Introduction
Lipopolysaccharide (LPS), the bacterial endotoxin,
is considered to be the primary factor responsible for multi-organ
failure in septic shock (1). The
myocardium is one of the organs affected by septic shock, which is
a major cause of mortality (2). It
has been previously demonstrated that LPS activates caspases in
cardiomyocytes, which induces end-stage nuclear apoptosis, cleavage
of cardiac myofilament proteins and sarcomere disorganization
(3). LPS-induced myocardial
dysfunction is mediated by various proinflammatory mediators,
including tumor necrosis factor (TNF)-α and interleukin 1 (IL-1),
which may be responsible for LPS-induced multiple organ failure,
including heart failure (4,5).
Toll-like receptor-4 (TLR4), which is a type of pattern recognition
receptor, is considered to be involved in the LPS-induced innate
immune response (6). The TLR4
predominantly expressed in cardiomyocytes recognizes LPS and
triggers the recruitment of adaptors, including myeloid
differentiation factor 88 and Toll/IL-1 receptor domain-containing
adaptor protein-inducing interferon-β (7). These two pathways result in the
activation of nuclear factor-κB (NF-κB), a key transcription factor
involved in inflammatory activation (7). Therefore, pharmacological
interventions that disrupt the TLR4-induced inflammatory response
in cardiomyocytes may be a promising approach for the treatment of
certain cardiovascular diseases.
Ampelopsis, a plant of the Vitaceae family
and widely used in Chinese traditional medicine for treating liver
disorders (8), is widely
distributed in tropical and subtropical regions (9). Dihydromyricetin (DHM) is a type of
flavonoid compound, which is extracted from the stems and leaves of
Ampelopsis grossedentata (10). Previous studies have reported that
DHM functions as an anti-insulin resistance (11), antitumor (12), anti-inflammatory (13) and antioxidative (14) agent. DHM is reported to suppress
the levels of proinflammatory cytokines and increase the level of
anti-inflammatory cytokines in macrophage cells (13). Recently, it has been reported that
DHM protects against angiotensin II-induced cardiomyocyte
hypertrophy by augmenting nitric oxide production (15). In addition, existing data also
indicates that DHM protects against Adriamycin-induced heart injury
by protecting myocardial cells from apoptosis (16). Therefore, the current study aimed
to investigate whether DHM protects against LPS-induced
cardiomyocyte injury and to identify the mechanisms involved.
Materials and methods
H9c2 cardiomyocyte culture
The H9c2 embryonic rat heart-derived cell line was
obtained from The Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). DHM was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and was dissolved at
a concentration of 50 mM in dimethysulfoxide (Sigma-Aldrich; Merck
KGaA) for storage at −20°C. Cells were cultured in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), penicillin (100 U/ml) and
streptomycin (100 mg/ml; Gibco; Thermo Fisher Scientific, Inc.) in
a humidified incubator with an atmosphere of 5% CO2 at
37°C. Cells were seeded at a density of 1×106 per well
onto 6-well culture plates for mRNA extraction, 5×103
cells per well in 96-well plates for terminal deoxynucleotidyl
transferase dUTP nick-end labeling (TUNEL) analysis and
1×107 per well onto culture dishes (100 mm) for protein
extraction. Cells were cultured in serum-free medium for 8 h at
37°C and pretreated with DHM (25, 50 and 100 µM) or PBS for 12 h
prior to LPS (10 µg/ml; L2630; Sigma-Aldrich; Merck KGaA)
stimulation for 12 h at 37°C.
Cell viability assay
Cell viability was evaluated by Cell Counting kit-8
(CCK-8; Sigma-Aldrich; Merck KGaA) assay according to the
manufacturer's instructions. Briefly, 10 µl CCK-8 solution was
added to each well of a 96-well plate (1×103 cells per
well) and, following 4 h incubation at 37°C, the absorbance was
measured at 450 nm using the BioTek Synergy HT reader (BioTek
Instruments, Inc., Winooski, VT, USA). The effect of DHM on cell
viability was expressed as the percentage cell viability compared
with the control group, which was set at 100%.
TUNEL staining
TUNEL assays were performed using the
ApopTag® Plus Fluorescein In Situ Apoptosis
Detection kit (EMD Millipore, Billerica, MA, USA) to label
apoptotic nuclei, according to the manufacturer's instructions.
Briefly, following 12 h pretreatment with DHM, cells were incubated
with LPS for 12 h and subsequently fixed on coverslips in 1%
paraformaldehyde in PBS (Sinopharm Chemical Reagent Co., Ltd.,
Shanghai, China) at room temperature for 5 min. After washing with
PBS three times, cells were stained with TUNEL reagents (EMD
Millipore) and 4′, 6-diamidino-2-phenylindole (DAPI; 0.3 mmol/l;
Invitrogen; Thermo Fisher Scientific, Inc.) for 1 min, and observed
under a fluorescence microscope (BX51; Olympus Corporation, Tokyo,
Japan). The index of cell apoptosis was calculated as the
percentage of apoptotic nuclei/total number of nuclei (n=10 fields
of view).
Western blot analysis
Cells in dishes (1×107 per well) were
harvested and lysed in radioimmunoprecipitation assay (RIPA) lysis
buffer with shaking for 15 min on ice. RIPA assay lysis buffer
contained the following per ml: RIPA (720 µl; Beyotime Institute of
Biotechnology, Haimen, China), PMSF (20 µl; 1 mM),
cOmplete™ Protease Inhibitor Cocktail (100 µl; Roche;
Sigma-Aldrich; Merck KGaA), PhosStop™ (100 µl; Roche;
Sigma-Aldrich; Merck KGaA), NaF (50 µl; 1 mM) and
Na3VO4 (10 µl). The protein concentration was
measured using a bicinchoninic acid protein assay kit with a BioTek
Synergy HT reader (BioTek Instruments, Inc.). The extracted protein
(50 µg) from each sample was separated by 8–12% SDS-PAGE and the
proteins were transferred onto polyvinylidene difluoride membranes.
The membranes were blocked with 5% non-fat milk powder for 1 h at
room temperature and were then incubated with primary antibodies
overnight at 4°C. The primary antibodies used were as follows:
B-cell lymphoma 2 apoptosis regulator (Bcl-2; cat. no. 2870;
1:1,000; Cell Signaling Technology, Inc.), Bcl-2-associated X
apoptosis regulator (Bax; cat. no. 2772; 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), total (T)-NF-κB p65 (cat. no.
8242; 1:1,000; Cell Signaling Technology, Inc.), phosphorylated
(p)-NF-κB p65 (cat. no. 3033; 1:1,000; Cell Signaling Technology,
Inc.), TLR4 (cat. no. sc-30002; 1:200; Santa Cruz Biotechnology,
Inc., Dallas, Texas, USA) and GAPDH (cat. no. sc-25778; 1:200;
Santa Cruz Biotechnology, Inc.). The membranes were subsequently
incubated with IRDye 800CW-conjugated secondary antibodies (LI-COR
Biosciences; cat. no. 926-32211; 1:200; Lincoln, NE, USA) for 1 h
at room temperature. The blots were scanned using an
Odyssey® Fc infrared scanner, allowing for simultaneous
detection of two targets (phosphorylated and total protein) within
the same experiment (LI-COR Biosciences). The specific protein
expression levels were normalized against the expression of GAPDH
by Quantity One software (version 4.6.2; Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cells in 6-well plates (1×106 per well)
were harvested and total RNA was extracted using TRIzol (Thermo
Fisher Scientific, Inc.). The yield and purity levels of the RNA
were spectrophotometrically estimated using A260/A280 and A230/A260
ratios, obtained via a SmartSpec Plus Spectrophotometer (Bio-Rad
Laboratories, Inc.). The RNA (2 mg each sample) was reverse
transcribed into cDNA using a Transcriptor First Strand cDNA
Synthesis kit (Roche Diagnostics, Basel, Switzerland), according to
the manufacturer's protocol. The PCR amplifications were quantified
using the LightCycler® 480 SYBR® Green I
Master Mix (Roche Diagnostics), according to the manufacturer's
protocol, in the LightCycler® 480 Real-Time PCR System
(Roche Diagnostics). Briefly, following a 5 min initial
denaturation at 95°C, a total of 42 primer-extension cycles were
carried out. Each cycle consisted of a 10 sec denaturation step at
95°C, a 20 sec annealing step at 60°C and a 20 sec incubation at
72°C for extension. A final extension step was performed at 72°C
for 10 min. The double standard curve was used to quantify the PCR
results. Calibrator normalized ratio = (concentration of sample
target/concentration of sample reference)/(concentration of
calibrator target/concentration of calibrator reference). The
results were normalized to the mRNA expression of GAPDH by using
the ∆∆Cq method (17). The
experiment was repeated three times. The sequences of the
oligonucleotide primers (Sangon Biotech Co., Ltd., Shanghai, China)
were as follows: TNF-α forward, 5′-AGCATGATCCGAGATGTGGAA-3′ and
reverse, 5′-TAGACAGAAGAGCGTGGTGGC-3′; IL-6 forward,
5′-GTTGCCTTCTTGGGACTGATG-3′, and reverse,
5′-ATACTGGTCTGTTGTGGGTGGT-3′; and GAPDH forward,
5′-GACATGCCGCCTGGAGAAAC-3′ and reverse
5′-AGCCCAGGATGCCCTTTAGT-3′.
Immunofluorescence staining
Briefly, the cells (5×103 per well) were
washed with PBS, fixed with 1% paraformaldehyde for 5 min at room
temperature, permeabilized in 0.1% Triton X-100 (Amresco, LLC,
Solon, OH, USA) in PBS for 5 min at room temperature and blocked
with 8% goat serum (Beyotime Institute of Biotechnology) for 1 h at
room temperature. Cells were then stained with anti-p-NF-κB p65
(cat. no. BS4135; Bioworld Technology, Inc., St. Louis Park, MN,
USA) overnight at a dilution of 1:100 in 1% goat serum at 4°C.
Following 5 washes in PBS, cells were incubated with Alexa Fluor
568 goat anti-rabbit IgG secondary antibody (cat. no. A-11011;
1:200; Invitrogen; Thermo Fisher Scientific, Inc.) for 60 min at
room temperature. Following 6 washes in PBS, cells on coverslips
were mounted onto glass slides using SlowFade® Gold
Antifade Mountant with DAPI (Invitrogen; Thermo Fisher Scientific,
Inc.).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Differences among the groups were determined by one way
analysis of variance followed by a post-hoc Tukey test. Comparisons
between two groups were performed using the unpaired Student's
t-test. Statistical analyses were performed using SPSS version 13.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
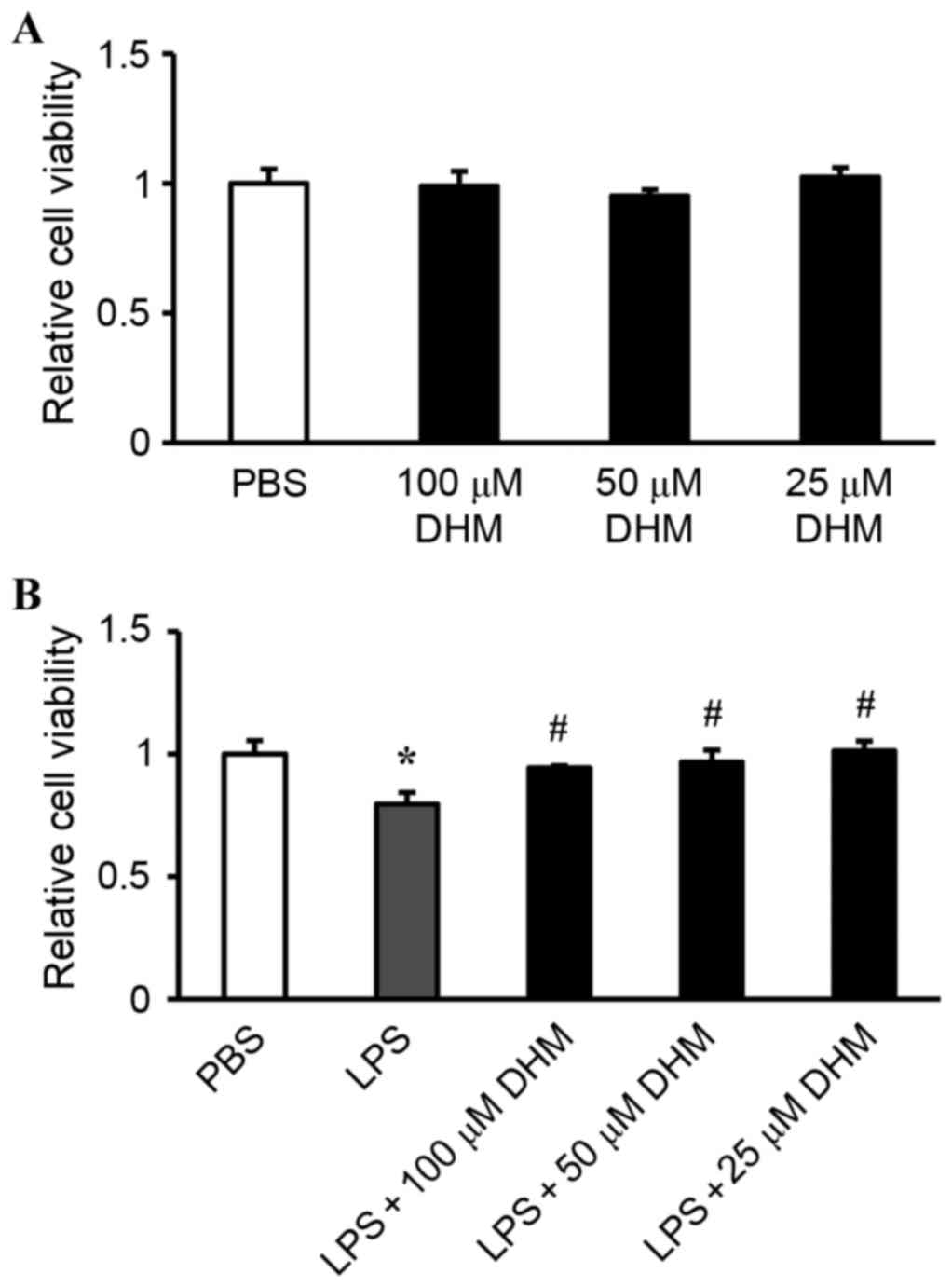
Effect of DHM on cell viability
The potential cytotoxicity of DHM was examined by
CCK-8 assay. H9c2 cells were incubated with varying concentrations
of DHM (100, 50 and 25 µM) for 12 h. Cell viability in DHM-treated
cells was not significantly different compared with that of control
cells treated with PBS, indicating that DHM (100, 50 and 25 µM) did
not cause cytotoxicity in H9c2 cells (Fig. 1A). Stimulation with LPS for 12 h
significantly reduced the cell viability, while 100, 50 and 25 µM
concentrations of DHM pretreatment attenuated LPS-induced
cytotoxicity (Fig. 1B).
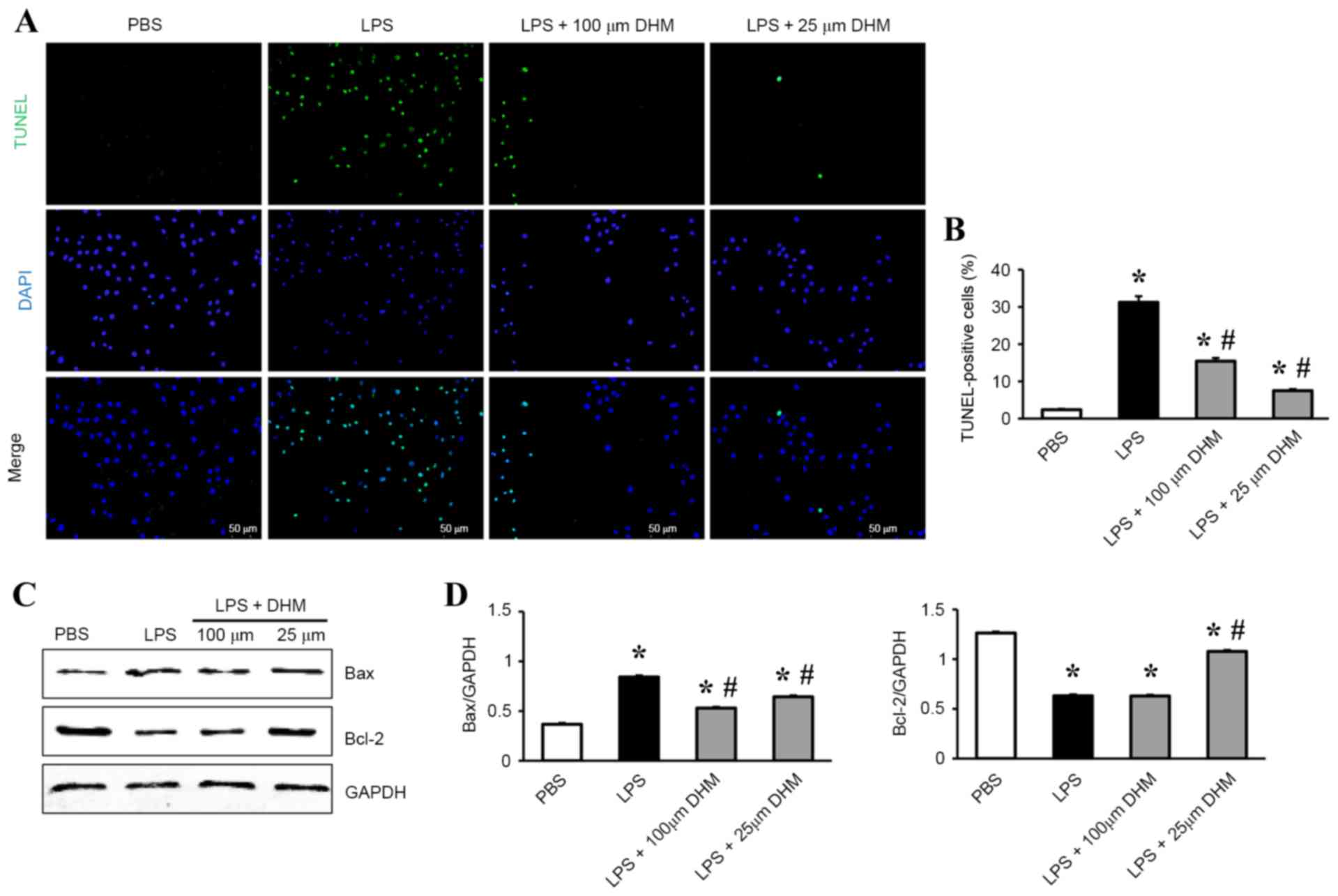
DHM attenuates LPS-induced apoptosis
in H9c2 cells
TUNEL staining was used to identify the potential
protective role of DHM on LPS-induced apoptosis in H9c2 cells. A
significant increase in the number of TUNEL-positive nuclei was
observed in cells incubated with LPS compared with control cells,
and DHM (100 and 25 µM) pretreatment significantly reduced
LPS-induced cell apoptosis (Fig. 2A
and B). In addition, DHM pretreatment decreased the levels of
expression of Bax protein, while increasing the Bcl-2 protein
expression levels in H9c2 cells following LPS stimulation (Fig. 2C and D).
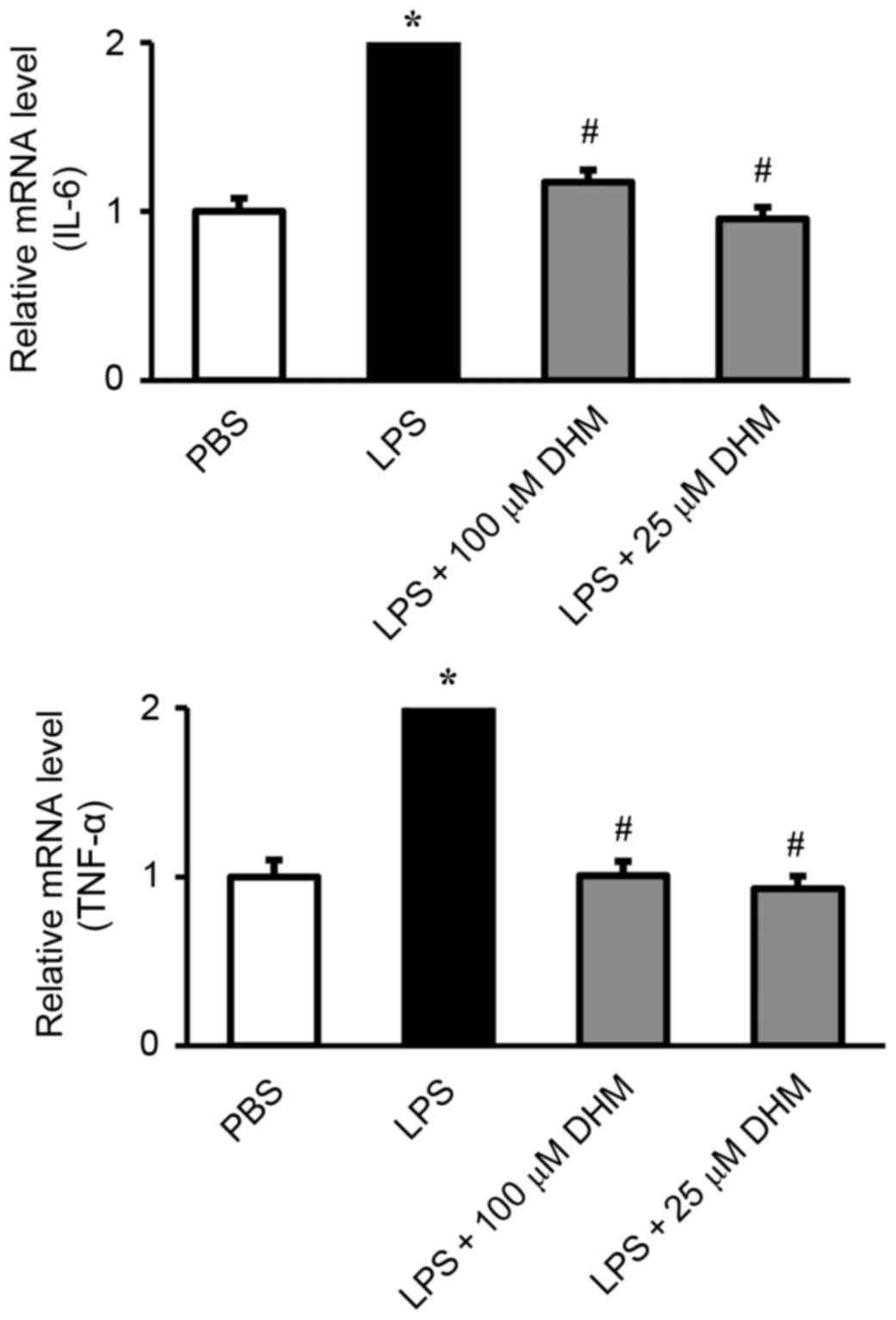
DHM inhibits the expression of
inflammatory genes induced by LPS in H9c2 cells
The effect of DHM on the induction of IL-6 and TNF-α
in response to LPS was measured by RT-qPCR. LPS significantly
stimulated the release of IL-6 and TNF-α. Pretreatment with DHM
(100 and 25 µM) significantly attenuated the LPS-induced increase
in IL-6 and TNF-α (Fig. 3).
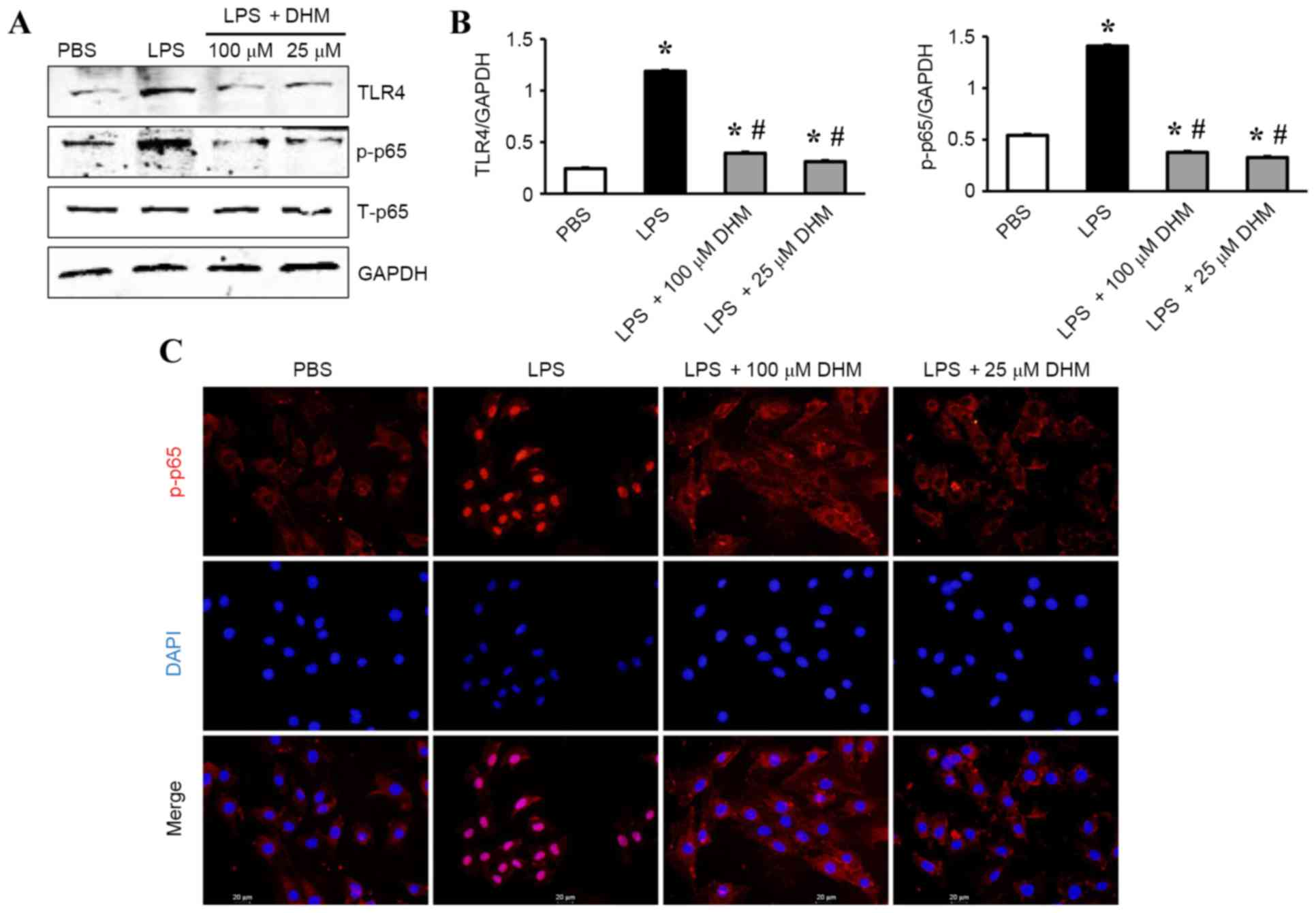
DHM reduces the activation of
TLR4/NF-κB signaling in response to LPS
The mechanisms underlying the anti-inflammatory
effects of DHM on LPS-treated H9c2 cells was investigated by
western blot analysis. The results demonstrated that LPS
significantly increased the expression level of TLR4 and
subsequently increased the levels of p-NF-κB p65 (Fig. 4A and B). While 100 and 25 µM DHM
decreased expression of TLR4 and subsequently decreased the levels
of p-NF-κB p65 (Fig. 4A and B). In
addition, treatment with 100 and 25 µM DHM reduced LPS-induced
increases in the nuclear translocation of NF-κB p65 as determined
by immunofluorescence staining (Fig.
4C).
Discussion
DHM has been previously demonstrated to be an
anti-inflammatory (13) and
antioxidative (14) compound. The
present study demonstrated a novel role of DHM in the protection of
cardiomyocytes from LPS-induced injury. DHM attenuated LPS-induced
apoptosis in cardiomyocytes by reducing Bax expression and the
upregulation of Bcl-2 expression. DHM also attenuated LPS-induced
inflammatory response by the inhibition of the TLR4/NF-κB signaling
cascade.
Sepsis is a major consequence of infectious
diseases, which causes injury to multiple organs, including injury
to the cardiovascular system (18). It has been demonstrated that
cardiomyocytes are the major local source of proinflammatory
cytokines in the myocardium during sepsis (19). These proinflammatory cytokines,
including TNF-α, IL-6 and IL-1β are responsible for LPS-induced
cardiac dysfunction and myocardial depression, which may cause
cardiomyocyte apoptosis and heart failure (20,21).
Therefore, blocking inflammatory signaling may produce beneficial
effects in the dysfunctional heart. Ampelopsis grossedentata
has been used for treating pharyngitis in traditional Chinese
medicine for hundreds of years. Hou et al (13) reported that DHM, a major bioactive
component of Ampelopsis grossedentata, suppresses the
release of TNF-α, IL-6 and IL-1β in macrophage cells (13). The present study demonstrated that
DHM downregulated the expression of TNF-α and IL-6 in
LPS-stimulated H9c2 cells, indicating an anti-inflammatory effect
of DHM in cardiomyocytes.
Inflammatory mediators cause cardiac cytotoxicity
and lead to cardiomyocyte apoptosis, thereby promoting cardiac
dysfunction (22). A well-balanced
interplay between anti- and proapoptotic Bcl-2 family members is
essential for the maintenance of mitochondrial integrity, which
determines cell survival (23).
Within the Bcl-2 family, BH3-only proteins, including Bcl-2-like
11, Bcl-2 binding component 3 or BH3 interacting domain death
agonist (also termed Bim, Puma and Bid, respectively), neutralize
antiapoptotic Bcl-2 proteins and directly activate rate-limiting
cell death effectors Bax and Bcl-2 antagonist/killer 1 (also termed
Bak). Upon activation, these cell death effectors form homodimers
and assemble into higher order oligomers that allow the activation
of caspase proteases lead to cell death (24). The present study demonstrated that
DHM attenuated LPS-induced cardiomyocyte apoptosis by
downregulating the expression of Bax, while upregulating Bcl-2
expression, in LPS-stimulated H9c2 cells. These results indicate a
potential therapeutic role for DHM in heart disease.
TLR4 has critical roles in mediating inflammatory
responses associated with heart diseases (7). TLR4 acts as a pattern recognition
receptor, which are receptors that recognize molecular patterns
associated with pathogens and damage (25). Once activated, TLR4 triggers an
intracellular signaling response and causes the activation and
translocation of NF-κB to the nucleus, which leads to an
inflammatory response and cell apoptosis. The present study
demonstrated that DHM treatment inhibited TLR4 expression and
blocked the phosphorylation and nuclear translocation of NF-κB p65
in response to LPS. This indicates that the alleviating effect of
DHM on LPS-induced inflammatory responses and apoptosis in
cardiomyocytes may be mediated by inhibition of the TLR4/NF-κB
pathway. In conclusion, the present study, to the best of our
knowledge, is the first to demonstrate a protective effect of DHM
on LPS-induced cardiomyocyte injury via inhibition of the
TLR4/NF-κB signaling pathway. These results may be important for
the development of strategies for the treatment of heart failure in
septic shock. However, further studies are required before DHM may
be considered for clinical usage in inflammatory disease.
References
|
1
|
Ramachandran G: Gram-positive and
gram-negative bacterial toxins in sepsis: A brief review.
Virulence. 5:213–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balija TM and Lowry SF: Lipopolysaccharide
and sepsis-associated myocardial dysfunction. Curr Opin Infect Dis.
24:248–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McDonald TE, Grinman MN, Carthy CM and
Walley KR: Endotoxin infusion in rats induces apoptotic and
survival pathways in hearts. Am J Physiol Heart Circ Physiol.
279:H2053–H2061. 2000.PubMed/NCBI
|
|
4
|
Ward PA: The sepsis seesaw: Seeking a
heart salve. Nat Med. 15:497–498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Xu X, Ceylan-Isik AF, Dong M, Pei
Z, Li Y and Ren J: Ablation of Akt2 protects against
lipopolysaccharide-induced cardiac dysfunction: Role of Akt
ubiquitination E3 ligase TRAF6. J Mol Cell Cardiol. 74:76–87. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liaunardy-Jopeace A and Gay NJ: Molecular
and cellular regulation of toll-like receptor-4 activity induced by
lipopolysaccharide ligands. Front Immunol. 6:4732014.
|
|
7
|
Liu L, Wang Y, Cao ZY, Wang MM, Liu XM,
Gao T, Hu QK, Yuan WJ and Lin L: Up-regulated TLR4 in
cardiomyocytes exacerbates heart failure after long-term myocardial
infarction. J Cell Mol Med. 19:2728–2740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie J, Liu J, Chen TM, Lan Q, Zhang QY,
Liu B, Dai D, Zhang WD, Hu LP and Zhu RZ: Dihydromyricetin
alleviates carbon tetrachloride-induced acute liver injury via
JNK-dependent mechanism in mice. World J Gastroenterol.
21:5473–5481. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia J, Guo S, Fang T, Feng D, Zhang X,
Zhang Q, Liu J, Liu B, Li M and Zhu R: Dihydromyricetin induces
autophagy in HepG2 cells involved in inhibition of mTOR and
regulating its upstream pathways. Food Chem Toxicol. 66:7–13. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu S, Liu B, Zhang Q, Liu J, Zhou W, Wang
C, Li M, Bao S and Zhu R: Dihydromyricetin reduced Bcl-2 expression
via p53 in human hepatoma HepG2 cells. PLoS One. 8:e768862013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi L, Zhang T, Liang X, Hu Q, Huang J,
Zhou Y, Chen M, Zhang Q, Zhu J and Mi M: Dihydromyricetin improves
skeletal muscle insulin resistance by inducing autophagy via the
AMPK signaling pathway. Mol Cell Endocrinol. 409:92–102. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang L, Zhang Q, Ren H, Ma S, Lu C, Liu
B, Liu J, Liang J, Li M and Zhu R: Dihydromyricetin enhances the
chemo-sensitivity of nedaplatin via regulation of the p53/Bcl-2
pathway in hepatocellular carcinoma cells. PLoS One.
10:e01249942015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou XL, Tong Q, Wang WQ, Shi CY, Xiong W,
Chen J, Liu X and Fang JG: Suppression of inflammatory responses by
dihydromyricetin, a flavonoid from ampelopsis grossedentata, via
inhibiting the activation of NF-κB and MAPK signaling pathways. J
Nat Prod. 78:1689–1696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hou X, Tong Q, Wang W, Xiong W, Shi C and
Fang J: Dihydromyricetin protects endothelial cells from hydrogen
peroxide-induced oxidative stress damage by regulating
mitochondrial pathways. Life Sci. 130:38–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng G, Yang S, Chen Y, Yao W, Zhu H and
Zhang W: Attenuating effects of dihydromyricetin on angiotensin
II-induced rat cardiomyocyte hypertrophy related to antioxidative
activity in a NO-dependent manner. Pharm Biol. 53:904–912. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu H, Luo P, Fu Y, Wang J, Dai J, Shao J,
Yang X, Chang L, Weng Q, Yang B and He Q: Dihydromyricetin prevents
cardiotoxicity and enhances anticancer activity induced by
adriamycin. Oncotarget. 6:3254–3267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liaudet L: Cardiovascular dysfunction in
sepsis: From basic mechanisms to clinical management. Curr Vasc
Pharmacol. 11:121–122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu X, Jia B, Wang F, Lv X, Peng X, Wang Y,
Li H, Wang Y, Lu D and Wang H: α1 adrenoceptor
activation by norepinephrine inhibits LPS-induced cardiomyocyte
TNF-α production via modulating ERK1/2 and NF-κB pathway. J Cell
Mol Med. 18:263–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang P, Han Y, Gui L, Sun J, Chen YL, Song
R, Guo JZ, Xie YN, Lu D and Sun L: Gastrodin attenuation of the
inflammatory response in H9c2 cardiomyocytes involves inhibition of
NF-κB and MAPKs activation via the phosphatidylinositol 3-kinase
signaling. Biochem Pharmacol. 85:1124–1133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Wang HY, Bassel-Duby R, Maass DL,
Johnston WE, Horton JW and Tao W: Role of interleukin-6 in cardiac
inflammation and dysfunction after burn complicated by sepsis. Am J
Physiol Heart Circ Physiol. 292:H2408–2416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Z, Liu Y, Deng W, Dai J, Li F, Yuan
Y, Wu Q, Zhou H, Bian Z and Tang Q: Hesperetin attenuates
mitochondria-dependent apoptosis in lipopolysaccharide-induced H9C2
cardiomyocytes. Mol Med Rep. 9:1941–1946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sochalska M, Tuzlak S, Egle A and
Villunger A: Lessons from gain- and loss-of-function models of
pro-survival Bcl2 family proteins: Implications for targeted
therapy. FEBS J. 282:834–849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heiserman JP, Chen L, Kim BS, Kim SC, Tran
AL, Siebenborn N and Knowlton AA: TLR4 mutation and HSP60-induced
cell death in adult mouse cardiac myocytes. Cell Stress Chaperones.
20:527–535. 2015. View Article : Google Scholar : PubMed/NCBI
|