Introduction
Gastric cancer is one of the most common malignant
cancers globally. It is estimated that gastric cancer alone causes
almost 10% of all cancer-associated mortality with a higher
(10–12%) incidence rate in Asia and Europe (1). Despite global reductions in
prevalence and mortality over the past 20 years, gastric cancer is
still ranked as the second major cause of cancer-associated
mortality and considered as the foremost gastrointestinal
infectious disease in eastern Asia (2). Understanding of the molecular
mechanisms underlying gastric cancer have improved, but there
remains a distinctive lack of targeted treatments in the clinical
development of the disease (3).
Therefore, there is a necessity to establish novel therapeutic
agents that diminish the mortality rate of patients with gastric
cancer, with minor side effects.
Plant-derived natural products have been used as a
source of medicinal agents useful to treat humans, and the hunt for
novel, effective therapeutic compounds continues. Bakuchiol is a
typical prenylated monoterpene phenolic compound separated from
Psoralea corylifolia, a member of the Leguminosae family.
Previous reports have demonstrated that molecular structures
containing styryl moieties in conjugation with other structural
features, including chromones, quinazolines and pyrenes, have
multiple biological uses including the treatment of cancer and HIV
(4–7). Bakuchiol has previously been
demonstrated to exhibit cytotoxicity in certain human cancer cell
lines (8). Based on these prior
examinations, the present study investigated the anticancer effects
of bakuchiol in gastric cancer, aiming to produce novel insights to
enhance understanding of the underlying molecular mechanism.
Materials and methods
Materials and reagents
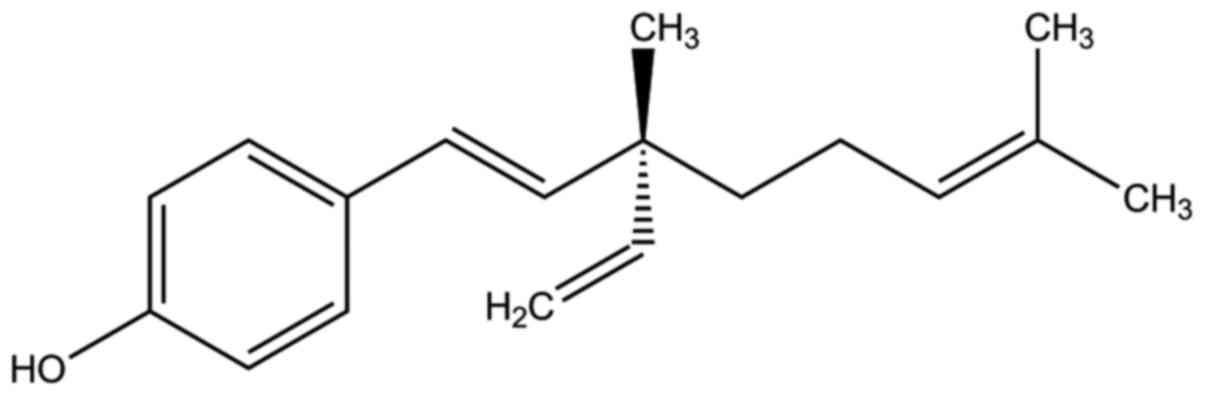
Bakuchiol (Fig. 1),
MTT reagent and dimethyl sulfoxide (DMSO) were ordered from Sigma
Aldrich; Merck KGaA (Darmstadt, Germany). Cell culturing RPMI-1640
medium, fetal bovine serum (FBS) and penicillin/streptomycin
antibiotics were obtained from Gibco; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). Anti-rabbit monoclonal B cell lymphoma-2
associated X, apoptosis regulator (Bax; cat. no. ab32503; 1:1,000),
anti-rabbit monoclonal B cell lymphoma-extra large (Bcl-xL; cat.
no. ab32370; 1:1,000), anti-rabbit monoclonal procaspase-3 (cat.
no. ab32150; 1:500), anti-rabbit monoclonal procaspase-6 (cat. no.
ab3263, 1:800), anti-mouse monoclonal procaspase-8 (cat. no.
ab38271; 1:1,000) and anti-rabbit polyclonal procaspase-9 (cat. no.
ab135544; 1:800), anti-rabbit monoclonal poly (ADP-ribose)
polymerase (PARP; cat. no. ab191217; 1;1,000), anti-rabbit
monoclonal cleaved PARP (cat. no. ab32064; 1:1,200), anti-rabbit
monoclonal protein kinase B (AKT; cat. no. ab183758; 1:1,000),
anti-rabbit monoclonal phosphorylated (p-AKT; cat. no. ab81283;
1;800), anti-rabbit monoclonal, p-c-Jun N-terminal kinase (JNK;
cat. no. ab179461; 1:1,200), anti-rabbit monoclonal extracellular
signal-regulated kinase 1/2 (ERK1/2; cat. no. ab184699; 1:1,000),
anti-rabbit polyclonal cleaved caspase-3 (cat. no. ab2302;
1:1,200), anti-rabbit monoclonal β-actin (cat. no. ab32575;
1;1,500) and anti-mouse monoclonal β-actin (cat. no. ab8226;
1:1,500) antibodies were purchased from Abcam (Cambridge, UK).
Horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G
(IgG; cat. no. ab97023; 1:10,000) and anti-rabbit IgG (cat. no.
ab6721; 1:10,000) were also obtained from Abcam (Cambridge, UK).
The Muse Annexin V and Dead Cell Assay kit (cat. no. MCH100105) and
the Muse Cell Cycle Assay kit (cat. no. MCH100106) were ordered
from EMD Millipore (Billerica, MA, USA). DAPI was obtained from
Vector Laboratories, Inc. (Burlingame, CA, USA). All other
chemicals and materials utilized for electrophoresis were acquired
from Bio-Rad Laboratories, Inc. (Hercules, CA, USA).
Cell culture
NUGC3 human gastric cancer cell lines were purchased
from the Japanese Health Science Research Resources Bank (Osaka,
Japan). The cells were cultured in RPMI-1640 medium and
supplemented with heat-inactivated 10% FBS (v/v) and 1% penicillin
and streptomycin in humidified conditions of 5% CO2 at
37°C.
MTT assay
To test the effect of bakuchiol on human gastric
cancer cell viability, NUGC3 cells were seeded in a 96 well plate
at a density of 6×104 cells/ml, then treated with 0, 20,
40, 60, 80, 100 or 120 µg/ml bakuchiol and incubated for 24 h at
37°C. The negative control cells were treated with DMSO. Following
incubation, 0.5 g/ml MTT was added to each well and the plates were
further incubated for 3 h at 37°C. The medium was then removed and
DMSO was added to dissolve the formazan crystals formed. The
absorbance was recorded at a 540 nm wavelength using an iMark
(model 550) ELISA microplate absorbance reader (Bio-Rad
Laboratories, Inc.).
Cell cycle distribution and cell
apoptosis analysis
NUGC3 cells were treated with 0, 50 and 100 µg/ml
bakuchiol and incubated for 24 h at 37°C. The cell lines were
collected and cleaned using cold PBS and centrifuged at 1,000 × g
for 10 min at 37°C. Cold ethanol 70% (v/v) was used to fix the
pellet at −20°C for 3 h, and the cells were then washed using PBS
and 200 µl was transferred to a fresh tube. Muse Cell Cycle Assay
kit solution (100 µl) was added to each well and incubated for 30
min in the dark at room temperature. The pellet was resuspended in
1 ml RPMI-1640 medium and 100 µl was transferred to a fresh tube
for the analysis of apoptosis. Again, 100 µl of Muse Annexin V and
Dead Cell Assay kit solution was added and incubated at room
temperature for 30 min in the dark at 37°C and analyzed using the
Muse cell analyzer (0500–3115) using Muse count and viable software
(version 1.05.0) from EMD Millipore (Billerica, MA, USA).
Morphological changes and DAPI
staining
NUGC3 cells were treated with 0, 50 and 100 µg/ml
bakuchiol and incubated for 24 h at 37°C. A total of
1×104 cells were then washed with cold PBS at room
temperature. Then the cells were fixed with 3.7% paraformaldehyde
(1 ml) and 95% ethanol at room temperature for 10 min and followed
by washing with PBS. The fixed cells were stained using DAPI (DAPI
staining kit solution). The morphological nuclear changes were
examined using fluorescence microscopy at ×400 magnification
(Model-DMLS) from Leica Microsystems (GmbH, Wetzlar, Germany).
Western blot analysis
NUGC3 cells were treated with 0, 50 and 100 µg/ml
bakuchiol at 37°C for 72 h and then lysed with NP-40 (1% w/w) ice
cold radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany), sodium deoxycholate (1% w/v), SDS (0.1%
w/v), NaCl (0.15 M), EDTA (2 mM) sodium phosphate buffer (0.01 M,
pH 7.2) and sodium fluoride (50 mM). The resultant cell lysates
were centrifuged at 3,000 × g for 10 min at 37°C and total cellular
protein concentration was determined by Bio-Rad Bradford protein
assay method (Bio-Rad Laboratories, Inc.) (9). Equal quantities of protein (50
µg/lane) was separated by SDS PAGE on 12% gel and then transferred
onto a polyvinyldene fluoride membrane. The membrane was blocked
with Tris-buffered saline containing Tween-20 and 5% skimmed milk
for 4 h at 37°C and probed with the primary antibodies overnight at
37°C, followed by incubation with secondary antibodies conjugated
to horseradish peroxidase for 2 h at 37°C. The blots were examined
using an enhanced chemiluminescence detection kit (GE Healthcare
Life Sciences, Chalfont, UK) and quantification was performed using
Image J software from the National Institutes of Health (version
2.8; Bethesda, MD, USA). All assays were performed in
triplicate.
Statistical analysis
The statistical analyses were performed using SPSS
software (version 20.0; SPSS, Inc., Chicago, IL, USA). The results
were expressed as the mean ± standard deviation of three
independent experiments. Differences between the experimental
groups were determined using one-way analysis of variance followed
by Dunnett's multiple comparison post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Bakuchiol inhibits NUGC3 cell
viability
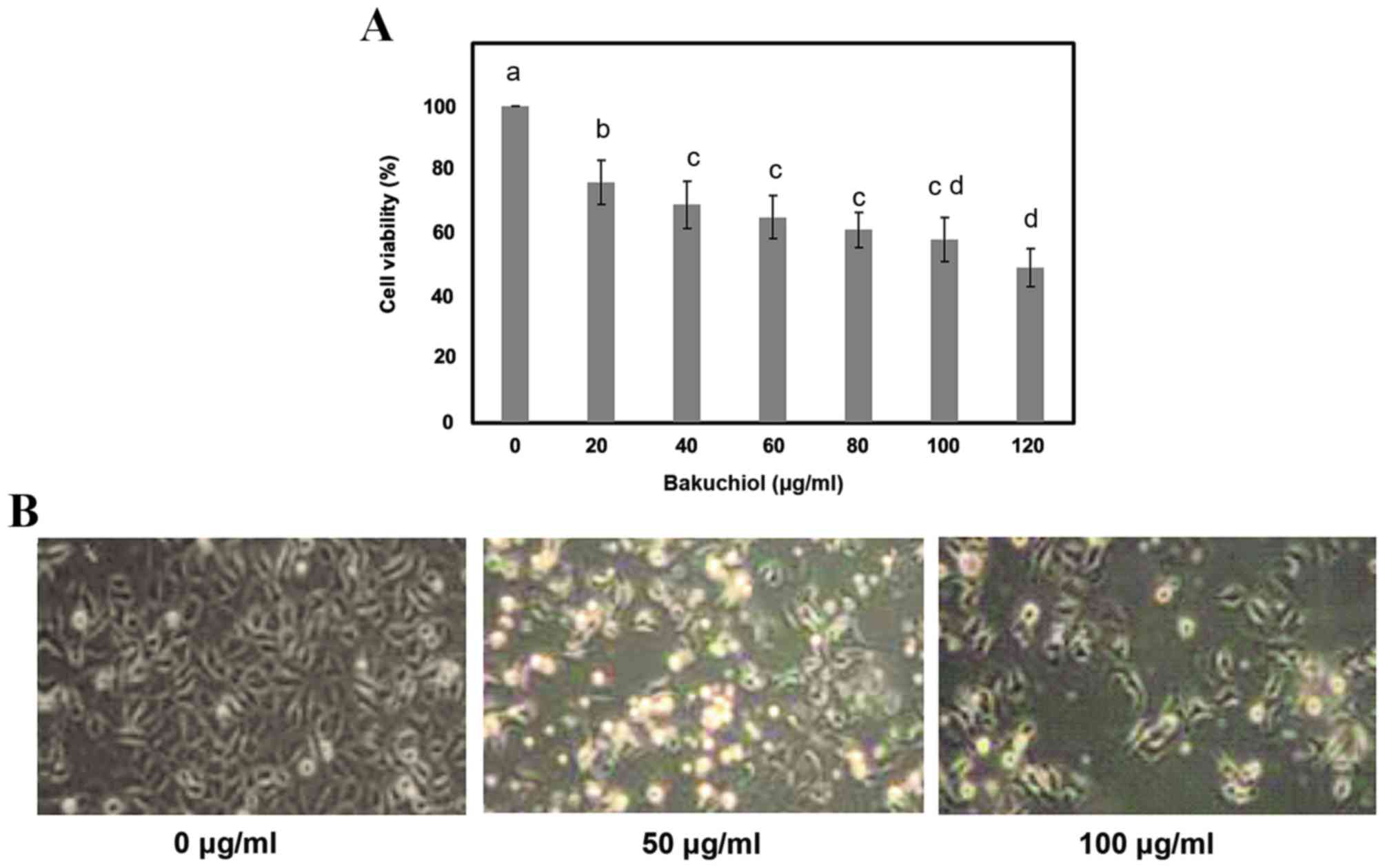
To determine the concentration of bakuchiol that
inhibits cell viability, NUGC3 cells were treated with 0, 20, 40,
60, 80, 100 or 120 µg/ml bakuchiol for ~24 h, and cell viability
was then measured by MTT assay. Following bakuchiol treatment and
24 h incubation, cell viability was inhibited in a
concentration-dependent manner when compared with the control,
without bakuchiol treatment (P<0.05; Fig. 2A). The concentration that produced
50% inhibition (IC50 value) was revealed to be ~120
µg/ml. Therefore, bakuchiol concentrations of 0, 50 and 100 µg/ml
were used for further examination. Microscopic study indicated
changes in the cell structure, including visible shrinkage of cells
and reduced cell counts, in bakuchiol treated cells compared with
controls (Fig. 2B).
Bakuchiol induces apoptosis in NUGC3
cells
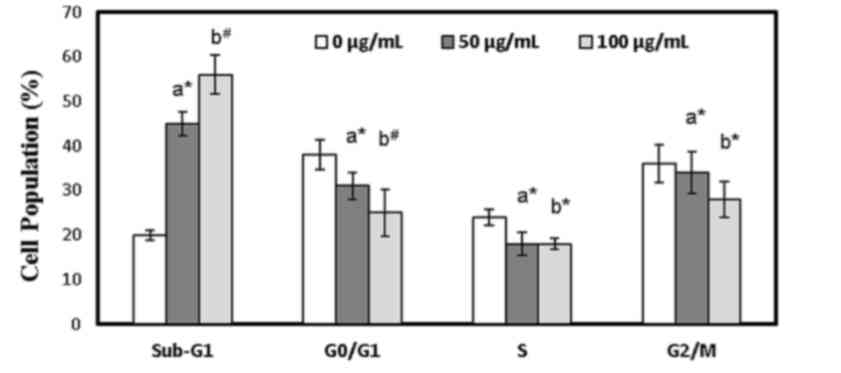
Cell cycle distribution and the apoptotic cell
population in bakuchiol-treated NUGC3 cells was determined using
flow cytometry analysis. Bakuchiol treatment elevated the
percentage of sub-G1 cells from 13% to 28 and 39% at 50 (P<0.05)
and 100 µg/ml (P<0.01), respectively (Fig. 3). Meanwhile, bakuchiol treatment
significantly reduced the cell population of G0/G1, S
and G2/M compared with the control (P<0.05; Fig. 3). In addition, the effect of
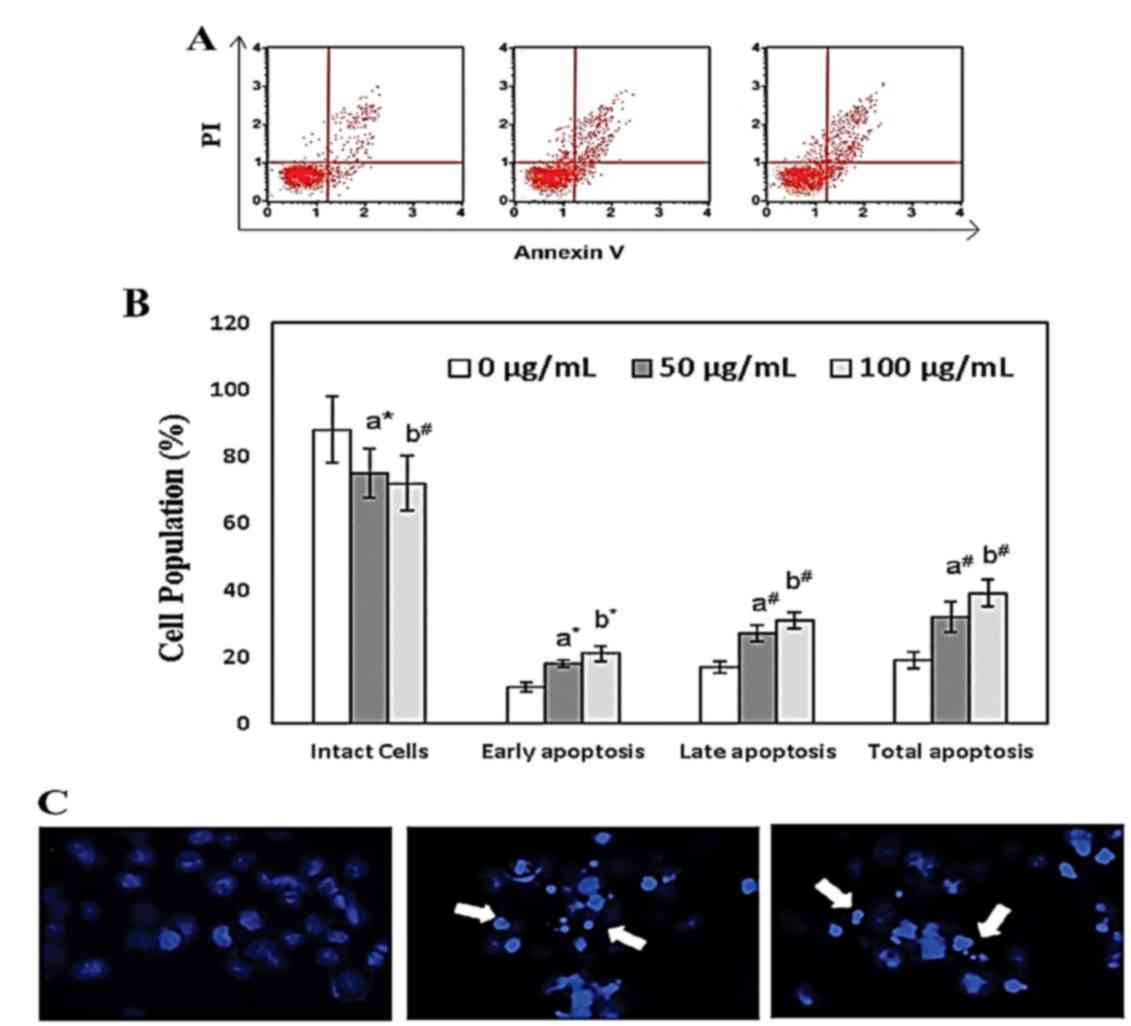
bakuchiol on apoptosis induction in NUGC3 cells was examined using
Annexin V-fluorescein isothiocyanate/propidium iodide double
staining and flow cytometry. The mild elevation of sub-G1 cells in
the untreated group was attributed to the effect of DMSO on NUGC3
cells.
Treatment with bakuchiol (50 µg/ml; P<0.05 and
100 µg/ml; P<0.01) significantly increased the percentage of
early apoptosis, late apoptosis and total apoptosis in NUGC3 cells
in a concentration dependent manner compared with the control
(Fig. 4A and B). Nuclear
fragmentation and apoptotic organelles were observed following 50
and 100 µg/ml bakuchiol treatment, using DAPI staining (Fig. 4C). These data suggested that
bakuchiol treatment induced cell death in NUGC3 cells.
Caspase activation and PARP cleavage
was induced by bakuchiol
To confirm whether bakuchiol-induced apoptosis was
caspase dependent, western blotting analysis was performed
(Fig. 5A). Furthermore, the levels
of apoptosis-associated proteins, Bax and Bcl-xL, were measured in
bakuchiol-treated NUGC3 cells. Levels of procaspase-3,6,8 and 9
were significantly decreased following treatment with 50 and 100
µg/ml bakuchiol treatment compared with the control (P<0.05;
Fig. 5B-E, respectively), while
cleaved caspase-3 and cleaved PARP levels were significantly
elevated following 50 and 100 µg/ml bakuchiol treatment compared
with the control (P<0.01; Fig. 5F
and G, respectively). The ratio of Bax/Bcl-xL in NUGC3 cells
was also significantly upregulated following bakuchiol (50 µg/ml;
P<0.05 and 100 µg/ml; P<0.01) treatment compared with the
control (Fig. 5H). These data
suggested that bakuchiol treatment induced caspase-dependent
apoptosis in NUGC3 cells.
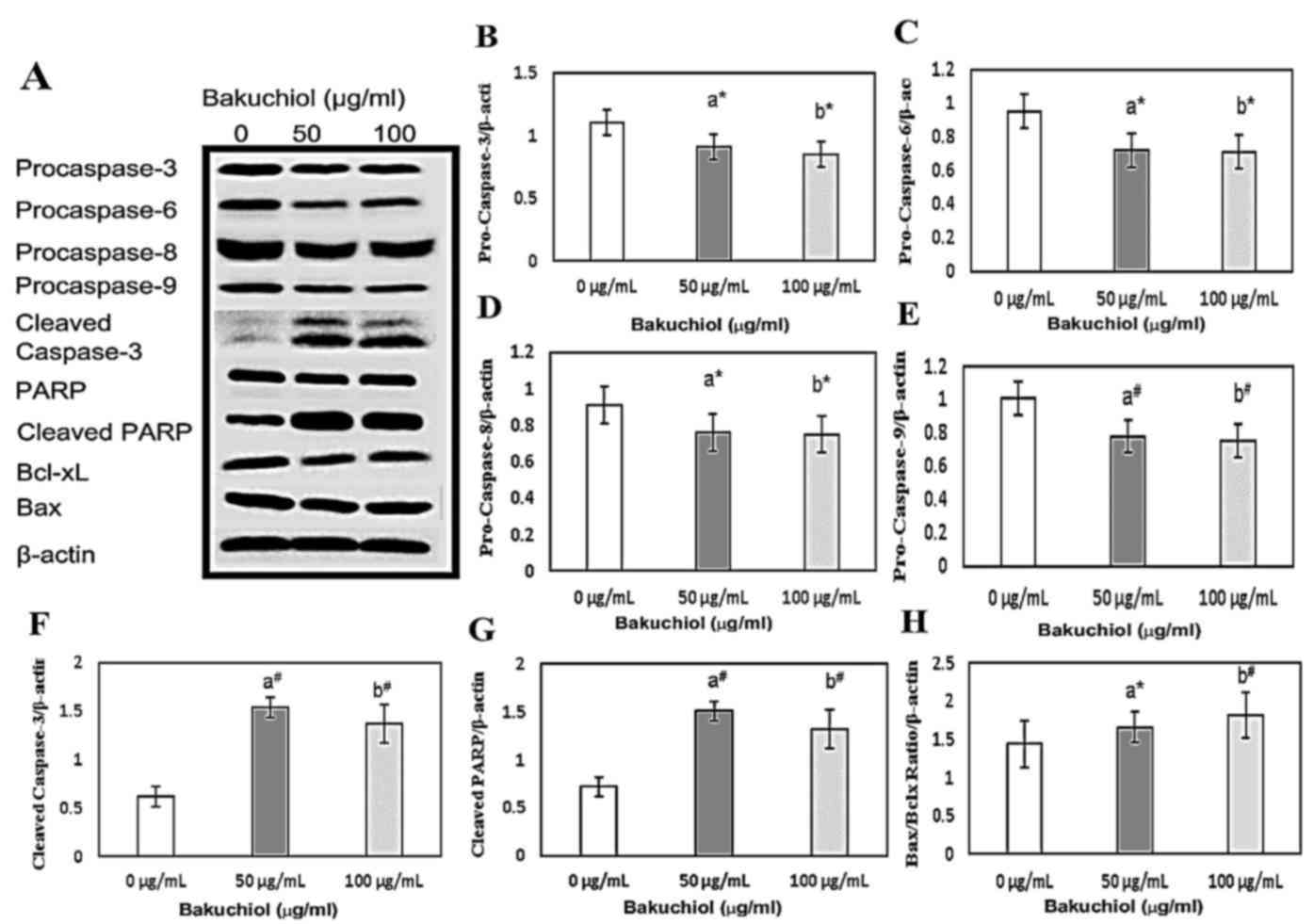 | Figure 5.(A) Western blot analysis of caspase
activation and PARP cleavage in NUGC3 cells treated with 0, 50 or
100 µg/ml bakuchiol for 24 h. Densitometric analyses of (B)
procaspase-3, (C) procaspase-6, (D) procaspase-8, (E) procaspase-9,
(F) cleaved caspase-3, (G) PARP and (H) Bax:Bcl-xL. *P<0.05;
#P<0.01. vs. 0 µg/ml. PARP, poly (ADP-ribose)
polymerase; Bax, B cell lymphoma-2 associated X, apoptosis
regulator; Bcl-xL, B cell lymphoma-extra large. |
Bakuchiol-induced apoptosis is
regulated by the phosphoinositide 3-kinase (PI3K)/AKT and
mitogen-activated protein kinase (MAPK) pathways in NUGC3
cells
Cell proliferation and apoptosis are regulated by
the PI3K/AKT and MAPK signaling pathways. As AKT activity is
maintained by phosphorylation, the phosphorylation of PI3K/AKT and
MAPK was analyzed using western blotting during bakuchiol-induced
apoptosis in NUGC3 cells (Fig.
6A). Bakuchiol treatment (50 and 100 µg/ml) resulted in
significant decrease (50 µg/ml; P<0.01 and 100 µg/ml; P<0.05)
in the levels of phosphorylated AKT (p-Akt) compared with the
control (Fig. 6B). Furthermore,
phosphorylated ERK1/2, JNK and p38 levels were significantly
upregulated upon treatment with bakuchiol compared with the control
(P<0.01; Fig. 6C-E,
respectively). These experimental findings suggested that bakuchiol
induced apoptosis in NUGC3 cells by regulation of the PI3K/AKT and
MAPK signaling pathways.
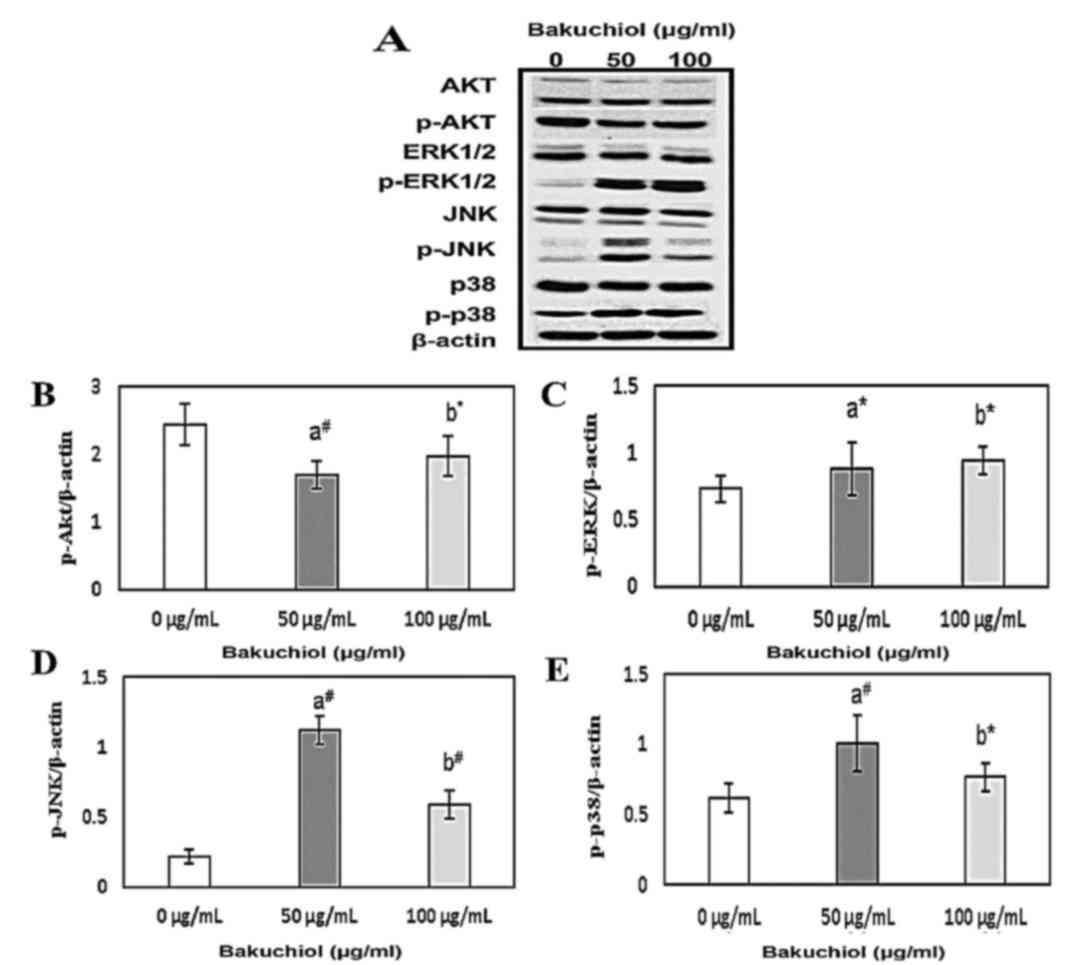 | Figure 6.(A) Effect of 0, 50 or 100 µg/ml
bakuchiol treatment for 24 h on PI3K/AKT and MAPK signaling
pathways in NUGC3 cells, analyzed by western blotting.
Densitometric analyses of (B) p-Akt, (C) p-ERK1/2, (D) p-JNK and
(E) p-p38. *P<0.05; #P<0.01. vs. 0 µg/ml. PI3K,
phosphoinositide 3-kinase; AKT, protein kinase B; MAPK,
mitogen-activated protein kinase; p-, phosphorylated; ERK1/2,
extracellular signal related kinase 1/2; JNK, c-Jun N-terminal
kinase. |
Discussion
The aim of the present study was to investigate
whether bakuchiol treatment induced gastric cancer cell death, and
to further determine the underlying molecular mechanisms of
bakuchiol-induced apoptosis in NUGC3 cells. Successful medicinal
treatment using chemotherapeutic agents is primarily dependent on
their potential to induce apoptosis in cancer cells (10). Isolated bakuchiol, which is a major
chemical component of Psoralea corylifolia, a member of the
Leguminosae family (11), is one
of the primary medicinal plants in Indian Ayurveda and Chinese
traditional medicinal treatments owing to its inhibitory properties
on DNA polymerase (12) and
topoisomerase II (13). A previous
study on bakuchiol observed that its antibacterial activity was
significantly enhanced via chemical modification to obtain
reduction of MIC up to 8-fold against Gram (+) and (−) bacteria
(14). The present study focused
on the anticancer effects of bakuchiol against human gastric cancer
cell lines.
The results of the MTT assay revealed that bakuchiol
significantly reduced NUGC3 cell viability in a concentration
dependent manner. Type I programmed cell death, or apoptosis, is
the primary mechanism by which different anti-tumor and
chemoprotective agents, inclusive of naturally derived products,
exert anti-cancer properties (15). Earlier reports demonstrated that
abnormalities in the cell cycle may result in apoptosis in various
cancer cell lines (16,17). Bakuchiol treatment resulted in
sub-G1 phase cell accumulation in NUGC3 cells. Annexin V-FITC/PI
double staining confirmed the induction of apoptosis and nuclear
fragmentation and apoptotic organelles were observed in
bakuchiol-treated NUGC3 cells. These data revealed that bakuchiol
suppressed NUGC gastric cancer cell viability and was responsible
for the induction of apoptosis.
To explore the involved molecular mechanisms,
western blotting was performed and the results indicated that
procaspase-3,6,8 and 9 expression levels were significantly
decreased. The vital caspase is caspase-3, which activates PARP
cleavage and results in the induction of the apoptosis process.
Elevated cleaved caspase-3 expression concomitantly induced the
cleavage of PARP. These data confirmed that bakuchiol induced
apoptosis in a caspase-3 dependent manner. Mitochondrial apoptotic
functions are regulated by apoptotic regulatory proteins of the
Bcl-2 family. Cell viability and death were determined by the
levels of Bcl-2 family pro-and anti-apoptotic proteins. The ratio
of Bax and Bcl-xL has been demonstrated to be an apoptosis
determining factor. In the present work, Bax protein expression
levels remained the same while Bcl-xL levels were significantly
downregulated, but following bakuchiol treatment the upregulation
of the Bax:Bcl-xL ratio was observed in NUGC3 cells. When released
from the mitochondria to the cytosol, cytochrome c has previously
been observed to interact with apoptotic peptidase activating
factor-1 and thereby lead to Bax/Bcl-xL ratio increment, thus
activating caspase-3 and resulting in apoptosis (18).
To further evaluate the molecular mechanisms and
involved signaling pathways in bakuchiol-induced apoptosis,
PI3K/AKT and MAPK pathway phosphorylation levels were analyzed by
western blotting. Bakuchiol inhibited levels of p-AKT, the
downstream target of PI3K, which is known to control proliferation
and cell apoptosis. Similar to the results of the present study,
PI3K/AKT signaling pathway inhibition induces apoptosis in
different cancer types (18,19).
The MAPK signaling pathway also participates in cell viability,
proliferation and apoptosis, and classified into 3 major divisions:
JNK, p83 and ERK MAPKs (20).
Although ERK1/2 pathway activation is associated with cell growth
and proliferation, it has also been reported to induce apoptosis in
T cells via the Fas expression ligand (21). ERK1/2 promotes apoptosis through
preventing inactivation of the pro-apoptotic Bcl-2 family member,
BCL2 associated agonist of cell death (22). JNK is a representative MAPK
downstream kinase family member, which has been reported to
maintain Fas and Fas ligand receptor expression in apoptosis
(23). JNK is also involved in the
apoptosis intrinsic pathway, where activated JNK controls the
pro-apoptotic proteins BH3 interacting domain death agonist and
Bax, and induces cytochrome c release into the cytosol from
mitochondria (24). JNK activation
results in anti-apoptotic Bcl-2 protein downregulation (25). It has previously been noted that
activated p38 induces apoptosis in different cell line (26,27).
The results of the present study exhibited similar patterns in
which p-ERK, p-JNK and p-p38 levels were increased following
bakuchiol treatment in er NUGC3 gastric cancer cells (Fig. 6), indicating the involvement of PI3
K/AKT and MAPKs in bakuchiol-induced apoptosis in NUGC3 cells.
In conclusion, bakuchiol was demonstrated to
decrease cell viability and induce caspase-dependent apoptosis in
NUGC3 gastric cancer cells. PI3K/AKT and MAPK signaling pathways
triggered apoptosis following bakuchiol treatment in the NUGC3
gastric cancer cell line. To the best of our knowledge, the present
study is the first to demonstrate the anticancer effect of
bakuchiol treatment in NUGC3 cells. Therefore, bakuchiol may be an
effective novel chemotherapeutic agent for treating human gastric
cancer.
Acknowledgements
The authors would like to thank Xiangyang Central
Hospital, Hubei University of Arts and Science for providing
funding support to the present study (grant no. XCH2212-15).
References
|
1
|
Guggenheim DE and Shah MA: Gastric cancer
epidemiology and risk factors. J Surg Oncol. 107:230–236. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herszényi L and Tulassay Z: Epidemiology
of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci.
14:249–258. 2010.PubMed/NCBI
|
|
3
|
Ren J, Huang HJ, Gong Y, Yue S, Tang LM
and Cheng SY: MicroRNA-206 suppresses gastric cancer cell growth
and metastasis. Cell Biosci. 4:262014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jaing JB, Thesson DP, Dusak BA, Dexter DL,
Kang GJ and Hamel E: Synthesis and biological evaluation of
2-styrylquinazolin-4(3H)-ones, a new class of antimitotic
anticancer agents which inhibit tubulin polymerization. J Med Chem.
33:1721–1728. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zouhiri F, Danet M, Bénard C, Normad-Boyle
M, Mouscadet JF, Leh H, Thomas CM, Mbemba G, d'Angelo J and
Desmaële D: HIV-1 replication inhibitors of the styrylquinoline
class: Introduction of an additional carboxyl group at the C-5
position of the quinolone. Tetrahedron Lett. 46:2201–2205. 2005.
View Article : Google Scholar
|
|
6
|
Eltahla AA, Lim KL, Eden JS, Kelly AG,
Mackenzie JM and White PA: Nonnucleoside inhibitors of norovirus
RNA Polymerase: Scaffolds for rational drug design. Antimicrob
Agents Chemother. 58:3115–3123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee IK, Han MS, Lee MS, Kim YS and Yun BS:
Styrylpyrones from the medicinal fungus Phellinus baumii and their
antioxidant properties. Bioorg Med Chem Lett. 20:5459–5461. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bapat SA, Mali AM, Koppikar CB and Kurrey
NK: Stem and progenitor like cells contribute to the aggressive
behaviour of human epithelial ovarian cancer. Cancer Res.
65:3025–3029. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vansteenkiste J: Improving patient
management in metastatic non-small cell lung cancer. Lung Cancer.
57 Suppl 2:S12–S17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chevalier A and Kindersley D: The
encyclopedia of medicinal plants. ISBN 9-780751-303148. London:
1996
|
|
12
|
Duke JA and Ayensu ES: Medicinal plants of
China Inc. ISBN 0-917256,. 20:41985.
|
|
13
|
Sun NJ, Woo SH, Cassady JM and Snapka RM:
DNA polymerase and topoisomerase II inhibitors from Psoralea
corylifolia. J Nat Prod. 61:362–366. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katsura H, Tsukiyama RI, Suzuki A and
Kobayashi M: In vitro antimicrobial activities of bakuchiol against
oral microorganisms. Antimicrob Agents Chemother. 45:3009–3013.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reddy MV, Thota N, Sangwan PL, Malhotra P,
Ali F, Khan IA, Chimni SS and Koul S: Novel bisstyryl derivatives
of bakuchiol: Targeting oral cavity pathogens. Eur J Med Chem.
45:3125–3134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi Y: Caspase activation: Revisiting the
induced proximity model. Cell. 117:855–858. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park SJ, Ahmad F, Philp A, Baar K,
Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, et al:
Resveratrol ameliorates aging-related metabolic phenotypes by
inhibiting cAMP phosphodiesterases. Cell. 148:421–433. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee DH, Park KI, Park HS, Kang SR,
Nagappan A, Kim JA, Kim EH, Lee WS, Hah YS, Chung HJ, et al:
Flavonoids isolated from Korea Citrus aurantium L. Induce G2/M
phase arrest and apoptosis in human gastric cancer AGS cells. Evid
Based Complement Alternat Med. 2012:5159012012.PubMed/NCBI
|
|
19
|
Hussain AR, Al-Rasheed M, Manogaran PS,
Al-Hussein KA, Platanias LC, Al Kuraya K and Uddin S: Curcumin
induces apoptosis via inhibition of PI3′-kinase/AKT pathway in
acute T cell leukemias. Apoptosis. 11:245–254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gururajan M, Dasu T, Shahidain S, Jennings
CD, Robertson DA, Rangnekar VM and Bondada S: Spleen tyrosine
kinase (Syk), a novel target of curcumin, is required for B
lymphoma growth. J Immunol. 178:111–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van den Brink MR, Kapeller R, Pratt JC,
Chang JH and Burakoff SJ: The extracellular signal-regulated kinase
pathway is required for activation-induced cell death of T cells. J
Biol Chem. 274:11178–11185. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Basu S, Bayoumy S, Zhang Y, Lozano J and
Kolesnick R: BAD enables ceramide to signal apoptosis via Ras and
Raf-1. J Biol Chem. 273:30419–30426. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Faris M, Kokot N, Latinis K, Kasibhatla S,
Green DR, Koretzky GA and Nel A: The c-Jun N-terminal kinase
cascade plays a role in stress-induced apoptosis in Jurkat cells by
up-regulating Fas ligand expression. J Immunol. 160:134–144.
1998.PubMed/NCBI
|
|
25
|
Dhanasekaran DN and Reddy EP: JNK
signaling in apoptosis. Oncogene. 27:6245–6225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang YJ, Zhou ZX, Wang GW, Buridi A and
Klein JB: Suppression by metallothionein of doxorubicin-induced
cardiomyocyte apoptosis through inhibition of p38 mitogen-activated
protein kinases. J Biol Chem. 275:13690–13698. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|