Introduction
Acute myocardial infarction (AMI) remains of primary
concern, resulting in a huge physical and economic burden around
the world, despite decades of extensive research. Heart failure
gradually develops due to loss of cardiomyocytes, which are
replaced with scar tissue post-AMI. It has been reported that there
are other intercellular alterations, including collagen and elastic
fibres in the infarcted region (1–3). At
present, research interests focus on alterations of cardiomyocytes
and the neogenesis of capillaries following AMI (4,5).
The extracellular matrix is an important component
of the heart, and includes collagen fibre, reticular fibre and
elastic fibres with distinctive functions. These fibres constitute
a complex network of cardiac fibre stents for maintaining heart
form and function (6). Myocardial
collagen fibre, elastic fibre and reticular fibre increase
gradually from embryonic stage to birth, and then to adulthood. The
fastest fibre growth is from childhood to youth, and tends to be
stable following the onset of adulthood (7). Elastic fibres exhibit an
indispensable role in maintaining cardiac morphology and function,
although the content of elastic fibre is relatively lower compared
with collagen fibre in the heart. In the events of disproportional
distribution between the collagen fibre and elastic fibre, normal
heart function will be compromised, resulting in
electrophysiological systolic and diastolic functional alterations,
which may eventually progress to heart failure (8). Intercellular collagen and elastic
fibre will have different degrees of deposition subject to damage
or reconstruction following AMI.
However, the kinetic alterations of interstitial
collagen and elastic fibres during AMI, and in particular, their
association with cardiac function, remain to be fully elucidated
(9,10). The present study aimed to explore
the kinetics of type I/III collagen fibre and elastic fibre in
infarcted tissue at different times following AMI and the
association with cardiac function. The data may provide a novel
insight for myocardial regeneration, which may be of interest to
researchers and clinicians.
Materials and methods
Rat model of AMI
A total of 50 healthy adult Sprague-Dawley rats
(age, 4–6 weeks), female only, weighing 230±20 g, were obtained
from the Laboratory Animal Centre, Xinxiang Medical University
(Xinxiang, China), animal number: SYXK (YU) 2009–0002. The rats
were separately maintained in an animal room between 20 and 23°C,
under a 12-h light/dark cycle, with free access to standard rat
feed and ordinary tap water.
These rats were randomly divided into non-infarcted
and infarcted week 1–4 groups (n=10). The experiment strictly
adhered to the Guidance for the Care and Use of Laboratory Animals
formulated by The Ministry of Science and Technology, China. The
present study was approved by the ethics committee of Xinxiang
Medical University.
Generation of myocardial infarction: Following
weighing, animals were anaesthetised with 10% chloral hydrate
intraperitoneally (i.p. 3 ml/kg). The thoracic cavity was exposed
following surgical area disinfection with iodine. An artificial
breathing machine (Shanghai Biowill Co., Ltd., Shanghai, China) was
used for artificial respiration. The left anterior descending
coronary artery was ligated following opening of the pericardium,
then the chest was closed. A dose of 1.60×107 units of
penicillin were administered i.p. immediately following surgery,
and 0.4×107 units of penicillin were administered daily
for three days post-operation, following myocardial infarction.
Heart function monitoring and
myocardial pathological examination
Intraoperative electrocardiogram (ECG) was used on a
BL-420 biological function experimental system, using a small
animal ultrasonic instrument MS-250 probe [Winsun (China) Limited,
Beijing, China]. Heart function was assessed using the following
parameters: left ventricular ejection fraction (LVEF), left
ventricular fraction shortening (LVFS), interventricular septal
(IVS) thickness, left ventricular internal diameter at
end-diastolic (LVIDd), end-systolic (LVIDs) and left ventricular
posterior wall (LVPW) thickness.
Immunohistochemistry of type I, III
collagen fibre and elastic fibre
The hearts were washed in normal saline and fixed in
4% paraformaldehyde at 25–28°C for 24 h following sacrificing. The
paraffin-embedded blocks (5 µm) were sectioned for Masson staining
at 25–28°C for 20 min for histopathology with an inverted
microscope (TE2000U-PH-E; Leica Microsystems GmbH, Wetzlar,
Germany) Immunohistochemistry was then performed as previously
described (11). Briefly, paraffin
sections were incubated in 0.01 mol/l citric acid salt boil buffer
for 15 min. After cooling at room temperature, the sections were
incubated with 3% H2O2 deionized water for 15
min and washed three times with PBS (5 min/wash), then dewaxed and
rehydrated. The sections were incubated with primary antibodies
[mouse anti-rat collagen I (Abcam, Cambridge, UK; catalog no.
ab90395; 1:1,000) or III (Abcam; catalog no. ab6310; 1:600) or,
rabbit polyclonal anti-rat elastin (EterLife, Tianjin, China;
catalog no. EL913879; 1:200) overnight at 4°C. PBS phosphate buffer
(dry powder) (catalog no. top0027; Beijing Biotopped Technology
Co., Ltd., Beijing, China) was used as an isotype control. Polymer
helper was added on the sections for 20 min at room temperature
following PBS washing, then followed by addition of
polyperoxidase-anti-mouse/rabbit IgG (ready to use; catalog no.
PV-9000; OriGene Technologies, Inc., Beijing, China) for 30 min at
room temperature. DAB was finally added for colour development. The
slides were lightly counterstained with haematoxylin at 25–28°C for
2 min, and dehydration and transparency-rendering were conducted
prior to observation of each section at ×100 magnification under an
inverted microscope (TE2000U-PH-E; Leica Microsystems GmbH).
Immunofluorescence detection of type
I, III collagen fibre and elastic fibre in the myocardial
infarction zone
Frozen sections (8 µm) from AMI and control heart
specimens, dried at room temperature, were fixed in cold acetone
for 10 min at 4°C, washed three times with PBS (5 min/wash), and
blocked with normal goat serum (New Zealand origin; 100 ml; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 25–28°C for 30 min.
The sections were incubated with primary antibodies [mouse anti-rat
collagen I (Abcam; catalog no. ab90395; 1:1,000) or III (Abcam;
catalog no. ab6310; 1:600) or rabbit polyclonal anti-rat elastin
(EterLife; catalog no. EL913879; 1:200) overnight at 4°C. PBS
phosphate buffer (dry powder) (catalog no. top0027; Beijing
Biotopped Technology Co., Ltd.) was used as an isotype control. A
fluorescein isothiocyanate-conjugated goat anti-rabbit CY3 secary
antibody (catalog no. A0516; Beyotime Institute of Biotechnology,
Haimen, China; 1:500) was incubated with sections for 1 h at room
temperature. Images were captured under an inverted fluorescence
microscope (DMI3000; Leica Microsystems GmbH).
Western blotting detection of type I,
III collagen fibre and elastic fibre
LV sections of hearts were collected from AMI and
normal animals. Total proteins were extracted using a
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology), and proteins were quantified using the
bicinchoninic acid method. Proteins (30 µg/lane) were separated by
10% SDS-PAGE. The protein was transferred to a polyvinylidene
fluoride membrane and blocked with 5% skimmed milk for 2 h at
25–28°C. Anti-collagen I (1:2,000); anti-collagen III (1:4,000); or
anti-elastin primary antibodies (1:200), as aforementioned, were
incubated with the membrane overnight at 4°C. Horseradish
peroxidase-labelled goat anti-mouse secondary antibody (Beyotime
Institute of Biotechnology; catalog no. A0216; 1:500) was added for
incubation at room temperature for 1 h, followed by addition of an
enhanced chemiluminescence luminous fluid (Beyotime Institute of
Biotechnology) at room temperature for 3 min. The gel was
photographed using a gel imaging system. GAPDH (catalog no. AG019;
Beyotime Institute of Biotechnology) was used as a house-keeping
reference. The density of the target(s) was determined as the ratio
between the target and GAPDH, which was expressed as
semi-quantitative.
Statistical analysis
Gel phase system (version 5.3; National Institutes
of Health; Bethesda, MD, USA) and Image J (version 2.1.4.7;
National Institutes of Health) were used to analyse photographs of
western blotting tests and Image-Pro Plus Analysis Systems (version
6.0; Media Cybernetics, Inc., Rockville, MD, USA) for analysis of
immunohistochemistry and immunofluorescence, five horizons of clear
and non-overlapping tissue in infarcted area were selected for
semi-quantitative analysis. The data were analysed by SPSS
software, version 19.0 (IBM SPSS, Armonk, NY, USA). One-way
analysis of variance followed by Turkey post hoc test were used to
assess differences between groups. Data are expressed as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
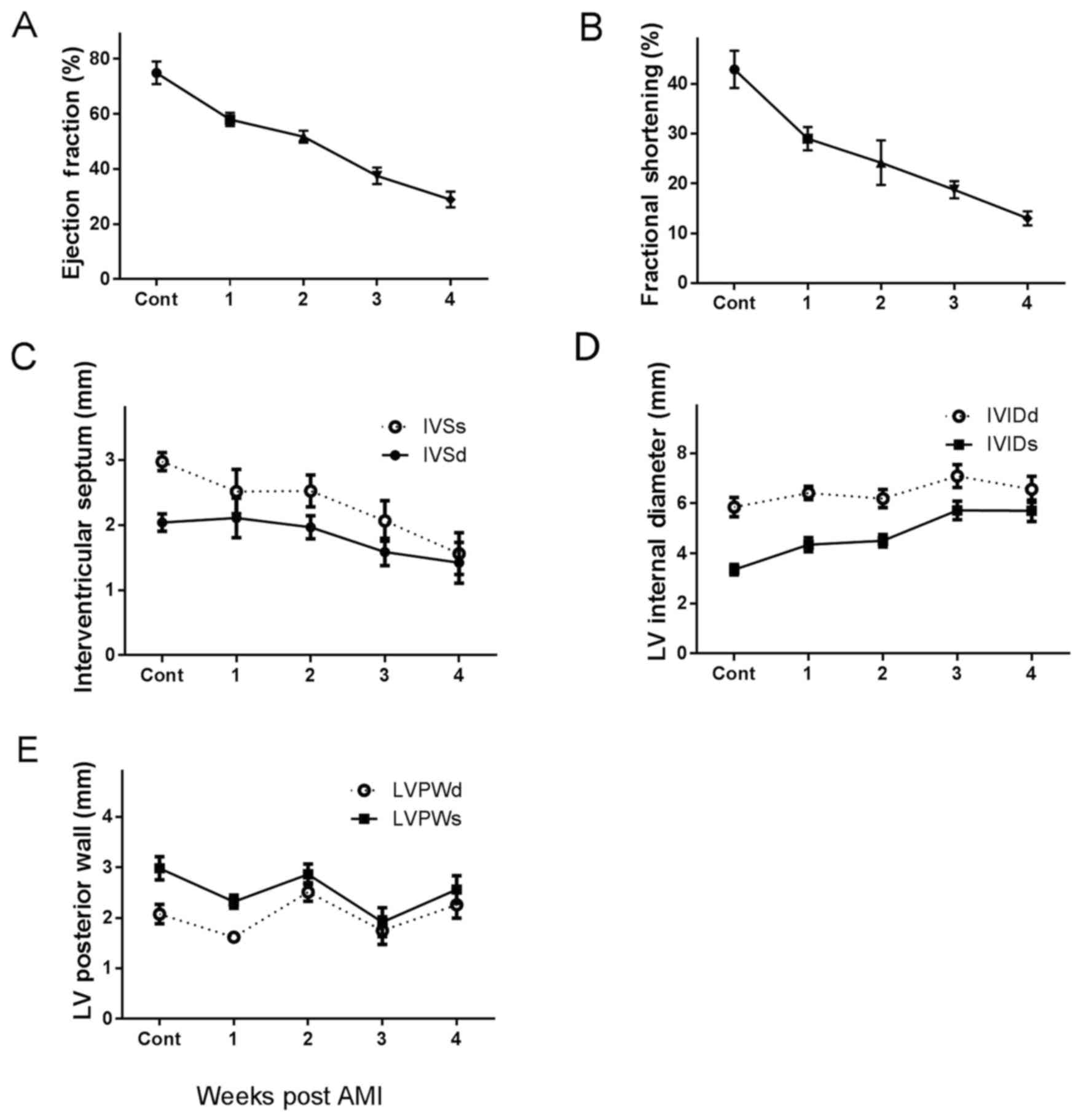
Cardiac function alterations at
different periods following AMI
LVEF, LVFS and IVS were gradually decreased, however
LVIDs and LVIDd increased with post-AMI time, compared with the
normal heart, demonstrating impairment of heart function. In
addition, cardiac function gradually decreased with time,
particularly 2–3 weeks following infarction. Myocardial infarction
models were successfully established and LVPW was not altered
(Fig. 1).
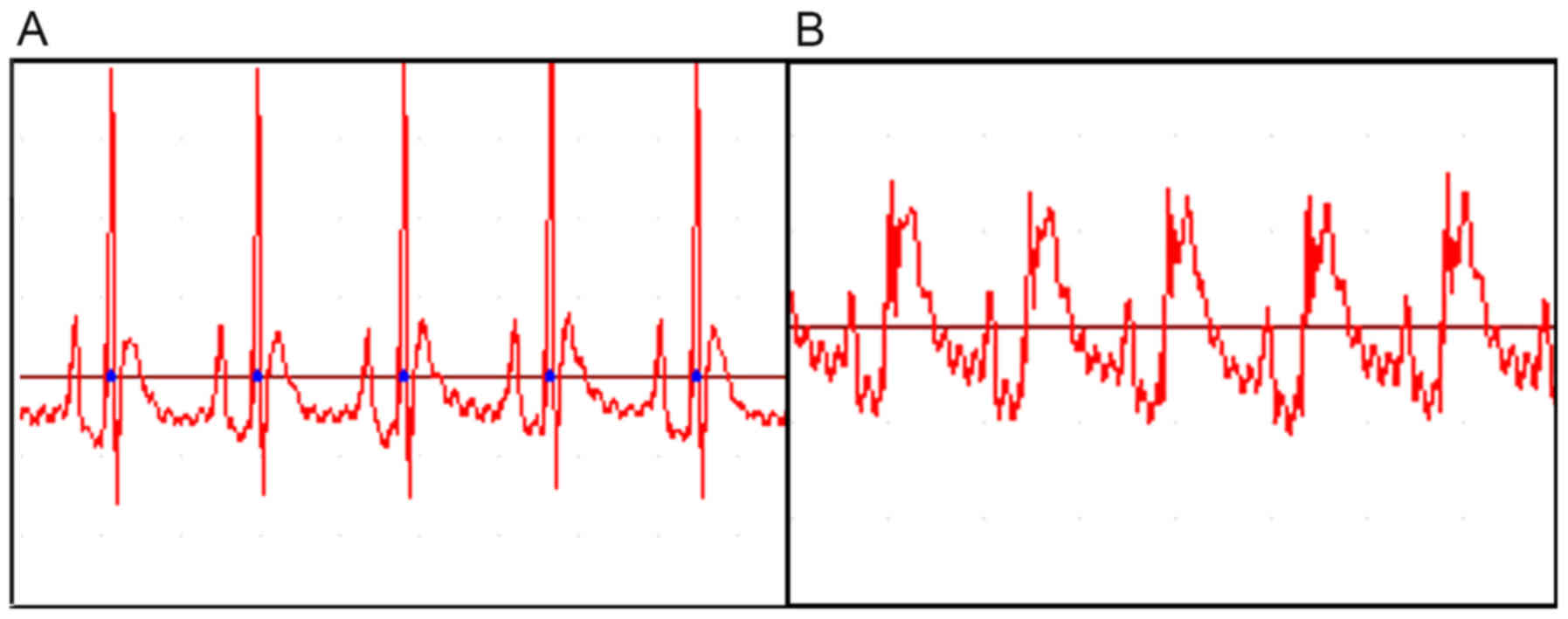
Verification of myocardial
infarction
Following ligation of the left anterior descending
coronary artery, the myocardium below the ligation turned purple or
pale, and the left auricle congested heart beat weakened. AMI was
diagnosed using an ECG, and the heart rate slowed, with varying
degrees of arrhythmia. II ST segment of the infarction group
shifted 20 min following ligation, leading to an ST segment arch
upward-push by 0.2 mV (Fig. 2),
which demonstrated that that the ligature was successful and the
myocardial infarction model had been successfully established.
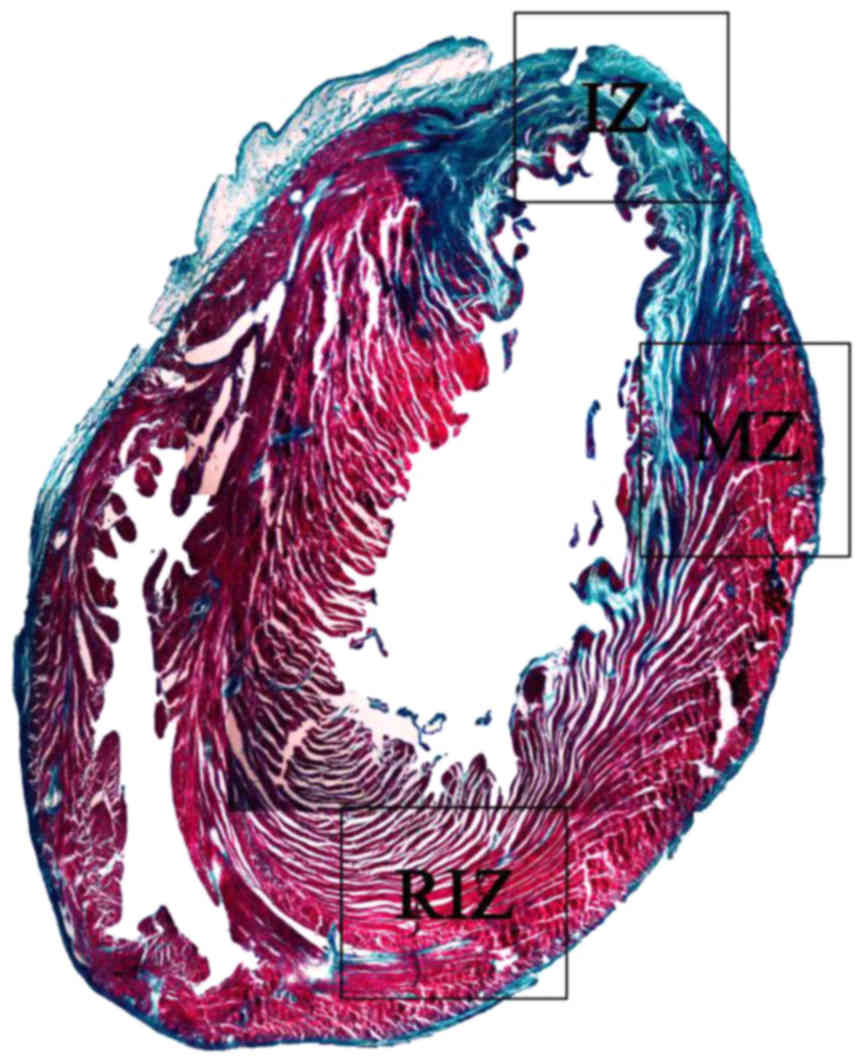
Histopathological verification of
AMI
Paraffin-embedded and Masson stained heart section
was evaluated for AMI. Undamaged heart tissue was presented as red,
however AMI damaged heart was blue, due to collagen fibre
replacement. The front wall of the left ventricle was significantly
thinner, infarcted myocardial tissue was replaced by fibrous scar
tissue (Fig. 3), confirming the
myocardial infarction model.
Immunohistochemistry and
immunofluorescence detection of collagen and elastic fibre in the
infarcted heart
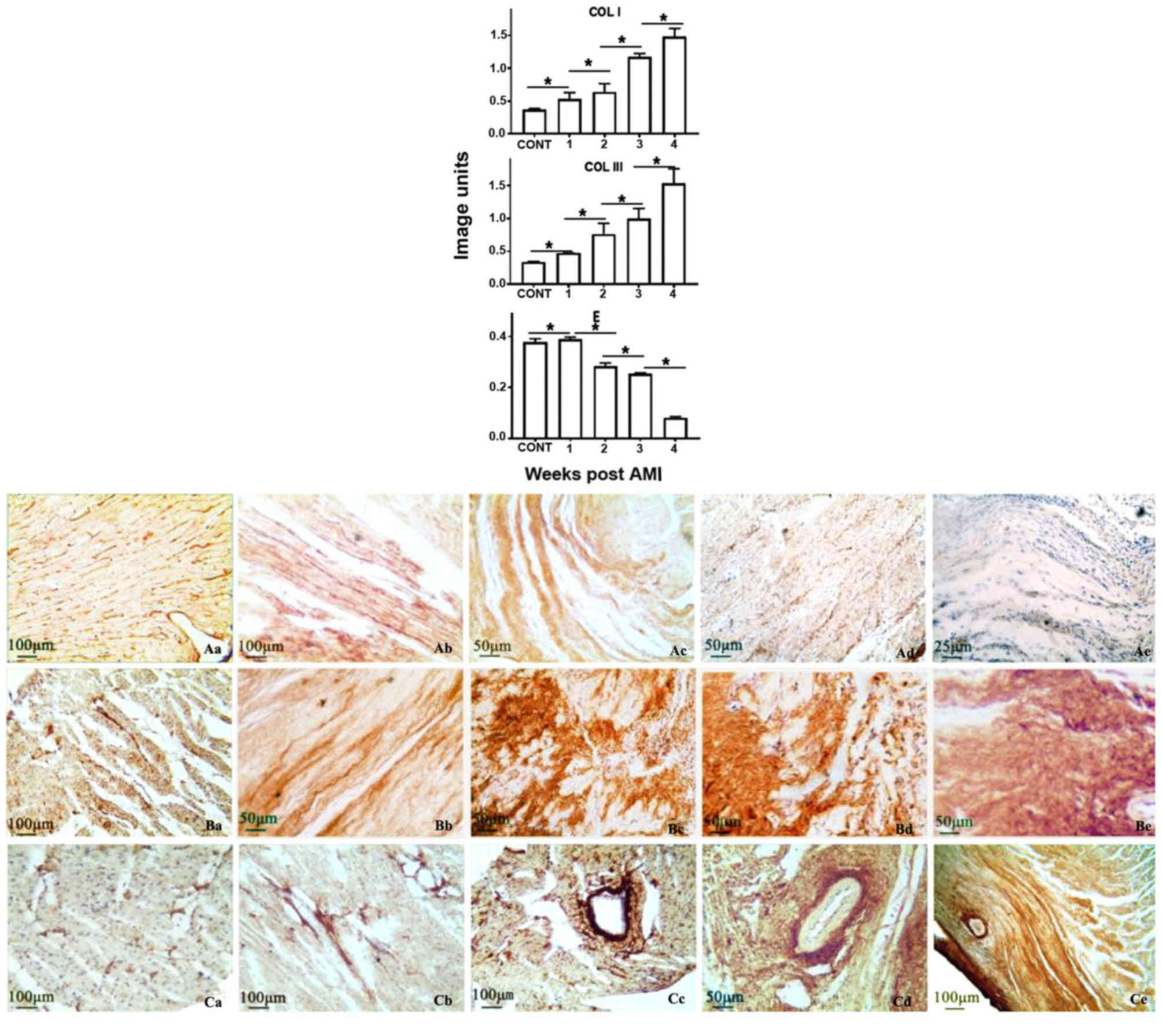
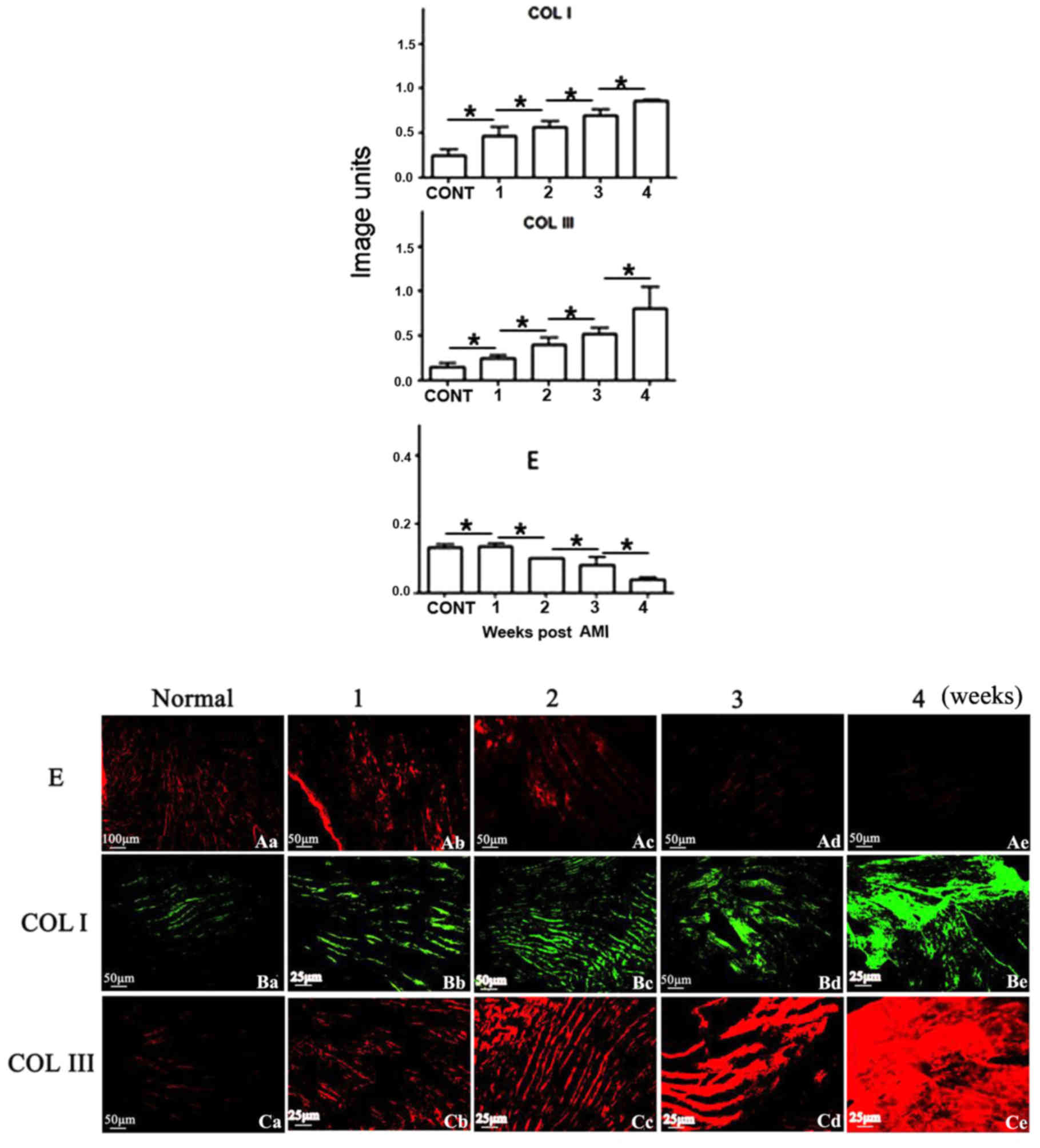
Using immunohistochemistry, production of collagen I
was demonstrated to be gradually increased with AMI time, and
revealed a 1.4-, 1.5-, 2.9- or 3.9-fold increase at the weeks 1, 2,
3 or 4, compared with the normal heart, respectively. Similarly,
collagen III was increased 1.2-, 1.9-, 2.5- or 3.9-fold,
respectively. In contrast, elastin was decreased gradually, and
revealed a 2, 77, 86 or 97% reduction, compared with the normal
heart, at weeks 1, 2, 3 or 4 post-AMI, respectively. Elastic fibre
was almost completely replaced by collagen fibre in the infarcted
zone, forming scar tissue, at week 4 post-AMI (Figs. 3 and 4). Immunofluorescence was additionally
utilised to verify the results obtained from immunohistochemistry
analysis. Similar trends were identified in elastin and collagen
III [identified in red (CY3)] production, or collagen I [identified
in green (FITC)] in the infarcted heart, post-AMI (Fig. 5).
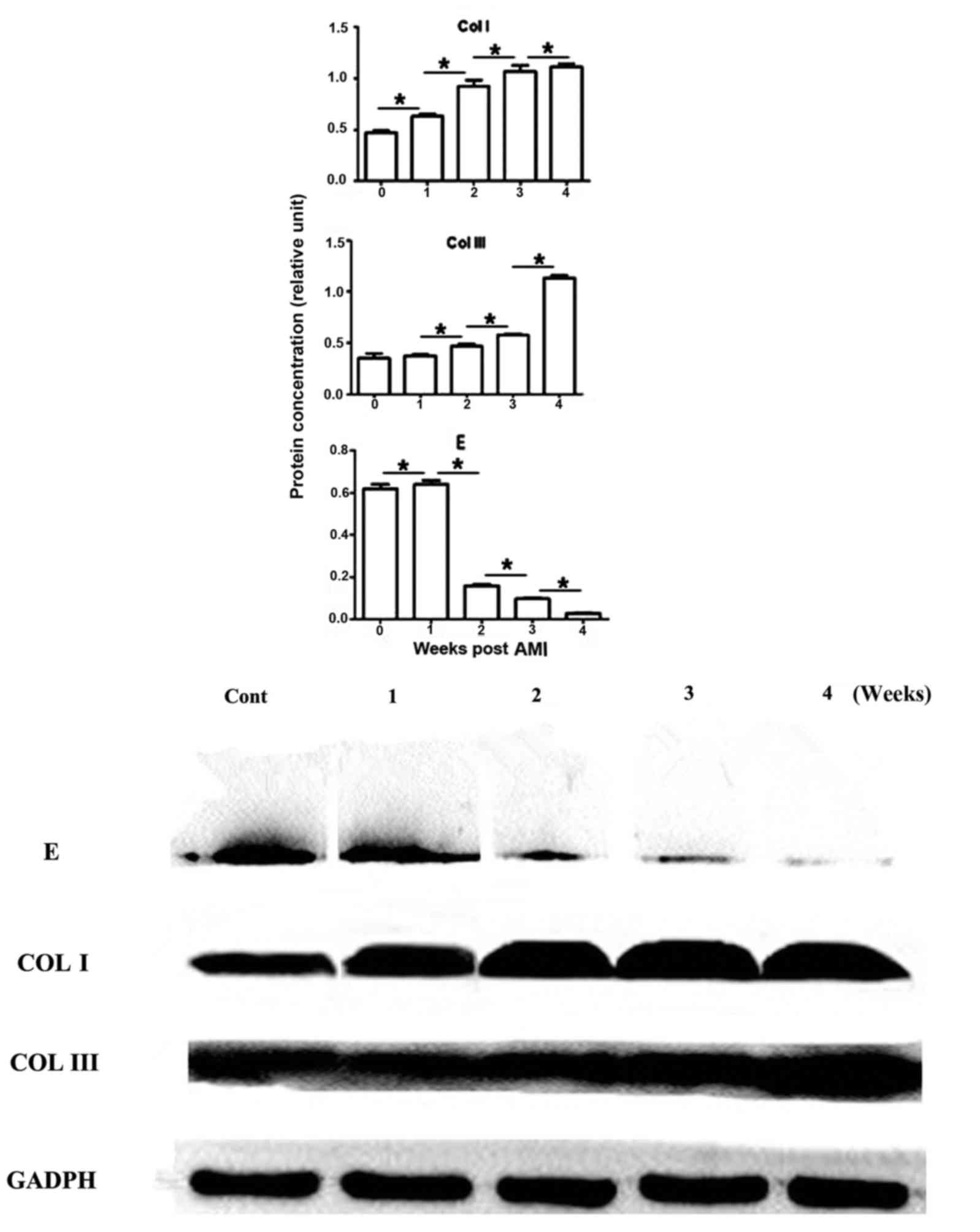
Collagen and elastic fibre protein
expression levels are varied in the infarcted heart
Collagen I was increased gradually following
increased AMI time, and revealed a 1.5-, 2.0-, 2.5- or 2.6-fold
increase at weeks 1, 2, 3 or 4, respectively, compared with the
normal heart. Collagen III demonstrated a similar trend, with a
1.0-, 1.3-, 1.5- or 2.5-fold increase at weeks 1, 2, 3 or 4,
respectively (Fig. 6). In
contrast, elastin was decreased gradually with AMI time, and
revealed a 4, 78, 87 or 98% reduction, compared with the normal
heart at weeks 1, 2, 3 or 4, respectively, the comparison between
groups was statistically significant (P<0.05). The results of
the western blotting assay were consistent with those obtained via
immunohistochemistry and immunofluorescence assays.
Discussion
The results of the present study demonstrated
gradually increased collagen I/III over the four weeks post-AMI,
which was consistent with gradually compromised cardiac function.
Furthermore, there was an inverse association between increased
collagen I/III and decreased elastin in the infarcted heart, which
suggested that elastic fibre is critical in maintaining correct
cardiac function.
Scar structure is variable at the different periods
following myocardial infarction. Following AMI, adjustment of
collagen I/III fibre and elastic fibre composition determines
cardiac function via ventricle wall compliance and tensile strength
(12). An increase in the collagen
I/III component augments heart stiffness, which subsequently
aggravates left ventricular diastolic and systolic dysfunction
(13,14). These previous findings are in
accordance with the results of the present study, and collectively
suggest that a compromised the function of the heart, resulting in
reconstruction of heart configuration, leads to cardiac dysfunction
and even heart failure.
Due to the uneven distribution of elastic fibre in
the myocardium, and its relatively small content compared with
collagen fibre, the literature regarding its specific functions is
sparse (12,15). However, it has previously been
demonstrated that adjustment of the content and structure of
cardiac elastic fibre continues to occur with the altering course
of the disease following myocardial infarction, indicating that
elastic fibre takes part in the ventricular remodelling that
follows myocardial infarction (16). In addition, elastic fibre markedly
reduces in size and quantity in the maturation of scarring
following AMI (17).
However, the dynamic alterations of collagen fibre
and elastic fibre, and the alterations in ratio of one counterpart
to the other in the context of AMI, remain to be elucidated and
have not been reported to date, particularly regarding their
association with cardiac function. The present study demonstrated
that the content of collagen I fibre increased gradually. There was
consistency between the upregulation of collagen I/III and the
downregulation of cardiac function when comparing alterations in
collagen with cardiac function. Reduction in cardiac function was
inversely associated with the greatest induction of collagen
following AMI. This finding is supported by the result from a
previous study, which demonstrates that a novel collagen-derived
matricryptin is generated post-MI, that mediates remodelling of the
left ventricle (3).
Furthermore, the data demonstrated that elastic
fibre decreased in the myocardial infarction zone gradually over
four weeks AMI, which was consistent with reduced cardiac function
over the period of AMI. The maximum reduction of elastic fibre was
in the 2–3 week period, which was associated with the fastest
reduction of cardiac function post AMI. According to Fung's Theory,
elastin is the most ‘linearly’ elastic biosolid material, which is
extensively distributed in animal tissues and organs. If a
cylindrical specimen of elastin is prepared and subjected to an
unaxial load in a tensile testing machine, the existence of an
energy dissipation in the material is small (18). From the view of biomechanics, the
increase or decrease of elastic fibres should not exert too much
influence on heart function. Therefore, whether there is a causal
association between the decrease of elastic fibres and the decline
of cardiac function requires further research. The data from the
present study is in accordance with previous studies, and suggested
that increased elastic fragments in the area of myocardial
infarction in rats altered the extracellular matrix of the
infarcted zone during ventricular remodelling. Injection of elastic
fragments into the AMI zone forms an organic extracellular matrix
structure, and ultimately suppresses adverse modification in
ventricular remodelling (15,17).
Finally, the ratio of elastic fibre and collagen increases, which
may significantly improve the heart function, reduce scar area, and
decrease ventricular dilatation (19,20).
The results of the present study demonstrated that
there was an association between the reduction of elastic fibre and
induction of collagen I/III during AMI, and in particular, these
alterations were consistent with impairment of cardiac function.
However, the specific molecular mechanism underlying the process of
pathological alteration following myocardial infarction, and the
association with the presence of elastic fibre and collagen fibre,
remains to be fully elucidated. The data suggested that decreased
elasticity occurred in conjunction with increased ‘hardness’ in the
formation of myocardial infarction scar tissue. Cardiac tissue
flexibility or stiffness may be determined by the composition of
elastic or collagen fibre during and/or post AMI (21). The data additionally suggested that
inhibition of collagen and/or induction of elastic fibre may act as
an effective approach to preserve or improve cardiac function
during and/or post AMI.
Under various circumstances, animal gender may
influence the research results. According to the author's previous
study, there were differences in cells, tissues and their
functions, between the different sexes, however there were almost
no differences of the intercellular substance between males and
females, including in collagen fibres and elastic fibres.
Therefore, the heart of female rats only were selected as the
research object of the present study (22).
In conclusion, the results of the present study
demonstrated that in myocardial infarction, not only were
cardiomyocytes damaged, but dynamic alterations in collagen and
elastic fibers were also detected in the interstitium of the
myocardium. Alterations in myocardial collagen and elastic fibers
may also affect myocardial function, which has not yet attracted
the attention of clinical diagnosis and treatment. Our future
studies aim to improve and adjust the structural alterations in
myocardial collagen and elastic fibers following myocardial injury,
and make it more suitable for myocardial repair process.
Acknowledgements
The present study was supported by The Innovation
Support Plan from Xinxiang Medical University (grant no.
YJSCX201302Z) and The National Natural Science Fund Project (grant
nos. 81400237 and 81570268), China. YY and YG performed the
experiment and interpreted the data; YY drafted the manuscript. SB
interpreted the data and proof-read the manuscript, GZ designed the
experiment and final proof-read the manuscript.
References
|
1
|
Guo J and Gao C: Significance of metabolic
changes of myocardial collagens in rat myocardial infarction model
during ventricular remodelling. Chin J of Clin Rehab. 9:56–57.
2005.(In Chinese).
|
|
2
|
Wang ZF, Wang NP, Harmouche S, Philip T,
Pang XF, Bai F and Zhao ZQ: Retraction note to: Postconditioning
promotes the cardiac repair through balancing collagen degradation
and synthesis after myocardial infarction in rats. Basic Res
Cardiol. 110:122015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu X: Physiological and pathological
significance of myocardial interstitial networks. Basic Clin Med.
12:17–21. 1999.(In Chinese).
|
|
4
|
Lindsey ML, Iyer RP, Zamilpa R,
Yabluchanskiy A, DeLeon-Pennell KY, Hall ME, Kaplan A, Zouein FA,
Bratton D, Flynn ER, et al: A novel collagen matricryptin reduces
left ventricular dilation post-myocardial infarction by promoting
scar formation and angiogenesis. J Am Coll Cardiol. 66:1364–1374.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zuo H, Li C and Guo Z: Relationship
between cardiac function and angiogenesis after rat myocardial
infarction. Acta Anatomica Sinica. 45:35–40. 2014.
|
|
6
|
Guo Z: Current Cardiac Histology. People
Health Publishing Company; Beijing: 2016
|
|
7
|
Cai XH, Guo ZK, Mao HL and Qin C:
Histologial architecture of reticular fibers and age-ralated
changes of myocardial tissue in rats. Acta Anatomica Sinica.
40:127–129. 2009.
|
|
8
|
Kong CH, Lin XY, Woo CC, Wong HC, Lee CN,
Richards AM and Sorokin VA: Characteristics of aortic wall
extracellular matrix in patients with acute myocardial infarction:
Itissue microarray detection of collagen I, collagen III and
elastin levels. Interact Cardiovasc Thorac Surg. 16:11–15. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li G, Liu X and Tian L: A study on
myocardial collagen synthesis and cardiac function in patients with
anterior acute myocardial infarction without early successful
reperfusion. J Postgrad Med. 28:27–29. 2005.
|
|
10
|
Serpooshan V, Zhao M, Metzler SA, Wei K,
Shah PB, Wang A, Mahmoudi M, Malkovskiy AV, Rajadas J, Butte MJ, et
al: The effect of bioengineered acellular collagen patch on cardiac
remodeling and ventricular function post myocardial infarction.
Biomaterials. 34:9048–9055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu H, Wise SG, Rnjak-Kovacina J, Kaplan
DL, Bilek MM, Weiss AS, Fei J and Bao S: Biocompatibility of
silk-tropoelastin protein polymers. Biomaterials. 35:5138–5147.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhai X, Wu J, Guo Z and Wang L:
Age-related changes of elastic fibers and elastic protein in rat
ventricular myocardium. Acta Anatomica Sinica. 40:127–129.
2009.
|
|
13
|
Voorhees AP, DeLeon-Pennell KY, Ma Y,
Halade GV, Yabluchanskiy A, Iyer RP, Flynn E, Cates CA, Lindsey ML
and Han HC: Building a better infarct: Modulation of collagen
cross-linking to increase infarct stiffness and reduce left
ventricular dilation post-myocardial infarction. J Mol Cell
Cardiol. 85:229–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hervas A, Ruiz-Sauri A, de Dios E, Forteza
MJ, Minana G, Nunez J, Gomez C, Bonanad C, Perez-Sole N, Gavara J,
et al: Inhomogeneity of collagen organization within the fibrotic
scar after myocardial infarction: Results in a swine model and in
human samples. J Anat. 228:47–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizuno T, Mickle DA, Kiani CG and Li RK:
Overexpression of elastin fragments in infarcted myocardium
attenuates scar expansion and heart dysfunction. Am J Physiol Heart
Circ Physiol. 288:H2819–H2827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vander Donckt C, Van Herck JL, Schrijvers
DM, Vanhoutte G, Verhoye M, Blockx I, van der Linden A, Bauters D,
Lijnen HR, Sluimer JC, et al: Elastin fragmentation in
atherosclerotic mice leads to intraplaque neovascularization,
plaque rupture, myocardial infarction, stroke and sudden death. Eur
Heart J. 36:1049–1058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li SH, Sun Z, Guo L, Han M, Wood MF, Ghosh
N, Vitkin IA, Weisel RD and Li RK: Elastin overexpression by
cell-based gene therapy preserves matrix and prevents cardiac
dilation. J Cell Mol Med. 16:2429–2439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fung YC: Mechanical properties of living
tissue. 7Springer Verlag; New York, NY: pp. 242–245. 1993
|
|
19
|
Lichtenauer M, Mildner M, Baumgartner A,
Hasun M, Werba G, Beer L, Altmann P, Roth G, Gyöngyösi M, Podesser
BK and Ankersmit HJ: Intravenous and intramyocardial injection of
apoptotic white blood cell suspensions prevents ventricular
remodelling by increasing elastin expression in cardiac scar tissue
after myocardial infarction. Basic Res Cardiol. 106:645–655. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uchinaka A, Kawaguchi N, Hamada Y,
Miyagawa S, Saito A, Mori S, Sawa Y and Matsuura N: Transplantation
of elastin-secreting myoblast sheets improves cardiac function in
infarcted rat heart. Mol Cell Biochem. 368:203–214. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Xu Y, Wang Z, Wen D, Zhang W,
Schmull S, Li H, Chen Y and Xue S: Electrospun nanofibrous sheets
of collagen/elastin/polycaprolactone improve cardiac repair after
myocardial infarction. Am J Transl Res. 8:1678–1694.
2016.PubMed/NCBI
|
|
22
|
Zhai XB, Wu JF, Guo ZK, et al: Aging
changes of elastic fibers and collagen fibers in normal myocardium
of rats. Acta Anatomica Sinica. 44:106–110. 2013.
|