Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most
common pancreatic neoplasm, and is highly aggressive and malignant
with a very low survival rate. Nowadays, as a devastating disease,
PDAC is the fourth most common disease-associated mortality
worldwide (1,2). The 5-year survival rate is ~6%, and
only 20% of patients present with resectable disease (3,4). The
high mortality of these patients is partly due to more than
three-quarters of the cases being diagnosed at too late a stage to
allow surgical resection, which is the only curative option.
Non-surgical patients are treated with standard chemotherapies
(5). Despite numerous promising
novel therapies approved by the Food and Drugs Administration, the
advances have been very slow for patients with PDAC (6). In addition, some cancer cells can
become progressively resistant to drugs during the course of
treatment, ultimately resulting in a non-responsive tumor to
chemotherapies (7). Therefore,
novel effective therapeutic methods are necessary.
Traditional Chinese medicine is appreciated for its
5000-year-old history and still holds an important position in
primary health care in China; it has also become more popular among
cancer patients in the western world (8). Recent reports also demonstrated that
Chinese herbs may be used for the development of functional foods,
the promotion of health, and as pharmaceutical agents for various
kinds of human cancers, such as oral, gastric, breast and
epithelial ovarian cancers (9–13).
Codonopsis (Campanulaceae, C.) is
represented in China by 39 species, some of which are commonly used
as herbal remedies due to their tonic effects, such as C.
pilosula and C. tangshen (14). In addition, the roots of some
Codonopsis species, including C. cordifolioidea,
C. bulleyana, C. micrantha and C. subglobosa,
are well-known vegetables in southwest China (15,16).
Codonopsis cordifolioidea (C. cordifolioidea) is
herbaceous plant found in Yunnan, Tibet, and Sichuan Provinces. Its
roots, locally known as Tsoong, have been used as a food in Yunnan
Province since ancient times (13). Meanwhile, this species has become
an important economic plant widely cultivated in several areas of
Yunnan Province (17,18). As major components isolated from
C. cordifolioidea, phenylpropanoids, lignans and flavonoids
are isolated and demonstrated to present biological effects of
anti-human immunodeficiency virus type 1 (HIV-1) activities and
cytotoxicity (13,19). Hu et al (13) have performed and finished the
isolation, structural elucidation and biological activities
assessing of the major compounds, termed cordifoliketones A-B. The
cytotoxicity tests for the isolates were performed in HL-60 (human
acute promyelocytic leukemia), Hep-G2 (human hepatocellular
carcinoma), KB (human oropharyngeal epidermoid carcinoma) and
MDA-MB-231 (human breast cancer cells) tumor cell lines. Their
results revealed that compounds 1–2 have significant potential
cytotoxic abilities against various tumor cell lines (13). However, the possible effects of
these new compounds isolated from the roots of C.
cordifolioidea, Tsoong, on the proliferation, apoptosis,
migration and invasion ability of PDAC, are still unknown.
In present study, an extraction of cordifoliketones
A from Tsoong was prepared, and the possible effects against PDAC
cells was explored. The human PDAC cell lines, AsPC-1, BxPC-3 and
PANC-1, were used. The cytotoxicity against PDAC cells and the
effects of cordifoliketones A on PDAC cell proliferation,
apoptosis, invasion and migration were examined in vitro and
in vivo. The effect of cordifoliketones A treatment on the
survival and apoptosis, invasion and migration of human PDAC cell
in vitro, were evaluated by resazurin reduction assay, flow
cytometry, and migration and invasion assays. Western blotting was
also used to determine the expression of cytokine associated with
apoptosis. A xenograft nude mouse model was established by
injection with PDAC cells followed by treatment with
cordifoliketones A.
On day 27 post treatment, mice were sacrificed, and
tumors were isolated for determination of weight and volume.
Materials and methods
Preparation of cordifoliketones A
Tsoong, roots of C. cordifolioidea, were
collected in Dali Prefecture and Yuxi (Yunnan, China) in September
2013. The identification of the plant material was verified by
Professors Chen. and Mei (Yunnan Nationalities University). A
voucher specimen (YNNI 09-9-13) was used. The air-dried and
powdered roots of C. cordifolioidea (1.5 kg) were repeatedly
subjected to column chromatography on Si gel, Sephadex LH-20, RP-18
and Preparative high-performance liquid chromatography systems to
obtain a 70% aqueous methanol extract, which contained compounds
1–11, including two novel phenylpropanoids, termed cordifoliketones
A-B, together with nine known phenylpropanoids (13). The combined extract was then
stepwise eluted by petroleum, ethyl acetate and n-butyl alcohol
(20). A total of 50 mg
phenylpropanoids, termed cordifoliketones A, was isolated and
extracted by vacuum column chromatography at 20–25°C. The combined
phenylpropanoids were subjected to a silica gel (Merck KGaA,
Darmstadt, Germany) vacuum liquid chromatography and eluted with
n-hexane (Sigma-Aldrich; Merck KGaA; diameter, 6.5 cm),
chloroform (Sigma-Aldrich; Merck KGaA), EtOAc (Sigma-Aldrich; Merck
KGaA), acetone (Sigma-Aldrich; Merck KGaA) and MeOH (Sigma-Aldrich;
Merck KGaA) to obtain a major product. The product was then
subjected to silica gel open column chromatography (diameter, 5 cm)
and eluted with n-hexane-EtOAc step gradients. Following
this, 23 fractions of 200 ml each were collected. These fractions
were then pooled into eleven new fractions (compounds 1–11). Nine
of them were known phenylpropanoids as described previously
(13). The novel phenylpropanoid,
was crystallized from n-hexane to obtain colorless crystals
and termed as cordifoliketones A. The physicochemical data
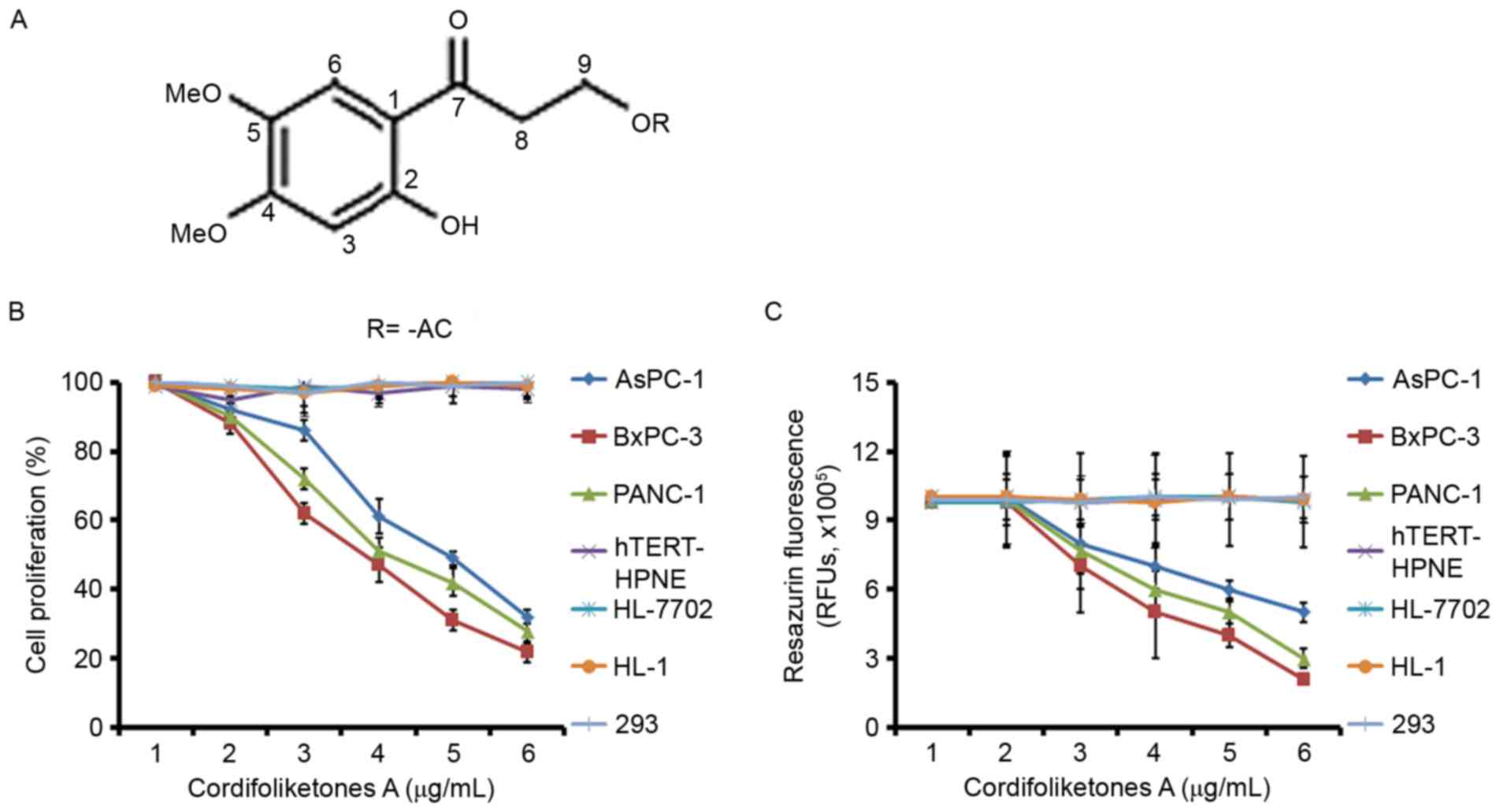
cordifoliketone A are presented in Fig. 1A and described as followed:
Obtained as pale yellow gum; UV (MeOH) λmax (log ε) 325 (2.42), 288
(4.22), 248 (3.12), 210 (4.89) nm; IR (KBr) νmax 3376, 2925, 2850,
1713, 1638, 1512, 1450, 1434, 1362, 1283, 1175, 1137, 1086, 1047,
971, 828 cm-1; 1H and 13C NMR data (C5D5N, 500 and 125 MHz,
respectively); positive ESIMS m/z 249 [M+Na]+; HRESIMS m/z 249.0746
[M+Na]+ (calculated 249.0739 for molecular formula determined as
C11H14NaO5). The cordifoliketones A was prepared and dissolved in
0.2% ethanol (v/v) in the present study.
Cell culture
Human PDAC cell lines AsPC-1, BxPC-3 and PANC-1, the
hTERT-HPNE and 293 normal human pancreatic cells, the HL-7702
hepatocyte cell line, and HL-1 cardiac myocytes were purchased from
Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
PDAC cell lines and hTERT-HPNE cells were cultured in specific
medium supplemented with 10% (v/v) fetal bovine serum (FBS) and 1%
antibiotics at 37°C in a humidified incubator under 5%
CO2 (3). The HL-7702
cells were cultured in RPMI 1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 8% dialyzed
FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) as
described previously (21). HL-1
cells were cultured in Claycomb medium supplemented with 10% FBS, 2
mM L-glutamine, 0.1 mM norepinephrine, 100 units/ml penicillin and
100 µg/ml streptomycin. The cells were cultured at 37°C and 5%
CO2. Twice a week, cells were split after reaching
confluence. 293a cells were grown in Dulbecco's modified Eagle's
medium supplemented with 10% FBS, 1% L-glutamine and 1%
penicillin-streptomycin stock solutions. All of the cells were
negative for: HIV-1, hepatitis B virus, hepatitis C virus as
detected by quantitative polymerase chain reaction; mycoplasma
using fluorescent monoclonal antibodies against major outer
membrane protein; bacteria under microscopy without in medium
antibiotics and varying pH values, yeast and fungi with a lack of
hyphae with microscopy prior to experimentation by the Cell Bank of
the Chinese Academy of Sciences (Shanghai, China).
Cytotoxicity assay
Resazurin reduction assay was performed to test the
cytotoxic effects of the natural product, cordifoliketone A, on
three PDAC cell lines (AsPC-1, BxPC-3 and PANC-1) and normal human
cells (hTERT-HPNE, HL-7702, HL-1 and 293 cells) as described before
(22,23). The contribution of the extracts (at
various tested concentrations, including 0, 0.5, 1, 1.5, 2, 2.5, 3,
3.5, 4, 4.5, 5, 5.5 and 6 µmol/ml, respectively) to the
fluorescence (Fs) was determined, both in the absence and presence
of resazurin, prior to any cell studies. A total of
2×104 cells/well were seeded in 96-well plates in a
total volume of 100 µl. Cordifoliketone A of varying concentrations
(0–6 µmol/ml) or the positive control Doxorubicin (3.5 µmol/ml),
was immediately added to additional 100 ml of culture medium to
obtain a total volume of 200 µl/well, respectively. After 24 h or
48 h, 20 ml resazurin (Sigma-Aldrich, Merck KGaA) 0.01% w/v in
ddH2O was added to each well and the plates were
incubated at 37°C for 4 h. Blank control were treated with 20 µl
ddH2O instead of resazurin. Fluorescence was measured on
a microplate reader using an excitation wavelength of 544 nm and an
emission wavelength of 590 nm. Doxorubicin was used as positive
control. After 72 h incubation and staining by resazurin
(Sigma-Aldrich, Merck KGaA), Fs was measured on a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, United States) using an
excitation wavelength of 544 nm and an emission wavelength of 590
nm. Each assay was performed at least three times, with six
replicates each. Half-maximal inhibitory concentration
(IC50) values represent the sample concentration
required to inhibit 50% of cell proliferation, and was calculated
from a calibration curve by linear regression as described
previously (24). The calculation
formula was as follows: Percentage (%) of cell proliferation=(Fs of
cells treated with cordifoliketone A/Fs of untreated cells)
×100.
Apoptosis assay
A propidium iodide (PI) and Annexin V-fluorescein
isothiocyanate-flow cytometry assay (BD Pharmingen; BD Biosciences,
Franklin Lakes, NJ, USA) was used to detect the apoptosis rate in
the AsPC-1, BxPC-3 and PANC-1 cells after treatment with various
concentrations of cordifoliketones A (0, 2, 4 or 6 µg/ml). In the 0
µmol/ml cordifoliketones A group, cells were treated with 0.2%
ethanol. Briefly, 1×106 cells per well were cultured in
6-well plates in the absence of 10% FBS for 48 h. Adherent cells
were detached with 0.25% trypsin without EDTA in 1X PBS. Cells were
harvested in complete RPMI 1640 medium and centrifuged at 350 × g
for 5 min. According to the manufacturer's protocol, each of the
cells were washed with 1X PBS and then stained with 50 ug/ml PI and
Annexin V-FITC at 25°C for 30 min (25).
Detection of apoptosis-associated
proteins by western blotting
The present study used western blotting to evaluate
the protein expression levels of caspase-3, caspase-8, caspase-9,
Bad, Bax, Bcl-2 and Bcl-xL in PDAC cell lines. Antibodies for
caspase-3 (sc-271759), caspase-8 (sc-5263), caspase-9 (sc-17784),
Bad (sc-8044), Bax (sc-20067), Bcl-2 (sc-509), Bcl-xL (sc-136207)
and GAPDH (sc-69778) were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). According to the manufacturer's protocols,
~40 mg total cell lysate protein [quantified with a Bicinchoinic
Acid Protein Assay kit (Beyotime Institute of Biotechnology,
Shanghai, China)] extracts from each cell line were separated using
15% SDS-PAGE and transferred to nitrocellulose membranes (Beyotime
Institute of Biotechnology). The membrane was blocked with PBS
containing 0.05% Tween-20 (PBST; (Beyotime Institute of
Biotechnology,) with 10% nonfat dry milk (Beyotime Institute of
Biotechnology) overnight at 4°C for 12 h and subsequently washed
three times for 10 min each time. The membrane was incubated with
monoclonal anti-Bax (1:500), anti-Bcl-2 (1:200), anti-Bcl-xL
(1:800), anti-Bad (1:800), and polyclonal anti-caspase-3 (1:1,000),
−8 (1:200), and −9 (1:500) antibodies at 4°C for 24 h. Following
three washes three times for 10 min each, the membrane was
incubated with a horseradish-peroxidase conjugated rabbit
anti-mouse IgG (1:2,500; Beyotime Institute of Biotechnology,
A0216) for 2 h at room temperature, and washing three times for 10
min each time. Then the membrane was developed in enhanced
chemiluminescence-based detection (GE Healthcare, Chicago, IL, USA)
as previously described (25). The
optical densities of the protein bands for the target proteins were
analyzed for each group in order to semi-quantity the protein
levels (26,27).
Cell migration and invasion assay
The present study determined the possible effect of
cordifoliketones A on the viability of PDAC cells. Following this,
the effect of cordifoliketones A on proliferation of PDAC cells
in vivo was also detected. BioCoat Matrigel invasion
chambers (BD Biosciences) were used to compare the effect of
cordifoliketones A treatment on in vitro invasion of AsPC-1,
BxPC-3 and PANC-1 cells, according to the manufacturer's protocol.
Briefly, for the invasion assay, Costar Transwell 8-µm inserts were
coated with 50 µg reduced serum Matrigel (BD Biosciences). Invasion
chambers were coated with Matrigel. AsPC-1, BxPC-3 and PANC-1 cells
(1×106) were treated with various concentrations of
cordifoliketones A (0.2% ethanol): 0, 2, 4 or 6 µg/ml were added
per chamber.
Dulbecco's modified Eagle's medium (BD Biosciences)
supplemented with 10% FBS was used in the lower chamber. Following
incubation, the cells that had invaded through the membrane were
fixed with polyoxymethylene at 25°C for 30 min (Sigma-Aldrich,
Merck KGaA) and stained with crystal violet (Sigma-Aldrich, Merck
KGaA), at room temperature for 0.5 h, before the membrane was
removed and mounted on a slide for microscopic assessment. Invasive
cells were visualized at ×40 magnification under a light microscope
(Leica Microsystems GmbH, Wetzlar, Germany) and the number of cells
in five random fields was counted and the average calculated
(average=sum number of cells in five random fields/five). For
migration assays, the same procedure was used excluding the
Matrigel.
After 12 h, non-invading cells and media were
removed, and cells on the lower surface of the membrane were fixed
with polyoxymethylene and stained with 0.1% crystal violet (both
from Sigma-Aldrich; Merck KGaA) for 0.5 h at room temperature.
Stained cells were counted under a microscope (Leica Microsystems
GmbH, inverted microscope, DMI3000B) in five randomly selected
fields, and the aforementioned average (as described above) was
used to indicate cell migration and invasion. All experiments were
performed in triplicate (8).
PDAC cells xenograft mouse model
A total of 36 BALB/c nude mice (female; age, 4–5
weeks; weight, 20–25 g; Beijing HFK Bioscience Co., Ltd., Beijing,
China; animal license no. SCXK 2009–000) were housed and raised in
the laboratory animal center of Yunnan University (Kunming, China).
The mice were maintained in a 20–25°C quiet, 40–60% humidified
vivarium under a 12 h light/dark cycle. They were allowed free
access to water and food prior to experimentation. The treatment
and use of animals during the study was approved by the Animal
Ethics Committee of Yunnan University.
Mice were randomly assigned to 6 groups (n=6/group):
Mice bearing human AsPC-1, BxPC-3 and PANC-1 cells alone (PDAC +
placebo groups, control), as well as mice bearing human AsPC-1,
BxPC-3 and PANC-1 cells and treatment with cordifoliketones A (PDAC
+ CA groups). In a preliminary experiment, different concentrations
(20, 80, 120, and 240 M/kg) of cordifoliketones A (ethanol extract,
80 M, supplied by Prof. Yu-Ming Chen of Shanghai Traditional
Medical University, Shanghai, China) were used to assess the
appropriate in vivo dose. IC50 values of
cordifoliketones A in PANC-1 cells were the highest among the three
PDAC cell lines in vitro. A volume of 100 µl of PANC-1 cells
suspended in PBS (1.0×107/ml) were injected
intradermally into the left axilla of the mice. On the following
day, mice bearing PDAC-1 cells received 20, 80, 120 or 240 M/kg
cordifoliketones A or an equal volume noncytotoxic placebo (0.2%
ethanol) treatment by gavage once a day. Following seeding, liquid
absorption at the injection site, tumor growth (volume and weight),
as well as mouse condition, status and survival were observed.
Tumor volume was measured on days 5, 7, 12, 16, 19, 21, 25 and 27
post-injection. A concentration of 240 M/kg was used for subsequent
experiments the most prominent effects in tumor growth inhibition.
A volume of 100 µl of each cell type suspended in PBS
(1.0×107/ml) was injected intradermally into the left
axilla of the mice. On the following day, mice bearing PDAC cells
received 240 M/kg cordifoliketones A or an equal volume 0.2%
ethanol (noncytotoxic placebo) treatment by gavage once a day.
After seeding, liquid absorption at the injection site, tumor
growth (volume and weight), and mouse survival were observed. Tumor
volume was measured on days 5, 7, 12, 16, 19, 21, 25 and 27
post-injection. On day 27, all mice were sacrificed, and tumors
were isolated for determination of weight and volume (28).
The largest (a) and smallest diameters (b) of each
tumor were measured twice on days 5, 7, 12, 16, 19, 21, 25 and 27
to estimate tumor volume (V) using the formula V=0.52 ×
a2 × b (31). Mean
tumor volumes were used to plot tumor growth curves for each group
of mice.
Statistical analysis
SPSS v13.0 (SPSS Inc., Chicago, IL, USA) was used
for statistical analysis. Data are presented as means ± standard
deviation. One-way analysis of variance with five levels was used
with a completely randomized design, and the homogeneity of
variance was tested. A q test (Student-Newman-Keuls) was used to
compare the differences between groups, and a rank sum test was
performed to randomly compare treatments. P<0.05 was considered
to indicate a statistically significant difference.
Results
Cytotoxic of cordifoliketones A on
PDAC cells
The cytotoxic abilities against AsPC-1, BxPC-3,
PANC-1 and normal human cells by resazurin reduction assay (with
doxorubicin as the positive control) are presented in Table I. The structure of cordifoliketones
A extracted from the roots of C. cordifolioidea is presented
in Fig. 1A. The results
demonstrated that cordifoliketones A has significant potential
cytotoxic abilities against various PDAC cell lines in a
dose-dependent manner, with IC50 values ranging from
4.18 to 5.56 µg/ml (Table I). The
lowest IC50 value of 4.18 µg/ml was obtained by treating
PANC-1 cells with cordifoliketones A. Compared to with PDAC cells,
normal human hTERT-HPNE cells, and 293, HL-7702 and HL-1 cells were
relatively resistant to cordifoliketones A (IC50 was
>6 µg/ml, the highest concentration tested in the study), which
implies the specific anticancer activities of cordifoliketones A
(Fig. 1B and C). Treatment with
cordifoliketones A (>3 µg/ml) revealed a significant inhibition
in cell viability of PDAC cells. In addition, cordifoliketones A
significantly reduced the resazurin fluorescence values from 3–6
µg/ml.
 | Table I.Cytotoxic effects of compounds on a
variety of pancreatic ductal adenocarcinoma cells. |
Table I.
Cytotoxic effects of compounds on a
variety of pancreatic ductal adenocarcinoma cells.
|
| PDAC cell lines
(IC50 µg/ml) |
|---|
|
|
|
|---|
| Compounds | AsPC-1 | BxPC-3 | PANC-1 |
|---|
| Cordifoliketones
A | 5.56±0.22 | 4.26±0.11 | 4.18±0.31 |
| Doxorubicin
(µM) | 2.86±0.21 | 2.69±0.14 | 3.49±0.18 |
Cordifoliketones A treatment induces
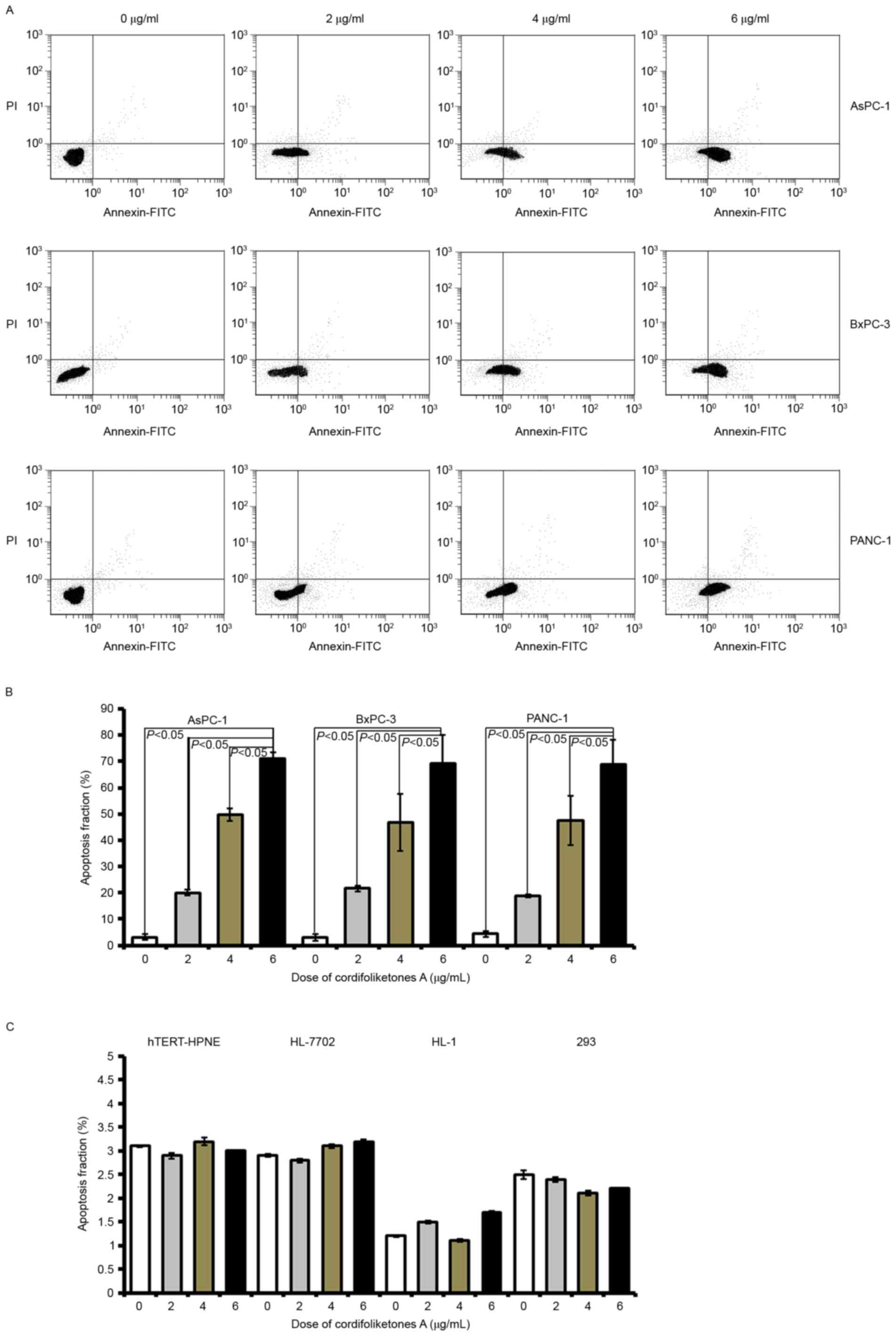
apoptosis of PDAC cells
In the three types of PDAC cells, there was a
significant increase in the apoptosis rate after treatment with 2
µg/ml cordifoliketones A, compared with 0 µg/ml (blank control;
Fig. 2). In AsPC-1, BxPC-3 or
PANC-1 cells, cordifoliketones A increased the apoptosis rate in a
dose-dependent manner. There were more apoptotic PDAC cells in the
6 µg/ml cordifoliketones A-treated groups, when compared with that
the 2 and 4 µg/ml treated groups, respectively (P<0.05, Fig. 2). However, cordifoliketones A
exhibited no significant effects on the apoptosis of normal human
cell lines (Fig. 2C,
P>0.05).
Effects of cordifoliketones A on the
expression of apoptosis-associated proteins
Quantitative analysis by western blotting
demonstrated that cordifoliketones A upregulated the expression of
Bax and Bad, and down-regulated the expression of Bcl-2 and Bcl-xL
in AsPC-1 (Fig. 3), BxPC-3
(Fig. 4) and PANC-1 (Fig. 5) cells in a dose-dependent manner
(P<0.05). For the other apoptosis-associated proteins tested,
caspase-3/8/9 expression were significantly upregulated after
treatment with 2, 4 or 6 µg/ml cordifoliketones A in AsPC-1, BxPC-3
and PANC-1 cells (P<0.05, Figs.
3, 4 and 5, respectively).
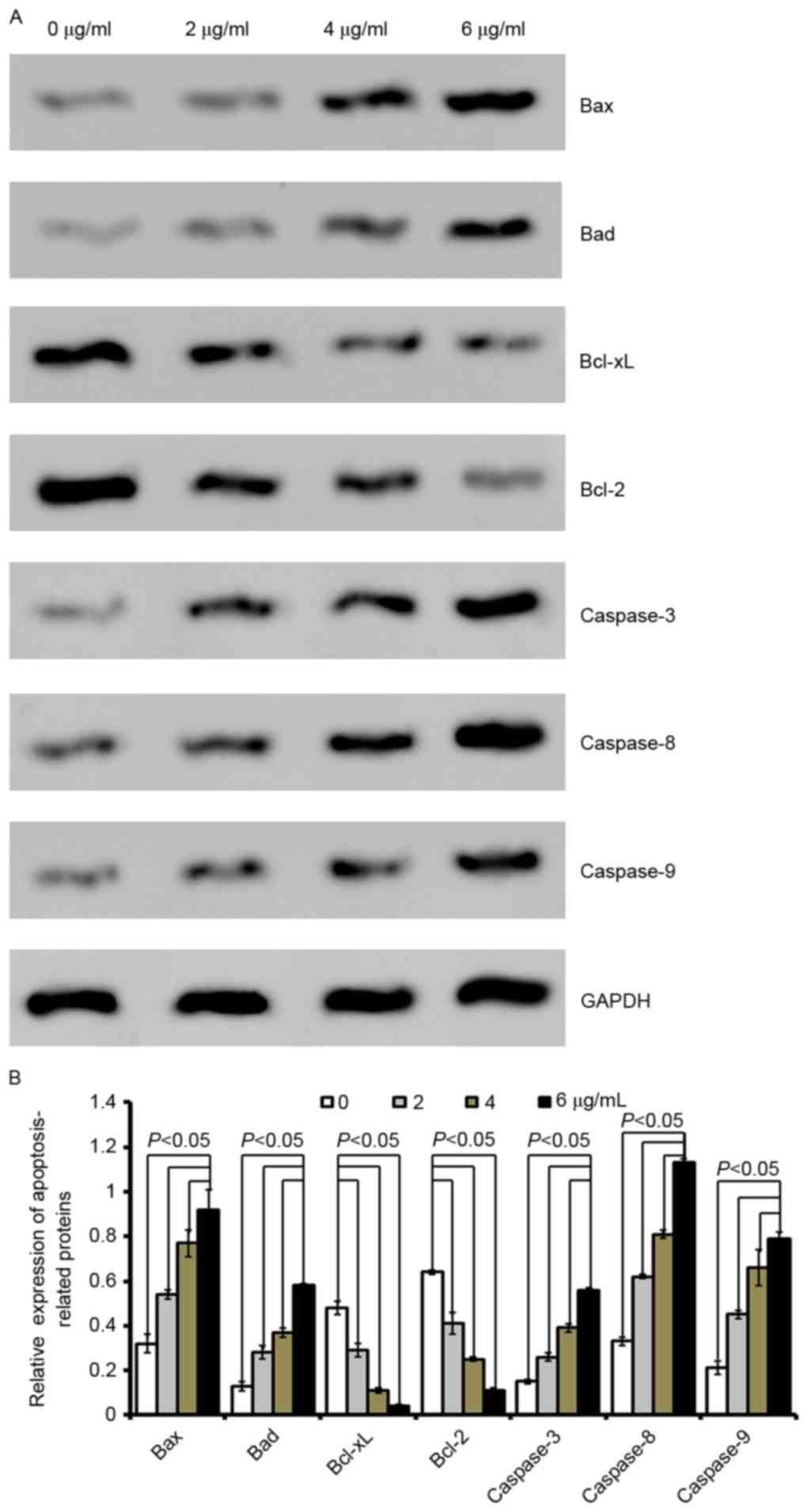 | Figure 3.Cordifoliketones A affects the
expression of apoptosis-associated proteins in AsPC-1 cells. (A)
Representative western blot images and (B) quantification of
protein expression levels of Bax, Bad, Bcl-xL, Bcl-2, caspase-3,
caspase-8 and caspase-9 following cordifoliketones A treatment (0,
2, 4, and 6 µg/ml). Average values are from three independent
experiments performed in duplicate. Data are presented as the mean
± standard deviation (n=3). Bax, Bcl-2-associated X protein; Bad,
Bcl-2-associated death promoter; Bcl-XL, B-cell lymphoma
extra-large; Bcl-2, B-cell lymphoma 2. |
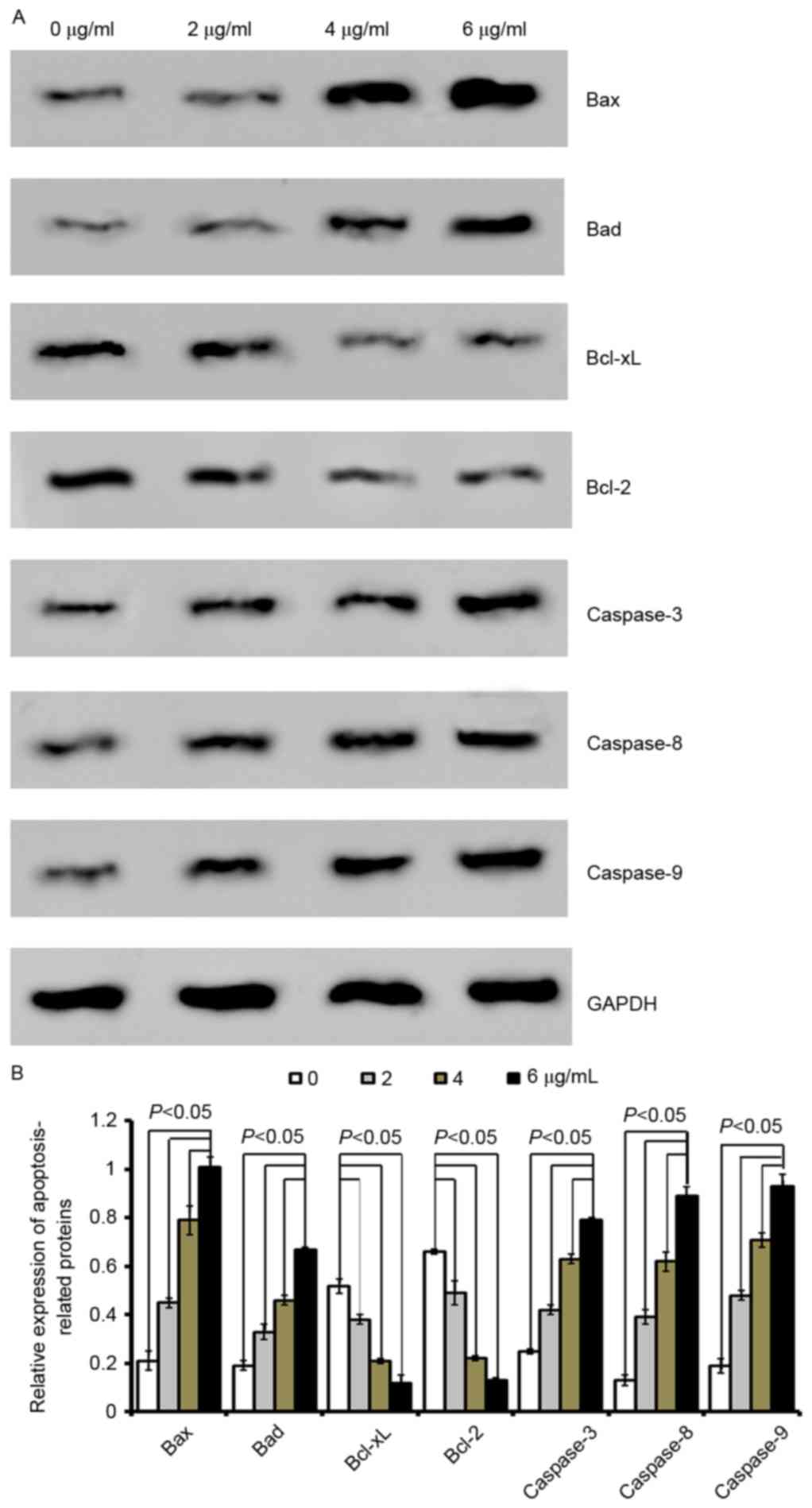 | Figure 4.Cordifoliketones A affects the
expression of apoptosis-associated proteins in BxPC-3 cells. (A)
Representative western blot images and (B) quantification of
protein expression levels of Bax, Bad, Bcl-xL, Bcl-2, caspase-3,
caspase-8 and caspase-9 following cordifoliketones A treatment (0,
2, 4, and 6 µg/ml). Average values are from three independent
experiments performed in duplicate. Data are presented as the mean
± standard deviation (n=3). Bax, Bcl-2-associated X protein; Bad,
Bcl-2-associated death promoter; Bcl-XL, B-cell lymphoma
extra-large; Bcl-2, B-cell lymphoma 2. |
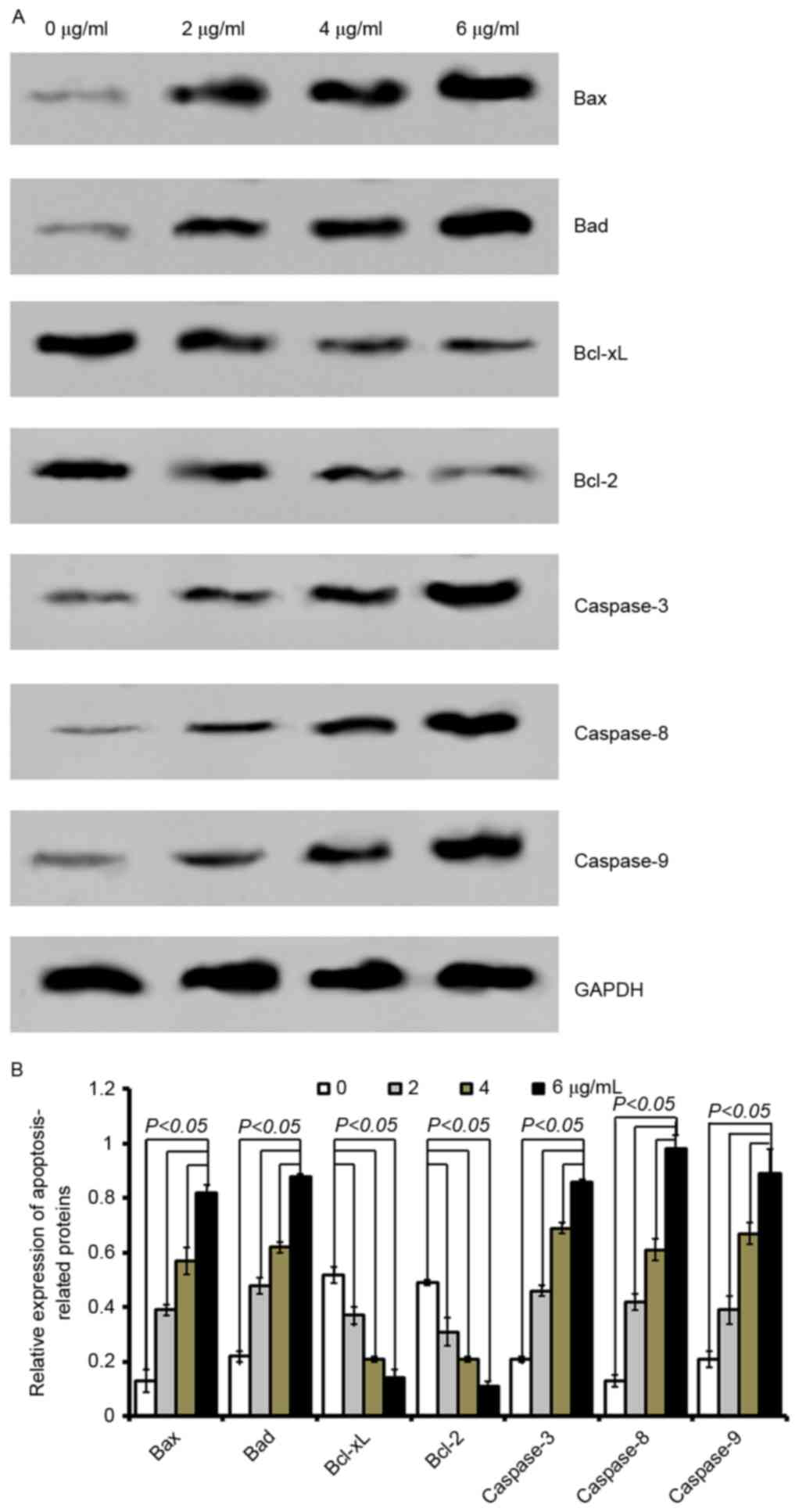 | Figure 5.Cordifoliketones A affects the
expression of apoptosis-associated proteins in PANC-1 cells. (A)
Representative western blot images and (B) quantification of
protein expression levels of Bax, Bad, Bcl-xL, Bcl-2, caspase-3,
caspase-8 and caspase-9 following cordifoliketones A treatment (0,
2, 4, and 6 µg/ml). Average values are from three independent
experiments performed in duplicate. Data are presented as the mean
± standard deviation (n=3). Bax, Bcl-2-associated X protein; Bad,
Bcl-2-associated death promoter; Bcl-XL, B-cell lymphoma
extra-large; Bcl-2, B-cell lymphoma 2. |
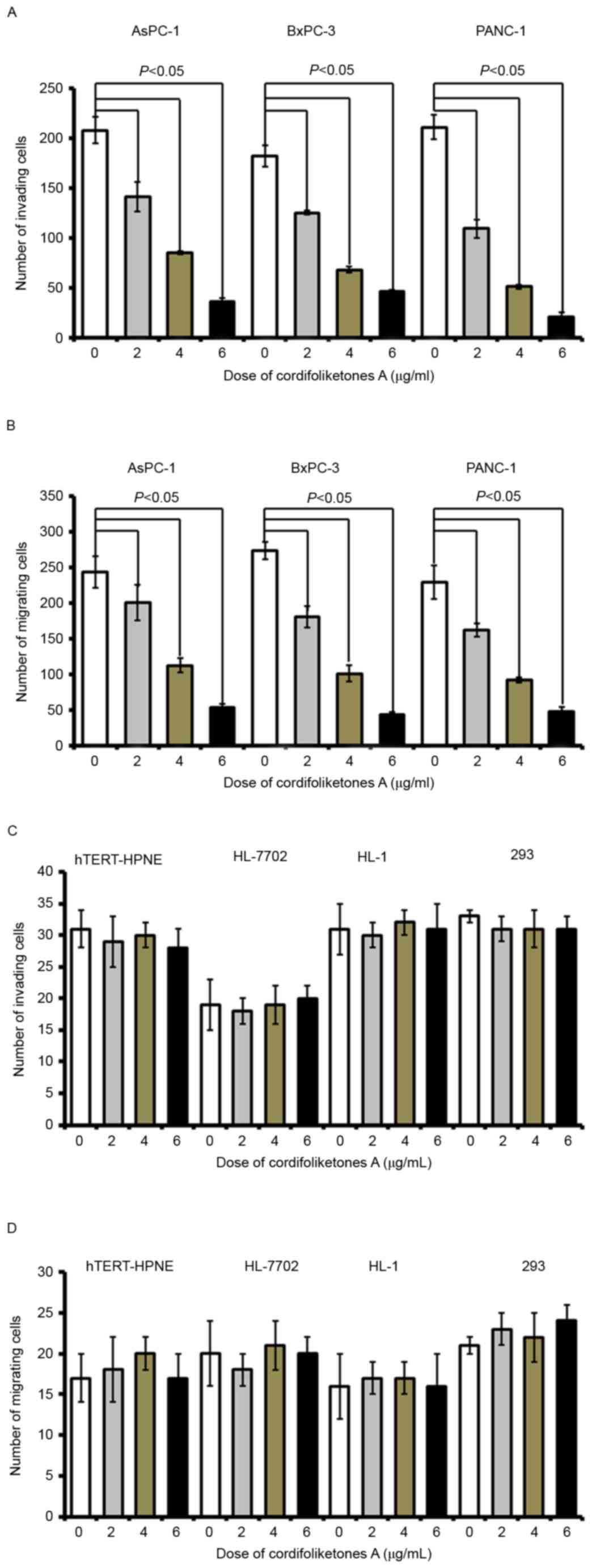
Cordifoliketones A inhibits migration
and invasion of PDAC cells
Following treatment with cordifoliketones A at 2, 4
or 6 µg/ml, there were significant reductions in the invasion of
AsPC-1, BxPC-3 and PANC-1 cells, in comparison with that of the
control cells (treated with 0 µg/ml; P<0.05; Fig. 6). Furthermore, cordifoliketones A
induced inhibitions in migration and invasion of PDAC cells in a
dose-dependent manner (Fig.
6).
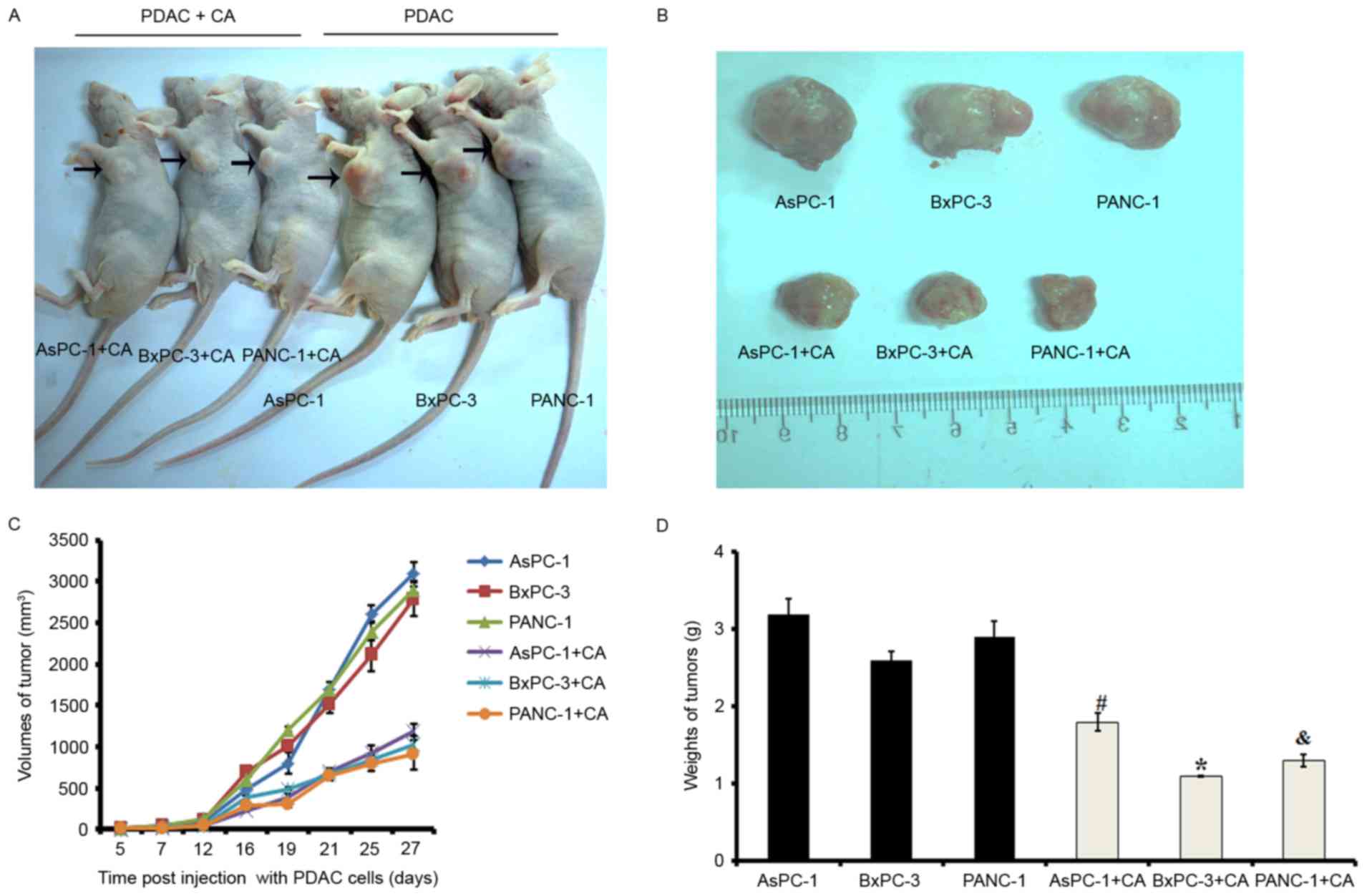
Cordifoliketones A treatment inhibits
the proliferation of PDAC in vivo
To explore the efficacy of cordifoliketones A
against PDAC in vivo, the xenograft models were established
via subcutaneous injection of cells, and tumor growth was
monitored. On day 27 post-injection, mice were sacrificed and the
tumors were dissected and weighed. Neoplasms in cordifoliketones
A-treated mice grew much more slowly than that in control mice
(Fig. 7).
Discussion
In present study, cordifoliketones A was extracted
from the roots of C. cordifolioidea, Tsoong. The effects of
cordifoliketones A on tumor cell growth, apoptosis, invasion, and
migration were examined. The data demonstrated that
cordifoliketones A: i) inhibited proliferation and promoted
apoptosis of PDAC cells without cytotoxicity against to normal
human cells; ii) significantly induced apoptosis and altered
expression of apoptosis-associated proteins in a dose-dependent
manner; iii) suppressed migration and invasion of PDAC cells in a
dose-dependent manner; and iv) restrained the growth of PDAC
neoplasms in nude mice. Therefore, these results indicate that
cordifoliketones A may be a potential candidate compound for the
prevention of PDAC proliferation and metastasis.
Natural products have been used in traditional and
folk medicine for therapeutic purposes. They are generally nontoxic
compared to synthetic chemical compounds, and thus provide
important sources of promising leads for the development of novel
therapeutic drugs (30). In this
regard, the search for novel chemopreventive and anti-tumor agents
that are more effective and less toxic has created a great deal of
interest in phytochemicals (30).
The roots of C. cordifolioidea, locally known as Tsoong,
have been used as a food or vegetable and herbal remedies due to
their tonic effects in Yunnan Province since ancient times
(17,30). Recently, phytochemical research
also revealed that the crude extract of Tsoong increased the
chemotherapeutic sensitivities, and attenuated and enhance immune
function of tumor-bearing mice (31). In light of this, the major
components from this plant were isolated and identified. In
particular, cordifoliketones A, as one of compounds of the roots of
Tsoong, was isolated and demonstrated to have anti-HIV and
cytotoxic properties (13). The
present study demonstrated that the cytotoxic properties against
PDAC cells of cordifoliketones A might be associated with apoptosis
promotion and proliferation inhibition.
Disordered apoptosis is important in many disease
processes, including oncologic and rheumatologic phenomena, and
thus the accurate control of apoptosis is important not just to the
normal development of an organism, but also to its health and
growth (32). Therefore, various
kinds of agents that can effectively induce apoptosis offer
promising strategies for the treatment of cancer. The present study
revealed that treatment with cordifoliketones A significantly
induced apoptosis and altered the expression of
apoptosis-associated proteins in a dose-dependent manner in PDAC
cells. Cordifoliketones A was cytotoxic to AsPC-1, BxPC-3 and
PANC-1 cells with an IC50 at most of 5.56 µmol/ml
(Table I). This cytotoxicity may
be attributable to the induction of apoptosis, as demonstrated by
an apoptosis assay and expressional evaluation of proteins
associated with apoptosis. The Ced-3/caspase-1 family, which is
comprised of caspase-1, caspase-2, caspase-3, caspase-4, caspase-6,
caspase-7, caspase-9 and caspase-10, functions as a key component
of apoptosis, and acts to destroy specific target proteins which
are critical to cellular longevity (33). As an initiator caspase, caspase-8
activates effector caspases by cleaving their inactive forms. In
the activation cascade responsible for apoptosis induced by tumor
necrosis factor receptor superfamily member 1A and mediated by
tumor necrosis factor receptor superfamily member 6, caspase-8 is
the most upstream protease. B-cell lymphoma 2 (Bcl-2) among
numerous key regulators of apoptosis, are essential for
development, tissue homeostasis and protection against foreign
pathogens. Human Bcl-2 is an anti-apoptotic, membrane-associated
oncoprotein that can promote cell survival via protein-protein
interactions with other Bcl-2-associated family members, such as
the death suppressors B-cell lymphoma extra-large (Bcl-xL), induced
myeloid leukemia cell differentiation protein (MCL-1), Bcl-2-like
protein 2 and A1, or the death agonists Bcl-2-associated X protein
(Bax), Bcl-2 homologous antagonist/kiler, Bcl-2 interacting killer,
Bcl-2-associated death promoter (Bad) and BH3-interacting domain
death agonist (33,34).
Cordifoliketones A upregulated the expression of
Bax, Bad and caspase-3/8/9, and downregulated the expression of
Bcl-2 and Bcl-xL. As a complex biochemistry process, apoptosis is
regulated by a variety of factors (35,36).
The two most important groups of proteins involved in apoptotic
cell death has been demonstrated as members of the Bcl-2 family and
caspases (37,38). It is well known that the Bcl-2
family functions as inhibitors (e.g. Bcl-2, Bcl-xL and MCL-1) or
promoting factors (e.g. Bax, Bcl-XS, Bad and Bak) in programmed
cell death, apoptosis. Otherwise, subsequent activation of
caspase-3 has been regarded as a primary mechanism of apoptosis
(38,39). Given this knowledge, it was
hypothesized that cytotoxic properties against PDAC cells of
cordifoliketones A might be associated with induction apoptosis.
Additionally, chemical agents with strong apoptosis-inducing
activity but minimal toxicity are expected to have potential
utility as anticancer drugs (22,40).
The present study additionally revealed that cordifoliketones A
exhibited no cytotoxicity to normal hTERT-HPNE cells, which
suggested that cordifoliketones A might be a promising anticancer
drug against PDAC. Furthermore, the present data also demonstrated
that cordifoliketones A treatment cells inhibited the migration and
invasion of PDAC cells in a dose-dependent manner. Therefore, the
results indicated that cordifoliketones A might prevent of PDAC
metastasis by inhibiting in invasion and migration.
Taking together, these results indicate that
cordifoliketones A may be a potential candidate compound for the
prevention of PDAC proliferation and metastasis, presumably by
induction apoptosis, inhibiting proliferation, invasion and
migration of PDAC cells, as well as minimal toxicity to normal
cells. However, the mechanisms underlying remain unknown and
require further investigation.
In conclusion, the present study demonstrated that
cordifoliketones A prepared from the roots of C.
cordifolioidea, Tsoong: i) inhibited proliferation and promoted
apoptosis of PDAC cells; ii) significantly induced apoptosis and
altered expression of apoptosis related protein in a dose-dependent
manner; iii) suppressed migration and invasion of PDAC cells in a
dose-dependent manner; and iv) restrained the growth of PDAC
neoplasm in nude mouse. Furthermore, cordifoliketones A exhibited
non-cytotoxic activity in normal human cells. Therefore, these
results indicated that cordifoliketones A may be a potential
candidate compound for the prevention of PDAC proliferation and
metastasis, presumably by induction of apoptosis, and inhibiting
invasion and migration of PDAC cells.
Acknowledgements
The present study was supported by Yunnan Provincial
Department of Education (grant no. 2015Y288).
References
|
1
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: Current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hariharan D, Saied A and Kocher HM:
Analysis of mortality rates for gallbladder cancer across the
world. HPB (Oxford). 10:327–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang SH, He P, Ma MZ, Wang Y, Li RK, Fang
F, Fu Y, Tian GA, Qin WX and Zhang ZG: PNMA1 promotes cell growth
in human pancreatic ductal adenocarcinoma. Int J Clin Exp Pathol.
7:3827–3835. 2014.PubMed/NCBI
|
|
4
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molejon MI, Tellechea JI, Moutardier V,
Gasmi M, Ouaissi M, Turrini O, Delpero JR, Dusetti N and Iovanna J:
Targeting CD44 as a novel therapeutic approach for treating
pancreatic cancer recurrence. Oncoscience. 2:572–575. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W, Yao JL, Dong SC, Hou FQ and Shi
HP: SLPI knockdown induced pancreatic ductal adenocarcinoma cells
proliferation and invasion. Cancer Cell Int. 15:372015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee YJ, Kim DB, Lee JS, Cho JH, Kim BK,
Choi HS, Lee BY and Lee OH: Antioxidant activity and
anti-adipogenic effects of wild herbs mainly cultivated in Korea.
Molecules. 18:12937–12950. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xin T, Zhang F, Jiang Q, Chen C, Huang D,
Li Y, Shen W, Jin Y and Sui G: The inhibitory effect of a
polysaccharide from Codonopsis pilosula on tumor growth and
metastasis in vitro. Int J Biol Macromol. 51:788–793. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin JA, Kim JS, Hong IS and Cho SD: Bak
is a key molecule in apoptosis induced by methanol extracts of
Codonopsis lanceolata and Tricholoma matsutake in HSC-2 human oral
cancer cells. Oncol Lett. 4:1379–1383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huo J, Qin F, Cai X, Ju J, Hu C, Wang Z,
Lu W, Wang X and Cao P: Chinese medicine formula ‘Weikang Keli’
induces autophagic cell death on human gastric cancer cell line
SGC-7901. Phytomedicine. 20:159–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu QF, Xuesen Li XS, Huang HT, Mu HX, Tu
PF and Li GP: Phenylpropanoids from the roots of codonopsis
cordifolioidea and their biological activities. Bull Korean Chem
Soc. 33:278–280. 2012. View Article : Google Scholar
|
|
14
|
Hong DY, Lian YS and Shen LD: Flora of
China. Science Press: Beijing. 73:321983.
|
|
15
|
Yunnan Corporation of Materia Medica, .
List of Chinese Herb Medicine Resources in Yunnan. Science
Publishing; pp. 5441993
|
|
16
|
Duang QF, Zhao H and Wang YQ: The
cytotoxicity of Nature medicine in Yunnan province. Chin J Yunnan
Med. 12:392003.
|
|
17
|
Chen ZJ, Wei QH and Zhou JY: The effects
of Codonopis bulleynana on the growth and proliferation of HL60
cells. Yunnan J Tradit Chin Med Mater. 27:492006.
|
|
18
|
Sun J, Wang L, Wang M, Wang Z and Li F:
Two new polyacetylene glycosides from the roots of Codonopsis
tangshen Oliv. Nat Prod Res. 30:2338–2343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen ZJ, Wei QH and Zhou JY: The research
development in Tsoong. Yunnan J Tradit Chin Med Mater. 27:49–50.
2006.
|
|
20
|
Luan YP, Zheng SQ and Li YM:
Identification of active ingredient of codonopsis cordifolioidea by
n-butyl alcohol. Adv Eng Res. 178–182. 2016.
|
|
21
|
Li C, Ni J, Liu YX, Wang H, Liang ZQ and
Wang X: Response of MiRNA-22-3p and MiRNA-149-5p to Folate
Deficiency and the Differential Regulation of MTHFR Expression in
Normal and Cancerous Human Hepatocytes. PLoS One. 12:e01680492017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee KW, Jung HJ, Park HJ, Kim DG, Lee JY
and Lee KT: Beta-D-xylopyranosyl-(1→3)-beta-D-glucuronopyranosyl
echinocystic acid isolated from the roots of Codonopsis lanceolata
induces caspase-dependent apoptosis in human acute promyelocytic
leukemia HL-60 cells. Biol Pharm Bull. 28:854–859. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuete V, Fankam AG, Wiench B and Efferth
T: Cytotoxicity and modes of action of the methanol extracts of six
Cameroonian medicinal plants against multidrug-resistant tumor
cells. Evid Based Complement Alternat Med. 2013:859032013.
View Article : Google Scholar
|
|
24
|
Kuete V, Tankeo SB, Saeed ME, Wiench B,
Tane P and Efferth T: Cytotoxicity and modes of action of five
Cameroonian medicinal plants against multi-factorial drug
resistance of tumor cells. J Ethnopharmacol. 153:207–219. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuete V, Sandjo LP, Mbaveng AT, Seukep JA,
Ngadjui BT and Efferth T: Cytotoxicity of selected Cameroonian
medicinal plants and Nauclea pobeguinii towards multi-factorial
drug-resistant cancer cells. BMC Complement Altern Med. 15:3092015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Skup M, Dwornik A, Macias M, Suleczak D,
Wiater M and Czarkoeska-Bauch J: Long-term locomotor training
upregulates TrkBFL receptor-like proteins, brain-derived
neurotrophic factor, and neurotrophin 4 with different topographies
of expression in oligodendroglia and neurons in the spinal cord.
Exp Neurol. 176:289–307. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin DX, Zou XL, Luo W, Zhang W, Zhang HT,
Li XL, Zhang H, Wang XY and Wang TH: Expression of some
neurotrophins in the spinal motoneurons after cord hemisection in
adult rats. Neurosci Lett. 410:222–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu T, Zhang C, Tang Q, Su Y, Li B, Chen L,
Zhang Z, Cai T and Zhu Y: Variant G6PD levels promote tumor cell
proliferation or apoptosis via the STAT3/5 pathway in the human
melanoma xenograft mouse model. BMC Cancer. 13:2512013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niu G, Heller R, Catlett-Falcone R,
Coppola D, Jaroszeski M, Dalton W, Jove R and Yu H: Gene therapy
with dominant-negative Stat3 suppresses growth of the murine
melanoma B16 tumor in vivo. Cancer Res. 59:5059–5063.
1999.PubMed/NCBI
|
|
30
|
Wang L, Xu ML, Hu JH, Rasmussen SK and
Wang MH: Codonopsis lanceolata extract induces G0/G1 arrest and
apoptosis in human colon tumor HT-29 cells-involvement of ROS
generation and polyamine depletion. Food Chem Toxicol. 49:149–154.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu ZY: Herbal in Yunnan. Science Press:
Beijing. 5:4871991.
|
|
32
|
Chen ZJ, Li YS, Wei QH, Chen SL and Chen
DX: Efects of Codonopis bulleynana Forest ex Diels on enhancing
sensitivity, reducing toxicity of chemotherapy and regulating
immune function in Sarcoma 180 tumor-bearing mice. Chin Trad Patent
Med. 34:1848–1851. 2012.
|
|
33
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hockenbery D, Nuñez G, Milliman C,
Schreiber RD and Korsmeyer SJ: Bcl-2 is an inner mitochondrial
membrane protein that blocks programmed cell death. Nature.
22:334–336. 1990. View Article : Google Scholar
|
|
35
|
Vaux D and Korsmeyer S: Cell death in
development. Cell. 96:245–254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qiang FX and Guo YJ: Apoptosis in
oncology. Cell Res. 11:1–7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Korsmeyer SJ: BCL-2 gene family and the
regulation of programmed cell death. Cancer Res. 59:1693–1700.
1999.
|
|
39
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem. J. 326:1–16. 1997.
|
|
40
|
Nagata S: Apoptosis by death factor. Cell.
88:355–365. 1997. View Article : Google Scholar : PubMed/NCBI
|