Introduction
Coronary heart disease (CHD) is a type of
cardiovascular disease causing by coronary artery atherosclerosis
and plaque rupture, leading to the arterial stenosis or occlusion,
then resulting in myocardial ischemia or necrosis, which is among
the top ten causes of human mortality. At present, the treatment
methods of CHD include: Surgical treatment, interventional therapy
(stent implantation) and medication (nitric acid ester, β-blocker,
antithrombotic and statins); however, medication is the basis of
all treatment (1). With the
improvement of the living standards, the incidence and mortality
rates of CHD has increased rapidly, particularly within young
people (≤40 years old) in China (2,3). The
earlier incidence of CHD is not only threat to human health, but
may burden a patient's families and society; CHD has gained
increasing attention of researchers (4). It is well-known that smoking,
drinking, obesity, hypertension and metabolic syndrome are risk
factors for CHD. For smokers, nicotine in tobacco may damage the
vascular endothelium, resulting in the vasospasm and plaques
rupture, accelerating the onset of coronary atherosclerosis
(5). In addition, previous studies
have demonstrated that the functional disturbance of endothelial
cells and early-stage atherosclerosis may be caused by reduced
levels of nitric oxide (NO), nitric oxide has an important role in
the pathogenesis of CHD (6–8).
Endothelial NO synthase (eNOS) is the enzyme responsible for the
generation of NO in endothelial cell (9–11).
Various studies have investigated the association between the eNOS
G894T polymorphism and the risks of CHD (12–15).
However, to the best of our knowledge, the association between eNOS
and the risk of CHD in young people is yet to be established.
Therefore, in the present study, the association between the eNOS
G894T polymorphism and CHD in young individuals, and the influence
of the polymorphism on the protein structure and function, were
analyzed and the potential biological mechanisms were preliminarily
investigated by a series of bioinformatics analyses.
Materials and methods
Study subjects
A total of 234 cases of young patients with CHD, who
received treatment between August 2013 and May 2016 at the
Department of Cardiology, The First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China) were included in the CHD
group, which comprised 198 males and 36 females between the ages of
18 and 40 years old with a mean age of 34.2±3.7 years old. All
cases were diagnosed with CHD through a coronary angiogram and
complied with the following CHD diagnostic criteria specified by
the World Health Organization (16): Exhibited symptoms typical of
angina; an electrocardiogram identified the previous occurrence of
a myocardial infarction; and confirmation by angiography, which is
considered the ‘gold standard’ for the assessment of coronary
artery disease. A comprehensive individualized treatment program
should be applied to suit the circumstances of each patient. A
total of 228 healthy individuals between the ages of 18 and 40
years old who visited the hospital for physical examination and
voluntarily agreed to participate in the study during the same
period were randomly collected as the control group, which
comprised 195 males and 33 females with a mean age of 35.7±2.6
years old. All of the participants lived in the Henan province of
China and had no blood relationship with each other. The present
study was reviewed and approved by the Ethical Inspection Committee
of Zhengzhou University and participants in the CHD and control
groups volunteered and signed the informed content.
Identifying genotypes by polymerase
chain reaction-restriction fragment length polymorphism
(PCR-RFLP)
Peripheral blood (4 ml) was collected from each
participant, 0.6 mg EDTA-Na2 was used as an
anticoagulant (final concentration was 1.5 g/l) and the genomic DNA
was extracted using a Genelute™ blood genomic DNA kit
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) according to the
manufacturer's protocols. The diagnostic primer for the genotype of
the G894T polymorphism was synthesized by Sangon Biotech Company
(Shanghai, China): Sense, 5′-GAGATGAAGGCAGGAGACAGT-3′; and
anti-sense, 5′-TCCATCCCACCCAGTCAAT-3′. The PCR reaction system (25
µl) constituted: Genomic DNA 1.0 µl, 10× buffer 2.5 µl, dNTP Mix
2.0 µl, sense primer 0.2 µl, anti-sense primer 0.2 µl, Taq DNA
polymerase 0.1 µl and deionized water 19 µl. The amplification
procedure: pre-denaturation at 95°C or 2 min; 35 cycles of
denaturation at 95°C for 30 sec, annealing at 58°C for 30 sec and
extension for 30 sec at 72°C and final extension at 72°C for 5 min.
The PCR products were purified with Gel PCR purification kit
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocols. PCR-amplified specific fragments of eNOS
were obtained. Amplified products (10 µl) were digested with 0.5 µl
restriction endonuclease Eco24I (Ban II) (Takara, Japan) at 37°C
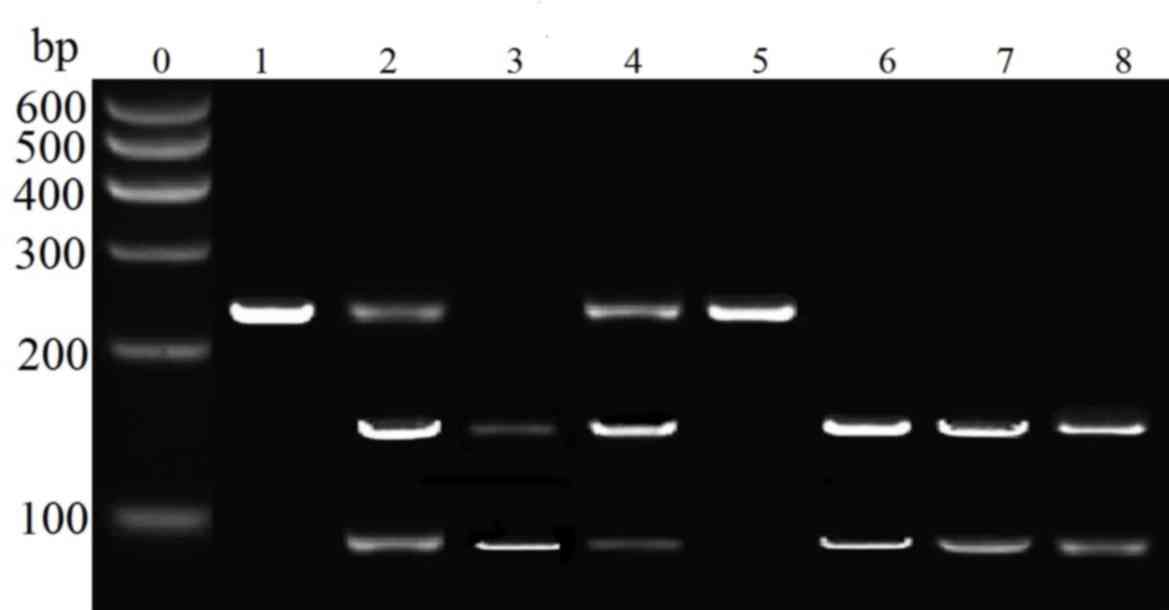
for 10 h. The results of enzyme digestion was performed by agarose
gel electrophoresis (Fig. 1) and
the results demonstrated that wild-type homozygote GG contained 169
and 94 bp fragments, variant homozygote TT contained a single 263
bp fragment and heterozygote GT contained 263 and 169 and 94 bp
fragments. The G8494T polymorphism from individuals of the present
study had been sequence by PCR-RFLP; in the GG genotype, the G849T
locus was at a guanine nucleotide, which could be digested by
Eco24I. Within the TT genotype, the G849T locus was at a thymine
nucleotide; however, within the GT type, the G849T locus was a
guanine or thymine nucleotide.
Bioinformatics analysis on the
polymorphism site
Alterations in the amino acid sequences of the eNOS
G894T gene polymorphism were analyzed by the Single Nucleotide
Polymorphisms and open reading frame databases (National Center for
Biotechnology Information (NCBI); https://www.ncbi.nlm.nih.gov/snp/; https://www.ncbi.nlm.nih.gov/orffinder/,
respectively), and alterations in the protein structure prior to
and following the amino acid sequence mutation were analyzed using
the protein modeling tool, QUARK (17). Amino acid sequences consisting of
20 amino acids around the G894T polymorphism site were entered into
the QUARK tool to perform local structural modeling of the protein,
and structural characteristics prior to and following the mutation
were analyzed and compared by Cn3D 4.3.1 viewer software (NCBI;
http://www.ncbi.nlm.nih.gov).
Characteristics of the eNOS structural domain were analyzed by
SMART v7 (18) and the NCBI
Conserved Domain Database (CDD) (19), and the potential biological
functions of the region where the polymorphism sites were located
was discussed. In addition, the potential eNOS signaling pathway
and the effects of structural changes on the pathway activity were
analyzed by using the Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway database (20)
(http://www.genome.jp/kegg/pathway.html).
Statistical analysis
All data were analyzed by SPSS 19.0 (IBM Corp.,
Armonk, NY, USA). Data are presented as the mean ± standard
deviation, and the comparisons between two groups were performed by
Student's t-test. The comparison of enumeration data in two groups
(including sex, smoking, hypertension and genotype, Tables I, II and III) was performed by Chi-squared
(χ2) test. P<0.05 was considered to indicate a
statistically significant difference. Hardy-Weinberg equilibrium
was used to analyze whether samples in each group were
representative of the population and P>0.05 was considered to
indicate that the sample was representative. The differences in the
distribution of genotype and alleles between the two groups were
compared by the χ2 test and binary logistic regression
was performed to analyze the association between genotype and
disease risk, and the odds ratio (OR) value was presented to
indicate the relative risk.
 | Table I.Comparison of subject characteristics
between the CHD and control groups. |
Table I.
Comparison of subject characteristics
between the CHD and control groups.
| Characteristic | CHD group
(n=234) | Control group
(n=228) | P-value |
|---|
| Age, years | 34.2±3.7 | 35.7±2.6 | 0.73 |
| Sex |
|
| 0.84 |
| Male | 198 | 195 |
|
|
Female | 36 | 33 |
|
| BMI,
kg/m2 | 25.1±3.2 | 24.6±3.4 | 0.41 |
|
Smoking | 37 | 34 | 0.69 |
|
Hypertension | 47 | 51 | 0.52 |
|
Diabetes | 24 | 22 | 0.61 |
| Family
history of CHD | 39 | 24 | 0.23 |
| Medication |
|
|
|
| Nitric
acid ester | 60 | NA | NA |
|
β-blocker | 72 | NA | NA |
|
Antithrombotic | 63 | NA | NA |
|
Statins | 87 | NA | NA |
|
Other | 28 | NA | NA |
 | Table II.Analysis of the distribution of
genotype frequency by the Hardy-Weinberg equilibrium. |
Table II.
Analysis of the distribution of
genotype frequency by the Hardy-Weinberg equilibrium.
| A, Comparison of
actual and theoretical genotype frequencies in the CHD group |
|---|
|
|---|
|
| Genotype |
|
|---|
|
|
|
|
|---|
| Actual/theoretical
genotype frequency | GG | GT | TT | Comparison |
|---|
| Actual frequency
(n) | 67.1 (157) | 29.1 (68) | 3.8 (9) |
χ2=0.160, |
| Theoretical
frequency | 66.7 | 30.0 | 3.3 | P=0.923 |
|
| B, Comparison of
actual and theoretical genotype frequencies in the control
group |
|
|
|
Genotype |
|
|
|
|
|
|
Actual/theoretical genotype
frequency | GG | GT | TT |
Comparison |
| Actual frequency
(n) | 79.4 (181) | 18.4 (42) | 2.2 (5) |
χ2=0.434, |
| Theoretical
frequency | 78.5 | 20.2 | 1.3 | P=0.805 |
 | Table III.The association between the eNOS
G894T polymorphism and the risk of CHD in young individuals. |
Table III.
The association between the eNOS
G894T polymorphism and the risk of CHD in young individuals.
| Genotype/allele
frequency | Control group
(n=228) | CHD group
(n=234) | OR (95% CI) | P-value |
|---|
| Genotype frequency
(%) |
|
|
|
|
| GG | 181 (79.4) | 157 (67.1) | 1 | NA |
| GT | 42 (18.4) | 68 (29.1) | 1.867
(0.892–3.05) | 0.034 |
| TT | 5 (2.2) | 9 (3.8) | 2.075
(1.132–4.865) | 0.011 |
| GT +
TT | 47 (20.6) | 77 (32.9%) | 1.889
(0.902–3.14) | 0.029 |
| Allele frequency
(%) |
|
|
|
|
| G | 404 (88.6) | 382 (81.6) | 1 | NA |
| T | 52 (11.4) | 86 (18.4) | 1.749
(0.847–2.874) | 0.013 |
Results
Subject characteristics
The characteristics of the control and CHD groups
are presented in Table I. There
were no statistically significant differences in age, sex, body
mass index, smoking, diabetes, hypertension or family history of
CHD between the CHD and control groups (P>0.05).
Distribution of genotype and allele
frequency of the G894T site
As demonstrated in Table II, the distribution of genotype in
the control and CHD groups conformed to the Hardy-Weinberg
equilibrium (P>0.05), which indicates that the samples were
typically representative. The results for logistic regression
following adjustment for other factors, including age, sex, body
mass index and smoking, are presented in Table III. Significant differences in
the distribution of genotype and allele frequency between the two
groups were observed (P<0.05), and the risk of CHD in the GT
genotype group was 1.867 times the risk in the GG genotype group,
while the risk of CHD in TT and GT + TT groups was 2.075 and 1.889
times the risk in the GG group, respectively (P<0.05; Table III). Furthermore, the risk of CHD
associated with the eNOS 894T allele was 1.749 times the risk
associated with the eNOS 894G allele (P<0.05; Table III).
Partial structural modeling of the
G894T locus
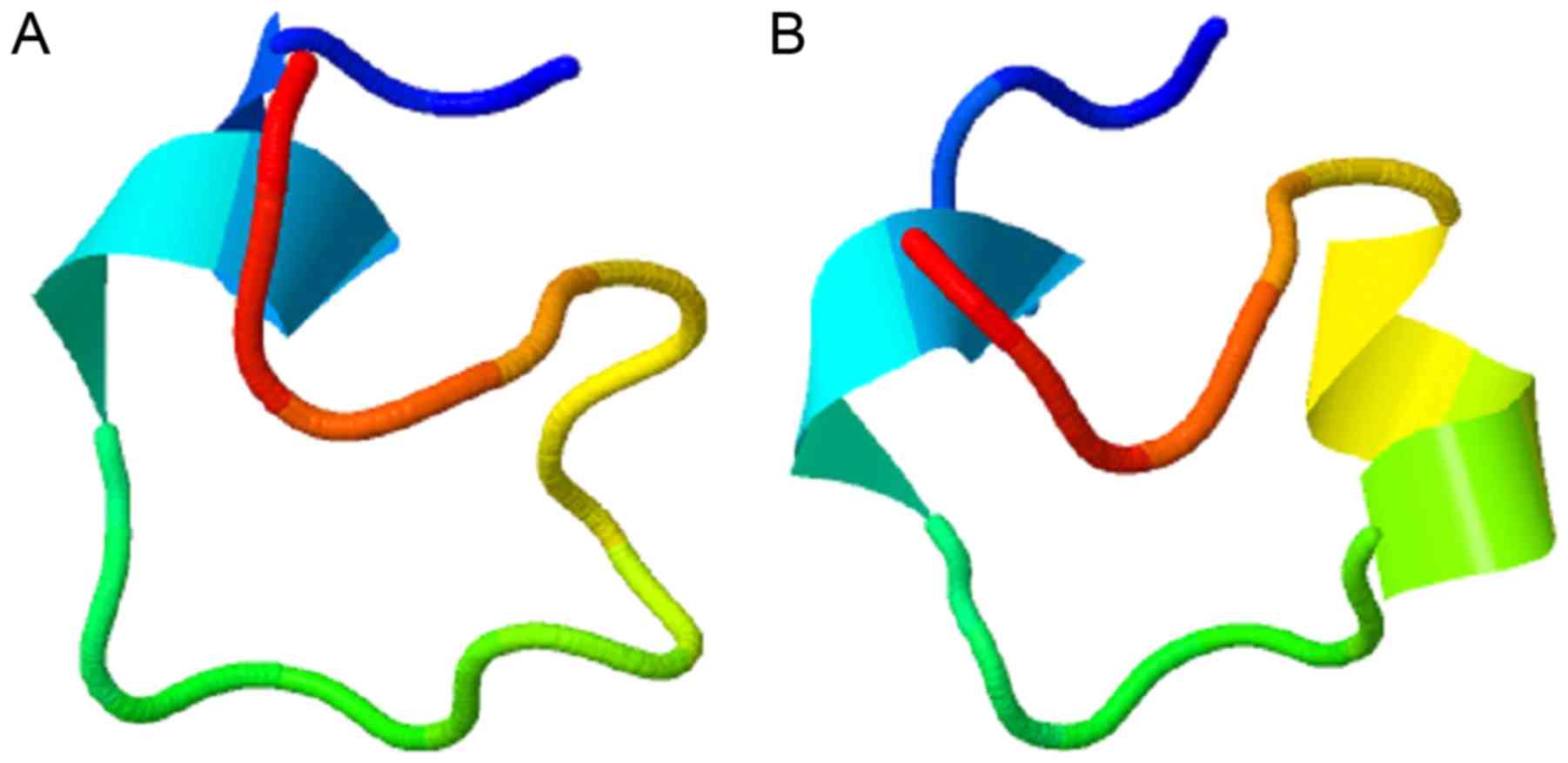
The eNOS G894T gene polymorphism leads to a change
in the amino acid located at the 289th amino acid position,
changing from glutamic acid (Glu/E) to aspartic acid (Asp/D), and
the result of modeling is presented in Fig. 2 as obtained from QUARK (14) and the Cn3D 4.3.1 viewer software
(http://www.ncbi.nlm.nih.gov). When the
289th amino acid was changed from Glu to Asp, the tertiary
structure of the short-peptide changed from a random coil to an
α-helix, indicating that the local structure of the protein in
different genotypes was different, which may result in alterations
to the protein function.
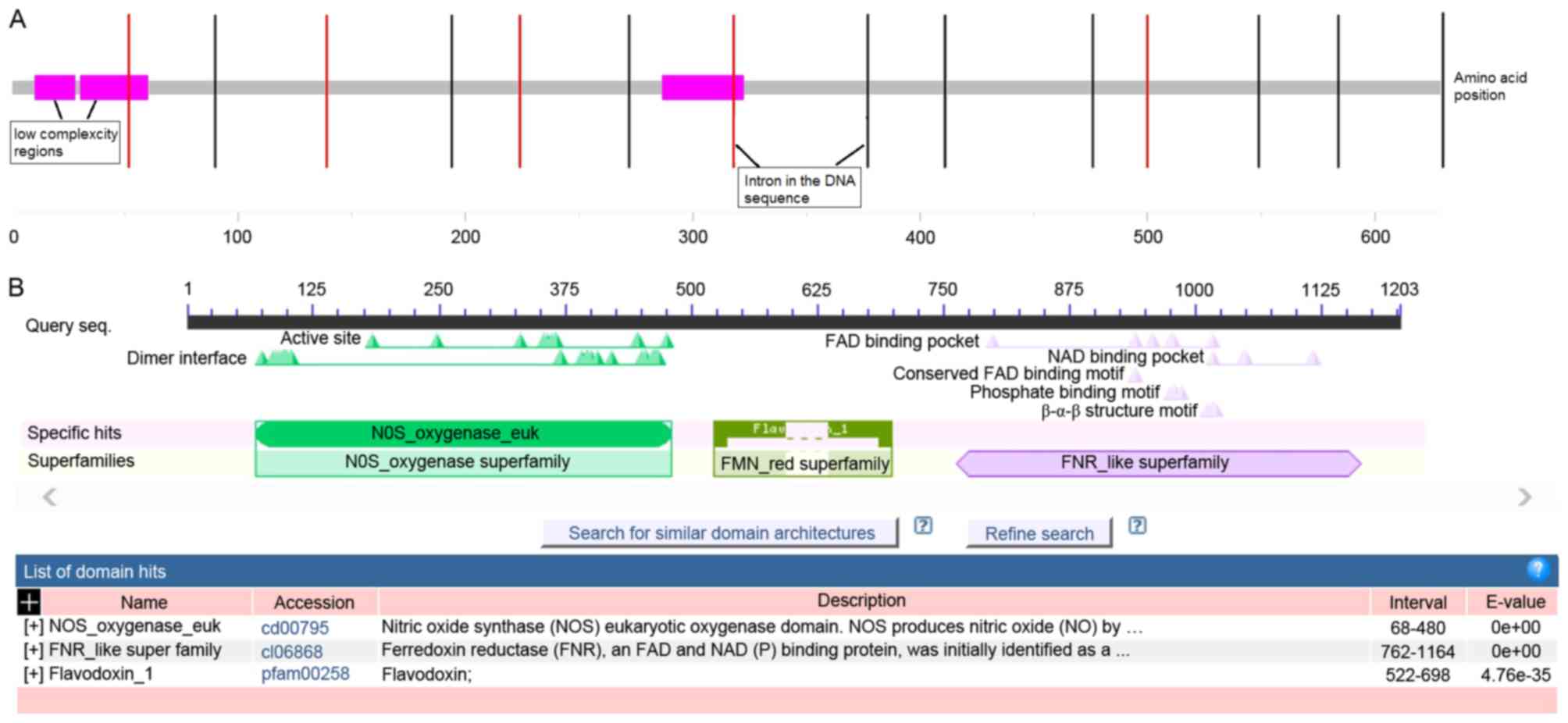
Analysis of structural domain
The structural domain of eNOS was analyzed by SMART
and results are presented in Fig.
3. The G894T site was located in the low complexity region at
the 287–321th amino acid of eNOS (Fig.
3A), which indicated that the region may be involved in
flexible binding associated with specific functions and important
in determining binding properties and biological roles (21). Furthermore, CDD analysis
demonstrated that G894T was located in the active region of the
eNOS enzyme, which binds to the substrate L-arginine, zinc, the
cofactor heme, tetrahydrobiopterin and the C-terminal electron
supplying reductase region. Therefore, it may be hypothesized that
the G894T polymorphism may change the partial structure of the
protein and be one of the major functional sites in the active
region of the eNOS enzyme. As a result of altered structure of the
active region of eNOS, the activity of enzyme may subsequently be
altered by affecting its ability to bind to substrates, thus
affecting biological functions.
Signaling pathway associated with eNOS
and CHD
KEGG pathway analysis demonstrated that eNOS is
involved in the processes of atherosclerosis and arginine and
proline metabolism. In addition, eNOS was demonstrated to be a key
enzyme involved in the conversion of L-arginine to NO in
endothelial cells (22); eNOS
catalyzes the generation of NO, which activates guanylate cyclase
in vascular smooth cells to increase cyclic (c)GMP levels, which
subsequently relaxes the vascular smooth muscle and leads to
vasodilation to regulate the blood pressure, and consequently
prevents cardiovascular diseases such as atherosclerosis (23). Therefore, it may be hypothesized
that, if the partial structure of eNOS is altered due to the eNOS
G894T polymorphism, the activity of the enzyme may be reduced. Upon
reduced enzymatic activity, the binding of L-arginine and eNOS may
be reduced, which would subsequently reduce the generation of NO
and lead to improper regulation of vasodilatation and
vasoconstriction, with altered blood pressure and blood flow, which
may reduce superoxide clearance (24). As a result, coronary vascular
atherosclerosis and thrombi may develop, which increase the risk of
CHD (Fig. 4). Therefore,
alterations in the activity of eNOS, levels of NO, activity of
guanylate cyclase and levels of cGMP in individuals with the
different genotypes for this polymorphism should be determined in
further studies to confirm the above hypothesis, which may aid the
development of novel drugs for the treatment of CHD at a molecular
level.
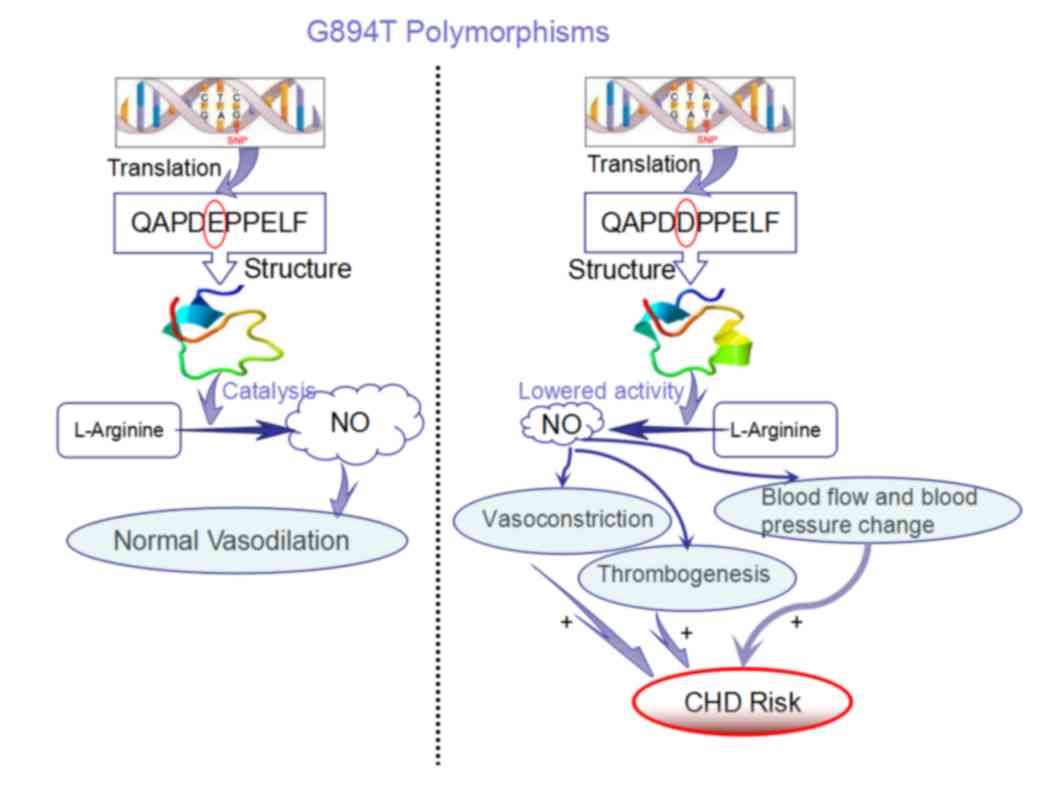 | Figure 4.Signaling pathway associated with eNOS
and CHD. The potential signaling pathway associated with the
development of CHD when the 894 G→T mutation is present, which
causes the 289th amino acid glutamic acid → aspartic acid missense
mutation in the protein sequence. This alteration may theoretically
alter the activity of eNOS by affecting its ability to bind to its
substrate, L-arginine, and reducing NO levels. Lower NO levels lead
to vasoconstriction, subsequently reduced blood flow, and increased
blood pressure and risk of thrombogenesis, which all contribute to
an increased risk of CHD. E and D highlighted by the red circles
indicate glutamic acid and aspartic acid amino acids, respectively,
at the 289th amino acid position. eNOS, endothelial nitric oxide
synthase; CHD, coronary heart disease; NO, nitric oxide; E,
glutamic acid; D, aspartic acid. |
Discussion
CHD may be caused by various factors, including
genetic and environmental factors (25). At present, CHD incidence is
increasing within the Chinese population and the number of young
patients with CHD is increasing annually (26). Studies have demonstrated that
during the process of atherosclerosis, eNOS gene polymorphisms may
affect the activities of the enzyme and be associated with the
incidence of CHD (27,28). However, to the best of our
knowledge, the association between eNOS gene polymorphisms and CHD
in young people has not been previously clarified. The present
study compared the distribution of genotypes and allele frequency
of the eNOS G894T gene polymorphism between CHD and control groups,
analyzed alterations in the protein structure by bioinformatics and
further discussed the association between the eNOS G894T
polymorphism and the risk of CHD in young individuals, in addition
to the potential mechanisms.
The association between the eNOS G894T gene
polymorphism and the risk of premature CHD has been reported by
various studies globally, however, results obtained in the same
region and for similar ethnic groups were different (15,29,30).
A study by Colombo et al (31) included 315 Italians with 201 CHD
cases and 114 controls, and the results demonstrated that the
incidence and severity of CHD was associated with the G894T
polymorphism. By contrast, a study on an Italian population by
Rossi et al (32) indicated
that CHD was independent of the eNOS G894T polymorphism. In China,
He et al (33) reported
that differences in the T allele frequency of the G894T
polymorphism between the CHD and control groups were statistically
significant (P<0.05) in a study where participants were from
Henan province. However, Liang et al (34) demonstrated that the genotype TT was
significantly different in the CHD and control groups (OR, 8.50,
P<0.05), while differences in the T allele frequency of the
G894T polymorphism between the CHD and control groups was not
statistically significant. The results of the present study
indicated that the differences in the distribution of genotype and
allele frequency of the eNOS G894T gene polymorphism between the
young CHD and control groups were statistically significant, which
indicated that the incidence of CHD in young people may be
associated with the eNOS G894T gene polymorphism. The risk of CHD
associated with the GT genotype was 1.867 times the risk associated
with the GG genotype, while the risk for TT and GT + TT genotypes
was 2.075 and 1.889 times the risk for the GG genotype,
respectively. In addition, the risk of CHD associated with the eNOS
894T allele was 1.749 times the risk associated with the eNOS 894G
allele (P<0.05). These results indicated that individuals with
the T allele of the G894T polymorphism may be at a higher risk of
CHD.
The result of structure modeling based on the amino
acid sequence demonstrated that the protein structure of the G894T
polymorphism was different prior to and following mutation, which
led to partial structures in the active structural domain of eNOS
being altered from a random coil to an α-helix. Therefore, it was
hypothesized that this altered structure may influence the activity
of eNOS and the binding of eNOS with L-arginine, consequently
decreasing the generation of NO. Low NO levels restrict
vasodilation, reduce blood flow, affect the clearing of superoxide
in the blood, promote blood platelet adherence and reduces the
oxidation of low-density lipoprotein cholesterol. Therefore,
reduced NO levels accelerates the formation of coronary
atherosclerosis and thrombi, consequently increasing the incidence
of CHD (35). Although the
observed structural difference would be present in all individuals
that possess the 894T allele, and not just young individuals with
this allele, 894T may be an important risk factor for early-onset
CHD. Therefore, it may be more obvious in young patients with CHD
that the eNOS G894T polymorphism is associated with the development
of CHD, compared with older patients.
In conclusion, the occurrence and development of CHD
in young people may be associated with the eNOS G894T gene
polymorphism. The potential mechanism may be that the 894 G→T
mutation, which causes the 289th amino acid Glu→Asp missense
mutation in the protein sequence, leads to alterations in the
functional structure domain of partial structures in eNOS,
influencing the activity of eNOS and the binding of substrates to
eNOS and reducing the generation of NO, consequently leading to the
incidence of CHD. However, there certain limitations are associated
with the present study. Firstly, only one polymorphism site was
analyzed in the current study, and the interaction between G894T
and other polymorphism sites in eNOS has not been investigated.
Previous studies have indicated that additional polymorphism sites
in eNOS, including rs2070744 T786C and eNOS4a/4b 27 bp variable
number tandem repeat, may also be associated with CHD (22,36,37);
therefore, whether the G894T polymorphism may lead to CHD via
combined action with other polymorphism sites in eNOS requires
further investigation. Additionally, the mechanism was analyzed by
a bioinformatics tool and, although structural alterations were
identified, the effect of these structural alterations on eNOS
activity requires verification, which may illustrate the
association between the G894T polymorphism and the risk of CHD more
clearly. Furthermore, although the risk of CHD was analyzed in the
current study, the association between the G894T and the efficacy
of drugs for the treatment of CHD was not determined. Therefore,
investigation into the association between the eNOS polymorphism
and the efficacy of drugs for the treatment of CHD is required to
determine whether the polymorphism affects drug sensitivity or
multi-drug resistance may be of clinical importance for the
treatment of CHD.
Glossary
Abbreviations
Abbreviations:
|
CHD
|
coronary heart disease
|
|
eNOS
|
endothelial nitric oxide synthase
|
|
NO
|
nitric oxide
|
References
|
1
|
Lv H and Guo Y: Progress in the treatment
of coronary heart disease. Jin Ri Jian Kang. 15:1492016.(In
Chinese).
|
|
2
|
Batty GD, Shipley M, Smith GD and Kivimaki
M: Long term risk factors for coronary heart disease and stroke:
Influence of duration of follow-up over four decades of mortality
surveillance. Eur J Prev Cardiol. 22:1139–1145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Che J, Li G, Shao Y, Niu H and Shi Y: An
analysis of the risk factors for premature coronary artery disease
in young and middle-age Chinese patients with hypertension. Exp
Clin Cardiol. 18:89–92. 2013.PubMed/NCBI
|
|
4
|
Yao HM, Sun TW, Wan YD, Zhang XJ, Fu X,
Shen DL, Zhang JY and Li L: Domestic versus imported drug-eluting
stents for the treatment of patients with acute coronary syndrome.
World J Emerg Med. 5:175–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tolstrup JS, Hvidtfeldt UA, Flachs EM,
Spiegelman D, Heitmann BL, Bälter K, Goldbourt U, Hallmans G, Knekt
P, Liu S, et al: Smoking and risk of coronary heart disease in
younger, middle-aged, and older adults. Am J Public Health.
104:96–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ben AM, Messaoudi S, Ezzine H and Mahjoub
T: Contribution of eNOS variants to the genetic susceptibility of
coronary artery disease in a Tunisian population. Genet Test Mol
Biomarkers. 19:203–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han Xu WG: Research progress in
pathogenesis of coronary heart disease and its correlation with
endothelin and nitric oxide. China Medical Herald. 1–169. 2014.
|
|
8
|
Rochette L, Lorin J, Zeller M, Guilland
JC, Lorgis L, Cottin Y and Vergely C: Nitric oxide synthase
inhibition and oxidative stress in cardiovascular diseases:
possible therapeutic targets? Pharmacol Ther. 140:239–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shu X, Keller TT IV, Begandt D, Butcher
JT, Biwer L, Keller AS, Columbus L and Isakson BE: Endothelial
nitric oxide synthase in the microcirculation. Cell Mol Life Sci.
72:4561–4575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levinsson A, Olin AC, Bjorck L, Rosengren
A and Nyberg F: Nitric oxide synthase (NOS) single nucleotide
polymorphisms are associated with coronary heart disease and
hypertension in the INTERGENE study. Nitric Oxide. 39:1–7. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang FJ, Yuan HY, Hu XX, Ou ZJ, Fu L, Lin
ZB, Wang ZP, Wang SM, Zhou L, Xu YQ, et al: High density
lipoprotein from patients with valvular heart disease uncouples
endothelial nitric oxide synthase. J Mol Cell Cardiol. 74:209–219.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Na Z and Jin-Fei Hu: A case-control study
on the association between single nucleotide polymorphism of eNOS
gene T-786C and G894T and the risk of coronary heart disease.
Zhejiang J Prevent Med. 28:9–12. 2016.
|
|
13
|
Azzam N, Zafrir B, Fares F, Smith Y,
Salman N, Nevzorov R and Amir O: Endothelial nitric oxide synthase
polymorphism and prognosis in systolic heart failure patients.
Nitric Oxide. 47:91–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vecoli C: Endothelial nitric oxide
synthase gene polymorphisms in cardiovascular disease. Vitam Horm.
96:387–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang K, Bai P, Shi S, Zhou B, Wang Y,
Song Y, Rao L and Zhang L: The G894T polymorphism on endothelial
nitric oxide synthase gene is associated with increased coronary
heart disease among Asia population: Evidence from a Meta analysis.
Thromb Res. 130:192–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dawber TR, Moore FE and Mann GV: II.
coronary heart disease in the framingham study. Int J Epidemiol.
44:1767–1780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu D and Zhang Y: Ab initio protein
structure assembly using continuous structure fragments and
optimized knowledge-based force field. Proteins. 80:1715–1735.
2012.PubMed/NCBI
|
|
18
|
Letunic I, Doerks T and Bork P: SMART:
Recent updates, new developments and status in 2015. Nucleic Acids
Res. 43(Database Issue): D257–D260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marchler-Bauer A, Bo Y, Han L, He J,
Lanczycki CJ, Lu S, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR,
et al: CDD/SPARCLE: Functional classification of proteins via
subfamily domain architectures. Nucleic Acids Res. 45:D200–D203.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45:D353–D361. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coletta A, Pinney JW, Solis DY, Marsh J,
Pettifer SR and Attwood TK: Low-complexity regions within protein
sequences have position-dependent roles. BMC Syst Biol. 4:432010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y, Du K, Liu Z and Lu X: Endothelial
nitric oxide synthase (eNOS) 4b/a gene polymorphisms and coronary
artery disease: Evidence from a meta-analysis. Int J Mol Sci.
15:7987–8003. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loukanov T, Hoss K, Tonchev P, Klimpel H,
Arnold R, Sebening C, Karck M and Gorenflo M: Endothelial nitric
oxide synthase gene polymorphism (Glu298Asp) and acute pulmonary
hypertension post cardiopulmonary bypass in children with
congenital cardiac diseases. Cardiol Young. 21:161–169. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yousry SM and Sedky Y: Relation of
Endothelial Nitric Oxide Synthase (eNOS) Genetic polymorphisms and
pulmonary hypertension in egyptian children with congenital heart
disease Sherif Mohamed. J Am Sci. 10:124–130. 2014.
|
|
25
|
Wang D, Li W, Cui X, Meng Y, Zhou M, Xiao
L, Ma J, Yi G and Chen W: Sleep duration and risk of coronary heart
disease: A systematic review and meta-analysis of prospective
cohort studies. Int J Cardiol. 219:231–239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang WX, Yang Z, Wu YJ, Qiao SB, Yang YJ
and Chen JL: Factors associated with coronary artery disease in
young population (age ≤40): Analysis with 217 cases. Chin Med Sci
J. 29:38–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seckin S, Emrah B, Biyik I, Emre A, Burak
T, Azmi S, Omer C and Sinan D: 786T/c endothelial nitric oxide
synthase gene polymorphism and coronary collateral circulation.
Postepy Hig Med Dosw (Online). 70:80–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xin W: A study on the association between
intracranial atherosclerosis and carbonyldiamide and the
polymorphism of genes related to nitric oxide synthetic pathway.
Southern Medical University. 12:37–40. 2013.
|
|
29
|
Xueping J: Study of eNOS Gene G894T
polymorphism in CHD patients of multi-ethnic group of xingjiang.
Chin J Health Lab Technol. 1–1461. 2010.
|
|
30
|
Chen C and Jiang X: Study on polymorphism
of eNOS gene G894T in Kazak patients with CHD in Xinjiang. J Clin
Exp Med. 9:451–499. 2010.
|
|
31
|
Colombo MG, Andreassi MG, Paradossi U,
Botto N, Manfredi S, Masetti S, Rossi G, Clerico A and Biagini A:
Evidence for association of a common variant of the endothelial
nitric oxide synthase gene (Glu298→Asp polymorphism) to the
presence, extent, and severity of coronary artery disease. Heart.
87:525–528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rossi GP, Cesari M, Zanchetta M, Colonna
S, Maiolino G, Pedon L, Cavallin M, Maiolino P and Pessina AC: The
T-786C endothelial nitric oxide synthase genotype is a novel risk
factor for coronary artery disease in Caucasian patients of the
GENICA study. J Am Coll Cardiol. 41:930–937. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He Y, Yang D and Yu H: Research on
Correlativity of 27 bpVNTR and G894T polymorphisms of endothelial
nitric oxide synthase gene and coronary heart disease in henan han
population. China J Modern Med. 1–527. 2008.
|
|
34
|
Liang Q, Dong Y and Yang X: The research
of AC angiotensinogen and endothelial nitric oxide synthase gene
polymorphisms in predisposition to CHD with gene chip technology.
Hebei Med. 1–406. 2006.
|
|
35
|
Faure C, Leveille P, Dupont C, Julia C,
Chavatte-Palmer P; Alifert Group, ; Sutton A and Levy R: Are
superoxide dismutase 2 and nitric oxide synthase polymorphisms
associated with idiopathic infertility? Antioxid Redox Signal.
21:565–569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kumar GR, Spurthi KM, Kumar GK, Aiyengar
TM, Chiranjeevi P, Nivas S, Anuradha C, Swathi B, Sahu SK, Ali A
and Rani HS: Genetic polymorphisms of eNOS (−786T/C, Intron 4b/4a
& 894G/T) and its association with asymptomatic first degree
relatives of coronary heart disease patients. Nitric Oxide.
60:40–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu D, Jiang Z, Dai L, Zhang X, Yan C and
Han Y: Association between the-786T>C 1polymorphism in the
promoter region of endothelial nitric oxide synthase (eNOS) and
risk of coronary artery disease: A systematic review and
meta-analysis. Gene. 545:175–183. 2014. View Article : Google Scholar : PubMed/NCBI
|