Introduction
Pneumonia is a respiratory condition in which lung
inflammation is caused by pathogens or other factors (1). In children aged <5 years,
pneumonia is a major cause of mortality and morbidity (2), and acute pneumonia poses a serious
risk to the life or health of children (3,4). It
has been estimated that ~800,000 deaths caused by pneumonia occur
each year among children <5 years of age (5). A better understanding of the
mechanisms underlying acute pneumonia in children may facilitate
the development of effective therapeutic strategy, thus reducing
the burden of this disease.
microRNAs (miRNAs or miRs) are small, noncoding RNAs
that have important functions in posttranscriptional regulation of
gene expression (6,7). miRNAs have roles in a number of
biological processes, such as cell proliferation, apoptosis, and
innate immunity (8–10). miRNAs have also been identified as
key regulators in the pathological processes of a number of
diseases, including cancers (11),
neurological diseases (12) and
inflammatory disease (13,14). In the present study miR-3941 was
investigated, which was recently determined to be associated with
the malignant progression of lung cancer via regulating
immunoglobulin (CD79a) binding protein 1 (15). miR-3941 has also been implicated in
the tumorigenesis of colorectal cancer via interaction with ATP
binding cassette subfamily A member 6 (16). miR-3941 is also associated with
acute lymphoblastic leukemia in Chinese children via controlling
insulin-like growth factor (IGF)1 (17). However, to the best of our
knowledge the potential roles of miR-3941 in acute pneumonia in
child patients have not yet been the subject of study.
In the present study, the expression of miR-3941 in
child patients with acute pneumonia was detected. In previous
studies, A549 cells treated with lipopolysaccharides (LPS) have
been used as a cellular model for exploring the key mechanisms
associated with respiratory diseases including acute lung injury
and pneumonia (18–20). Therefore, to investigate the
potential roles of miR-3941 in regulating the development of acute
pneumonia, the effects of miR-3941 in LPS-induced A549 cell injury
were investigated by assessing cell viability, apoptosis and
inflammation. In addition, the regulatory relationship between
miR-3941 and IGF2 was explored, as well as the association between
miR-3941 and the phosphatidylinositol-4,5-bisphosphate
3-kinase/protein kinase B (PI3K/AKT) pathway. It was hypothesized
that down-regulation of miR-3941 may inhibit cell viability, induce
cell apoptosis and enhance the production of cytokines via
targeting IGF2 and activating the PI3K/AKT pathway, thus
aggravating LPS-induced cell injury in A549 cells. The findings of
the present study may provide new insights for the diagnosis and
treatment of acute pneumonia in child patients.
Materials and methods
Subjects
Between May 2015 and April 2016, a total of 20 child
patients with acute pneumonia (female:male, 8:12; age range 0.5–12
years; mean age 6.26±1.46 years) admitted to the Department of
Pediatrics (Huangshi Central Hospital, Huangshi, China) were
enrolled in the present study. Patients with nervous system
diseases, endocrine system disease, diseases of the circulatory
system, or failure of the heart, liver, kidney or other organs were
excluded. In addition, a total of 20 health controls (female:male,
7:13; age range 0.4–12.3 years; mean age 6.38±1.29 years) who
underwent a physical examination between April 2015 and March 2016
were also recruited. Variables including age, gender, height,
weight and body mass index (BMI) between patients with acute
pneumonia and healthy controls were comparable. The present study
was approved by the Ethics Committee of the Huangshi Central
Hospital (Hubei, China) and written informed consent was obtained
from the parent(s) of each patient.
Collection of blood samples
A total of 3 ml fasting peripheral venous blood was
harvested from each patient and control subject. Serum was obtained
following centrifugation of blood samples at 4,000 × g at 4°C for
10 min and stored at −80°C.
Cell culture and treatment
The human pulmonary epithelial cell line A549
(American Type Culture Collection, Manassas, VA, USA) was cultured
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and 1% glutamine (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified incubator
with 5% CO2 for 24–48 h. Cells were treated with 1 µg/ml
LPS (0.5 ng; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or
dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA) for 24, 48 and
72 h. DMSO was used as the control and its concentration in the
medium was kept at 0.1% to avoid toxicity.
Cell transfection
Cells (1×104 cells/well) were seeded in a
6-well plate. miR-3941 mimics (50 nM), normal control (NC) mimics
(50 nM), miR-3941 inhibitors (150 nM), NC inhibitors (150 nM), IGF2
specific small interfering RNA (siRNA) (100 nM) or si-NC (100 nM)
were all obtained from Shanghai GenePharma Co., Ltd. (Shanghai,
China) and then transfected into A549 cells using HiPerFect
transfection reagent (Qiagen GmbH, Hilden, Germany) according to
the manufacturer's protocol. Primer sequences were as follows:
miR-3941 mimics sense, 5′-UUACACACAACUGAGGAUCAUA-3′ and mimic
antisense 5′-UGAUCCUCAGUUGUGUGUAAUU-3′; miR-3941 inhibitors
5′-UAUGAUCCUCAGUUGUGUGUAA-3′; si-IGF2 5′-UCGUUGAGGAGUGCUGUUUdTdT3′
and si-NC 5′-GGCAUAGAUGUAGCUGUAAdTdT3′. A total of 48 h following
transfection, the cells were collected for further experiments.
MTT assay
Cell viability was determined via an MTT assay.
Briefly, A549 cells were plated in a 96-well plate
(1×104 cells/well; 37°C) containing serum-free
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) overnight. Following treatment with LPS or
transfection, cells were cultured with DMEM containing 10% FBS and
0.5 mg/ml MTT at 37°C for a further 4 h. To dissolve the formazan
crystals, DMSO was added. Cell viability was finally determined by
measuring the absorbance at 550 nm with a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Flow cytometry
Cell apoptosis was assessed using flow cytometry.
Briefly, A549 cells were cultured in a 96-well plate
(1×104 cells/well) at 37°C for 24–48 h in DMEM.
Following treatment with LPS or transfection, cells were suspended
in HEPES buffer containing annexin V-FITC and propidium iodide (PI)
(all, BD Biosciences, Franklin Lakes, NJ, USA) for 15 min at room
temperature. Following double staining for 1 h in the dark at 4°C,
cell apoptosis was assessed by flow cytometry using a FACScan flow
cytometer (Beckman Coulter, Inc., Brea, CA, USA). Apoptotic cells
were analyzed using BD CellQuest 3.0 software (BD Biosciences,
Franklin Lakes, NJ, USA).
ELISA assay
To determine levels of interleukin (IL)-6, IL-8 and
tumor necrosis factor (TNF)-α, an ELISA assay was conducted. ELISA
kits for IL-6 (cat. no. D6050), IL8 (cat. no. D8000C) and TNF-α
(cat. no. DTA00C) were purchased from R&D Systems, Inc.
(Minneapolis, MN, USA). Briefly, samples from A549 cells were
incubated with diluted antibody solution for 1 h at room
temperature, horseradish peroxidase (HRP) solution for another 1 h
and 3,3′,5,5′-tetramethylbenzidine solution for 15 min in the dark.
Within 5 min following termination, the optical density at 450 nm
was determined using a microplate reader (BioTek Instruments,
Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from A549 cells from
different treatment groups using the microRNA Extraction and
Purification kit (Shanghai Novland Co., Ltd., Shanghai, China). The
relative expression levels of miR-3941 and IGF2 were detected using
qPCR on an iQ5 Real-Time PCR system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) using a Two-Step Stemaim-it miR qRT-PCR
Quantitation kit (SYBR Green; Shanghai Novland Co., Ltd.). The
following thermocycling conditions were used for the PCR: Initial
denaturation for 2 min at 95°C, 40 cycles of 94°C for 30 sec, 60°C
for 30 sec, and 72°C for 2 min, and a final extension at 72°C for 5
min. The following primer sequences were used for the PCR: miR-3941
5′-TTACACACAACTGAGGATCATA-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′, reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; IGF2 forward:
5′-ATGTCACCCATGTCACCAAG-3′, reverse: 5′-GGCTTGTGCCAATTAGGTTCT-3′.
β-actin forward, 5′-GGGCACAGTGTGGGTGAC-3′, reverse
5′-CTGGCACCACACCTTCTAC-3′. U6 and β-actin were used as internal
controls for quantitative normalization of miRNA and mRNA,
respectively. The relative expression levels of miR-3941 and IGF2
were determined using the 2−ΔΔCq method (21).
Bioinformatics analysis
TargetScan Human 6.2 software (http://www.targetscan.org) was used to predict
biological targets of miRNAs.
Luciferase reporter assay
To determine whether IGF2 is a direct target of
miR-3941, complementary oligonucleotide containing the miR-3941
target site from the 3′-untranslated region (3′-UTR) downstream of
wild-type IGF2 (IGF2 3′UTR-WT) was synthesized by Invitrogen
(Thermo Fisher Scientific, Inc.). Following transfection, the DNA
samples from the A549 cells were extracted. The miR-3941-predicted
target sequence was then cloned in the p-MIR-REPORT luciferase
plasmid (Sangon Biotech Co., Ltd., Shanghai, China). A mutated
miR-3941 target sequence with identical flanking nucleotides of
IGF2 (IGF2 3′UTR-MUT) Invitrogen (Thermo Fisher Scientific, Inc.)
was used as the control. Cell transfection was performed using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
Following 48 h of transfection, the lysate was analyzed using a
Dual-Glo luciferase assay system (cat. no. E1960; Promega
Corporation, Madison, WI, USA) and the luciferase activity was
detected via a luminometer (Berthold Technologies GmbH & Co.,
KG, Wildbad, Germany). Firefly luciferase activity was normalized
to Renilla luciferase activity.
Western blot analysis
Total protein from the A549 cells was extracted
using RIPA lysis buffer (Invitrogen; Thermo Fisher Scientific,
Inc.), and the concentration of protein extracts was determined
using Bradford reagent (Bio-Rad Laboratories, Inc.). Following
separation by (20 µg/lane) 10% SDS-PAGE, protein bands were
transferred onto polyvinylidene difluoride membrane (EMD Millipore,
Billerica, MA, USA). The membranes were blocked in 1% TBS-Tween-20
(TBST) containing 5% skimmed milk for 1 h at room temperature and
washed with PBS three times. Thereafter, the blots in membranes
were incubated with primary antibodies against rabbit polyclone
IGF2 (cat. no. sc-5622), mouse monoclonal phosphorylated (p)-PI3K
(cat. no. sc-293172), mouse monoclonal PI3K (cat. no. sc-365290),
rabbit polyclonal AKT (cat. no. sc-7985-R), rabbit polyclonal p-AKT
(cat. no. sc-8312), mouse monoclonal B cell lymphoma 2 (Bcl-2)
(cat. no. sc-7382), mouse monoclonal Bcl-2-associated X protein
(Bax) (cat. no. sc-7480), mouse monoclonal pro-caspase-3 (cat. no.
sc-7272), rabbit polyclone caspase-3 (cat. no. sc-7148), mouse
monoclonal pro-caspase-9 (cat. no. sc-17784), mouse monoclonal
caspase-9 (cat. no. sc-56073) and mouse monoclonal GAPDH (cat. no.
sc-47724) at 4°C overnight (all 1:1,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and subsequently probed with anti
rabbit/mouse horseradish peroxidase-conjugated secondary antibodies
(1:5,000; cat. nos. 5571 and 7074, respectively; both Cell
Signaling Technology, Inc., Danvers, MA, USA). The protein bands
were finally detected using an enhanced chemiluminescence detection
kit (EMD Millipore).
Statistical analysis
All experiments were repeated three times and data
obtained from multiple experiments were presented as the mean ± or
+ standard deviation. The normal distribution of data was assessed
via one-sample Kologrov-Smirnov test. Statistical analysis of data
was performed using one-way analysis of variance followed by a
Tukey-Kramer's post hoc test or Student's t-test using SPSS 19.0
(IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
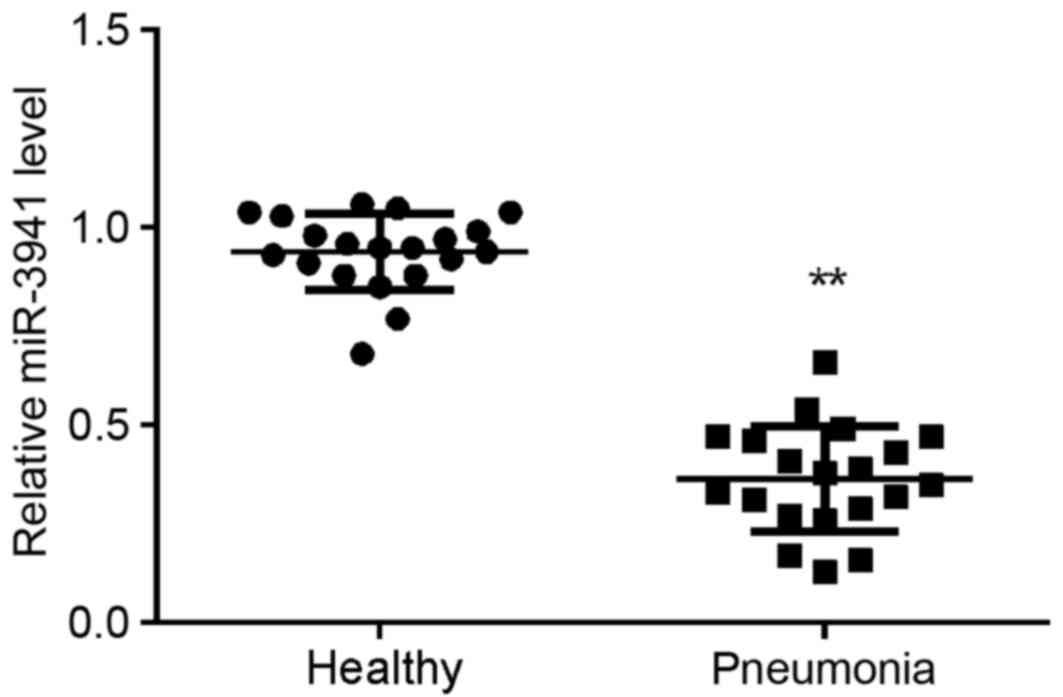
miR-3941 is downregulated in the serum
of child patients with acute pneumonia
A total of 20 child patients with acute pneumonia
and 20 healthy controls were enrolled in the present study. As
demonstrated in Fig. 1, miR-3941
expression was significantly decreased in the serum of children
with acute pneumonia compared with that in the serum of controls
(P<0.01).
LPS induces A549 cell injury and
decreases miR-3941 levels
A549 cells were treated with 1 µg/ml LPS to
establish a cellular model of acute pneumonia. During the 72 h
experimental period, LPS treatment significantly inhibited cell
viability at all time points (P<0.05; Fig. 2A). In addition, compared with the
control group, treatment with 1 µg/ml LPS for 48 h significantly
induced cell apoptosis (P<0.01; Fig. 2B) and markedly altered the
expression of apoptosis-relation proteins: Bcl-2, pro-caspase-3 and
pro-caspase-9 were down-regulated, whereas Bax, cleaved-caspase-3
and cleaved-caspase-9 were markedly upregulated (Fig. 2C). Furthermore, compared with the
control group, LPS treatment was demonstrated to significantly
increase the production of IL-6, IL-8 and TNF-α (P<0.01;
Fig. 2D) in A549 cells, which
indicated that the cellular model of acute pneumonia was
successfully established. Therefore, the expression of miR-3941 was
explored. The results demonstrated that LPS treatment resulted in
the significantly decreased expression of miR-3941 in A549 cells
(P<0.01; Fig. 2E), which
suggested that miR-3941 may have a key role in the development of
pneumonia.
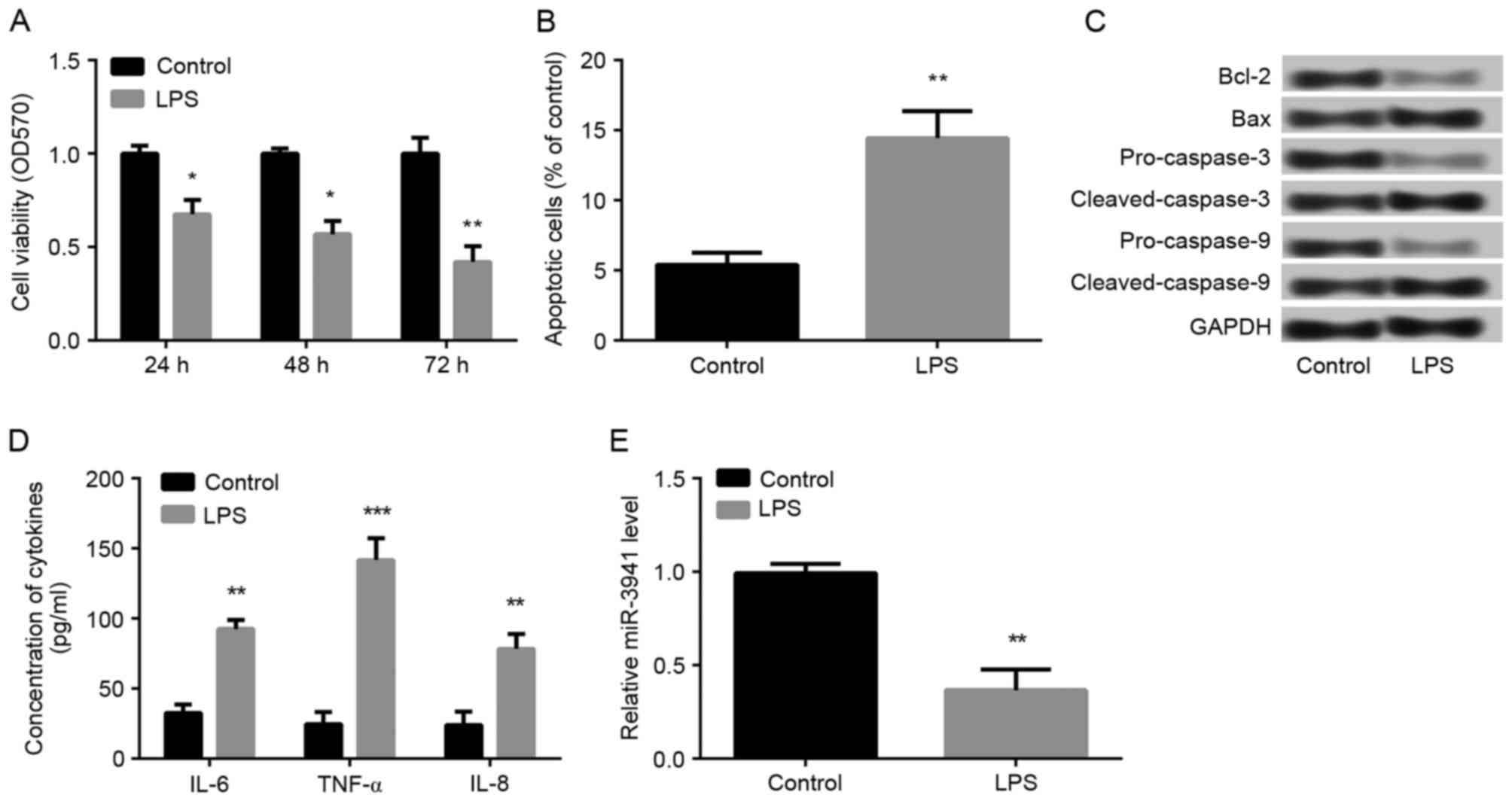 | Figure 2.LPS induced A549 cell injury and
decreased miR-3941 levels. Cells were treated with 1 µg/ml LPS or
dimethyl sulfoxide (control). (A) MTT assay demonstrated that cell
viability was significantly inhibited following treatment of LPS
for 24, 48 and 72 h. (B) Flow cytometry revealed that cell
apoptosis was significantly increased following treatment with 1
µg/ml LPS for 48 h. (C) Western blot analysis demonstrated the
expression changes of the apoptosis-related proteins Bcl-2, Bax,
pro-caspase-3, cleaved-caspase-3, pro-caspase-9 and
cleaved-caspase-9 following treatment with 1 µg/ml LPS for 48 h.
(D) ELISA analysis determined the enhanced production of IL-6, IL-8
and TNF-α in A549 cells following treatment with 1 µg/ml LPS for 48
h. (E) Reverse transcription-quantitative polymerase chain reaction
showed that the expression of miR-3941 was significantly decreased
following treatment with 1 µg/ml LPS for 48 h. Data are presented
as the mean + standard deviation (n=3). *P<0.05, **P<0.01,
***P<0.001 vs. control. LPS, lipopolysaccharides; miR, microRNA;
Bcl-2, B cell lymphoma 2; Bax, Bcl-2-associated X protein; IL,
interleukin; TNF, tumor necrosis factor; OD, optical density. |
Overexpression of miR-3941 alleviated
LPS-induced cell injury
To further investigate the effects of miR-3941,
miR-3941 was overexpressed in A549 cells by transfection with
miR-3941 mimic. As expected, it was demonstrated that miR-3941
expression was significantly increased in cells transfected with
miR-3941 mimic compared with cells transfected with mimic NC and
control cells (P<0.01; Fig.
3A). Furthermore, overexpression of miR-3941 ameliorated the
LPS-induced cell injury in LPS-treated cells via significantly
promoting cell viability (P<0.01; Fig. 3B) and inhibiting cell apoptosis
(P<0.01; Fig. 3C), markedly
reversing the LPS-induced expression changes of apoptosis-related
proteins (Fig. 3D), and
significantly suppressing the production of IL-6, IL-8 and TNF-α
(P<0.01; Fig. 3E).
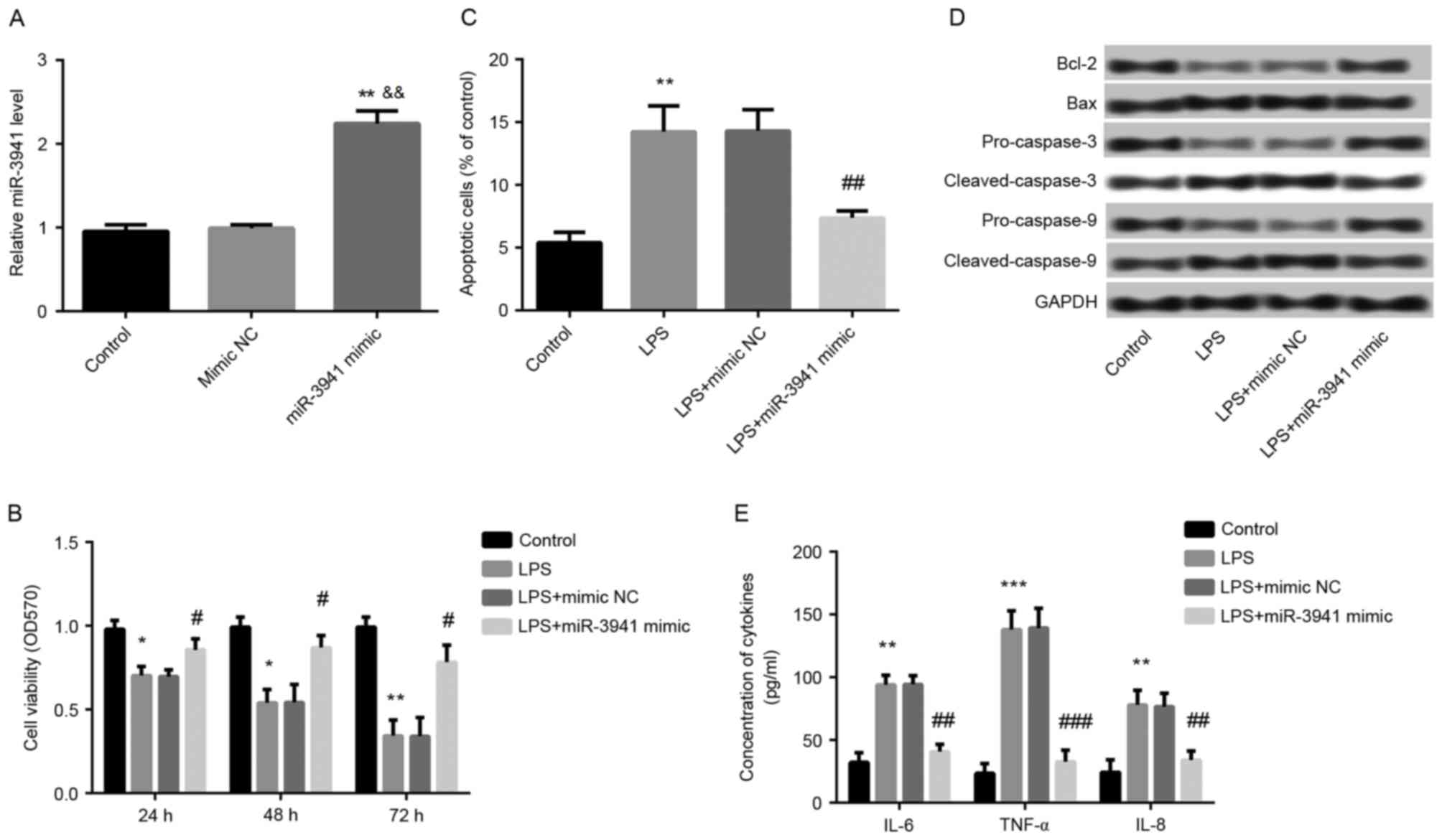 | Figure 3.Overexpression of miR-3941 alleviated
LPS-induced cell injury. Cells were treated with 1 µg/ml LPS or
dimethyl sulfoxide (control). A part of LPS-induced cells were
transfected with miR-3941 mimic or mimic NC. (A) Reverse
transcription-quantitative polymerase chain reaction revealed the
expression of miR-3941 in different treated groups. Compared with
controls, miR-3941 expression was significantly increased in
miR-3941 mimic transfected cells. (B) MTT assay showed cell
viability in different treated groups. The LPS-induced decreased
cell ability was significantly ameliorated by miR-3941 mimic. (C)
Flow cytometry demonstrated cell apoptosis in different treated
groups. The LPS-induced cell apoptosis was significantly reversed
by miR-3941 mimic. (D) Western blotting revealed the expression
changes of the apoptosis-related proteins Bcl-2, Bax,
pro-caspase-3, cleaved-caspase-3, pro-caspase-9 and
cleaved-caspase-9 in different treated groups. The LPS-induced
decreased cell ability was significantly reversed by miR-3941
mimic. (E) ELISA indicated the production of cytokines (IL-6, IL-8
and TNF-α). The LPS-induced production of cytokines was reduced by
miR-3941 mimic. Data are presented as the mean + standard deviation
(n=3). *P<0.05, **P<0.01, ***P<0.001 vs. control;
&&P<0.01 vs mimic NC; #P<0.05,
##P<0.01, ###P<0.001 vs. LPS group.
miR, microRNA; LPS, lipopolysaccharides; Bcl-2, B cell lymphoma 2;
Bax, Bcl-2-associated X protein; IL, interleukin; TNF, tumor
necrosis factor; NC, normal control; OD, optical density. |
IGF2 is a direct target gene of
miR-3941
By means of TargetScanHuman, the predicted binding
sequences of IGF2 and miR-3941 were obtained (Fig. 4A). The results of luciferase
reporter analysis demonstrated that the relative luciferase
activity containing the IGF2 3′UTR-WT was significantly decreased
in miR-3941 mimic-transfected cells compared with controls
(P<0.05; Fig. 4B). However, the
relative luciferase activity containing the IGF2 3′UTR-MUT was not
significantly different between miR-3941 mimic-transfected cells
and control cells (Fig. 4B).
Furthermore, IGF2 expression was significantly downregulated in the
miR-3941 mimic group compared with mimic NC group, and upregulated
in the miR-3941 inhibitor group (P<0.05; Fig. 4C). This was supported by similar,
marked differences in IGF2 protein expression (Fig. 4D). LPS treatment was also
demonstrated to significantly promote the mRNA expression
(P<0.05; Fig. 4E) and markedly
increase the protein expression (Fig.
4F) of IGF2 in A549 cells. These findings indicated that IGF2
was the direct target of miR-3941.
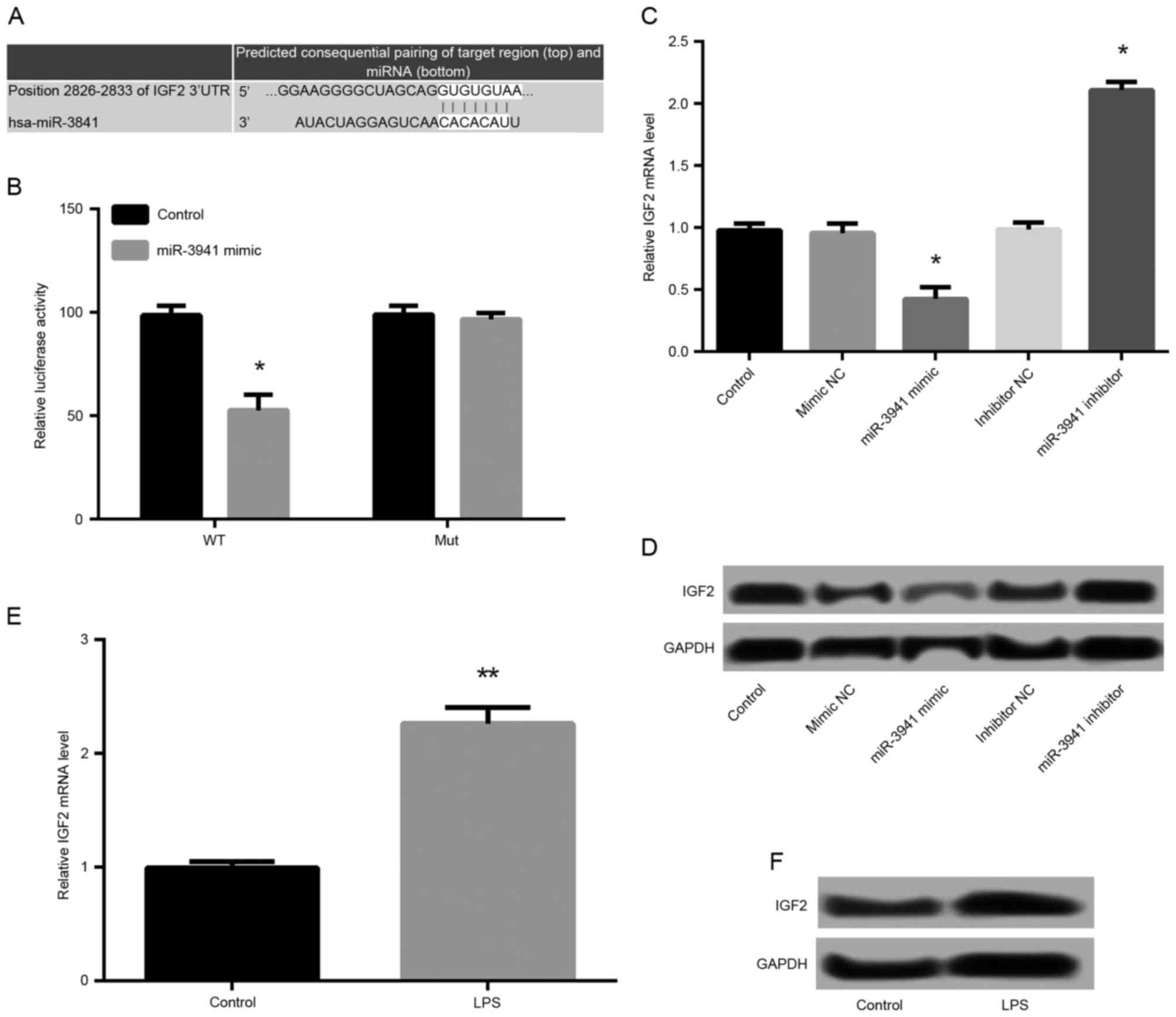 | Figure 4.IGF2 was a direct target gene of
miR-3941. (A) The predicted binding sequences of IGF2 and miR-3941.
(B) Luciferase reporter analysis confirmed that the relative
luciferase activity containing WT were significantly decreased in
miR-3941 mimic-transfected cells compared with control. *P<0.05
vs. the control group. (C and D) Cells were transfected with
miR-3941 mimic, mimic NC, miR-3941 inhibitor, or inhibitor NC. The
mRNA and protein expression of IGF2 in different transfected groups
was measured. IGF2 was downregulated in the miR-3941 mimic group
compared with mimic NC group and upregulated in the miR-3941
inhibitor group. *P<0.05 vs. the mimic NC group. (E and F) The
mRNA and protein expression of IGF2 were increased following LPS
treatment. Data are presented as the mean + standard deviation
(n=3). **P<0.01 vs. the control group. IGF2, insulin-like growth
factor 2; miR, microRNA; 3′UTR, 3′-untranslated region; WT,
miR-3941 target site from the 3′-UTR downstream of wild-type IGF2;
NC, normal control; LPS, lipopolysaccharides; MUT, mutated miR-3941
target sequence with identical flanking nucleotides of IGF2. |
Effects of IGF2 suppression on cell
viability, apoptosis, and expression of cytokines
The effects of IGF2 on the development of pneumonia
were subsequently investigated. As demonstrated in Fig. 5A, the IGF2 protein expression was
markedly decreased and mRNA expression was significantly decreased
in the si-IGF2 group compared with the si-NC group (P<0.05),
which indicated that IGF2 was successfully knocked down in A549
cells. In addition, compared with the LPS + si-NC + inhibitor NC
group, significantly increased cell viability (P<0.05; Fig. 5B), significantly inhibited cell
apoptosis (P<0.01; Fig. 5C),
and significantly decreased production of IL-6, IL-8 and TNF-α
(P<0.01; Fig. 5E) were observed
in the LPS+si-IGF2 group, which demonstrated that knockdown of IGF2
significantly alleviated LPS-induced cell injury. In addition,
suppression of miR-3941 by transfection with miR-3941 inhibitor
significantly (P<0.05; Fig. 5B, C
and E) and markedly (Fig. 5D)
ameliorated the effects of IGF2 knockdown on LPS-induced cell
injury.
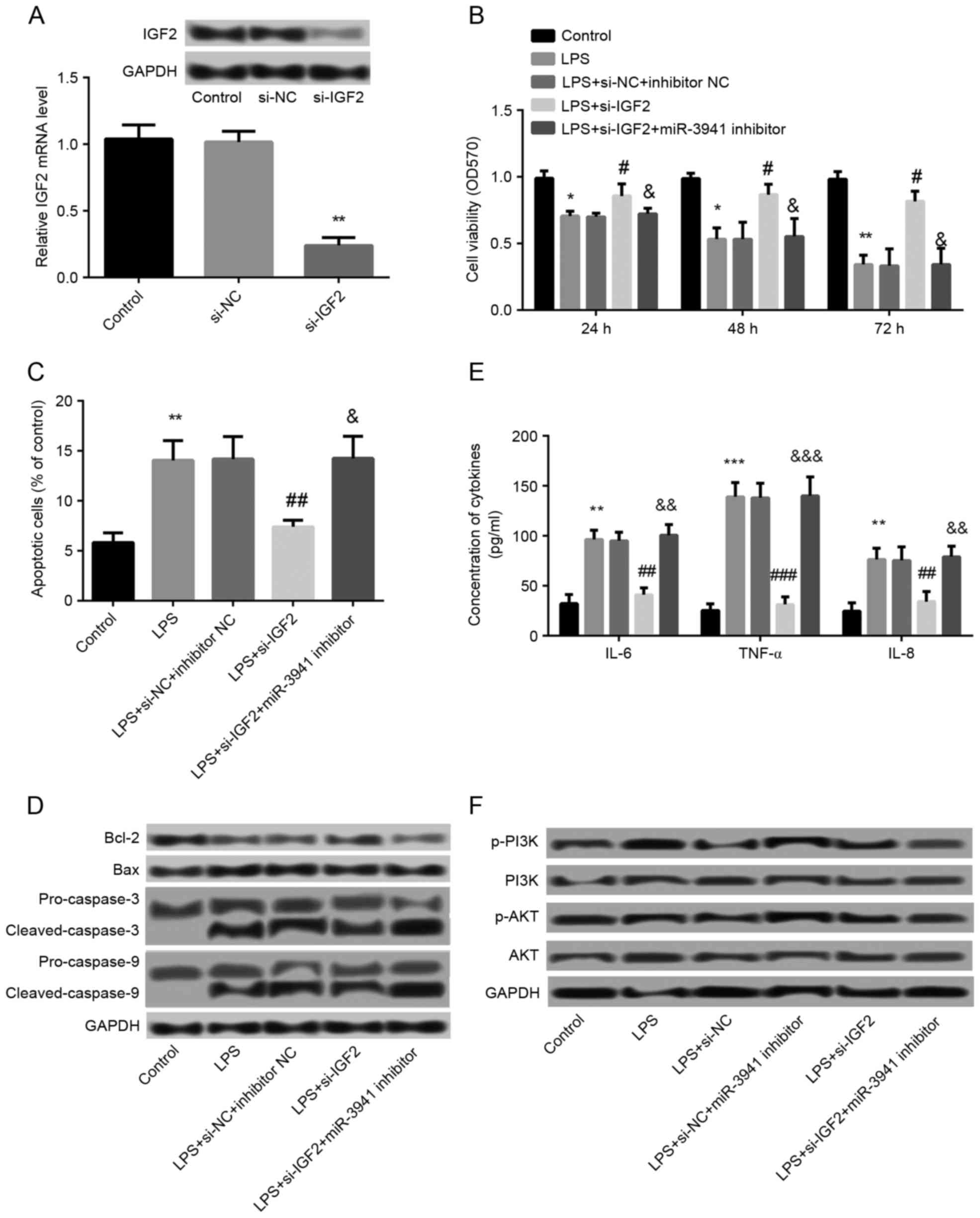 | Figure 5.Effects of IGF2 suppression on cell
viability, apoptosis, and expression of cytokines. (A) Cells were
transfected with si-IGF2 and si-NC. The mRNA and protein expression
of IGF2 was decreased in the si-IGF2 group compared with the si-NC
and control groups. **P<0.01 vs. the si-NC group. (B) MTT assay
showed cell viability in different groups which were treated with
LPS and then transfected with si-IGF2, si-NC, miR-3941 inhibitor or
inhibitor NC. (C) Flow cytometry revealed the cell apoptosis in
different treated groups. (D) Western blotting demonstrated the
expression changes of the apoptosis-related proteins Bcl-2, Bax,
pro-caspase-3, cleaved-caspase-3, pro-caspase-9 and
cleaved-caspase-9 in different treated groups. (E) ELISA indicated
the production of cytokines IL-6, IL-8 and TNF-α in different
treated groups. (F) Western blotting revealed the expression of
PI3K, p-PI3K, AKT and p-AKT in different treated groups. Data are
presented as the mean + standard deviation (n=3). *P<0.05,
**P<0.01, ***P<0.001 vs. control; #P<0.05,
##P<0.01, ###P<0.001 vs. LPS + si-NC +
inhibitor NC group; &P<0.05,
&&P<0.01,
&&&P<0.001 vs. LPS+si-IGF2 group. IGF2,
insulin-like growth factor 2; siRNA, small interfering RNA; NC,
normal control; LPS, lipopolysaccharides; miR, microRNA; Bcl-2, B
cell lymphoma 2; Bax, Bcl-2-associated X protein; IL, interleukin;
TNF, tumor necrosis factor; NC, normal control; PI3K,
phosphatidylinositol-4,5-bisphosphate 3-kinase; p, phosphorylated;
AKT, protein kinase B; OD, optical density, si-NC, small
interfering-normal control. |
Association between miR-3941 and the
PI3K/AKT pathway
To further explore the regulatory mechanism of
miR-3941, the association between miR-3941 and the PI3K/AKT pathway
was investigated. As shown in Fig.
5F, LPS treatment led to the markedly increased phosphorylation
level of p-PI3K and p-AKT compared with controls. Compared with the
LPS group, the expression of p-PI3K and p-AKT were decreased in the
LPS+si-NC+miR-3941 inhibitor group and increased in the LPS+si-IGF2
group. In addition, the expression of p-PI3K and p-AKT was markedly
decreased in the LPS+si-IGF2+miR-3941 inhibitor group. These
findings indicated that inhibition of miR-3941 may be able to
activate the PI3K/AKT pathway, whereas knockdown of IGF2 may
inhibit the activation of the PI3K/AKT pathway.
Discussion
miRNAs have been identified as regulators in the
pathological process of a number of inflammatory diseases (22). In the present study, miR-3941 was
demonstrated to be downregulated in children with acute pneumonia.
In the cellular model of acute pneumonia, LPS treatment
significantly induced cell injury via inhibiting cell viability,
inducing cell apoptosis and enhancing the production of cytokines.
LPS treatment also resulted in a significantly decreased expression
of miR-3941 in A549 cells and overexpression of miR-3941 alleviated
LPS-induced cell injury. In addition, IGF2 was confirmed as a
direct target gene of miR-3941. Knockdown of IGF2 significantly
alleviated LPS-induced cell injury, which was reversed by
suppression of miR-3941. Furthermore, inhibition of miR-3941 was
demonstrated to activate the PI3K/AKT pathway, whereas knockdown of
IGF2 inhibited activation of the PI3K/AKT pathway, which suggested
the regulatory functions of miR-3941 and IGF2 with the PI3K/AKT
pathway. These findings merit further discussion.
miRNAs typically have crucial roles in several
biological processes via regulating their target genes (23). In the present study, IGF2 was
identified as a direct target gene of miR-3941. IGF2 is one of the
most intricately regulated of all growth factors that has a crucial
role in epigenetic regulation (24). In a previous study, the IGF axis
consisting of two IGFs and six IGF-binding proteins was
demonstrated to have important roles in children with inflammatory
bowel disease (25). Furthermore,
IGF2 has been shown to be associated with the development of lung
adenocarcinoma (26,27). In addition, it has previously been
reported that serum IGF2 levels have important prognostic values to
predict the treatment outcome in children with bronchopneumonia
(28,29). In the present study, knockdown of
IGF2 significantly alleviated LPS-induced cell injury by increasing
cell viability, inhibiting cell apoptosis, and decreasing
production of cytokines, which was reversed by suppression of
miR-3941. Furthermore, overexpression of miR-3941 alleviated
LPS-induced cell injury. Taken together, these findings suggest
that miR-3941 may serve a crucial role in LPS-induced acute
pneumonia via regulating IGF2. However, IGF2 is known as a potent
mitogen (30), and the possible
mechanisms of IGF2 in regulating cell viability and apoptosis
remain to be elucidated. Further study is required to investigate
the functional regulatory mechanism of IGF2 and miR-3941.
Furthermore, the association between miR-3941 and
the PI3K/AKT pathway was investigated to explore the regulatory
mechanism of miR-3941. Pneumonia results from bacteria in the
alveoli and type I alveolar epithelial cells are able to activate
innate immune responses to defend pneumonia (31). The PI3K/AKT pathway has been
demonstrated to have a role in innate immune cells (32,33).
In addition, the PI3K/AKT pathway has been previously identified to
be associated with epithelial-to-mesenchymal transition in human
lung cancer A549 cells (34). The
PI3K/AKT signaling pathway has also been confirmed to regulate the
matrix metalloproteinase-2-induced VEGF-mediated angiogenesis in
A549 lung cancer cells (35). In
the present study, the activation of the PI3K/AKT pathway was
increased following the inhibition of miR-3941, and was markedly
inhibited following knockdown of IGF2, which suggests that IGF2 is
able to inhibit the miR-3941-induced activation of the PI3K/AKT
pathway. Therefore, it was speculated that miR-3941 may target IGF2
to regulate the activation of the PI3K/AKT pathway to control the
development of LPS-induced acute pneumonia.
In conclusion, the present study indicates that
miR-3941 is downregulated in child patients with acute pneumonia
and that the downregulation of miR-3941 may promote LPS-induced
cell injury in A549 cells via targeting IGF2 to regulate the
activation of the PI3K/AKT pathway. These findings provide a
potential therapeutic strategy and target for the treatment of
acute pneumonia in children.
References
|
1
|
Li W, An X, Fu M and Li C: Emergency
treatment and nursing of children with severe pneumonia complicated
by heart failure and respiratory failure: 10 case reports. Exp Ther
Med. 12:2145–2149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen CH, Wen HJ, Chen PC, Lin SJ, Chiang
TL, Hsieh IC and Guo YL: Prenatal and postnatal risk factors for
infantile pneumonia in a representative birth cohort. Epidemiol
Infect. 140:1277–1285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin LJ, Wang YC and Liu XM: Clinical and
immunological features of common variable immunodeficiency in
China. Chin Med J (Engl). 128:310–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pancer KW: Problem of immunoglobulin M
co-detection in serological response to bacterial and viral
respiratory pathogens among children suspected of legionellosis.
Cent Eur J Immunol. 40:174–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Brien KL, Wolfson LJ, Watt JP, Henkle E,
Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS and
Cherian T; Hib and Pneumococcal Global Burden of Disease Study
Team, : Burden of disease caused by Streptococcus pneumoniae in
children younger than 5 years: Global estimates. Lancet.
374:893–902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chapman CG and Pekow J: The emerging role
of miRNAs in inflammatory bowel disease: A review. Therap Adv
Gastroenterol. 8:4–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meijer HA, Kong YW, Lu WT, Wilczynska A,
Spriggs RV, Robinson SW, Godfrey JD, Willis AE and Bushell M:
Translational repression and eIF4A2 activity are critical for
microRNA-mediated gene regulation. Science. 340:82–85. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schickel R, Boyerinas B, Park Sm and Peter
M: MicroRNAs: Key players in the immune system, differentiation,
tumorigenesis and cell death. Oncogene. 27:5959–5974. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget.
6:8474–8490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer-a brief overview. Adv Biol Regul. 57:1–9.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao DD, Li L and Chan WY: MicroRNAs: Key
regulators in the central nervous system and their implication in
neurological diseases. Int J Mol Sci. 17:8422016. View Article : Google Scholar :
|
|
13
|
Oglesby IK, Mcelvaney NG and Greene CM:
MicroRNAs in inflammatory lung disease-master regulators or target
practice? Respir Res. 11:1482010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Plank M, Maltby S, Mattes J and Foster PS:
Targeting translational control as a novel way to treat
inflammatory disease: The emerging role of microRNAs. Clin Exp
Allergy. 43:981–999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato T, Shiba-Ishii A, Kim Y, Dai T, Husni
RE, Hong J, Kano J, Sakashita S, Iijima T and Noguchi M: miR-3941:
A novel microRNA that controls IGBP1 expression and is associated
with malignant progression of lung adenocarcinoma. Cancer Sci.
108:536–542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berillo O, Régnier M and Ivashchenko A:
Binding of intronic miRNAs to the mRNAs of host genes encoding
intronic miRNAs and proteins that participate in tumourigenesis.
Comput Biol Med. 43:1374–1381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang HM, Lu JH, Chen WY and Gu AQ:
Upregulated lncRNA-UCA1 contributes to progression of lung cancer
and is closely related to clinical diagnosis as a predictive
biomarker in plasma. Int J Clin Exp Med. 8:11824–11830.
2015.PubMed/NCBI
|
|
18
|
Li W, Qiu X, Jiang H, Han Y, Wei D and Liu
J: Downregulation of miR-181a protects mice from LPS-induced acute
lung injury by targeting Bcl-2. Biomed Pharmacother. 84:1375–1382.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ke XF, Fang J, Wu XN and Yu CH:
MicroRNA-203 accelerates apoptosis in LPS-stimulated alveolar
epithelial cells by targeting PIK3CA. Biochem Biophys Res Commun.
450:1297–1303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Z, Dong D, Chen X, Huang H and Wen S:
MicroRNA-381 negatively regulates TLR4 signaling in A549 cells in
response to LPS stimulation. Biomed Res Int. 2015:8494752015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai R and Ahmed SA: MicroRNA, a new
paradigm for understanding immunoregulation, inflammation and
autoimmune diseases. Transl Res. 157:163–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamasaki T, Kim EJ, Cerutti H and Ohama T:
Argonaute3 is a key player in miRNA-mediated target cleavage and
translational repression in Chlamydomonas. Plant J. 85:258–268.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chao W and D'Amore PA: IGF2: Epigenetic
regulation and role in development and disease. Cytokine Growth
Factor Rev. 19:111–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Corkins MR, Gohil AD and Fitzgerald JF:
The insulin-like growth factor axis in children with inflammatory
bowel disease. J Pediatr Gastroenterol Nutr. 36:228–234. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matouk IJ, Halle D, Gilon M and Hochberg
A: The non-coding RNAs of the H19-IGF2 imprinted loci: A focus on
biological roles and therapeutic potential in lung cancer. J Transl
Med. 13:1132015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jang HJ, Boo HJ, Lee HJ, Min HY and Lee
HY: Chronic stress facilitates lung tumorigenesis by promoting
exocytosis of IGF2 in lung epithelial cells. Cancer Res.
76:6607–6619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li P: Clinical significance of changes of
serum IGF-II, IL-2, IL-10 and TNF-α levels after treatment in
children with bronchopneumonia. J Radioimmunol. 19:288–289.
2006.
|
|
29
|
Qiu-qing D: Measurement of changes of
serum IGF-II, hs-CRP and M-CSF levels after treatment in pediatric
patients with bron-chopneumonia. J Huaihai Med. 6:112011.
|
|
30
|
McLaughlin KJ, Kochanowski H, Solter D,
Schwarzkopf G, Szabó PE and Mann JR: Roles of the imprinted gene
Igf2 and paternal duplication of distal chromosome 7 in the
perinatal abnormalities of androgenetic mouse chimeras.
Development. 124:4897–4904. 1997.PubMed/NCBI
|
|
31
|
Yamamoto K, Ferrari JD, Cao Y, Ramirez MI,
Jones MR, Quinton LJ and Mizgerd JP: Type I alveolar epithelial
cells mount innate immune responses during pneumococcal pneumonia.
J Immunol. 189:2450–2459. 2011. View Article : Google Scholar
|
|
32
|
Weichhart T and Säemann MD: The
PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic
applications. Ann Rheum Dis. 67 Suppl 3:iii70–iii74. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie S, Chen M, Yan B, He X, Chen X and Li
D: Identification of a role for the PI3K/AKT/mTOR signaling pathway
in innate immune cells. PLoS One. 9:e944962014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen XF, Zhang HJ, Wang HB, Zhu J, Zhou
WY, Zhang H, Zhao MC, Su JM, Gao W, Zhang L, et al: Transforming
growth factor-β1 induces epithelial-to-mesenchymal transition in
human lung cancer cells via PI3K/Akt and MEK/Erk1/2 signaling
pathways. Mol Biol Rep. 39:3549–3556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chetty C, Lakka SS, Bhoopathi P and Rao
JS: MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated
PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer.
127:1081–1095. 2010. View Article : Google Scholar : PubMed/NCBI
|