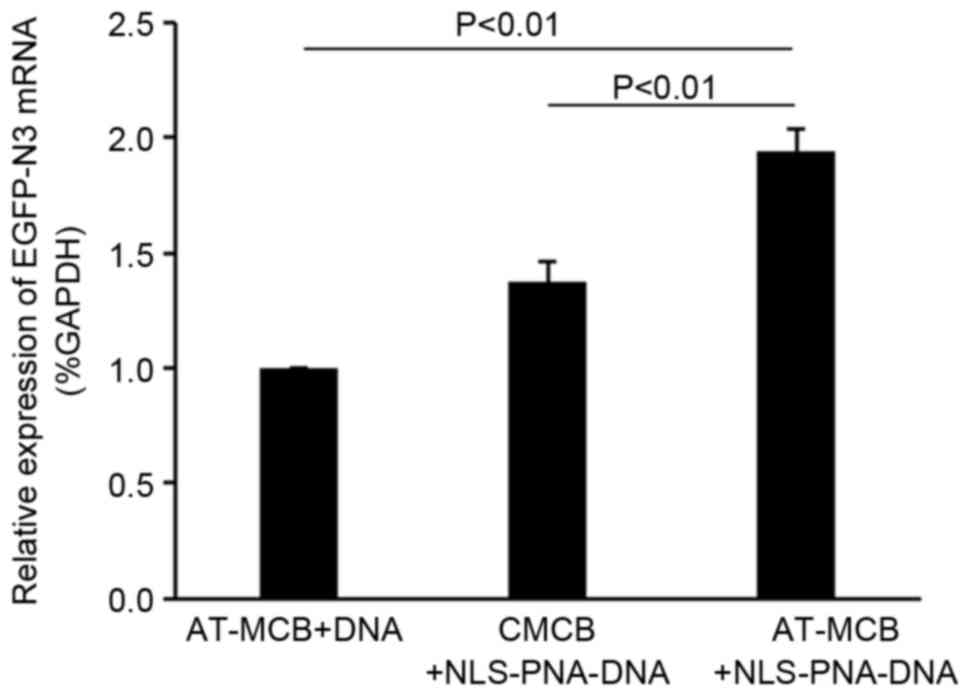
The mRNA expression levels of EGFP-N3 were greater
in the AT-MCB + NLS-PNA-DNA group compared with the AT-MCB + DNA
and CMCB + NLS-PNA-DNA groups (P<0.01; Fig. 5).
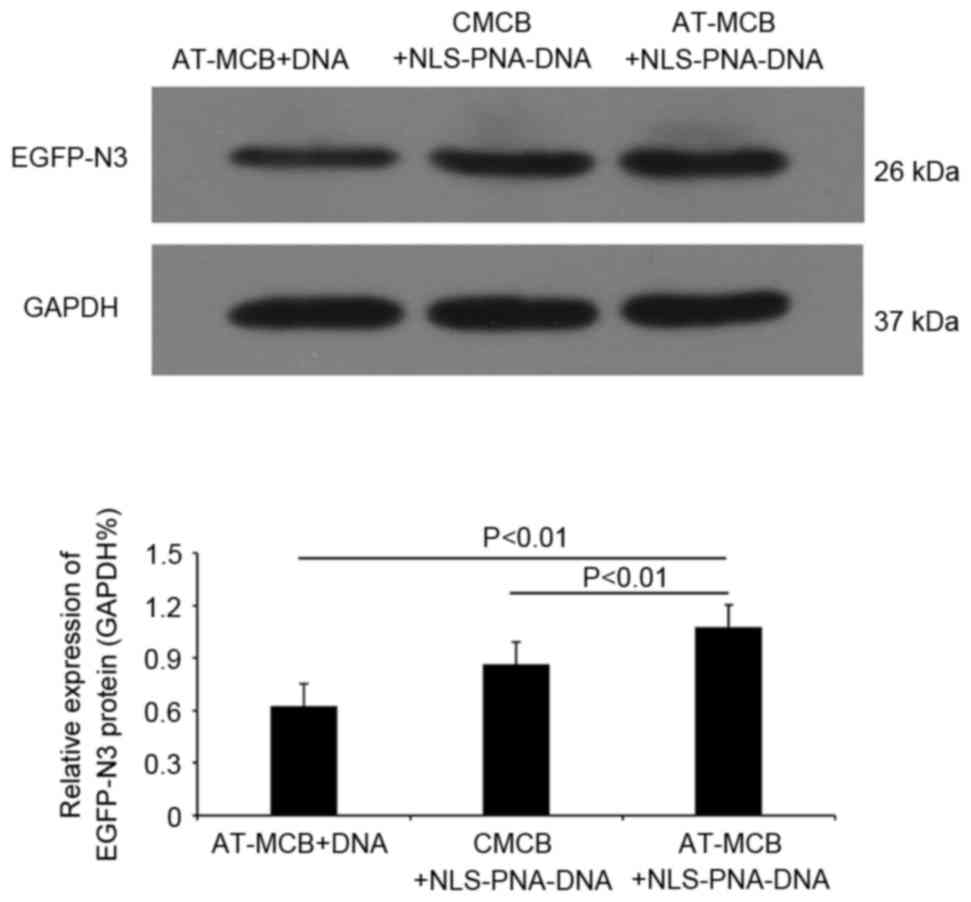
Following 48 h of transfection, western blot
analysis revealed that the protein expression levels of EGFP were
significantly greater in the AT-MCB + NLS-PNA-DNA group compared
with the AT-MCB + DNA and CMCB + NLS-PNA-DNA groups (P<0.01;
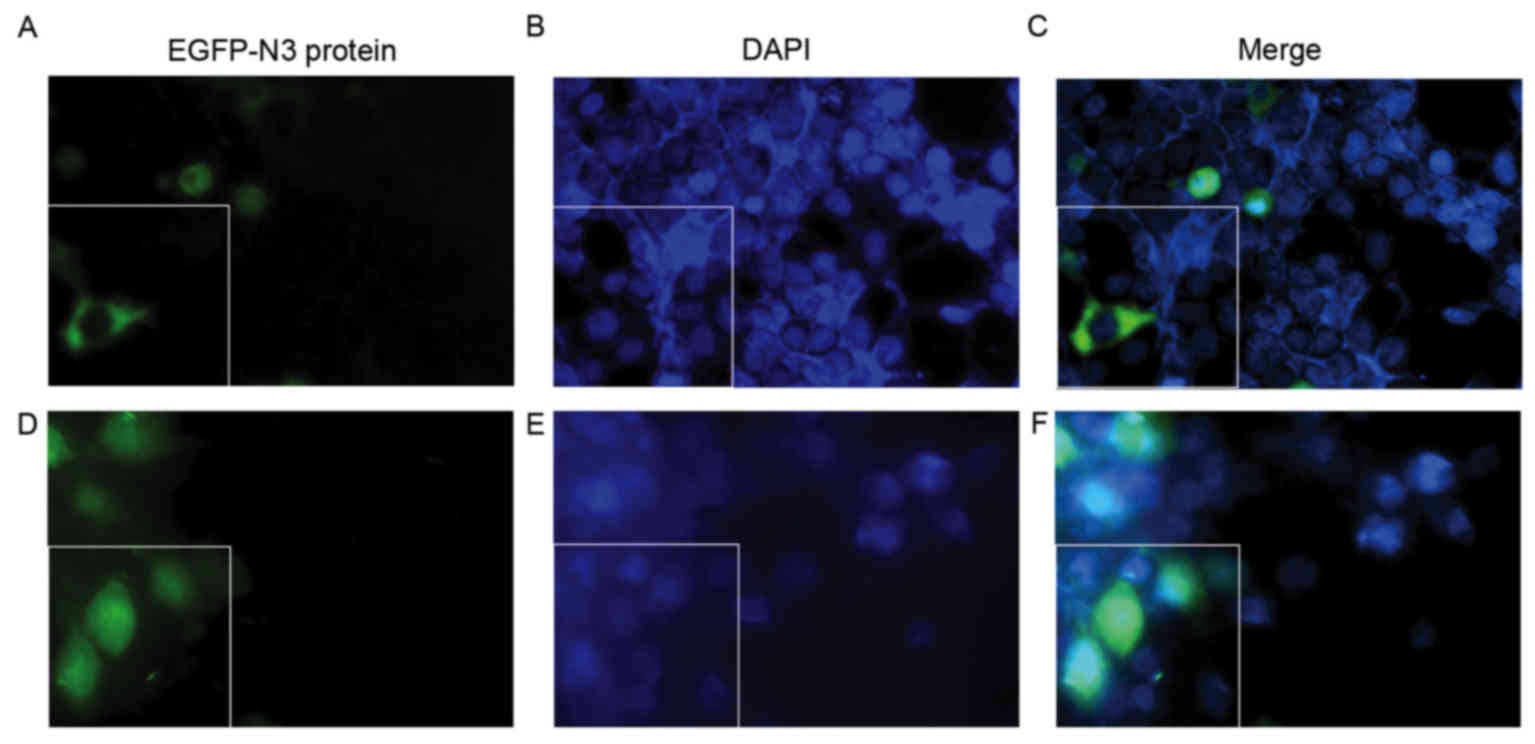
Fig. 6). Fig. 7 presents the subcellular
localization of NLS-free DNA and NLS-DNA plasmids, which were both
transfected with AT-MCB.
Nuclear uptake of pDNA is increased by
AT-MCB combined with PNA-binding NLS
Following 48 h of transfection, the fluorescence of
the cells following gene transfection in the three groups were
observed. As shown in Fig. 7, the
subcellular localization of NLS-free DNA and NLS-DNA plasmids were
presented under the fluorescence microscope, which were both
transfected with AT-MCB. The fluorescence intensity in the nuclei
of the NLS-PNA-DNA group was markedly greater compared with the
NLS-free DNA group (>5.0-fold).
Discussion
Transfection efficiency is a critical issue in gene
therapy. Poor transfection efficiency remains a major obstacle to
non-viral gene therapy, and is due to genes being unable to cross
the cytoplasmic and nuclear membranes (17). In the present study UTMD was
combined with PNA-binding NLS to promote transportation through
cytoplasmic and nuclear membranes. This strategy significantly
increased the entrance of pDNA into the cytoplasm and nucleus, with
the expression of the transfected gene at least 4.5-fold greater
compared with the control group (CMCB). UTMD is considered to be an
important method of gene delivery. Numerous studies have suggested
that microbubbles, which serve as cavitation nuclei, facilitate
cavitation during ultrasound exposure (5,18,19).
To achieve good transfection efficiency, it is important for the
pDNA to translocate from the cytoplasm to the nucleus. The
cavitation of microbubbles leads to the transient formation of
nanopores on the cell membrane that release large amounts of
energy. These reversible pores are formed by sonoporation and may
be observed by scanning electron microscopy (20). Therefore, exogenous pDNA or other
molecules may enter cells through these nanopores, without causing
irreversible cellular damage. Various types of microbubbles and
ultrasound parameters have been investigated to improve
transfection efficiency (21). In
the present study, commercially available SonoVue lipid
microbubbles were used and the optimized ultrasound exposure
parameters were as follows: Microbubble concentration,
1×107/ml; acoustic intensity, 1.5 W/cm2; and
exposure time, 45 sec. Ultrasound alone only slightly increased
gene delivery efficiency, with a transfection rate of <5%,
despite cell viability of >80%, suggesting that UTMD-mediated
gene delivery is safe but inefficient.
To increase the uptake of exogenous genes into the
cytoplasm, AT-MCB were used, which specifically recognized an
antigen present on the cell membrane (22). Thus, microbubbles were localized to
the cell membrane in greater numbers compared with CMCB. Following
UTMD, more nanopores were formed for the entry of exogenous pDNA.
However, the high cytoplasmic uptake of exogenous genes did not
result in high expression, as the nuclear membrane prevents
translocation of these genes from the cytoplasm to the nucleus.
NLS are special signal peptides that contain
arginine, lysine and other basic amino acids, including the SV40
large T antigen, which was the first NLS demonstrated to stimulate
gene translocation into the nuclei of non-dividing cells (23,24).
NLS guide their sequence to the nucleus, and may bind to nucleic
acid molecules to promote nuclear uptake. Under the guidance of
NLS, the combined DNA and nuclear membrane receptor of NLS may form
a complex called the nuclear pore targeting complex (25–27).
PNA is a polynucleic acid analog that has the deoxyribose-phosphate
backbone replaced with a peptide backbone (28). PNA may hybridize with cDNA in a
double-stranded helix, resulting in a greater affinity compared
with DNA-DNA. This hybrid is one of the most successful known DNA
or RNA analogs that, due to the neutral charge of the PNA backbone,
does not introduce a repulsive force to drive the hybridized
strands apart. PNA has demonstrated the potential to invade duplex
DNA and hybridize to plasmids to form a stable PNA-DNAPNA triplex
hybrid. Using this strategy, NLS were connected to PNA (29).
In the present study, a specific NLS was designed
and inserted into pDNA by combining PNA to promote nuclear import
(30). Combining the NLS with
genes and subsequently to an ultrasound-mediated AT-MCB increased
the nuclear membrane permeability, which further improved nuclear
gene transduction. These results indicated that PNA-binding NLS
significantly promoted the entry of the plasmid from the cytoplasm
to the nucleus. In addition, the protein expression levels of
EGFP-N3 were ~1.5-fold greater in cells transfected with PNA-NLS
compared with those transfected with normal pDNA. This result was
consistent with increased cytoplasmic and nuclear import.
In conclusion, the present study demonstrated the
efficiency of exogenous pDNA in delivering genes to cells. The
findings of the present study indicate that UTMD combined with
AT-MCB and a PNA-binding NLS plasmid may improve the import of
exogenous genes by enhancing cytoplasmic and nuclear intake, thus
providing an experimental basis for gene therapy for in vivo
experiments and clinical application.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81201109
and 81471674).
References
|
1
|
Jafari M, Soltani M, Naahidi S,
Karunaratne DN and Chen P: Nonviral approach for targeted nucleic
acid delivery. Curr Med Chem. 19:197–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dijkmans PA, Juffermans LJ, Musters RJ,
van Wamel A, ten Cate FJ, van Gilst W, Visser CA, de Jong N and
Kamp O: Microbubbles and ultrasound: From diagnosis to therapy. Eur
J Echocardiogr. 5:245–256. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bouakaz A, Versluis M and de Jong N:
High-speed optical observations of contrast agent destruction.
Ultrasound Med Biol. 31:391–399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Jong N, Emmer M, van wamel A and
Versluis M: Ultrasonic characterization of ultrasound contrast
agents. Med Biol Eng Comput. 47:861–873. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bekeredjian R, Chen S, Frenkel PA,
Grayburn PA and Shohet RV: Ultrasound-targeted microbubble
destruction can repeatedly direct highly specific plasmid
expression to the heart. Circulation. 108:1022–1026. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Juffermans LJ, van Dijk A, Jongenelen CA,
Drukarch B, Reijerkerk A, de Vries HE, Kamp O and Musters RJ:
Ultrasound and microbubble-induced intra- and intercellular
bioeffects in primary endothelial cells. Ultrasound Med Biol.
35:1917–1927. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miller AM, Munkonge FM, Alton EW and Dean
DA: Identification of protein cofactors necessary for
sequence-specific plasmid DNA nuclear import. Mol Ther.
17:1897–1903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zabner J, Fasbender AJ, Moninger T,
Poellinger KA and Welsh MJ: Cellular and molecular barriers to gene
transfer by a cationic lipid. J Biol Chem. 270:18997–19007. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Young JL and Dean DA: Nonviral gene
transfer strategies for the vasculature. Microcirculation. 9:35–49.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma H, Zhu J, Maronski M, Kotzbauer PT, Lee
VM, Dichter MA and Diamond SL: Non-classical nuclear localization
signal peptides for high efficiency lipofection of primary neurons
and neuronal cell lines. Neuroscience. 112:1–5. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klibanov AL: Targeted delivery of
gas-filled microspheres, contrast agents for ultrasound imaging.
Adv Drug Deliv Rev. 37:139–157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki R, Takizawa T, Negishi Y, Hagisawa
K, Tanaka K, Sawamura K, Utoguchi N, Nishioka T and Maruyama K:
Gene delivery by combination of novel liposomal bubbles with
perfluoropropane and ultrasound. J Control Release. 117:130–136.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suzuki R, Takizawa T, Negishi Y, Utoguchi
N, Sawamura K, Tanaka K, Namai E, Oda Y, Matsumura Y and Maruyama
K: Tumor specific ultrasound enhanced gene transfer in vivo with
novel liposomal bubbles. J Control Release. 125:137–144. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng Q, Chen JL, Zhou Q, Hu B, Chen Q,
Huang J and Guo RQ: Ultrasound microbubbles combined with the NFκB
binding motif increase transfection efficiency by enhancing the
cytoplasmic and nuclear import of plasmid DNA. Mol Med Rep.
8:1439–1445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Liu Y, Xiang G, Lv Q, Huang G,
Yang Y, Zhang Y, Song Y, Zhou H and Xie M: Ultrasound-triggered
microbubble destruction in combination with cationic lipid
microbubbles enhances gene delivery. J Huazhong Univ Sci Technolog
Med Sci. 31:39–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan Y, Yan L, Wu QQ, Zhou H, Jin YG, Bian
ZY, Deng W, Yang Z, Shen DF, Zeng XF, et al: Mnk1
(mitogen-activated protein kinase-interacting kinase 1) deficiency
aggravates cardiac remodeling in mice. Hypertension. 68:1393–1399.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pérez-Martínez FC, Guerra J, Posadas I and
Ceña V: Barriers to non-viral vector-mediated gene delivery in the
nervous system. Pharm Res. 28:1843–1858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mehier-Humbert S, Bettinger T, Yan F and
Guy RH: Plasma membrane poration induced by ultrasound exposure:
Implication for drug delivery. J Control Release. 104:213–222.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wan C, Li F and Li H: Gene therapy for
ocular diseases meditated by ultrasound and microbubbles (Review).
Mol Med Rep. 12:4803–4814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Newman CM and Bettinger T: Gene therapy
progress and prospects: Ultrasound for gene transfer. Gene Ther.
14:465–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duvshani-Eshet M and Machluf M:
Therapeutic ultrasound optimization for gene delivery: A key factor
achieving nuclear DNA localization. J Control Release. 108:513–528.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsutsui JM, Xie F and Porter RT: The use
of microbubbles to target drug delivery. Cardiovasc Ultrasound.
2:232004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kimmelman J: Protection at the cutting
edge: The case for central review of human gene transfer research.
CMAJ. 169:781–782. 2003.PubMed/NCBI
|
|
24
|
Benimetskaya L, Guzzo-Pernell N, Liu ST,
Lai JC, Miller P and Stein CA: Protamine-fragment peptides fused to
an SV40 nuclear localization signal delivery oligonucleotides that
produce antisense effects in prostate and bladder carcinoma cells.
Bioconjug Chem. 13:177–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Imamoto N: Diversity in nucleocytoplasmic
transport pathways. Cell Struct Funct. 25:207–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakanishi M, Akuta T, Nagoshi E, Eguchi A,
Mizuguchi H and Senda T: Nuclear targeting of DNA. Eur J Phar Sci.
13:17–24. 2001. View Article : Google Scholar
|
|
27
|
Lange A, Mills RE, Lange CJ, Stewart M,
Devine SE and Corbett AH: Classical nuclear localization signals:
Definition, function, and interaction with importin alpha. J Biol
Chem. 282:5101–5105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nielsen PE, Egholm M, Berg RH and Buchardt
O: Sequence-selective recognition of DNA by strand displacement
with athymine-substituted polyamide. Science. 254:1497–1500. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zelphati O, Liang X, Nguyen C, Barlow S,
Sheng S, Shao Z and Felgner PL: PNA-dependent gene chemistry:
Stable coupling of peptides and oligonucleotides to plasmid DNA.
Biotechniques. 28:304–310. 2000.PubMed/NCBI
|
|
30
|
Munkonge FM, Amin V, Hyde SC, Green AM,
Pringle IA, Gill DR, Smith JW, Hooley RP, Xenariou S, Ward MA, et
al: Identification and functional characterization of cytoplasmic
determinants of plasmid DNA nuclear import. J Biol Chem.
284:26978–26987. 2009. View Article : Google Scholar : PubMed/NCBI
|