Introduction
Renal tubulointerstitial fibrosis is a prominent
pathological characteristic of chronic kidney disease, and is
characterized by extensive interstitial myofibroblast activation
and excessive matrix protein accumulation, which are the common
attributes of numerous types of chronic kidney disease leading to
end-stage renal disease (1,2).
Previous studies have demonstrated that transforming growth factor
(TGF)-β1 serves an important role in mediating chronic
inflammation, myofibroblast activation and extracellular matrix
(ECM) accumulation (3–5). At present, there are no effective
treatments for renal interstitial fibrosis or ways to impede the
progress of the associated chronic kidney disease. Therefore, it is
of importance to investigate potential strategies to prevent the
development of renal interstitial fibrosis in the diseased
kidneys.
Although studies have focused on renal interstitial
fibrosis, the mechanism underlying its prevalence remains to be
elucidated. Among the putative mechanisms, epithelial-mesenchymal
transition (EMT) has become a popular hypothesis (6). The primary events of EMT include the
adoption of a mesenchymal-like cellular phenotype and migration
through the tubular basement membrane towards the tubular
interstitium. It has been established that a number of factors are
involved in the process of EMT, which include the profibrotic
factor TGF-β1 and the antifibrotic cytokine bone morphogenetic
protein-7 (BMP-7). These are the important molecules that determine
the fate of the kidney as they hold the potential to initiate and
complete the process of EMT (7,8).
It was previously demonstrated that the product of
uterine sensitization-associated gene 1 (USAG-1) acts as a
kidney-specific BMP antagonist, which binds to and inhibits the
biological activity of BMP-7 (9–14). A
previous study revealed USAG-1-deficient mice to be resistant to
in vivo kidney injury, while genetic ablation of USAG-1 in
the chronic renal injury model of unilateral uretral obstruction
(UUO) resisted kidney injury and therefore prolonged survival
(15). However, following
administration of USAG-l−/− mouse anti-BMP-7
neutralizing antibody, the USAG−/− renal protective
effect in mice was inhibited. Since USAG-1 may serve a notable role
in the regulation of BMP-7 renal protection, inhibiting USAG-1
expression may represent a promising therapeutic measure for the
treatment or management of renal interstitial fibrosis.
Artemisinin, extracted and isolated from
Artemisia annua compositae leaves, is a sesquiterpene
compound with a peroxide bridge. In addition to exhibiting
anti-malarial effects, artemisinin has anti-inflammatory,
anti-tumor and anti-fibrotic effects (16,17).
Artesunate (ART) is an artemisinin derivative, and has been
demonstrated to exhibit a therapeutic effect in the treatment of
pulmonary fibrosis, liver fibrosis and myocardial fibrosis
(18,19). Additionally, a previous study
confirmed that ART was able to effectively alleviate renal fibrosis
caused by UUO (20); however,
whether ART inhibits the occurrence of EMT by inhibiting the
activity of USAG-1 or activating the activity of BMP-7 remains
unclear.
In the present study, the effect of ART on
TGF-β1-induced EMT was investigated with the aim of examining the
potential underlying mechanism. The mechanism may be used to
elucidate the association between the effect of ART and the
abnormal expression of BMP-7 and USAG-1.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM)/high
glucose was purchased from Gibco® (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), Ausbian fetal bovine serum
(FBS) was purchased from Thermo Fisher Scientific, Inc., and
penicillin-streptomycin solution was purchased from Beyotime
Institute of Biotechnology (Haimen, China). Trypsin/EDTA solution
was purchased from Beijing Solarbio Science and Technology Co.,
Ltd. (Beijing, China) and the semi-quantitative polymerase chain
reaction (sq-PCR) kit was obtained from Tiangen Biotech Co., Ltd.
(Beijing, China). For western blotting, BMP-7 (cat. no. ab56023),
USAG-1 (cat no. ab99340), E-cadherin (cat. no. 5409-1) and α-smooth
muscle actin (α-SMA) (cat. no. 1184-1) antibodies were all
purchased from Abcam (Cambridge, UK), GAPDH antibody was obtained
from Zhongshan Jinqiao Biotechnology Co., Ltd. (Beijing, China),
and for immunofluorescence staining, USAG-1 (cat. no. sc-162253)
antibody was obtained from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA), the secondary antibodies were from Zhongshan Jinqiao
Biotechnology Co., Ltd. (Beijing, China).
Cell culture and treatment
Normal rat kidney tubular epithelial cells (NRK-52E)
were purchased from the Chinese Academy of Sciences Cell Bank
(Shanghai, China) and maintained in DMEM/high-glucose medium
supplemented with 1% penicillin-streptomycin and 10% FBS, in a
humidified incubator containing 95% air and 5% CO2 at
37°C atmosphere. For EMT experiments, cells were treated with 5
ng/ml TGF-β1 (Peprotech, Inc., Rocky Hill, NJ, USA) for 48 h
(21). ART (>98% purity;
molecular weight, 384.43) was purchased from TCI (Shanghai)
Development Co., Ltd. (Shanghai, China). In order to determine the
effect of ART on the activity and proliferation of NRK-52E cells
induced by TGF-β1, cells were seeded at 60–70% confluence in
complete medium containing 10% FBS for 24 h, and subsequently
serum-starved for 12 h. The cells were subsequently induced with
TGF-β1 (5 ng/ml), treated with different ART concentrations (0,
0.01, 0.1 and 1 µg/ml) containing 1% FBS and incubated for 48 h.
The groups for the experiment were as follows: Control group,
TGF-β1 group (normal NRK-52E cells treated with 5 ng/ml TGF-β1),
ART-L group (0.01 µg/ml ART), ART-M group (0.1 µg/ml ART), and
ART-H group (1 µg/ml ART). Cells were harvested, following which
the expression of associated proteins and genes was determined.
MTT assay for cell viability
NRK-52E cells were seeded into 96-well culture
plates and treated with various ART concentrations (0, 0.01, 0.1 1,
5 and 10 µg/ml) for 48 h. Cell viability was determined via an MTT
assay and cells were incubated with 20 µl MTT solution (0.5 mg/ml)
for 4 h at 37°C. The purple formazan crystals derived from the MTT
were dissolved in 150 µl dimethyl sulfoxide and agitated for 10
min. The absorbance at 490 nm was measured with a microplate reader
(Elx808; BioTek Instruments, Inc., Winooski, VT, USA).
Morpholopy observation
After treatment with TGF-β1 combined with or without
ART for 48 h, cells were washed with PBS twice. The morphological
changes of NRK-52E cells were observed under a phase-contrast
photomicroscope (Leica Microsystems GmbH, Wetzlar, Germany) and
photographed using a digital camera.
Reverse transcription (RT)-sqPCR
analysis
Total RNA was extracted using TRIzol reagent
(Tiangen Biotech Co., Ltd.), according to manufacturer's
instructions, and the cDNA was synthesized using a TIANScript RT
kit (Tiangen Biotech Co., Ltd.) (22). Primers were synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China). sqPCR was performed as
previously described (22). The
sequences of the primers used were as follows: α-SMA forward,
5′-GCATCCACGAAACCACCT-3′ and reverse, 5′-CGCCGATCCAGACAGAAT-3′ (210
bp); E-cadherin forward, 5′-TTCAACCCAAGCACGTACCA-3′ and reverse,
5′-CAGAATGCCCTCGTTGGTCT-3′ (186 bp); BMP-7 forward,
5′-GCACCTCCAGGGAAAAC-3′ and reverse, 5′-AAGCCCAGATGGTACGG-3′ (443
bp); USAG-1 forward, 5′-AGATTTGATTGCGTGGAAGAC-3′ and reverse,
5′-GGTTCGGAGGGATTTAGTTTG-3′ (311 bp); collagen type I (Col I)
forward, 5′-TCAGGGGCGAAGGCAACAGT-3′ and reverse,
TTGGGATGGAGGGAGTTTACACGA-3′ (219 bp); and GAP DH forward,
5′-TGCTGAGTATGTCGTGGAGT-3′ and reverse, 5′-AGTCTTCTGAGTGGCAGTGAT-3′
(289 bp). PCR amplification was performed with initial denaturation
at 94°C for 5 min, followed by 30 consecutive cycles of
denaturation at 94°C for 30 sec, annealing at 58–62°C for 30 sec
and extension at 72°C for 1 min, with a final extension at 72°C for
7 min. The amplified products were analyzed by electrophoresis on a
1.5% (w/v) agarose gel and stained with fluorescence staining dye
Goldview (Beijing Solarbio Science and Technology Co., Ltd.)
alongside a DNA marker (Tiangen Biotech Co., Ltd.) The relative
mRNA levels of various genes were calculated following
normalization to GAPDH. The signal intensity of the images was
analyzed using ImageJ version 1.48 software (National Institutes of
Health, Bethesda, MD, USA).
Western blot analysis
Cells were lysed with radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology), supplemented with
phenylmethylsulfonyl fluoride. The protein samples were quantified
using bicinchoninic acid protein assay reagents (Beyotime Institute
of Biotechnology). Western blot analysis was performed as
previously described (23).
Following boiling for 10 min in a 5X loading buffer (Beyotime
Institute of Biotechnology), protein samples (45 µg) were separated
by SDS-PAGE on an 8% gel and transferred onto nitrocellulose
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
subsequently blocked in PBS containing 3% BSA (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and incubated with primary antibodies at
a 1:1,000 dilution overnight at 4°C. The primary antibodies used
were as follows: Anti-α-SMA, anti-E-cadherin, anti-BMP-7 and
anti-USAG-1. Following washing with PBS-Tween-20, the membrane was
incubated with alkaline phosphatase-conjugated goat anti-rabbit and
horse anti-mouse secondary antibodies at a 1:1,000 dilution at room
temperature for 2 h. Signal detection was performed using an
enhanced chemiluminescence kit (Shanghai Tianneng Technology Co.,
Ltd., Shanghai, China). The band density was measured using ImageJ
software.
Immunofluorescent staining
The expressions of E-cadherin, α-SMA, BMP-7 and
USAG-1 in NRK-52E cells were analyzed by immunofluorescence
staining. Cells (2×104 cells/ml) were cultured on glass
coverslips and treated with TGF-β1 with or without 1 µg/ml ART for
48 h. Following treatment for 48 h, cells in the basement layer
were washed three times with PBS and fixed in 4% paraformaldehyde
for 15 min at room temperature. Following three extensive washes
with PBS, the cells were treated with 0.5% Triton X-100 for 10 min
and blocked with 2% normal donkey serum (Sigma-Aldrich; Merck KGaA)
for 1 h at room temperature, and subsequently incubated with
primary antibodies (1:200) at 4°C overnight. Cells were washed with
PBS for 3–5 min and incubated with goat anti-rabbit and rabbit
anti-goat secondary antibodies (Zhongshan Jinqiao Biotechnology
Co., Ltd., Beijing, China) at a dilution of 1:200 for 2 h.
Following washing in PBS, cells were stained with DAPI (Beyotime
Institute of Biotechnology) at room temperature to visualize the
nuclei. Following washing in PBS, the device was mounted with
anti-fluorescence quenching agent (Beyotime Institute of
Biotechnology) and cover-slipped; digital images were captured
using an inverted fluorescent microscope (magnification, ×400).
Statistical analysis
All data were reported as the mean ± standard
deviation. Statistical analysis was performed using the statistical
package SPSS for Windows, version 13.0 (SPSS, Inc., Chicago, IL,
USA) and GraphPad Prism v. 5.0 (GraphPad Software, Inc., La Jolla,
CA, USA). The comparisons among different groups were made using
one-way analysis of variance followed by least significant
difference/Dunett-T3 tests. P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
≥3 times.
Results
Effect of TGF-β1 on NRK-52E cells
In order to investigate the effect of TGF-β1 on
NRK-52E cells, cells were treated with different concentrations of
TGF-β1 (2, 5 and 10 µg/ml) for 48 h. The results demonstrated that
5 and 10 µg/ml TGF-β1 was able to significantly increase the
expression of α-SMA mRNA and protein. In addition, TGF-β1 (5 and 10
µg/ml) was able to downregulate the expression of E-cadherin mRNA,
while a concentration of 2 µg/ml TGF-β1 had no significant effect
on α-SMA mRNA and protein and E-cadherin mRNA compared with the
control group. Additionally, TGF-β1 was able to decrease cell-cell
contact and the cells adopted a more elongated morphology at a
concentration of 5 ng/ml. However, TGF-β1 did not influence cell
morphology at a concentration of 2 ng/ml. Therefore, 5 ng/ml TGF-β1
was selected as the concentration to be used in the following
experiment (data not shown). Subsequently, NRK-52E cells were
treated with 5 ng/ml TGF-β1 for 24, 48 and 72 h, and the results
demonstrated that the expression of α-SMA mRNA was significantly
upregulated compared with the control group when treated for 48 h,
while TGF-β1 had no marked effect on α-SMA mRNA expression when
cells were treated for 24 h. In addition, the cellular morphology
gradually altered from oval to a long spindle shape over time, and
this alteration was apparent at 48 h; however, cellular morphology
was almost unaltered compared with the control group at 24 h (data
not shown). According to the above results, 5 ng/ml TGF-β1 for 48 h
was selected as the treatment to be used for the remainder of the
experiments.
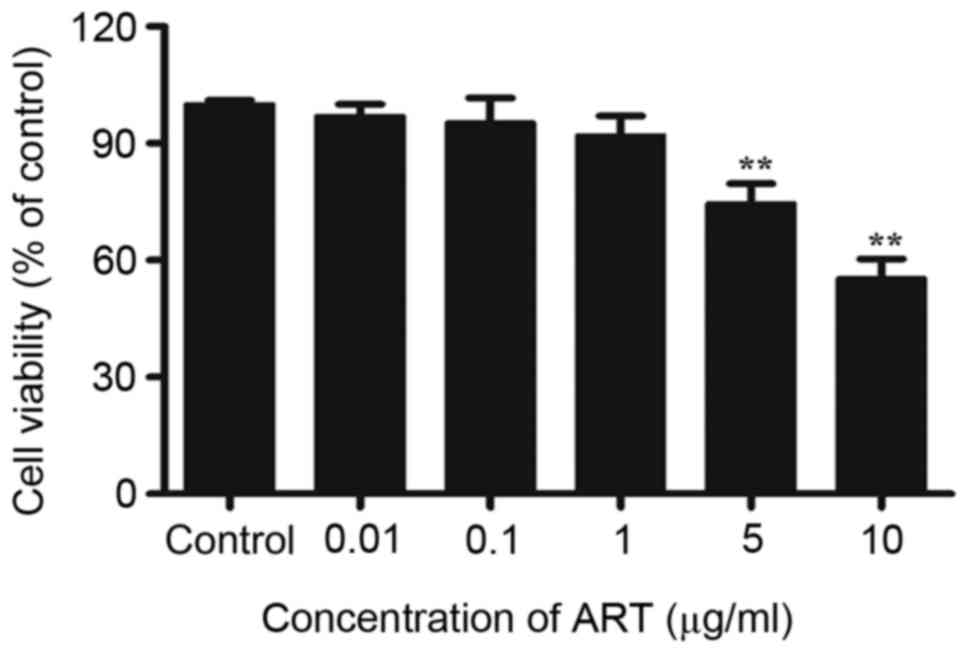
Effect of ART on cellular
viability
In order to determine the effect of ART on cell
viability, NRK-52E cells (2.5×104 cells/ml) were seeded
into 96-well culture plates and treated with different
concentrations of ART (0.01, 0.1, 1, 5 and 10 µg/ml) for 48 h,
following which cell viability was determined. The results
demonstrated that there was little or no effect on cell viability
low-doses of ART (0.01, 0.1 and 1 µg/ml). However, treatment with 5
or 10 µg/ml ART resulted in a significant decrease in cell
viability (Fig. 1). Therefore,
0.01, 0.1 and 1 µg/ml were used for the following experiments.
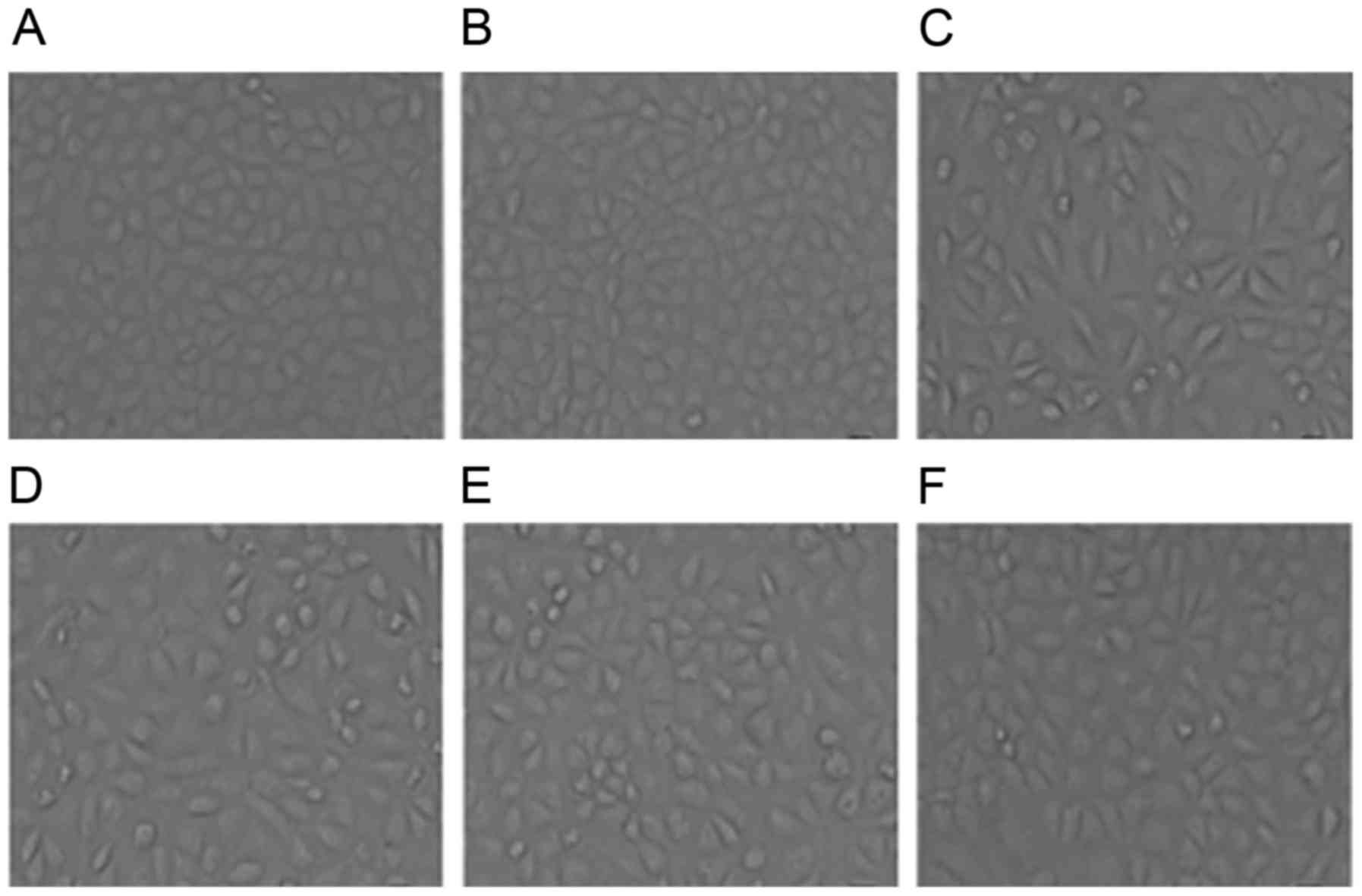
Effect of ART on cell morphology
In order to observe the alterations in cellular
morphology caused by treatment with ART, the morphological
alterations in NRK-52E cells were observed under a phase-contrast
photomicroscope (Leica Microsystems GmbH) and photographed using a
digital camera. The control cells exhibited a typical epithelial
cuboidal shape with cobblestone morphology (Fig. 2A). Treatment with ART alone (ART
group) had no effect on morphology compared with the control group
(Fig. 2B); therefore, this group
was not included in the following experiment. By contrast, NRK-52E
cells exposed to TGF-β1 (5 ng/ml) exhibited a decrease in cell-cell
contacts and adopted a more elongated morphological shape. Notably,
treatment with TGF-β1 resulted in a morphology which was
fibroblast-like in nature and identifiable by the presence of
elongated lamellipodia and a spindle shape, as presented in
Fig. 2C. However, ART was able to
improve the morphology of cells to different degrees and a dose of
ART at 1 µg/ml rendered the cellular morphology close to the normal
cellular morphology (Fig. 2D-F).
These results indicated that ART was able to improve TGF-β1-induced
morphological alterations in cells.
Effects of ART on the levels of
E-cadherin, α-SMA and Col I in NRK-52E cells
In order to evaluate the regulatory effects of ART
in TGF-β1-induced EMT, the expression of E-cadherin and α-SMA was
examined at the mRNA and protein levels in epithelial cells. The
level of α-SMA expression was low, while E-cadherin expression was
high in normal renal tubular epithelial cells. The results
suggested that TGF-β1-induced cells exhibited a significant
decrease in the gene expression of E-cadherin (Fig. 3A; P<0.01), and ART-L had no
significant effect on the expression of E-cadherin. The expression
of E-cadherin was significantly increased in the ART-M and ART-H
groups, particularly at the highest concentration of ART (Fig. 3A). The mRNA expression of α-SMA in
TGF-β1-induced EMT was significantly upregulated compared with the
control group. Treatment with ART significantly attenuated the
TGF-β1-induced increase in the mRNA expression of α-SMA, and this
was more apparent at moderate and high doses of ART (Fig. 3B). These results confirmed that EMT
served a role in tubulointerstitial fibrosis, and that ART may
improve renal fibrosis by inhibiting the process of EMT. The
occurrence of EMT ultimately leads to deposition of the ECM, and
Col I is the ECM deposit which results from EMT occurrence. To
evaluate the regulatory effects of ART in TGF-β1-induced ECM
protein accumulation, the mRNA expression of Col I was examined.
The results demonstrated that the expression of Col I was increased
in cells undergoing EMT, which was pathologically associated with
fibrosis, while treatment with ART in the low concentration group
decreased the level of Col I and the difference was statistically
significant with moderate and high concentrations of ART (Fig. 3C). The results demonstrated that
the ECM was significantly accumulated in renal fibrosis, and that
ART was able to decrease the accumulation of the ECM.
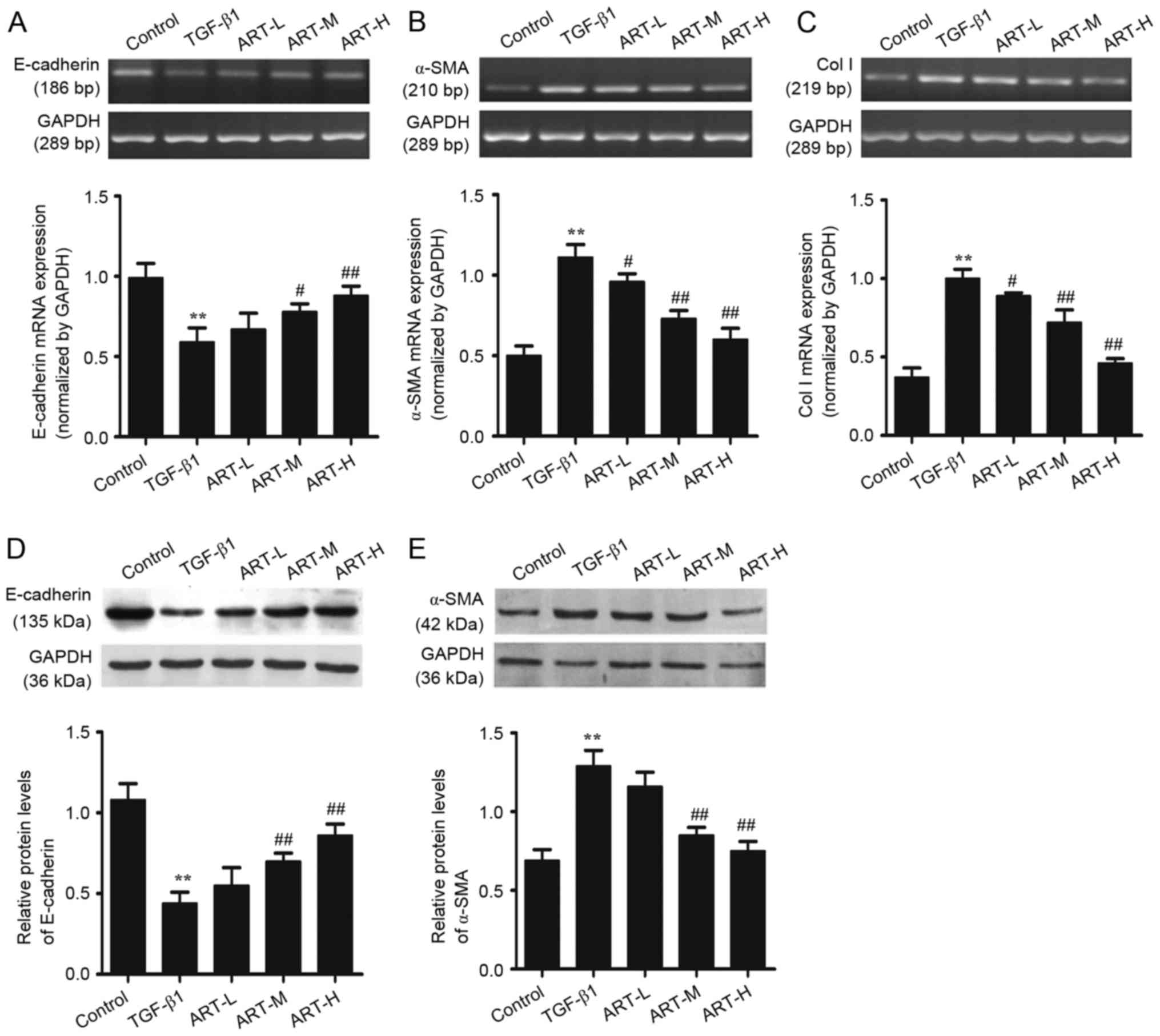 | Figure 3.Effect of ART on the expression of
E-cadherin, α-SMA and Col I at the mRNA and protein levels in
NRK-52E cells. Incubation of NRK-52E cells with different
concentrations of ART with or without TGF-β1 for 48 h. Reverse
transcription-semi-quantitative polymerase chain reaction analysis
was used to detect the expression of (A) E-cadherin, (B) α-SMA and
(C) Col I mRNA. Western blotting was applied to examine the protein
levels of (D) E-cadherin and (E) α-SMA. GAPDH was used as the
internal loading control. All data are presented as the mean ±
standard deviation. n=3. **P<0.01 vs. control;
#P<0.05, ##P<0.01 vs. TGF-β1. ART,
artesunate; ART-L, low-dose ART; ART-M, moderate-dose ART; ART-H,
high-dose ART; α-SMA, α-smooth muscle actin; Col I, collagen type
I; TGF-β1, transforming growth factor-β1. |
The expression of E-cadherin and α-SMA protein was
examined in total cell lysates using western blotting. The results
demonstrated that TGF-β1 was able to increase the expression of
α-SMA and decrease the E-cadherin expression in NRK-52E cells,
demonstrating that TGF-β1 stimulated EMT in tubular cells, which is
consistent with previous reports (6). However, treatment with ART
significantly increased E-cadherin expression and downregulated
α-SMA protein expression, which is in line with previous findings
in the UUO model (20). In
addition, the effect was more marked at moderate and high doses of
ART (Fig. 3D and E).
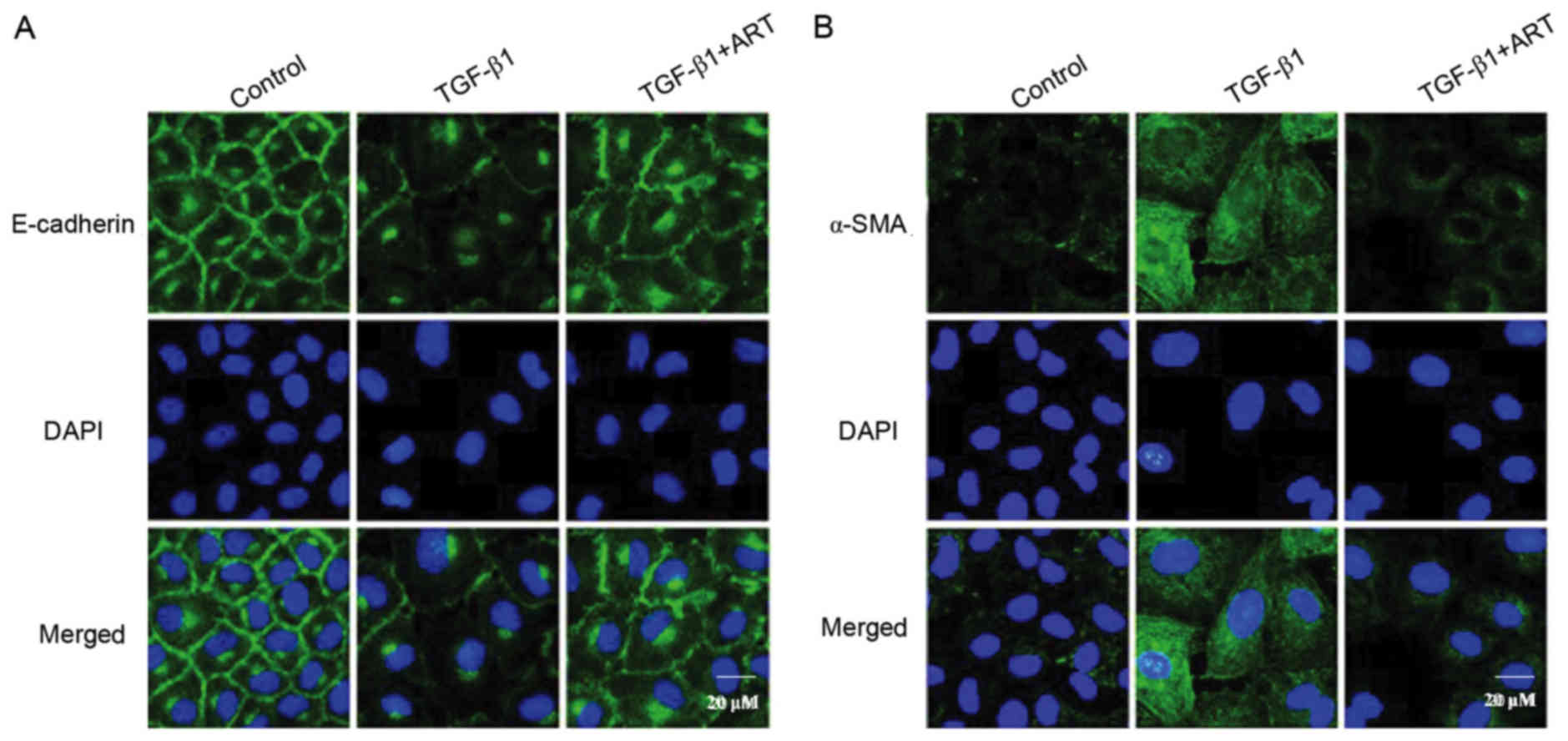
The involvement of α-SMA and E-cadherin in
TGF-β1-induced EMT was further investigated via a fluorescence
confocal assay. Under basal condition, the expression of E-cadherin
was abundant in renal tubular epithelial cells, and was primarily
localized to the plasma membrane (Fig.
4A). When the renal tubular cells were stimulated by TGF-β1,
the expression of E-cadherin was markedly decreased, as exhibited
by decreased green fluorescence; when cells were pretreated with
ART, the TGF-β1-induced E-cadherin delocalization was inhibited
(Fig. 4A). By contrast, the
abundance of the mesenchymal marker α-SMA was low in renal tubular
cells under control conditions, as indicated by the weak
fluorescence in confocal images (Fig.
4B). When the cells were stimulated by TGF-β1, the expression
of α-SMA was markedly increased, as exhibited by the increased
fluorescence in the confocal images (Fig. 4B). When renal tubular cells were
pretreated with ART, TGF-β1 failed to increase α-SMA expression
(Fig. 4B). These results further
confirmed that ART may improve TGF-β1-induced EMT progression.
Effect of ART on the expression of
USAG-1 and BMP-7 at the mRNA and protein levels in NRK-52E
cells
TGF-β1 is considered to be an important cytokine for
the induction of renal interstitial fibrosis, and BMP-7 is a member
of the TGF-β1 superfamily. A previous study reported that BMP-7 may
be able to reverse the renal structural alterations and the degree
of fibrosis in UUO rats by regulating the downstream mothers
against decapentaplegic homolog (Smad)1/5/8 signaling pathway
(24). USAG-1 serves as the
endogenous antagonist of BMP-7. Therefore, the present study
examined the expression of USAG-1 and BMP-7, and the underlying
mechanism.
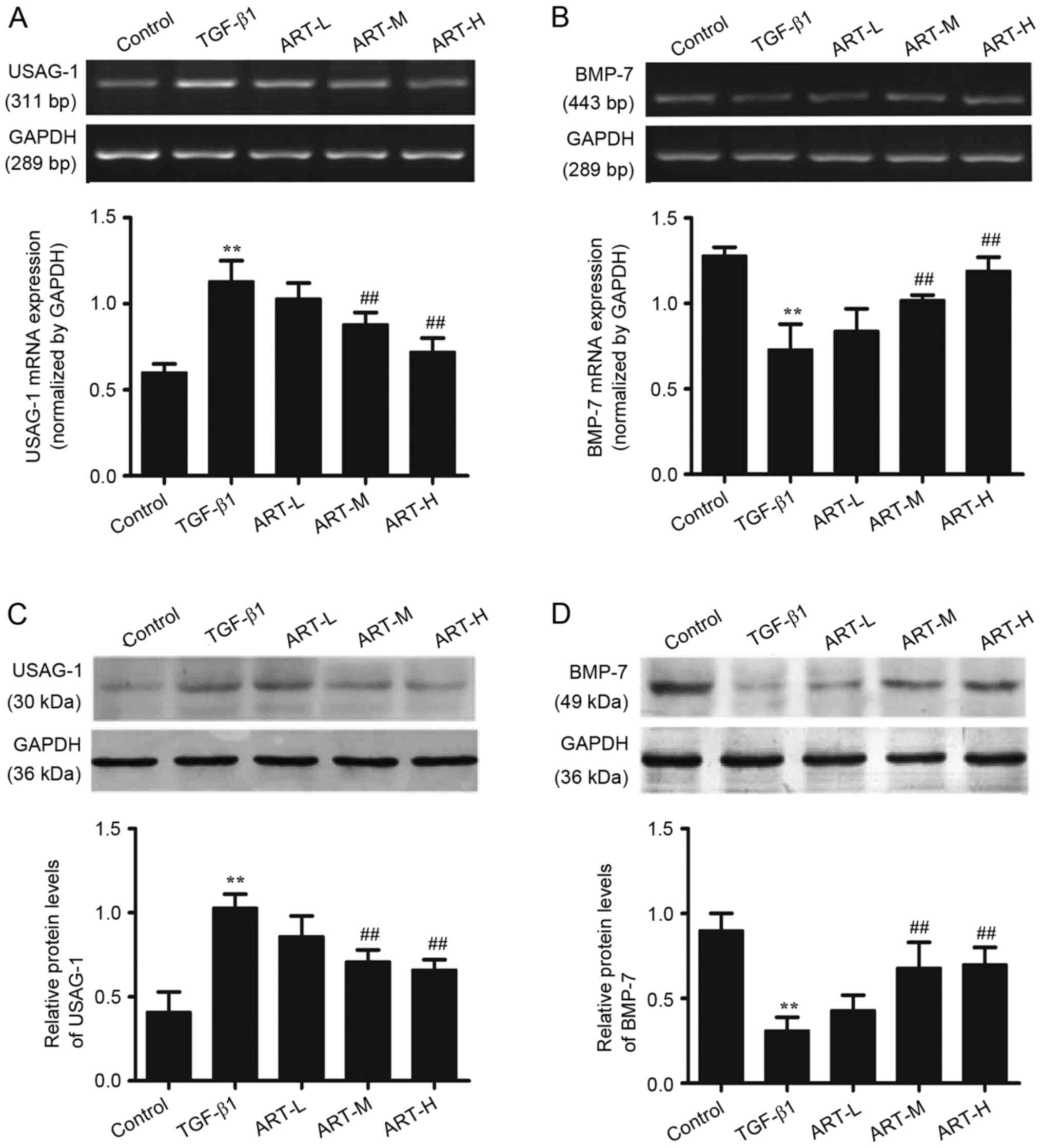
In order to determine the regulatory effects of ART
in TGF-β1-induced BMP-7 and USAG-1 expression, RT-sqPCR analysis
and western blotting were performed (Fig. 5). TGF-β1 upregulated the expression
of USAG-1 in NRK-52E cells as presented in Fig. 5A and C. Treatment with ART (0.1 and
1 µg/ml) significantly decreased the expression of USAG-1 in a
dose-dependent manner compared with the TGF-β1 treated group
(Fig. 5A and C). The effect of ART
on the expression of BMP-7 was additionally detected. Compared with
the control group, the expression of BMP-7 significantly decreased
when treated with TGF-β1; however, ART was able to restore the
expression of BMP-7, and the highest dose of ART exerted the most
marked effect (Fig. 5B and D). The
results of the RT-sqPCR analysis of USAG-1 and BMP-7 were
consistent with those of the western blot analysis.
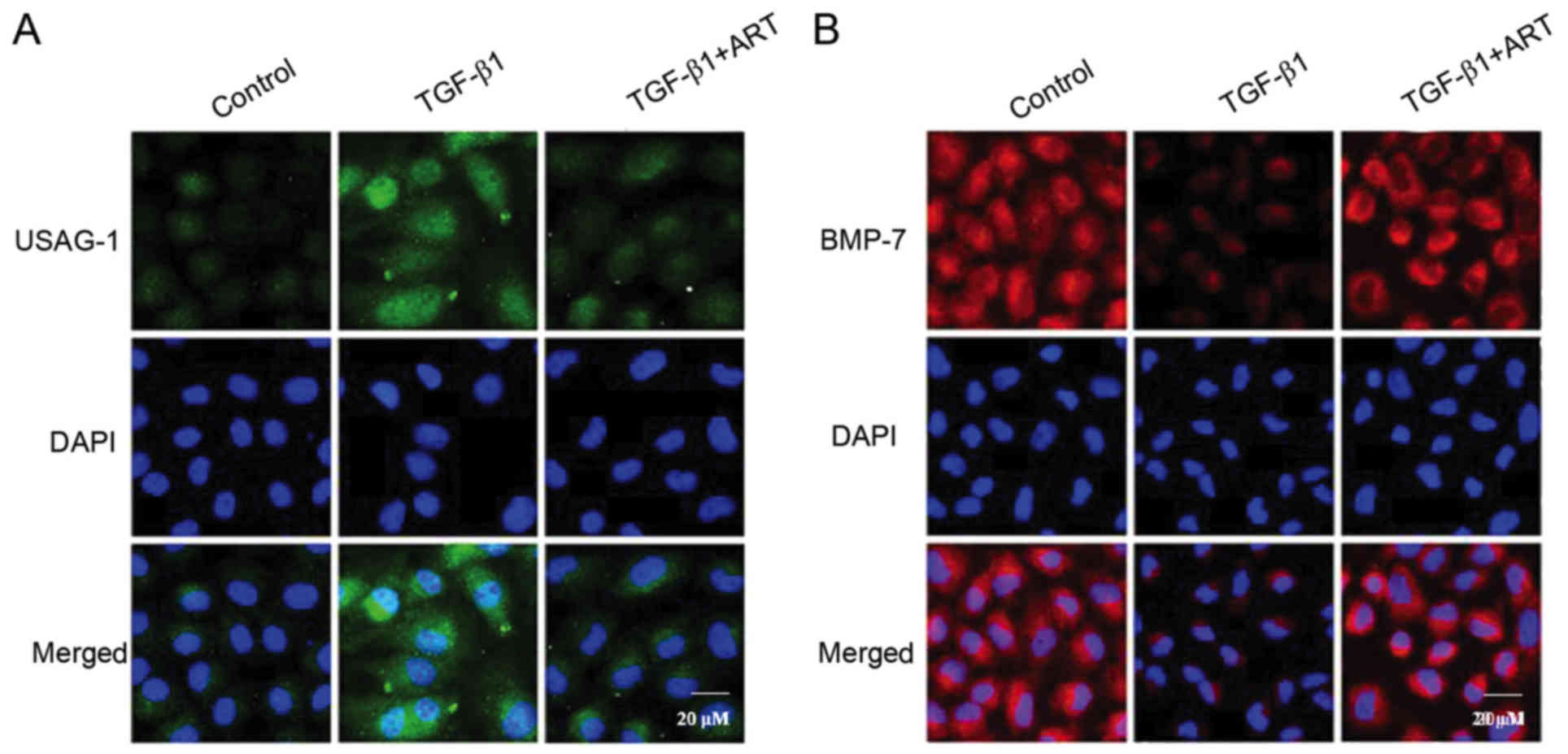
In order to observe the localization of BMP-7 and
USAG-1 in NRK-52E cells, immunofluorescent staining was performed
to detect the two proteins (Fig.
6). The results demonstrated that the expression of USAG-1 in
NRK-52E cells was minimal, while the expression of USAG-1 was
markedly increased when treated with 5 ng/ml TGF-β1. Notably, ART
inhibited the increased expression of USAG-1 (Fig. 6A). Additionally, BMP-7 was
abundantly expressed in NRK-52E cells; however, the expression of
BMP-7 was significantly decreased when induced by TGF-β1, and ART
was able to reverse this effect (Fig.
6B). The results further clarified that the expression of BMP-7
and USAG-1 may alter when induced by TGF-β1, and suggested that ART
may reverse these effects.
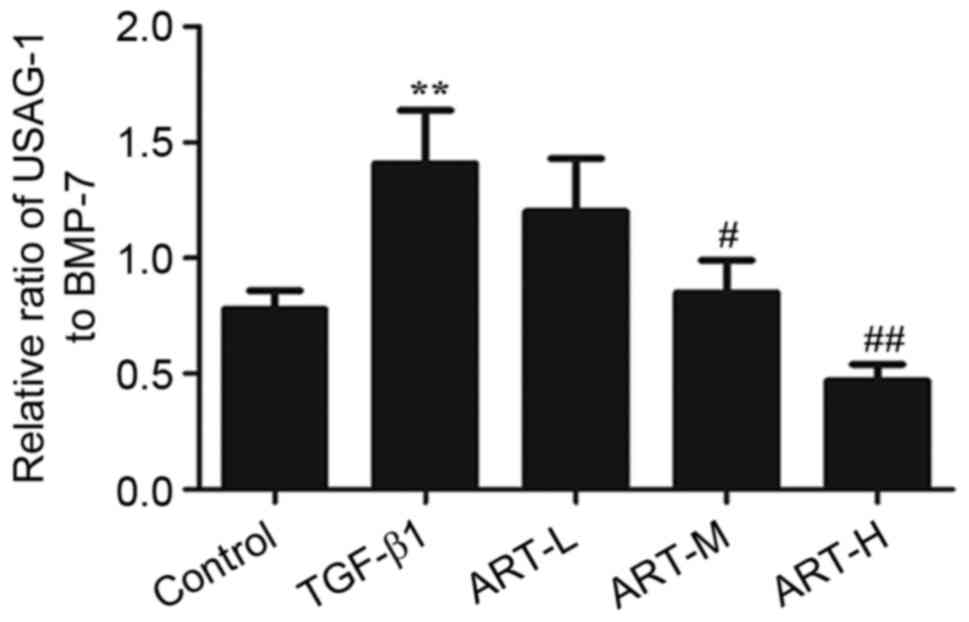
Effect of ART on the ratio of USAG-1
to BMP-7
In order to demonstrate the role of BMP-7 and USAG-1
in EMT, the effects of ART on the protein ratio of USAG-1 to BMP-7
were evaluated following treatment with TGF-β1. The ratio increased
in the TGF-β1 group compared with the control group (Fig. 7). However, the three doses of ART
in the experiment inhibited the elevation of the ratio, and this
was significant in the ART-H and ART-M groups, demonstrating that
the ameliorative effect of ART in EMT may be achieved by restoring
the ratio of USAG-1 and BMP-7.
Discussion
The primary aim of the present study was to
investigate the role of ART in EMT induced by TGF-β1. In the
present study, 2 days of exposure to TGF-β1 in renal proximal
tubular cells significantly increased the levels of α-SMA and
decreased the expression of E-cadherin, suggesting that TGF-β1 was
able to induce the occurrence of EMT (25). In addition, it was demonstrated
that ART was able to ameliorate TGF-β1-induced renal interstitial
fibrosis. Notably, ART reversed the TGF-β-induced increase in
USAG-1 and decrease in BMP-7. The downregulation of USAG-1
expression resulted in the inhibition of EMT. These results
provided evidence for a possible antifibrotic mechanism in renal
interstitial fibrosis.
Renal interstitial fibrosis, which is characterized
by the accumulation of the ECM, is the principal underlying
pathology in the progression of chronic kidney diseases, and is a
common pathway of chronic kidney disease progression to end-stage
renal failure (1). The
pathogenesis of renal fibrosis is characterized by tubular atrophy,
tubular cell loss, myofibroblast accumulation and excessive ECM
protein deposition (26). EMT is a
process through which epithelial cells lose their
epithelial-specific biomarkers, undergo cytoskeletal remodeling,
and gain a mesenchymal phenotype (4). It has been reported that the
TGF-β-Smad signaling pathway may promote the occurrence of EMT.
Studies have demonstrated that tubular EMT is an important resource
of fibrogenic myofibroblasts and serves a central role in
tubulointerstitial fibrosis, including in diabetes nephropathy. In
the present study, ART-treated cells displayed a significant
decrease in the levels of α-SMA and an increase the expression of
E-cadherin, and ART was able to improve the morphological
alterations induced by TGF-β1 and restore the morphology of the
epithelial cells; these results indicated that ART may improve
renal fibrosis induced by TGF-β1. Additionally, ART reversed the
process of EMT induced by TGF-β1.
BMP-7, a morphogenetic protein, has been
hypothesized to serve a role in antifibrosis in renal tubular
epithelial cells (27). Under
normal circumstances, there is abundant BMP-7 expression in renal
tubular epithelial cells, and BMP-7 is able to maintain the normal
functioning of renal tubular epithelial cells (28). The expression of BMP-7 has been
demonstrated to be significantly downregulated under disease
conditions, including hypertension, aristolochic acid nephropathy,
diabetic nephropathy and other disease states (29,30).
In addition, it was reported that exogenous administration of
recombinant human BMP-7 was able to reverse the alterations in
renal structure and the degree of fibrosis in UUO rats by
regulating the downstream Smad1/5/8 signaling pathway (24). A previous study demonstrated that
BMP-7 inhibited TGF-β1-induced fibrogenesis and EMT, and induced
EMT in vitro (31). In the
present study, the levels of BMP-7 mRNA and protein levels
decreased during the process of EMT, which was further confirmed by
the immunofluorescent staining results. However, ART was able to
inhibit the process of EMT and significantly increase the levels of
BMP-7, indicating that ART may inhibit the EMT process via an
upregulation of BMP-7 expression.
It has been demonstrated that the local activity of
endogenous BMP-7 is controlled by the regulation of its expression,
in addition to certain classes of molecules termed BMP antagonists
(13). USAG-1, a dominant
antagonist of BMP-7, is primarily expressed in renal tubular
epithelial cells and contributes to renal injury (9,10).
In previous studies, it was demonstrated that USAG-1-knockout mice
exhibited reduced tubulointerstitial fibrosis in a UUO model, and
improved renal function in acute and chronic kidney injury. The
above results illustrated that the upregulation of USAG-1 may serve
an important role in the process of renal interstitial fibrosis
(12). In the present study, the
expression of USAG-1 was upregulated when induced by TGF-β1,
although it significantly decreased when treated with ART,
particularly the high dose of ART. In addition, the reduction of
USAG-1 expression may improve other indicators of fibrosis. The
results stated above suggested that one of the possible mechanisms
underlying the antifibrotic effects of ART may be associated with
inhibition of the expression of USAG-1 in NRK-52E cells.
In conclusion, the results above demonstrate that
ART was able to inhibit EMT induced by TGF-β1. These effects may be
associated with the upregulation of BMP-7 or the inhibition of
USAG-1 expression, although the exact mechanisms require further
investigation.
Acknowledgements
The present study were funded by grants from the
Jiangsu Key Laboratory of New Drug Research and Clinical Pharmacy
(grant no. ZR-XY201408), and the College of Pharmacy, Xuzhou
Medical University (grant no. 2015YKYCX009).
References
|
1
|
Boor P, Ostendorf T and Floege J: Renal
fibrosis: Novel insights into mechanisms and therapeutic targets.
Nat Rev Nephrol. 6:643–656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jepson RE: Current understanding of the
pathogenesis of progressive chronic kidney disease in cats. Vet
Clin North Am Small Anim Pract. 46:1015–1048. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y: Cellular and molecular mechanisms
of renal fibrosis. Nat Rev Nephrol. 7:684–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barnes JL and Glass WF II: Renal
interstitial fibrosis: A critical evaluation of the origin of
myofibroblasts. Contrib Nephrol. 169:73–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akhurst RJ and Padgett RW: Matters of
context guide future research in TGFβ superfamily signaling. Sci
Signal. 8:re102015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y: New insights into
epithelial-mesenchymal transition in kidney fibrosis. J Am Soc
Nephrol. 21:212–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meng XM, Chung AC and Lan HY: Role of the
TGF-β/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond).
124:243–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang D, Wang Y, Zhu Z, Yang G, An G, Li
X, Niu P, Chen L and Tian L: BMP-7 attenuated silica-induced
pulmonary fibrosis through modulation of the balance between
TGF-β/Smad and BMP-7/Smad signaling pathway. Chem Biol Interact.
243:72–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yanagita M, Okuda T, Endo S, Tanaka M,
Takahashi K, Sugiyama F, Kunita S, Takahashi S, Fukatsu A,
Yanagisawa M, et al: Uterine sensitization-associated gene-1
(USAG-1), a novel BMP antagonist expressed in the kidney,
accelerates tubular injury. J Clin Invest. 116:70–79. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yanagita M, Oka M, Watabe T, Iguchi H,
Niida A, Takahashi S, Akiyama T, Miyazono K, Yanagisawa M and
Sakurai T: USAG-1: A bone morphogenetic protein antagonist
abundantly expressed in the kidney. Biochem Biophys Res Commun.
316:490–500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka M, Endo S, Okuda T, Economides AN,
Valenzuela DM, Murphy AJ, Robertson E, Sakurai T, Fukatsu A,
Yancopoulos GD, et al: Expression of BMP-7 and USAG-1 (a BMP
antagonist) in kidney development and injury. Kidney Int.
73:181–191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kiso H, Takahashi K, Saito K, Togo Y,
Tsukamoto H, Huang B, Sugai M, Shimizu A, Tabata Y, Economides AN,
et al: Interactions between BMP-7 and USAG-1 (uterine
sensitization-associated gene-1) regulate supernumerary organ
formations. PLoS One. 9:e969382014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakamura J and Yanagita M: Bmp modulators
in kidney disease. Discov Med. 13:57–63. 2012.PubMed/NCBI
|
|
14
|
Simmons DG and Kennedy TG: Uterine
sensitization-associated gene-1: A novel gene induced within the
rat endometrium at the time of uterine receptivity/sensitization
for the decidual cell reaction. Biol Reprod. 67:1638–1645. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Collette NM, Yee CS, Murugesh D, Sebastian
A, Taher L, Gale NW, Economides AN, Harland RM and Loots GG: Sost
and its paralog Sostdc1 coordinate digit number in a Gli3-dependent
manner. Dev Biol. 383:90–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chong CM and Zheng W: Artemisinin protects
human retinal pigment epithelial cells from hydrogen
peroxide-induced oxidative damage through activation of ERK/CREB
signaling. Redox Biol. 9:50–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tu Y: The discovery of artemisinin
(qinghaosu) and gifts from Chinese medicine. Nat Med. 17:1217–1220.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang C, Xuan X, Yao W, Huang G and Jin J:
Anti-profibrotic effects of artesunate on bleomycin-induced
pulmonary fibrosis in Sprague Dawley rats. Mol Med Rep.
12:1291–1297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lai L, Chen Y, Tian X, Li X, Zhang X, Lei
J, Bi Y, Fang B and Song X: Artesunate alleviates hepatic fibrosis
induced by multiple pathogenic factors and inflammation through the
inhibition of LPS/TLR4/NF-κB signaling pathway in rats. Eur J
Pharmacol. 765:234–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao J, Wang W, Li Y, Xia J, Peng Y, Zhang
Y and Xia A: Artesunate attenuates unilateral ureteral
obstruction-induced renal fibrosis by regulating the expressions of
bone morphogenetic protein-7 and uterine sensitization-associated
gene-1 in rats. Int Urol Nephrol. 48:619–629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pang M, Wang H, Rao P, Zhao Y, Xie J, Cao
Q, Wang Y, Wang YM, Lee VW, Alexander SI, et al: Autophagy links
β-catenin and Smad signaling to promote epithelial-mesenchymal
transition via upregulation of integrin linked kinase. Int J
Biochem Cell Biol. 76:123–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanaka T, Doi K, Maeda-Mamiya R, Negishi
K, Portilla D, Sugaya T, Fujita T and Noiri E: Urinary L-type fatty
acid-binding protein can reflect renal tubulointerstitial injury.
Am J Pathol. 174:1203–1211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meng XM, Huang XR, Chung AC, Qin W, Shao
X, Igarashi P, Ju W, Bottinger EP and Lan HY: Smad2 protects
against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol.
21:1477–1487. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Manson SR, Niederhoff RA, Hruska KA and
Austin PF: The BMP-7-Smad1/5/8 pathway promotes kidney repair after
obstruction induced renal injury. J Urol. 185 Suppl 6:S2523–S2530.
2011. View Article : Google Scholar
|
|
25
|
Lian YG, Zhou QG, Zhang YJ and Zheng FL:
VEGF ameliorates tubulointerstitial fibrosis in unilateral ureteral
obstruction mice via inhibition of epithelial-mesenchymal
transition. Acta Pharmacol Sin. 32:1513–1521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lawson JS, Syme HM, Wheeler-Jones CP and
Elliott J: Urinary active transforming growth factor β in feline
chronic kidney disease. Vet J. 214:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeisberg M: Bone morphogenic protein-7 and
the kidney: Current concepts and open questions. Nephrol Dial
Transplant. 21:568–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lim AI, Chan LY, Tang SC, Yiu WH, Li R,
Lai KN and Leung JC: BMP-7 represses albumin-induced chemokine
synthesis in kidney tubular epithelial cells through
destabilization of NF-κB inducing kinase. Immunol Cell Biol.
92:427–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bramlage CP, Tampe B, Koziolek M, Maatouk
I, Bevanda J, Bramlage P, Ahrens K, Lange K, Schmid H, Cohen CD, et
al: Bone morphogenetic protein (BMP)-7 expression is decreased in
human hypertensive nephrosclerosis. BMC Nephrol. 11:312010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ivanac-Janković R, Ćorić M, Furić-Čunko V,
Lovičić V, Bašić-Jukić N and Kes P: BMP-7 protein expression is
downregulated in human diabetic nephropathy. Acta Clin Croat.
54:164–168. 2015.PubMed/NCBI
|
|
31
|
Zeisberg M, Hanai J, Sugimoto H, Mammoto
T, Charytan D, Strutz F and Kalluri R: BMP-7 counteracts
TGF-beta1-induced epithelial-to-mesenchymal transition and reverses
chronic renal injury. Nat Med. 9:964–968. 2003. View Article : Google Scholar : PubMed/NCBI
|