Introduction
Gastric cancer is the fourth most common malignant
disease and second leading cause of cancer-related deaths worldwide
(1). Approximately 850,000 newly
diagnosed gastric cancer cases and 650 000 deaths occur per year
(2). Incidence and mortality of
gastric cancer are the highest in East Asia (particularly in Korea,
Mongolia, Japan and China); this disease became the second most
lethal cancer in China (3). Major
contributory risk factors to gastric cancer include Helicobacter
pylori infection, dietary factors, tobacco use, alcohol
consumption and obesity (4,5).
Despite considerable improvements in innovations in clinical
diagnostics, surgical techniques and development of new
chemotherapy regimens, five-year survival rates with advanced
gastric cancer increased minimally in the past few years (6). Poor prognosis of gastric cancer
patients mainly involves unlimited growth and strong metastatic
capacities of gastric cancer cells (7,8).
Mechanism of gastric cancer oncogenesis remains largely unclear in
spite of extensive clinical and basic research efforts (9,10).
Therefore, studies should focus on elucidating molecular mechanisms
underlying gastric cancer occurrence and development and exploring
novel therapeutic targets for gastric cancer treatments.
MicroRNAs (miRNAs) represent a large group of highly
conserved and small RNA molecules of spanning 17–25 nucleotides
(11). miRNAs regulate expression
of their target genes in a post-transcriptional manner through
interacting with 3′-untranslated regions (3′-UTRs) of targeted mRNA
and causing mRNA degradation and suppression of translation
(12). miRNAs regulate more than
60% of protein translation (13).
Increasing studies demonstrated that miRNAs are aberrantly
expressed in various types of human cancers and play important
roles in tumorigenic processes, including cell proliferation,
cycle, apoptosis, angiogenesis, invasion and metastasis (14,15).
Depending on their target genes, miRNAs may serve as either tumour
suppressors or oncogenes (16). A
large number of miRNAs contribute to gastric cancer tumourigenesis
and tumour development by regulating expression of specific target
genes; this condition suggests that miRNAs can be developed as
therapeutic strategies for patients with gastric cancer (17).
miR-28 was studied with regard to its expression and
biological functions in various types of human cancer (18–20).
However, no previous research studied expression patterns, roles
and associated molecular mechanisms of miR-28 in gastric cancer.
This study detected expression levels of miR-28 in gastric cancer
and determined its roles in regulation of aggressive behaviours of
gastric cancer cells and its underlying mechanisms.
Materials and methods
Tissue samples and cell lines
A total of 31 paired gastric cancer tissues and
adjacent normal gastric tissues were obtained from patients who had
undergone radical gastrectomy at Zhujiang Hospital of Southern
Medical University between January 2013 to December 2015. Normal
adjacent tissues were collected at sites more than 4 cm away from
the tumor margin. None of these gastric cancer patients had been
treated with radiotherapy or chemotherapy before surgery. These
tissues were immediately frozen in liquid nitrogen and then stored
at −80°C. This study was approved by the Medical Ethics Committees
of Zhujiang Hospital of Southern Medical University. Written
informed consent was obtained from the patients enrolled in this
research.
Five human gastric cancer cell lines (SGC-7901,
MGC-803, MKN-1, BGC-823, AGS) and the normal gastric epithelium
GES-1 cell line were all bought from the American Type Culture
Collection (ATCC; Rockville, MD, USA). Cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS) (both from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin in a humidified atmosphere with 5% CO2 at
37°C.
Cell transfection
The miR-28 inhibitor and corresponding scramble
miRNA inhibitor negative control (NC inhibitor) were purchased from
GenePharma (Shanghai, China). Small interference RNA (siRNA)
targeting PTEN (PTEN siRNA) and non-target control siRNA (NC siRNA)
were synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
Cells were seeded into 6-well plates at a density of
6×105 each well. Cell transfection was performed when
the cell density reached a confluence of 90%. Cells were
transfected with miR-28 inhibitor, NC inhibitor, phosphatase and
tensin homolog (PTEN) siRNA or NC siRNA using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) following to the
manufacturer's instructions. Culture medium was replaced with fresh
medium containing 10% FBS at 6 h post-transfection.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from gastric cancer tissue
samples or cells using TRIzol reagent (Ambion; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
concentration and quality of the total RNA was evaluated using the
ND-2000 spectrophotometer (NanoDrop Technologies; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). For miR-28 expression, cDNA
synthesis was performed with TaqMan® MicroRNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Quantitative real-time PCR was conducted using the TaqMan
MicroRNA Assay kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Relative expression level of miR-28 was normalized by U6
expression. For PTEN mRNA expression, reverse transcription was
performed using PrimeScript RT Reagent kit (Takara Biotechnology
Co., Ltd., Dalian, China). SYBR Premix Ex Taq Master Mix (Takara
Biotechnology Co., Ltd.) was utilized to detect PTEN mRNA
expression levels. GAPDH was used as an internal control for PTEN
mRNA level. The relative expression was calculated using the
2−∆∆Ct method (21).
Cell Counting Kit 8 (CCK8) assay
Cell proliferation was determined using CCK8 assay
according to the manufacturer's instructions. Briefly, transfected
cells were collected and seeded into 96-well plates
(3×103 cells/well). 0, 24, 48, and 72 after incubation,
cell proliferation was measured by the addition of 10 µl of CCK8
solution (Dojindo Molecular Technologies, Kumamoto, Japan) into
each well. After incubation at 37°C for 2 h, absorbance was
measured at a wavelength of 450 nm using a microplate reader
(SpectraMAX Plus; Molecular Devices, LLC, Sunnyvale, CA, USA). The
assays were performed in triplicates and repeated three times.
Matrigel invasion assay
Matrigel invasion assay was performed using 24-well
Transwell chambers (8-mm pore size; EMD Millipore, Billerica, MA,
USA) coated with Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA). In briefly, transfected cells were collected at 48 h
posttransfection and suspended in DMEM without FBS. Transfected
cells (5×104) were added to the top chamber, while the
DMEM supplemented with 10% FBS used as a chemoattractant was added
in the lower chamber. After 24 h incubation at 37°C with 5%
CO2, non-invasive cells on the top chambers were removed
using cotton swabs. The invasive cells were fixed with methanol and
stained with 0.1% crystal violet. Subsequent to washing three times
with PBS, invasive cells in five randomly selected visual fields
were photographed and counted under an inverted microscope (IX71;
Olympus Corporation, Tokyo, Japan).
Bioinformatic analysis and luciferase
reporter assay
Bioinformatic analysis was performed to predicate
the potential targets of miR-28 with TargetScan (http://www.targetscan.org/) and PicTar (http://pictar.mdcberlin.de/).
The pGL3-wild type-PTEN-3′-UTR (pGL3-Wt-PTEN-3′-UTR)
containing the putative binding site of miR-28 and
pGL3-mutant-PTEN-3′-UTR (pGL3-Mut-PTEN-3′-UTR) were chemically
synthesized and obtained from GenePharma. For luciferase reporter
assay, the plasmid (pGL3-Wt-PTEN-3′-UTR or pGL3-Mut-PTEN-3′-UTR)
together with miR-28 inhibitor or NC inhibitor were transfected
into cells using Lipofectamine 2000 reagent, according to the
manufacturer's instructions. Luciferase activities were measured 48
h later with a dual-luciferase reporter system (Promega
Corporation, Madison, WI, USA). Renilla luciferase activity
was used for normalization.
Western blot analysis
Tissue samples or cells were lysed using a
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) in the presence of a protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
A bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology) was used to detect protein concentration. Equal
amounts of protein were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinylidene fluoride membrane (EMD Millipore). After blocking
with 5% non-fat milk at room temperature in TBS, the membranes were
incubated overnight at 4°C with specific primary antibodies for
PTEN antibody (sc-133197; 1:1,000 dilution) and GAPDH antibody
(sc-47724; 1:1,000 dilution) (both from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). Subsequent to washing three times with
TBST, the membranes were incubated with goat anti-mouse horseradish
peroxidase-conjugated secondary antibody (sc-2005; 1:5,000
dilution; Santa Cruz Biotechnology, Inc.) at room temperature for 1
h. Positive signals were developed by enhanced chemiluminescence
solution (ECL; Pierce; Thermo Fisher Scientific, Inc.) and analyzed
with ImageJ 1.49 (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Data are presented as the mean ± standard deviation,
and compared with Student's t-test or one-way analysis of variance
(ANOVA) using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). Correlation
of miR-28 expression with that of PTEN mRNA was conducted with the
Spearman's correlation analysis. Two-tailed p-value <0.05 was
considered to indicate a statistically significant difference.
Results
miR-28 is upregulated in gastric
cancer tissues and cell lines
To elucidate whether miR-28 correlates with
progression of gastric cancer, its expression levels in 31 paired
gastric cancer tissues and adjacent normal gastric tissues were
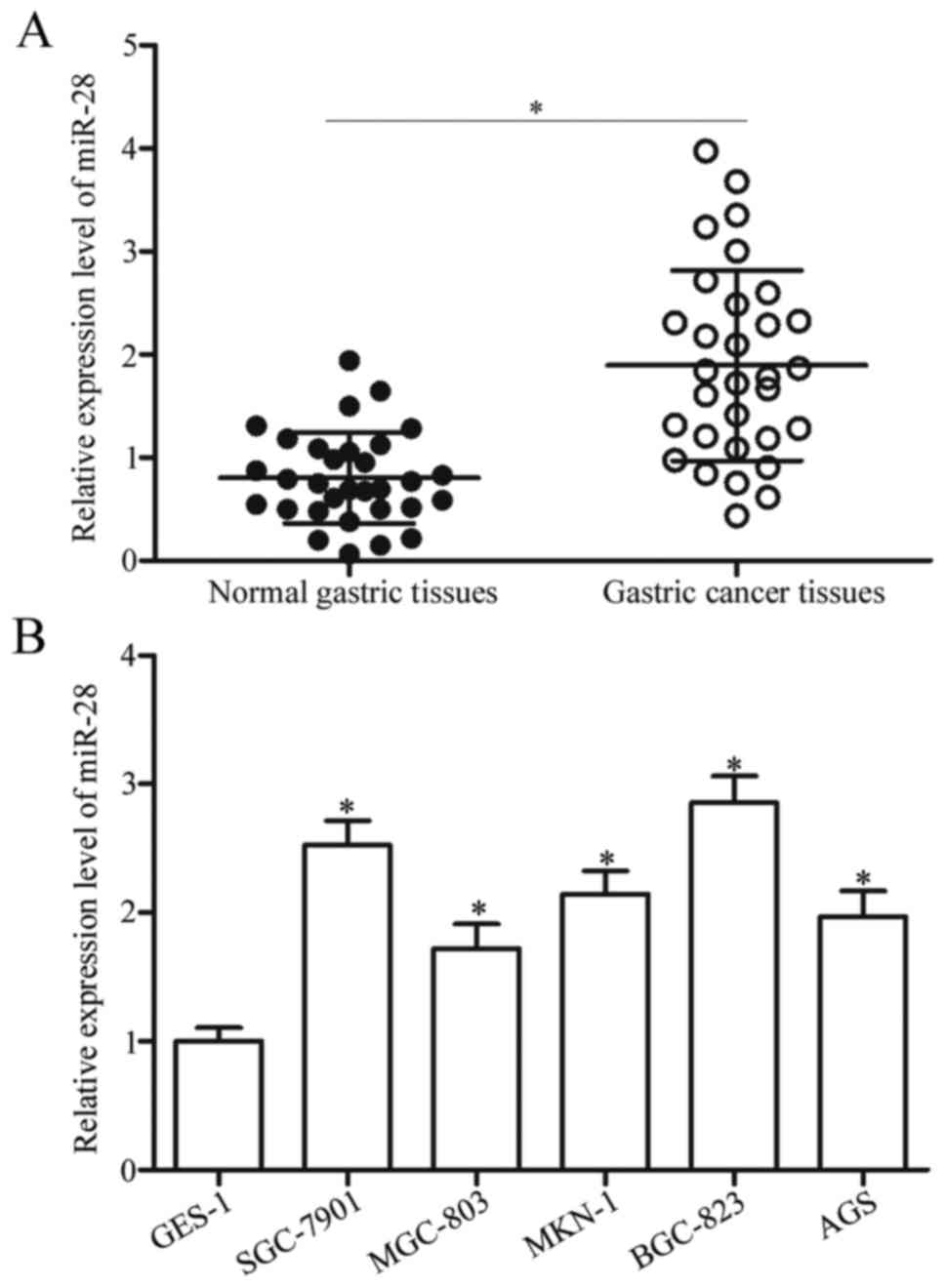
determined using RT-qPCR. Data showed that miR-28 expression was
upregulated in gastric cancer tissues compared with that in
adjacent normal gastric tissues (Fig.
1A, P<0.05). Expression of miR-28 in a normal gastric cell
line (GES-1) was compared with those of a panel of gastric cancer
cell lines (SGC-7901, MGC-803, MKN-1, BGC-823 and AGS); and gastric
cancer cell lines showed generally increased miR-28 expression
(Fig. 1B, P<0.05). These
results suggested that upregulation of miR-28 is a common event in
gastric cancer and may play essential roles in gastric cancer
progression.
Downregulation of miR-28 inhibits cell
proliferation and invasion in gastric cancer
To investigate biological roles of miR-28 in
development and progression of gastric cancer, miR-28 inhibitor was
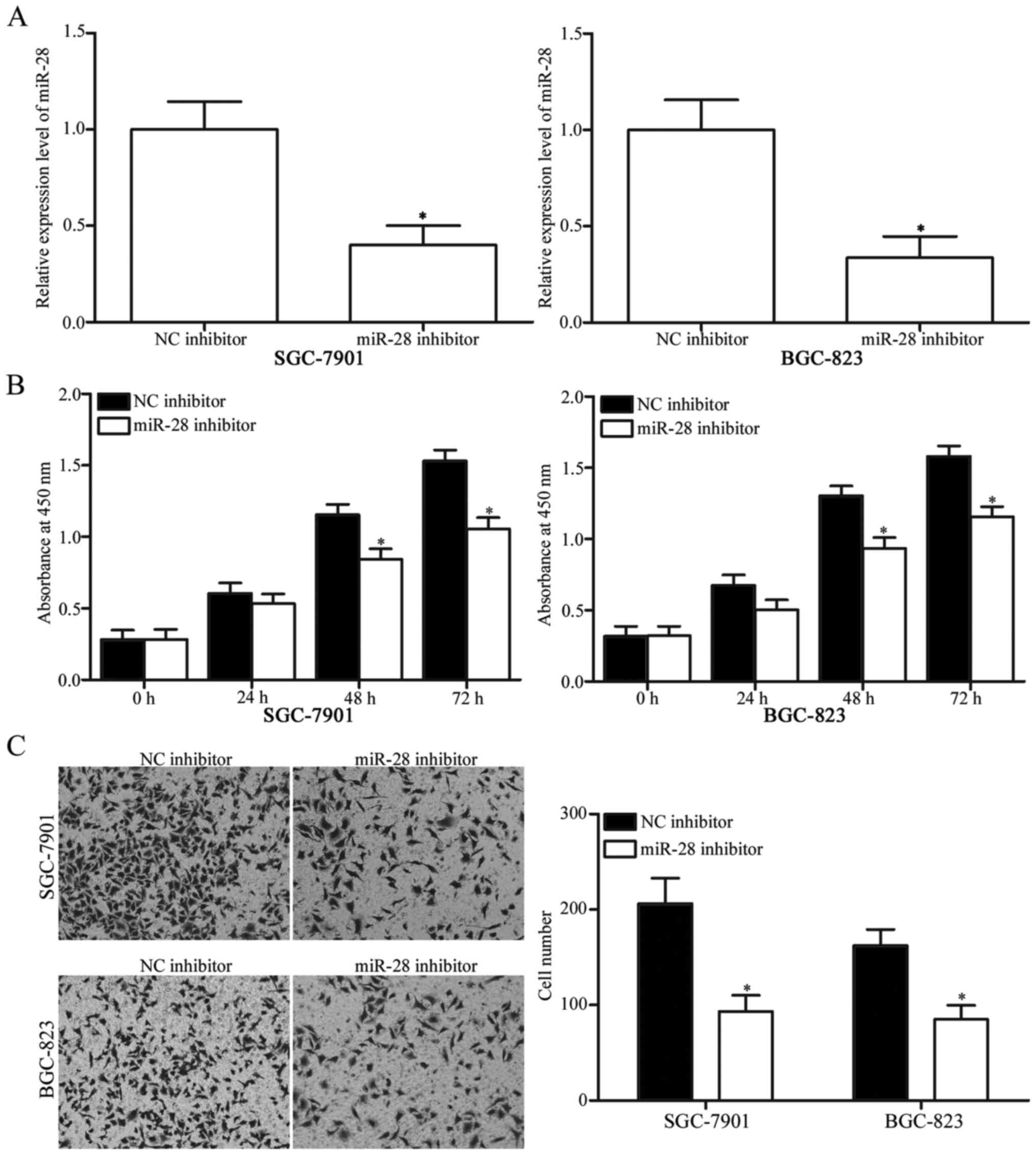
transfected into SGC-7901 and BGC-823 cells. RT-qPCR confirmed
significant downregulation of miR-28 in SGC-7901 and BGC-823 cells
after transfection with miR-28 inhibitor (Fig. 2A, P<0.05). Based on CCK8 assay,
miR-28 inhibitor transfection suppressed proliferation of SGC-7901
and BGC-823 cells compared with NC inhibitor group (Fig. 2B, P<0.05). Matrigel invasion
assay revealed that miR-28 knockdown decreased invasion abilities
of SGC-7901 and BGC-823 cells (Fig.
2C, P<0.05). These results demonstrated that miR-28 may
serve as an oncogene in gastric cancer.
PTEN is a direct target of miR-28
To elucidate underlying molecular mechanisms of
miR-28 in gastric cancer, potential targets of miR-28 were
predicted using bioinformatics analysis. Among candidates, PTEN was
selected for further validation because it was downregulated in
gastric cancer and participate in gastric cancer progression
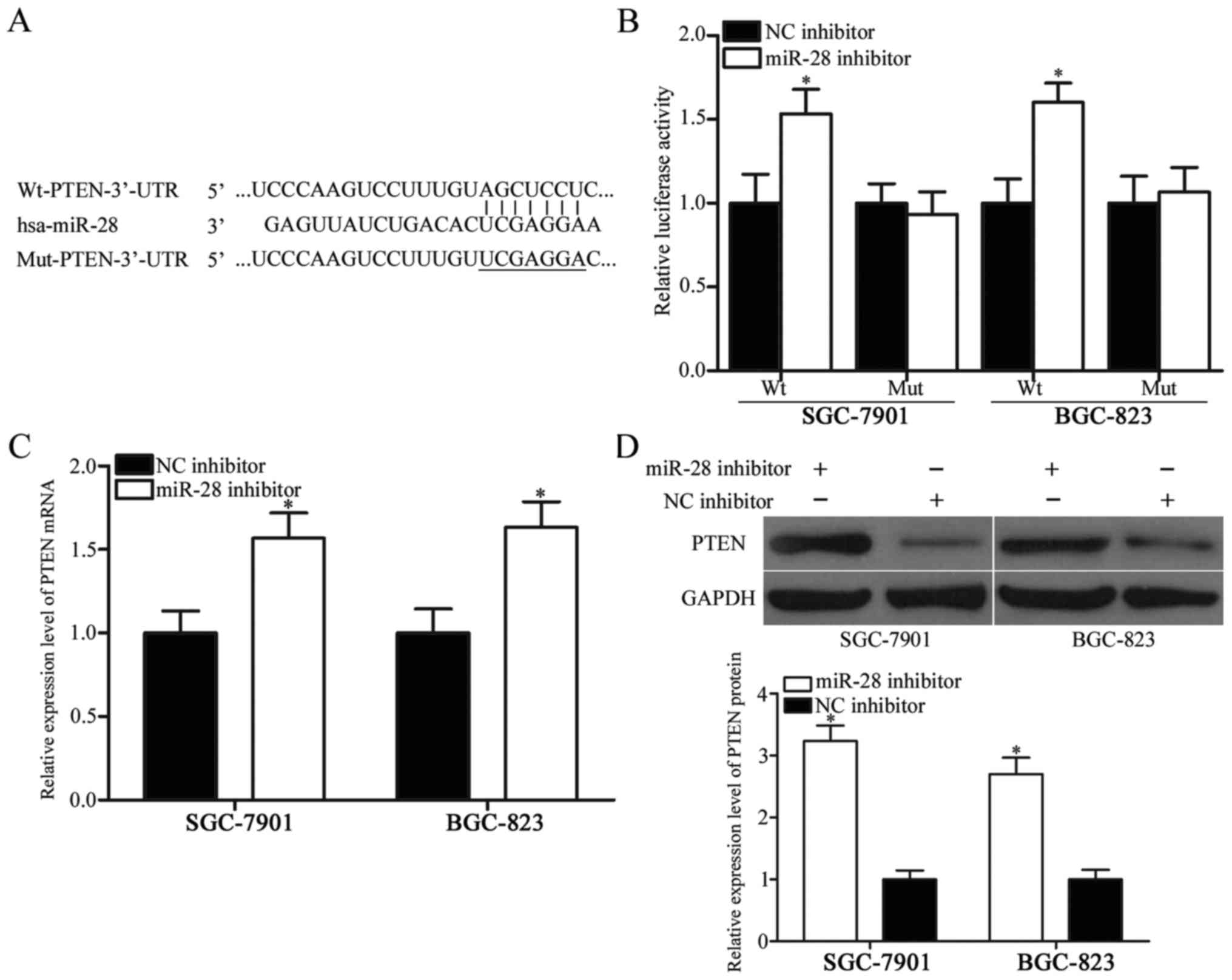
(22–24). Fig.
3A shows putative target sites of miR-28 in 3′-UTR of PTEN. To
confirm whether miR-28 directly targets the 3′-UTR of PTEN,
luciferase reporter assays were performed in SGC-7901 and BGC-823
cells transfected with plasmids (pGL3-Wt-PTEN-3′-UTR or
pGL3-Mut-PTEN-3′-UTR) along with miR-28 inhibitor or NC inhibitor.
Results showed that downregulation of miR-28 increased relative
luciferase activities of wild-type PTEN 3′-UTR (Fig. 3B, P<0.05), whereas luciferase
activities of mutant PTEN 3′-UTR remained unchanged.
mRNA and protein levels of PTEN levels in SGC-7901
and BGC-823 cells transfected with miR-28 inhibitor or NC inhibitor
were examined to confirm regulatory roles of miR-28 in PTEN in
gastric cancer. As shown in Fig. 3C
and D, transfection of miR-28 inhibitor into SGC-7901 and
BGC-823 cells resulted in remarkable upregulation of PTEN
expression at both mRNA and protein levels (P<0.05). Overall,
these results demonstrated that PTEN is a direct target gene of
miR-28 in gastric cancer.
An inverse correlation exists between
PTEN and miR-28 in gastric cancer tissues
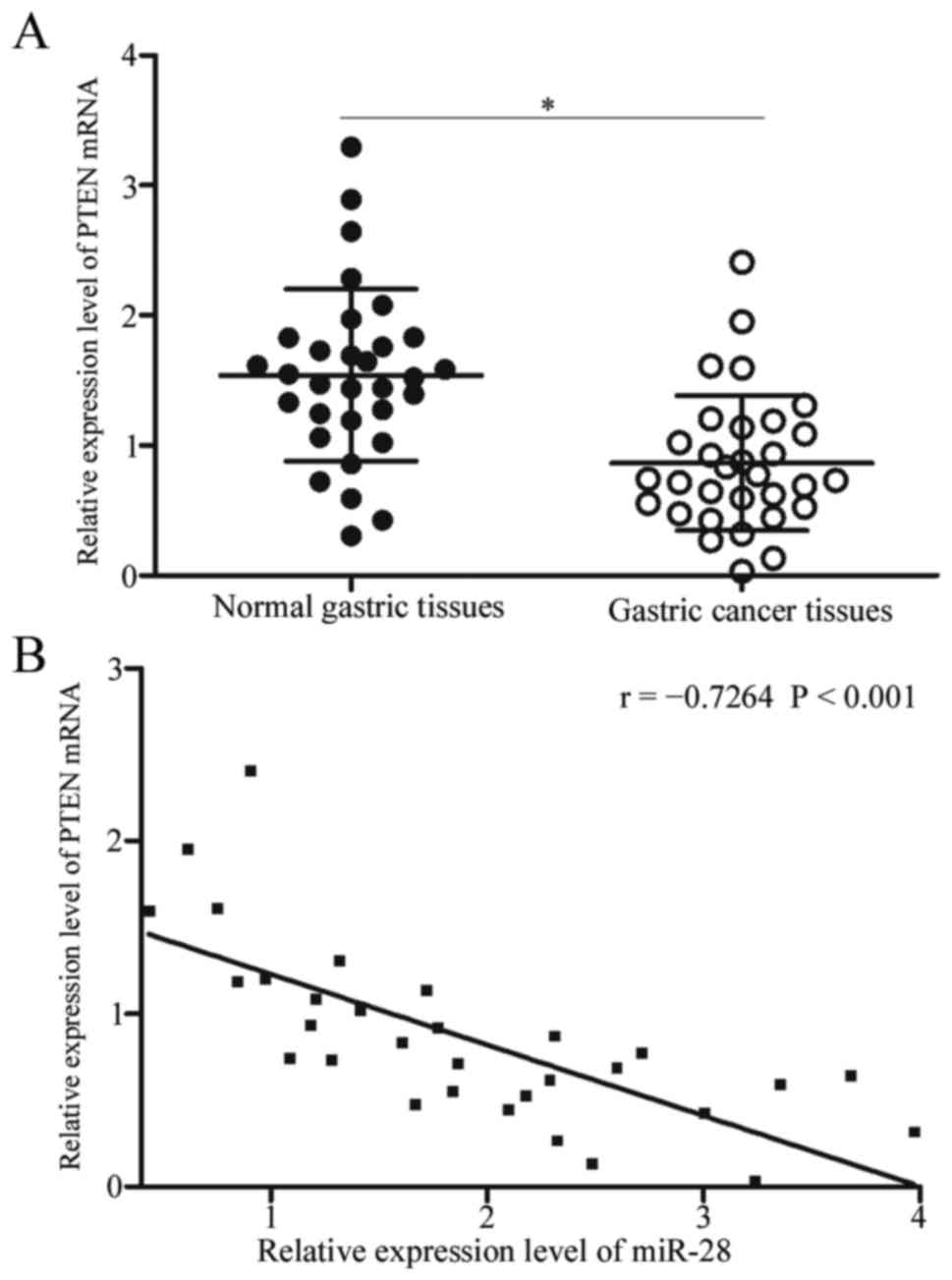
Subsequent examinations detected PTEN expression in
gastric cancer tissues and adjacent normal gastric tissues. RT-qPCR
analysis revealed upregulated expression of PTEN mRNA in gastric
cancer tissues in comparison with adjacent normal gastric tissues
(Fig. 4A, P<0.05). Spearman's
correlation analysis was used to evaluate association between PTEN
mRNA and miR-28. As shown in Fig.
4B, miR-28 was strongly correlated with PTEN mRNA expression in
gastric cancer specimens (r=−0.7264, P<0.001), suggesting that
upregulation of miR-28 in gastric cancer may primarily cause
downregulation of PTEN.
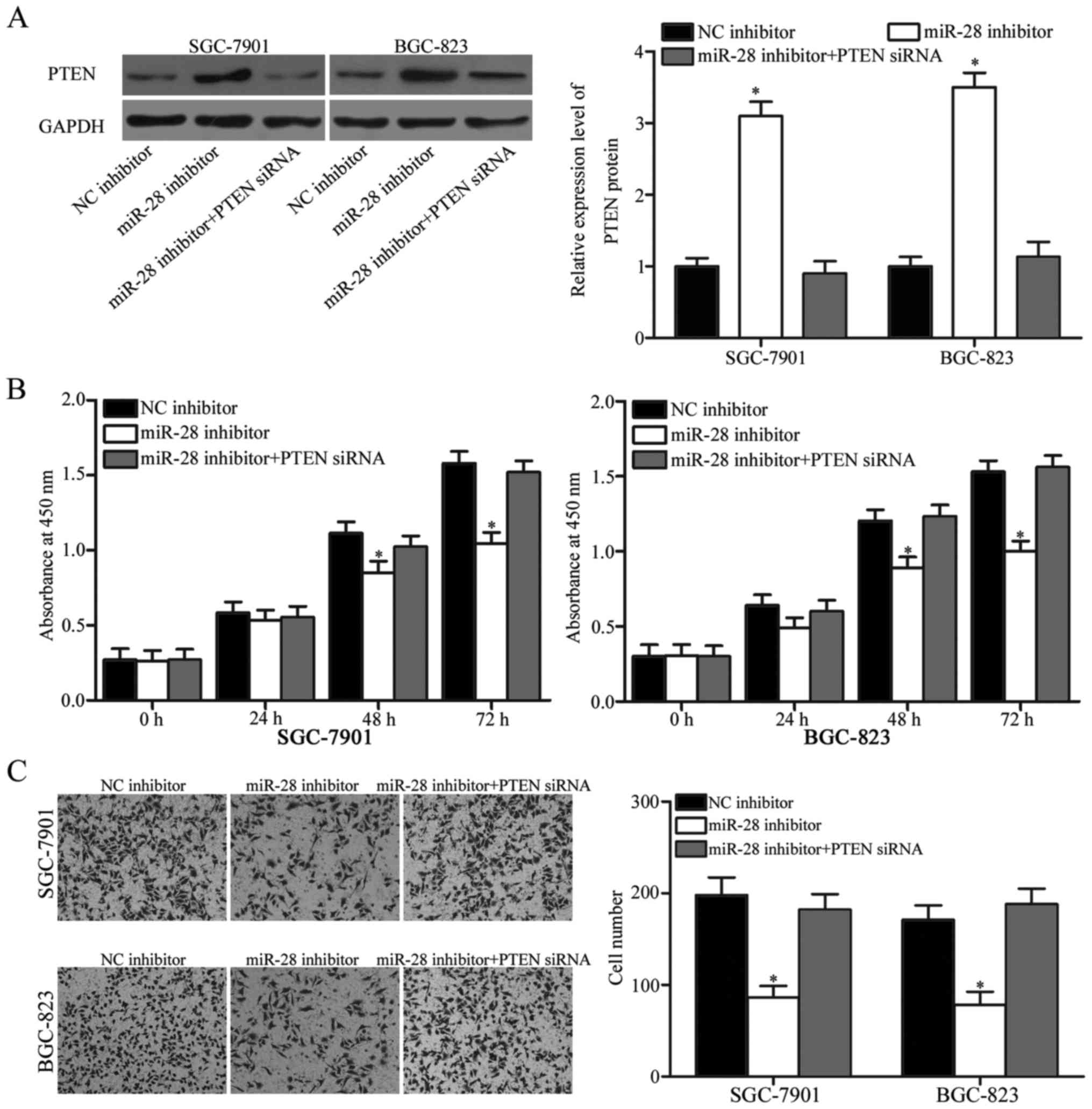
PTEN knockdown restores functional
effects of miR-28 on cell proliferation and invasion in gastric
cancer
Rescue experiments were performed to evaluate
whether PTEN is responsible for functional roles of miR-28 in
gastric cancer cells. Firstly, SGC-7901 and BGC-823 cells were
transfected with miR-28 inhibitor with or without PTEN siRNA. After
transfection, Western blot analysis confirmed that PTEN expression
was recovered in miR-28 inhibitor-transfected SGC-7901 and BGC-823
cells after transfection with PTEN siRNA (Fig. 5A, P<0.05). Subsequently, CCK8
assay and Matrigel invasion assays demonstrated that
co-transfection of PTEN siRNA restored functional effects of miR-28
inhibitor on gastric cancer cell proliferation (Fig. 5B, P<0.05) and invasion (Fig. 5C, P<0.05) in SGC-7901 and
BGC-823 cells. These results demonstrated that miR-28 plays
oncogenic roles in gastric cancer, at least in part, by targeting
PTEN.
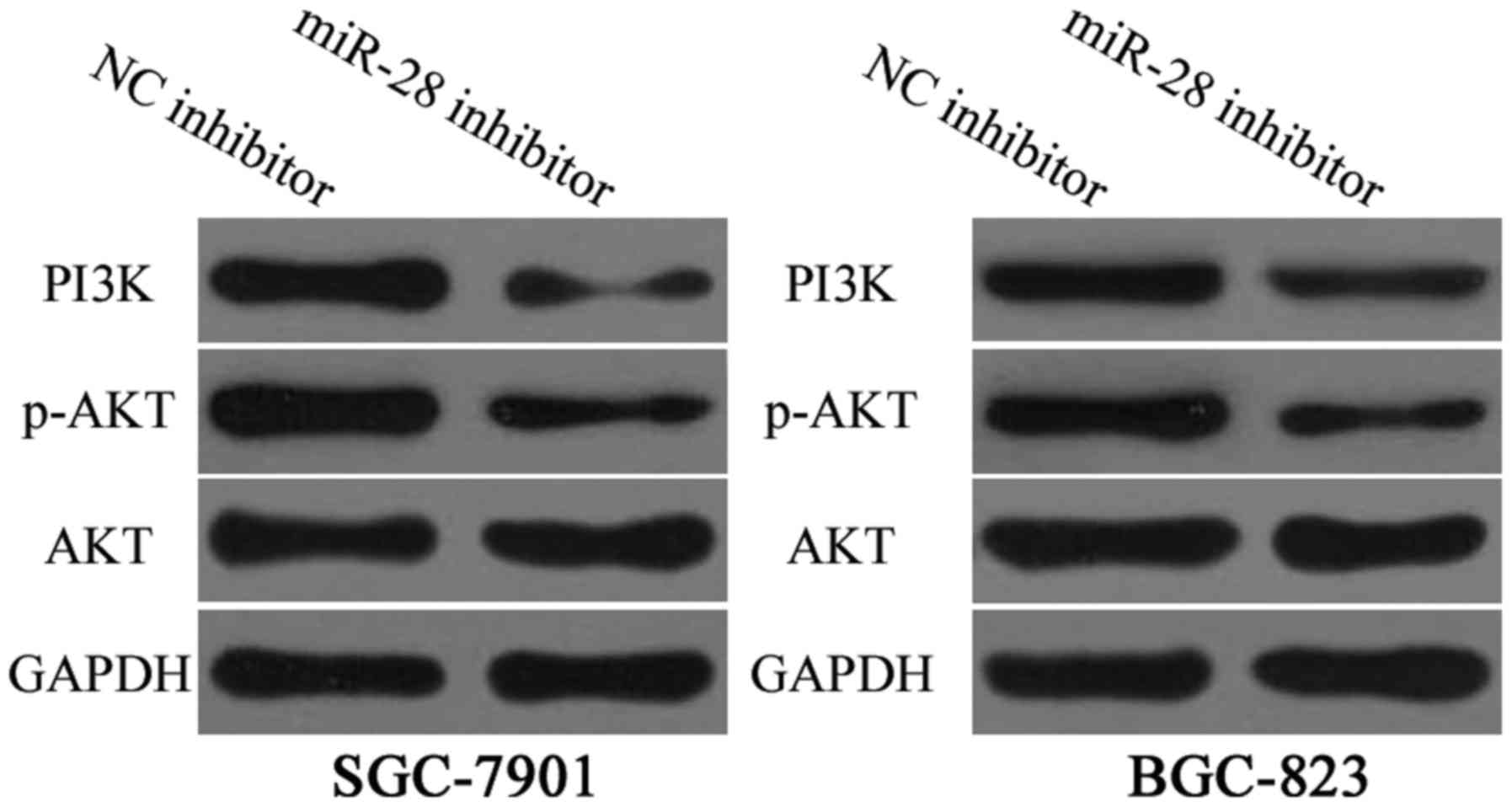
miR-28 inhibitor regulates
PTEN/PI3K/AKT signalling in gastric cancer
Previous studies reported that PTEN is a master
negative regulator of the PI3K/AKT signalling pathway in gastric
cancer (25,26). Hence, PI3K, AKT and p-AKT
expression levels in SGC-7901 and BGC-823 cells were measured after
transfection with miR-28 inhibitor or NC inhibitor. Results
revealed that downregulation of miR-28 decreased protein levels of
PI3K and p-AKT (Fig. 6, P<0.01)
in SGC-7901 and BGC-823 cells but did not affect total AKT
expression. These results suggested that miR-28 inhibits gastric
cancer progression by regulation of PTEN/PI3K/AKT signalling
pathway.
Discussion
Previous studies demonstrated aberrantly expressed
miRNAs in various types of human cancers, and these miRNAs play
important roles in tumourigenesis and tumour development (27,28).
miRNAs feature considerable potential for novel therapeutic
approaches for treating human cancers (29). Therefore, investigation of novel
miRNAs involved in gastric cancer progression provides
opportunities to improve prognosis of gastric cancer patients. In
this study, miR-28 expression was upregulated in gastric cancer
specimens and cell lines. Downregulation of miR-28 inhibited
gastric cancer cell proliferation and invasion through regulation
of PTEN/PI3K/AKT signalling pathway. These results suggested that
miR-28 plays a crucial role in gastric cancer and may be developed
as a therapeutic target for patients with such disease.
Recent studies showed that miR-28 expression is
deregulated in several human cancers. For example, miR-28 is lowly
expressed in hepatocellular carcinoma. Low miR-28 level was
correlated with tumour metastasis, recurrence and poor survival of
patients with hepatocellular carcinoma (30). Downregulation of miR-28 was also
observed in colorectal cancer (18), renal cell carcinoma (19) and B-cell lymphoma (20). However, miR-28 expression is
upregulated in ovarian cancer tissues (31). These conflicting findings suggested
tissue-specific expression of miR-28.
Tumour-suppressing roles of miR-28 were studied in
multiple kinds of human cancer. For example, Zhou et al
reported that miR-28 underexpression promoted tumour growth and
metastasis of hepatocellular carcinoma in vivo (30). Almeida et al showed that
upregulation of miR-28 inhibited colorectal cancer cell
proliferation, migration and invasion (18). A study by Wang et al
revealed that miR-28 suppressed cell proliferation and migration of
renal cell carcinoma in vitro (19). Another study by Schneider et
al demonstrated that resumption expression of miR-28 diminished
cell proliferation and clonogenic properties of B-cell lymphoma
cells (20). miR-28 was proven to
serve as an oncogene in ovarian cancer by regulation of cell
proliferation, cell cycle, apoptosis, colony forming and motility.
These contradicting findings indicated that miR-28 acts as a tumour
suppressor in certain cancers and an oncogene in others.
Several targets of miR-28 were validated; these
targets include interleukin-34 (30), insulin-like growth factor-1
(32) in hepatocellular carcinoma,
Ras-related protein Rap-1b in renal cell carcinoma (19), MAD2L1 (20) and BCL2 associated athano-gene 1
(20) in B-cell lymphoma and
Nedd4-binding partner-1 (31) in
ovarian cancer. In our study, PTEN was identified as a novel direct
target of miR-28 in gastric cancer. Firstly, bioinformatics
analysis predicted that PTEN gene contained a miR-28 seed match at
the 3′-UTR of PTEN. Luciferase reporter assay confirmed that zinc
finger E-box-binding homeobox 1 directly targeted the 3′-UTR of
PTEN gene. Subsequent RT-qPCR and western blot analysis indicated
negative regulatory effects of miR-28 on PTEN expression at both
mRNA and protein levels. PTEN was also highly expressed in gastric
cancer tissues and negatively correlated with miR-28 expression
level. PTEN knockdown restored functional roles of miR-28 in
gastric cancer cells. Collectively, these data demonstrated that
PTEN is a direct and functional downstream target of miR-28 in
gastric cancer.
PTEN, located in 10q23.3, is a well-known
tumour suppressor (33). Emerging
evidence revealed reduced expression levels of PTEN in various
human cancers, such as bladder cancer (34), colorectal cancer (35), glioma (36), lung cancer and prostate cancer
(37). PTEN deletion was reported
to be correlated with aggressive tumour phenotype and adverse
prognosis in human cancers (38–40).
Accumulated evidence confirmed roles of PTEN in biological
processes, such as cell proliferation, cell cycle, apoptosis,
migration, invasion, metastasis, metabolism, differentiation,
transcription and translation, through inhibition of multiple cell
signalling pathways (41–43). In gastric cancer, PTEN is
downregulated and negatively correlated with lymph node metastasis,
invasion depth, growth pattern, histological classification and age
of gastric cancer patients (22,23,44).
Functional assays indicated tumour suppressive roles for PTEN in
cell apoptosis, cell cycle arrest, proliferation, invasion and
metastasis in gastric cancer cells (45–47).
Considering importance and role of PTEN in gastric cancer, this
homolog may be developed as a therapeutic target for patients with
gastric cancer.
In conclusion, miR-28 is upregulated in gastric
cancer and plays oncogenic roles through regulation of
PTEN/PI3K/AKT signalling pathway. Therefore, this research proposes
that miR-28 can be targeted for development of novel treatment for
gastric cancer in the future. In following experiments, we will
explore the effect of miR-28 on gastric cancer cell proliferation
and invasion in vivo.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferro A, Peleteiro B, Malvezzi M, Bosetti
C, Bertuccio P, Levi F, Negri E, La Vecchia C and Lunet N:
Worldwide trends in gastric cancer mortality (1980–2011), with
predictions to 2015, and incidence by subtype. Eur J Cancer.
50:1330–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kato M and Asaka M: Recent knowledge of
the relationship between Helicobacter pylori and gastric cancer and
recent progress of gastroendoscopic diagnosis and treatment for
gastric cancer. Jpn J Clin Oncol. 40:828–837. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li L, Ying XJ, Sun TT, Yi K, Tian HL, Sun
R, Tian JH and Yang KH: Overview of methodological quality of
systematic reviews about gastric cancer risk and protective
factors. Asian Pac J Cancer Prev. 13:2069–2079. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moon YW, Jeung HC, Rha SY, Yoo NC, Roh JK,
Noh SH, Kim BS and Chung HC: Changing patterns of prognosticators
during 15-year follow-up of advanced gastric cancer after radical
gastrectomy and adjuvant chemotherapy: A 15-year follow-up study at
a single korean institute. Ann Surg Oncol. 14:2730–2737. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marano L, Polom K, Patriti A, Roviello G,
Falco G, Stracqualursi A, De Luca R, Petrioli R, Martinotti M,
Generali D, et al: Surgical management of advanced gastric cancer:
An evolving issue. Eur J Surg Oncol. 42:18–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Markar SR, Karthikesalingam A, Jackson D
and Hanna GB: Long-term survival after gastrectomy for cancer in
randomized, controlled oncological trials: Comparison between West
and East. Ann Surg Oncol. 20:2328–2338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Orditura M, Galizia G, Sforza V,
Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J,
Savastano B, Mabilia A, et al: Treatment of gastric cancer. World J
Gastroenterol. 20:1635–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu J, Wang R, Chen J, Wu J, Dang Z, Zhang
Q and Li B: miR-340 inhibits proliferation and induces apoptosis in
gastric cancer cell line SGC-7901, Possibly via the AKT Pathway.
Med Sci Monit. 23:71–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
14
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garzon R and Marcucci G: Potential of
microRNAs for cancer diagnostics, prognostication and therapy. Curr
Opin Oncol. 24:655–659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu D, Niu X, Pan H, Zhou Y, Zhang Z, Qu P
and Zhou J: Tumor-suppressing effects of microRNA-429 in human
renal cell carcinoma via the downregulation of Sp1. Oncol Lett.
12:2906–2911. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shrestha S, Hsu SD, Huang WY, Huang HY,
Chen W, Weng SL and Huang HD: A systematic review of microRNA
expression profiling studies in human gastric cancer. Cancer Med.
3:878–888. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Almeida MI, Nicoloso MS, Zeng L, Ivan C,
Spizzo R, Gafà R, Xiao L, Zhang X, Vannini I, Fanini F, et al:
Strand-specific miR-28-5p and miR-28-3p have distinct effects in
colorectal cancer cells. Gastroenterology. 142:886–896.e9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang C, Wu C, Yang Q, Ding M, Zhong J,
Zhang CY, Ge J, Wang J and Zhang C: miR-28-5p acts as a tumor
suppressor in renal cell carcinoma for multiple antitumor effects
by targeting RAP1B. Oncotarget. 7:73888–73902. 2016.PubMed/NCBI
|
|
20
|
Schneider C, Setty M, Holmes AB, Maute RL,
Leslie CS, Mussolin L, Rosolen A, Dalla-Favera R and Basso K:
MicroRNA 28 controls cell proliferation and is down-regulated in
B-cell lymphomas. Proc Natl Acad Sci USA. 111:pp. 8185–8190. 2014;
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fei G, Ebert MP, Mawrin C, Leodolter A,
Schmidt N, Dietzmann K and Malfertheiner P: Reduced PTEN expression
in gastric cancer and in the gastric mucosa of gastric cancer
relatives. Eur J Gastroenterol Hepatol. 14:297–303. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng HC, Li YL, Sun JM, Yang XF, Li XH,
Jiang WG, Zhang YC and Xin Y: Growth, invasion, metastasis,
differentiation, angiogenesis and apoptosis of gastric cancer
regulated by expression of PTEN encoding products. World J
Gastroenterol. 9:1662–1666. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu WT, Yang Z and Lu NH: Roles of PTEN
(phosphatase and tensin homolog) in gastric cancer development and
progression. Asian Pac J Cancer Prev. 15:17–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jing X, Cheng W, Wang S, Li P and He L:
Resveratrol induces cell cycle arrest in human gastric cancer
MGC803 cells via the PTEN-regulated PI3K/Akt signaling pathway.
Oncol Rep. 35:472–478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang SQ, Wang C, Chang LM, Zhou KR, Wang
JW, Ke Y, Yang DX, Shi HG, Wang R, Shi XL, et al: Geridonin and
paclitaxel act synergistically to inhibit the proliferation of
gastric cancer cells through ROS-mediated regulation of the
PTEN/PI3K/Akt pathway. Oncotarget. 7:72990–73002. 2016.PubMed/NCBI
|
|
27
|
Adams BD, Kasinski AL and Slack FJ:
Aberrant regulation and function of microRNAs in cancer. Curr Biol.
24:R762–R776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mirnezami AH, Pickard K, Zhang L, Primrose
JN and Packham G: MicroRNAs: Key players in carcinogenesis and
novel therapeutic targets. Eur J Surg Oncol. 35:339–347. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou SL, Hu ZQ, Zhou ZJ, Dai Z, Wang Z,
Cao Y, Fan J, Huang XW and Zhou J: miR-28-5p-IL-34-macrophage
feedback loop modulates hepatocellular carcinoma metastasis.
Hepatology. 63:1560–1575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu J, Jiang N, Shi H, Zhao S, Yao S and
Shen H: (Corrigendum) miR-28-5p promotes the development and
progression of ovarian cancer through inhibition of N4BP1. Int J
Oncol. 50:22362017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi X and Teng F: Down-regulated miR-28-5p
in human hepatocellular carcinoma correlated with tumor
proliferation and migration by targeting insulin-like growth
factor-1 (IGF-1). Mol Cell Biochem. 408:283–293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maehama T and Dixon JE: The tumor
suppressor, PTEN/MMAC1, dephosphorylates the lipid second
messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem.
273:13375–13378. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tanaka M, Koul D, Davies MA, Liebert M,
Steck PA and Grossman HB: MMAC1/PTEN inhibits cell growth and
induces chemosensitivity to doxorubicin in human bladder cancer
cells. Oncogene. 19:5406–5412. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sawai H, Yasuda A, Ochi N, Ma J, Matsuo Y,
Wakasugi T, Takahashi H, Funahashi H, Sato M and Takeyama H: Loss
of PTEN expression is associated with colorectal cancer liver
metastasis and poor patient survival. BMC Gastroenterol. 8:562008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ermoian RP, Furniss CS, Lamborn KR, Basila
D, Berger MS, Gottschalk AR, Nicholas MK, Stokoe D and Haas-Kogan
DA: Dysregulation of PTEN and protein kinase B is associated with
glioma histology and patient survival. Clin Cancer Res.
8:1100–1106. 2002.PubMed/NCBI
|
|
37
|
Mithal P, Allott E, Gerber L, Reid J,
Welbourn W, Tikishvili E, Park J, Younus A, Sangale Z, Lanchbury
JS, et al: PTEN loss in biopsy tissue predicts poor clinical
outcomes in prostate cancer. Int J Urol. 21:1209–1214. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cordes I, Kluth M, Zygis D, Rink M, Chun
F, Eichelberg C, Dahlem R, Fisch M, Höppner W, Wagner W, et al:
PTEN deletions are related to disease progression and unfavourable
prognosis in early bladder cancer. Histopathology. 63:670–677.
2013.PubMed/NCBI
|
|
39
|
Wise HM, Hermida MA and Leslie NR:
Prostate cancer, PI3K, PTEN and prognosis. Clin Sci (Lond).
131:197–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tao J, Xiong J, Li T, Yang Z, Li X, Li K,
Wu H and Wang C: Correlation between protein expression of PTEN in
human pancreatic cancer and the proliferation, infiltration,
metastasis and prognosis. J Huazhong Univ Sci Technolog Med Sci.
26:444–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stambolic V, Suzuki A, de la Pompa JL,
Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM,
Siderovski DP and Mak TW: Negative regulation of PKB/Akt-dependent
cell survival by the tumor suppressor PTEN. Cell. 95:29–39. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kohnoh T, Hashimoto N, Ando A, Sakamoto K,
Miyazaki S, Aoyama D, Kusunose M, Kimura M, Omote N, Imaizumi K, et
al: Hypoxia-induced modulation of PTEN activity and EMT phenotypes
in lung cancers. Cancer Cell Int. 16:332016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nogueira C, Kim KH, Sung H, Paraiso KH,
Dannenberg JH, Bosenberg M, Chin L and Kim M: Cooperative
interactions of PTEN deficiency and RAS activation in melanoma
metastasis. Oncogene. 29:6222–6232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou YJ, Xiong YX, Wu XT, Shi D, Fan W,
Zhou T, Li YC and Huang X: Inactivation of PTEN is associated with
increased angiogenesis and VEGF overexpression in gastric cancer.
World J Gastroenterol. 10:3225–3229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zheng T, Meng X, Wang J, Chen X, Yin D,
Liang Y, Song X, Pan S, Jiang H and Liu L: PTEN- and p53-mediated
apoptosis and cell cycle arrest by FTY720 in gastric cancer cells
and nude mice. J Cell Biochem. 111:218–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang LL, Liu J, Lei S, Zhang J, Zhou W
and Yu HG: PTEN inhibits the invasion and metastasis of gastric
cancer via downregulation of FAK expression. Cell Signal.
26:1011–1020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
He RF, Hu ZL and Wen JF: Biological
implication of PTEN gene expression in human gastric cancer and
related molecular mechanisms. Zhonghua Bing Li Xue Za Zhi.
36:324–328. 2007.(In Chinese). PubMed/NCBI
|