Introduction
According to its pathogenesis, spinal cord injury
(SCI) is divided into primary and secondary injuries. The former is
the result of an initial force applied directly or indirectly to
the spinal cord (1). Secondary
injuries are self-destructive lesions that occur on tissues
surrounding the lesions through a series of physiological and
biochemical mechanisms, including oxidative stress, inflammation
and the excessive release of excitatory amino acids. This leads to
an increase in the degree of damage and expansion of the damaged
area (2). The inflammatory
response following SCI is complex, and involves the immune and
nervous systems (3).
The positive expression of TGF-β in rats is observed
in the endochylema of chondrocytes in the articular cartilage
(4). Compared with the expression
in chondrocyte in articular cartilage of normal rats, the
expression on the surface layer of the cartilage articularis is
high, and is significantly higher than that observed in the middle
and deep layers, while expression in additional regions is low
(5). It has been reported that
during the chondrogenesis process, TGF-β1 is highly expressed in
the surface, transition and cellular layers with low maturity
(6,7). In articular chondrocytes of rats with
SCI, the surface, middle and deep surface layers exhibit relatively
high expression levels (2).
However, its expression decreases in different layers of
chondrocytes. It has been demonstrated that TGF-β1 is expressed in
hematomas that develop at the site of injury following SCI, and the
expression increases in the endochylema and karyon of astrocytes,
capillary endothelial cells present within and outside of the
marrow, as well as in motor neurons (3).
As spinal cord tissues are rich in lipids that are
sensitive to lipid peroxidation, free-radical mediated oxidative
stress serves an important role in secondary SCI (8). Antioxidant defense mechanisms are
activated primarily through the antioxidant response element, which
is a cis-acting element located upstream of antioxidant genes
(9). At present, the nuclear
factor (erythroid-derived 2)-like 2 (Nrf2) signaling pathway is
considered to be the most important defense mechanism in the
prevention of oxidative stress (9). Nrf2 possesses extensive
cytoprotective functions in preventing tumors, atherosclerosis and
neurodegenerative diseases (8).
Inflammation is a self-protective reaction that
occurs when organisms are confronted with damaging stimuli.
Excessive inflammation results in an excessive immune response,
which leads to the development of lesions (10). Serious inflammation may even induce
tumors (10). When inflammation
occurs, various cytokines induce the expression of nitric oxide
synthase (NOS) (11). Inducible
(i)NOS rapidly synthesizes excessive nitric oxide (NO), while
peroxynitrite, a strong oxidant, is rapidly produced following a
reaction between NO and a free oxygen radical (12). As a consequence, oxidative stress
occurs, which leads to plasma effusion at sites of inflammation, as
well as tissue damage and edema (12).
A previous study demonstrated that oxyresveratrol
exhibits anti-inflammatory and detumescence functions (13). Oxyresveratrol may inhibit the
expression of NOS and the accumulation of nitrous acid (13). It is therefore possible that they
possess protective functions in cells that have been injured by
inflammation. In addition, these compounds may increase the
antioxidative capacities of cells with significant
anti-inflammatory properties (14,15).
As an effective tyrosine kinase inhibitor, oxyresveratrol may
prevent herpes virus infection, inflammation and oxidation, as well
as protect nerves (14,15). In addition, it is known to inhibit
cell apoptosis following cerebral ischemia (16,17).
Previous studies have suggested that oxyresveratrol is antitussive,
anti-asthmatic, antioxidative, anti-inflammatory, and that they
possess analgesic properties and inhibit tyrosinase activity
(14,15). In addition, they may protect from
free radicals (14,15). In the present study, the
anti-inflammatory effects of oxyresveratrol and its associated
mechanisms were investigated using a rat model of SCI.
Materials and methods
Animals and generation of the SCI
model
A total of 32 adult female Sprague-Dawley rats (6–8
weeks, 160-180 g) were purchased from Experimental Animal Center of
Hebei Medical University (Hebei, China) and maintained in a
temperature-controlled room (23±1°C) with 12 h light/dark cycles
and with access to water and food ad libitum. The present
study was approved by the Ethics Committee of Hebei Cangzhou
Central Hospital (Cangzhou, China). All rats were anesthetized with
an intraperitoneal injection of 90 mg/kg ketamine (Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) and 10 mg/kg xylazine
(Sinopharm Chemical Reagent Co., Ltd.). Following shaving and
cleansing of the skin, the paraspinal fascia, T7-10 spinous process
and lamina were fully revealed and the T8-9 spinous process was
removed to expose the subdural space, whilst maintaining its
integrity. A 10 g object was dropped from 2.5 cm above the subject
to collide with the subdural space. The wound was washed with
hydrogen peroxide and incisions were sutured. Sham-operated rats
were anesthetized with an intraperitoneal injection of 90 mg/kg
ketamine and 10 mg/kg xylazine and their skin was shaved and
cleansed, the paraspinal fascia.
Experimental groups
The animals were randomly divided into the following
4 groups (n=8): The negative control group (sham-operated); the SCI
model group; the 10 mg/kg oxyresveratrol (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany)-treated SCI group; and the 20 mg/kg
oxyresveratrol-treated SCI group. Rats in the 10 and 20 mg/kg
oxyresveratrol-treated SCI groups were administered with
intraperitoneal injections of 10 and 20 mg/kg oxyresveratrol once a
day for 4 weeks, respectively. Rats from the negative control or
SCI model groups were administered with intraperitoneal injections
of 100 µl normal saline once a day for 4 weeks.
Motor function assessment
At 1, 2, 3 and 4 weeks following oxyresveratrol or
saline treatment, rats were evaluated at 8:00 a.m. using the Basso,
Beattie, and Bresnahan (BBB) locomotor rating scale from 0 to 21
(4).
Determination of spinal cord water
content
After oxyresveratrol treatment for 4 weeks, spinal
cord tissue samples were immediately acquired and washed with
phosphate-buffered saline (PBS). Spinal cord tissue samples were
weighed, and the values obtained were considered to be the wet
weight. Tissue samples were then dried at 68°C for 48 h. Dried
tissue samples were weighed and the values obtained were considered
to be the dried weight. The percentage spinal cord water content
was calculated using the following formula: (wet weight/dried
weight) ×100.
Biochemical analysis
After oxyresveratrol treatment for 4 weeks, rats
were anesthetized with intraperitoneal injection of 90 mg/kg
ketamine and 10 mg/kg xylazine. Venous blood was obtained from the
eye socket of every rat and was immediately centrifuged at 2,000 ×
g for 10 min at 4°C. Serum was collected to measure the expression
of GM-CSF (cat no. H060), nuclear factor-κB (NF-κB)/p65 (cat no.
H202), tumor necrosis factor (TNF)-α (cat no. H052), interleukin
(IL)-1β (cat no. H002), IL-6 (cat no. H007), malondialdehyde (MDA,
cat no. A003-1), superoxide dismutase (SOD, cat no. A001-3),
glutathione (GSH, cat no. A006-2) and GSH peroxidase (PX, cat no.
A005) using rat ELISA kits (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). The expression of these factors was
measured using the Spectramax M2 Microplate Reader (Molecular
Devices, LLC, Sunnyvale, CA, USA) at a wavelength of 450 nm.
Western blot analysis
After oxyresveratrol treatment, spinal cord tissue
samples were immediately acquired and washed with PBS. The tissue
samples were then lysed in radioimmunoprecipitation assay buffer
(Thermo Fisher Scientific, Inc.). Protein concentrations were
determined using a bicinchoninic protein assay kit (Pierce; Thermo
Fisher Scientific, Inc.). Proteins (50 µg) were separated on an
8–12% SDS-PAGE gel (with 60 V constant voltage for 3.5 h, wet-turn
14 V constant voltage for 14 h) and transferred to a polyvinylidene
difluoride membrane (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Membranes were subsequently incubated with the following
primary antibodies at 4°C overnight: TGF-β1 antibody (cat. no.
3711; dilution, 1:3,000; Cell Signaling Technology, Inc., Danvers,
MA, USA), iNOS antibody (cat. no. 2982; dilution, 1:2,000; Cell
Signaling Technology, Inc.), cyclo-oxygenase (COX)-2 antibody (cat.
no. 4842; dilution 1:5,000; Cell Signaling Technology, Inc.), Nrf2
antibody (cat. no. 12721; dilution, 1:4,000; Cell Signaling
Technology, Inc.) and β-actin (cat. no. 3700; dilution, 1:5,000;
Cell Signaling Technology, Inc.). This was followed by incubation
with a horseradish peroxidase-conjugated antibody (cat no. A0239,
dilution 1:2,000; Beyotime Institute of Biotechnology, Haimen,
China) at 37°C for 1 h. Protein bands were visualized using Pierce
ECL Plus™ Western Blotting Substrate (GE Healthcare Life Sciences,
Chalfont, UK). Protein expression was quantified using sodium
Image_Lab_3.0 software (Bio-Rad Laboratories, Inc.).
Statistical analysis
The results are expressed as the mean ± standard
error using SPSS version 17.0 software (SPSS, Inc., Chicago, IL,
USA). Differences among groups were analyzed by one-way analysis of
variance followed by a post hoc Tukey's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
The effects of oxyresveratrol improve
locomotor recovery in a rat model of SCI
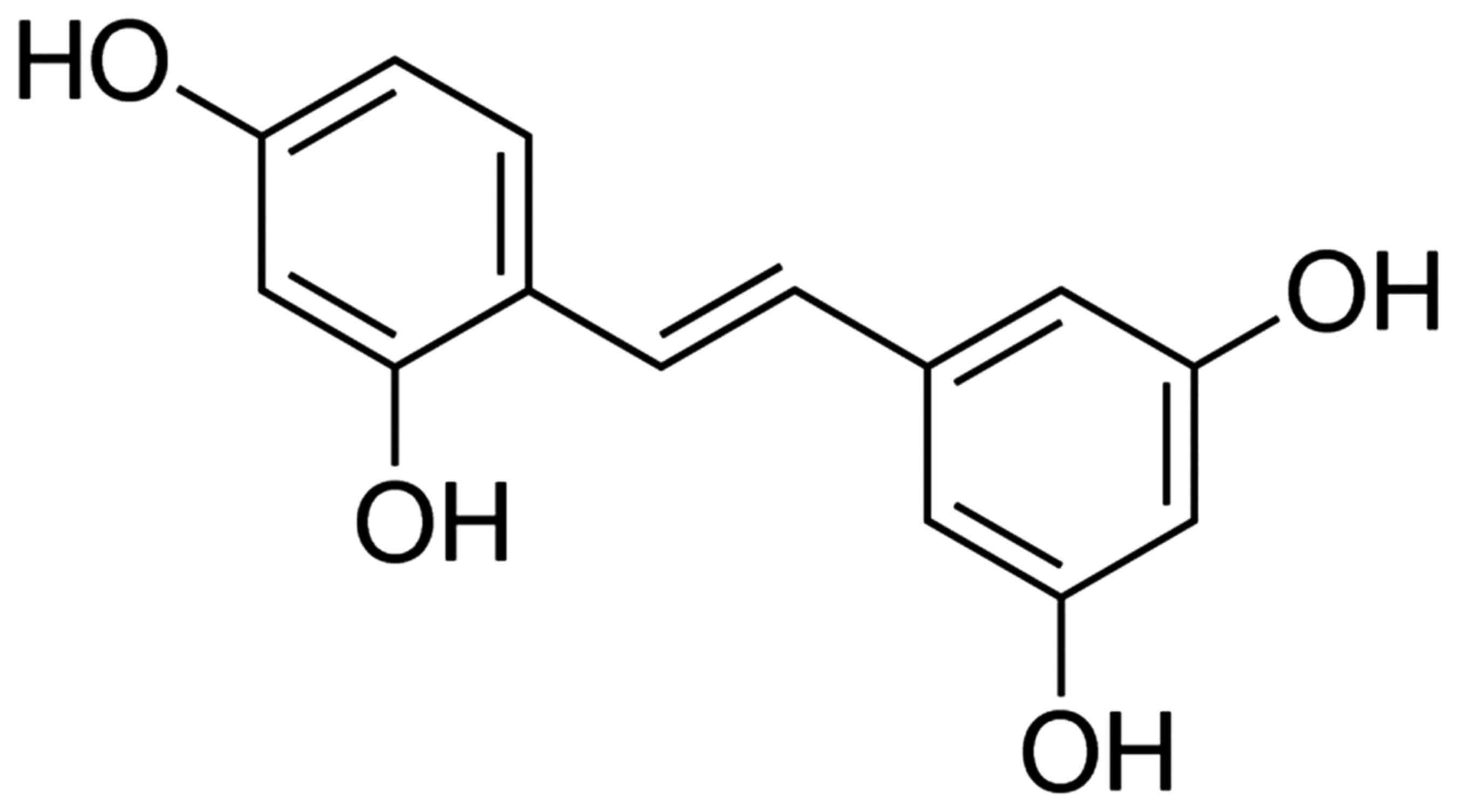
The chemical structure of oxyresveratrol is depicted
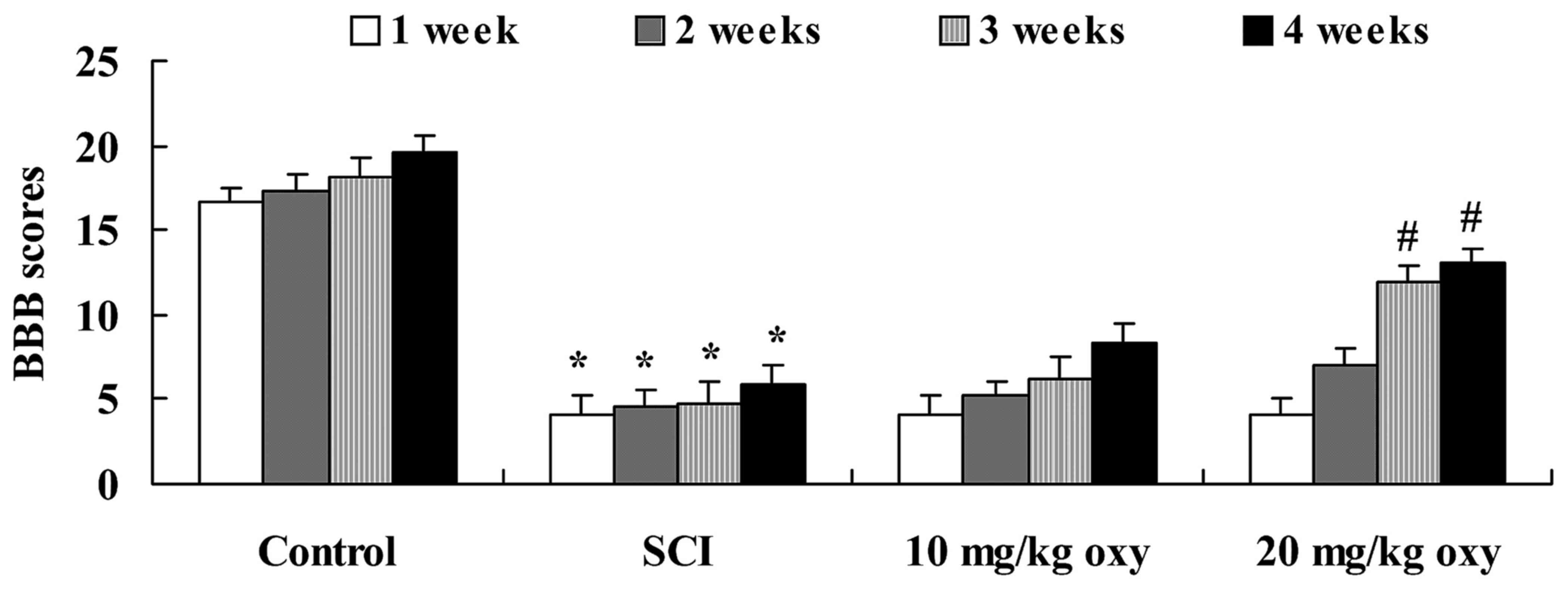
in Fig. 1. As demonstrated in
Fig. 2, the BBB scores of rats in
the SCI group were significantly lower when compared with those of
the sham-operated control group at 1, 2, 3 and 4 weeks following
induction of the SCI model. Treatment with oxyresveratrol (20
mg/kg) significantly reversed the SCI-mediated reduction in the BBB
scores of rats at 3 and 4 weeks following induction of the SCI
model (Fig. 2).
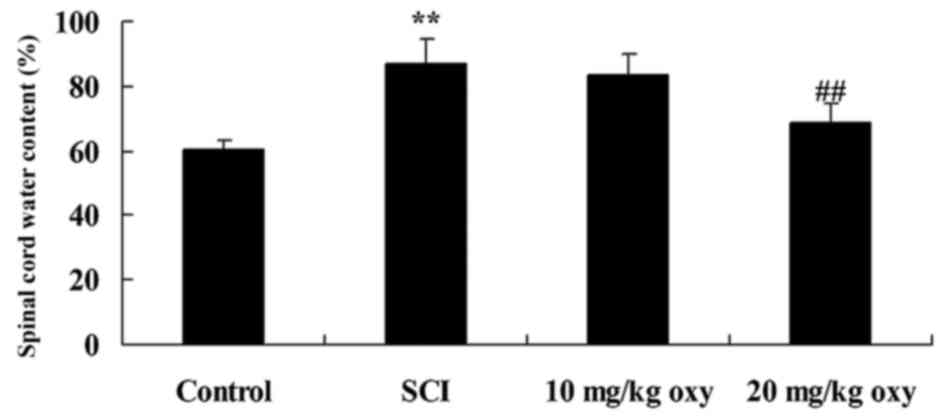
Oxyresveratrol abrogates the
SCI-induced increase in spinal cord water content
At 4 weeks following induction of the SCI model, a
significant increase in the spinal cord water content of SCI rats
was observed when compared with rats in the control group (Fig. 3). By contrast, treatment with 20
mg/kg oxyresveratrol significantly reduced the SCI-induced increase
in spinal cord water content (Fig.
3).
Oxyresveratrol reduces SCI-induced
inflammation
The levels of NF-κB/p65, TNF-α, IL-1β and IL-6
activity were used to evaluate the effects of oxyresveratrol on
inflammation in SCI rats. As demonstrated in Fig. 4, the levels of NF-κB/p65, TNF-α,
IL-1β and IL-6 were significantly increased in SCI rats when
compared with the control group. By contrast, treatment with 20
mg/kg oxyresveratrol significantly suppressed SCI-induced
NF-κB/p65, TNF-α, IL-1β and IL-6 activity in rats (Fig. 4).
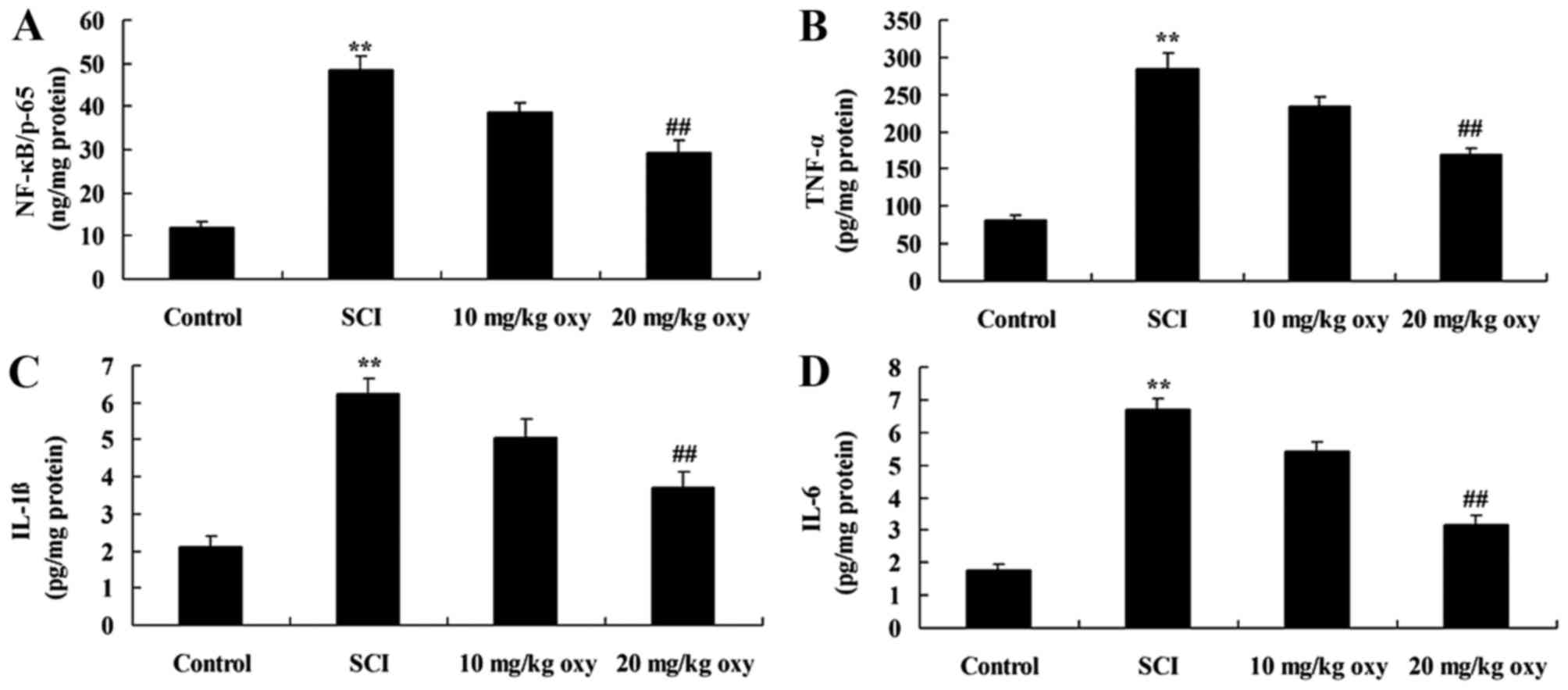 | Figure 4.Oxyresveratrol treatment reduces
inflammation in a rat model of SCI. The levels of (A) NF-κB/p65,
(B) TNF-α, (C) IL-1β and (D) IL-6 activity in a rat model of SCI.
**P<0.01 vs. control group; ##P<0.05 vs. SCI model
group. Control, sham-operated group; SCI, SCI model group; 10 mg/kg
oxy, 10 mg/kg oxyresveratrol-treated SCI group; 20 mg/kg oxy, 20
mg/kg oxyresveratrol-treated SCI group. SCI, spinal cord injury;
NF-κB, nuclear factor-κB; TNF-α, tumor necrosis factor-α; IL,
interleukin. |
Oxyresveratrol abrogates SCI-induced
oxidative stress
The levels of MDA, SOD, GSH and GSH-PX activity were
measured in order to investigate the anti-inflammatory effects of
oxyresveratrol in rats with SCI further. As demonstrated in
Fig. 5, an increase in MDA levels
and a reduction in SOD, GSH and GSH-PX levels was observed in SCI
model rats when compared with the control group. However, treatment
with 20 mg/kg oxyresveratrol significantly reversed MDA, SOD, GSH
and GSH-PX levels when compared with the SCI model group (Fig. 5).
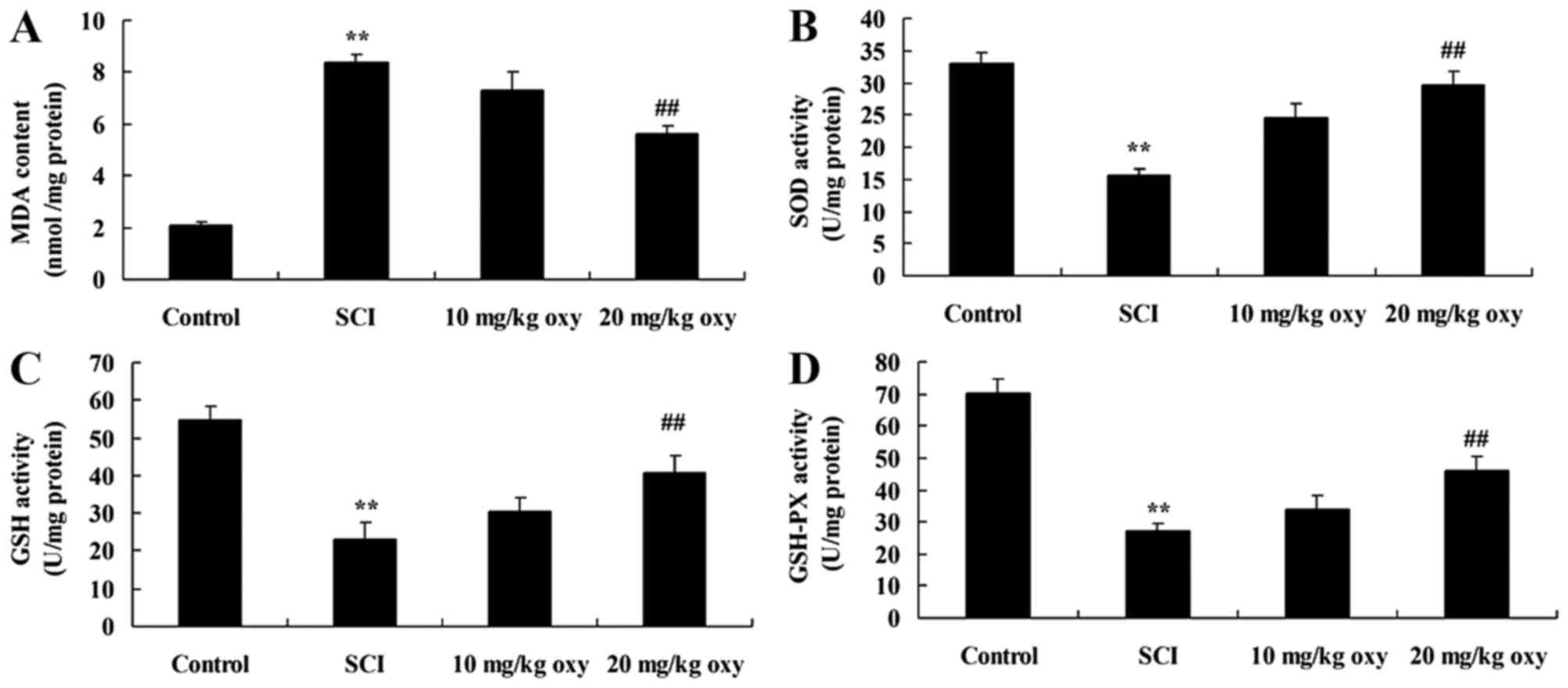 | Figure 5.Oxyresveratrol reduces oxidative
stress in a rat model of SCI. The levels of (A) MDA, (B) SOD, (C)
GSH and (D) GSH-PX activity in a rat model of SCI. **P<0.01 vs.
control group; ##P<0.05 vs. SCI model group. Control,
sham-operated group; SCI, SCI model group; 10 mg/kg oxy, 10 mg/kg
oxyresveratrol-treated SCI group; 20 mg/kg oxy, 20 mg/kg
oxyresveratrol-treated SCI group. SCI, spinal cord injury; MDA,
malondialdehyde; SOD, superoxide dismutase; GSH, glutathione; PX,
peroxidase. |
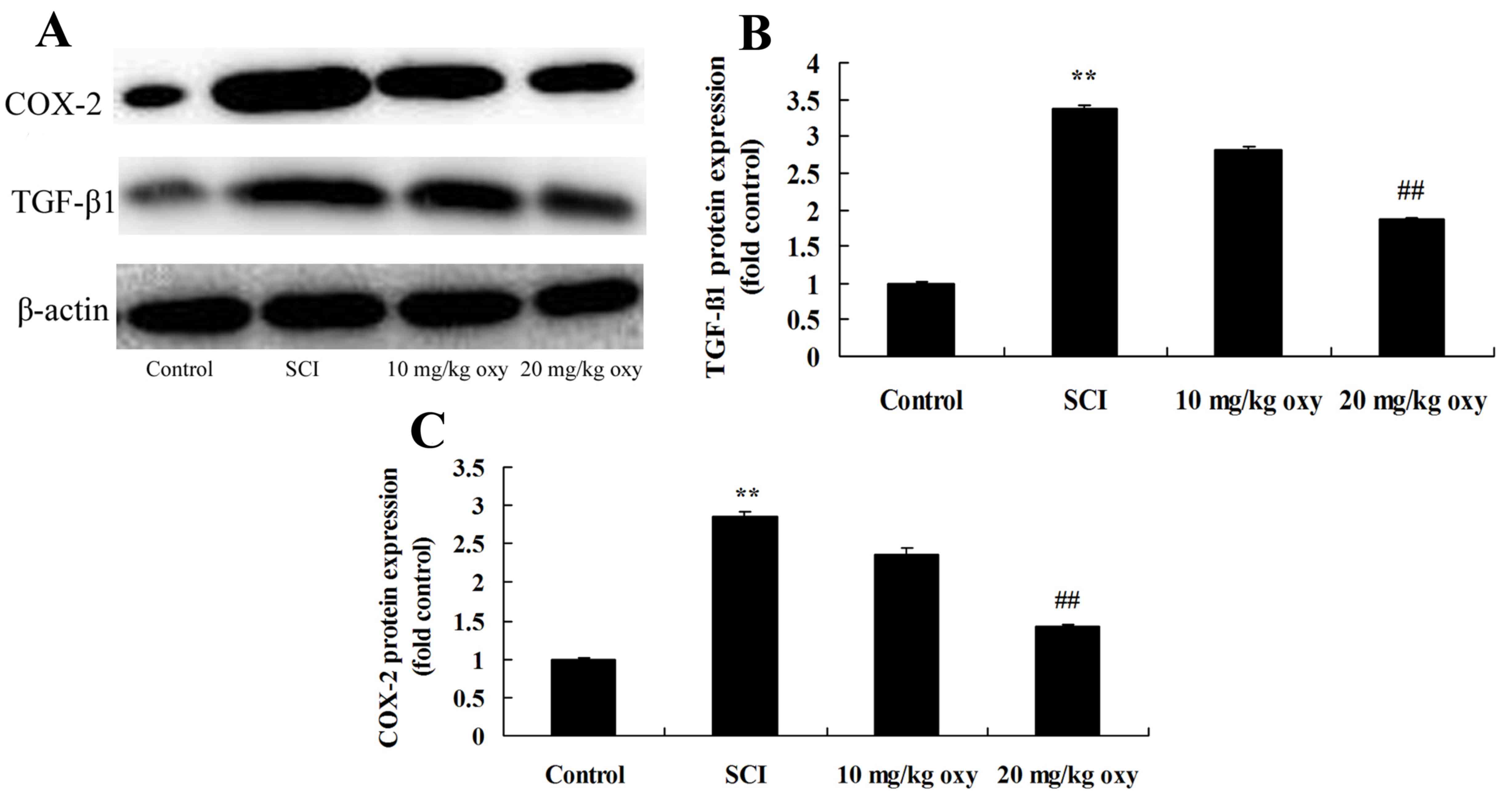
Oxyresveratrol abrogates SCI-induced
TGF-β1 and COX-2 expression
As demonstrated in Fig.
6, SCI significantly induced TGF-β1 and COX-2 protein
expression in SCI model rats when compared with the control group.
By contrast, treatment with 20 mg/kg oxyresveratrol significantly
suppressed the protein expression levels of TGF-β1 and COX-2 in SCI
rats when compared with the SCI model group (Fig. 6).
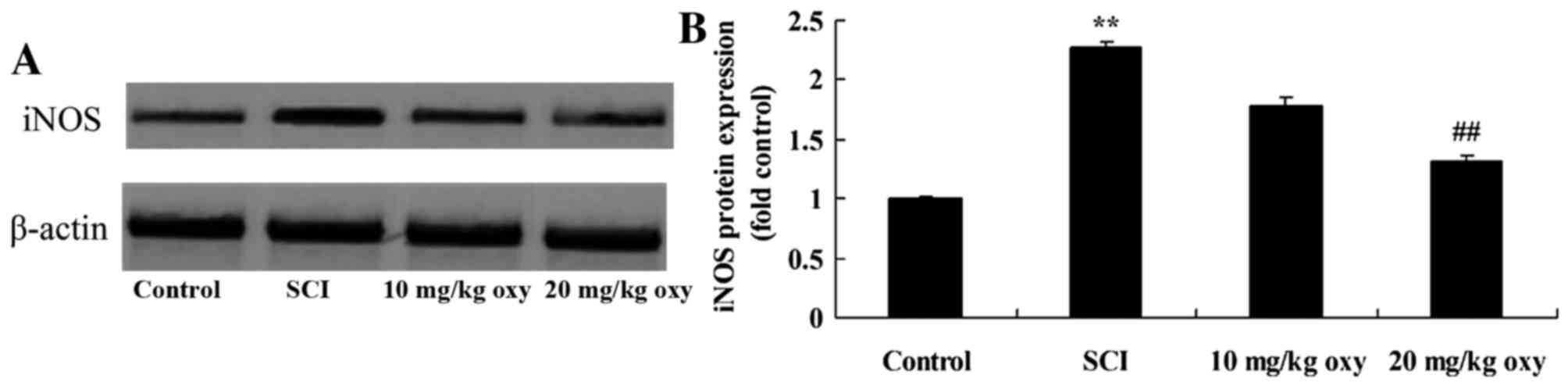
Oxyresveratrol abrogates SCI-induced
iNOS expression
In order to examine the effect of oxyresveratrol
treatment on iNOS expression in SCI rats, iNOS protein expression
was measured by western blot analysis. As demonstrated in Fig. 7, a significant increase in the
protein expression levels of iNOS was observed in SCI rats when
compared with the control group. Treatment with 20 mg/kg
oxyresveratrol significantly reduced the SCI-induced increase in
iNOS protein expression (Fig.
7).
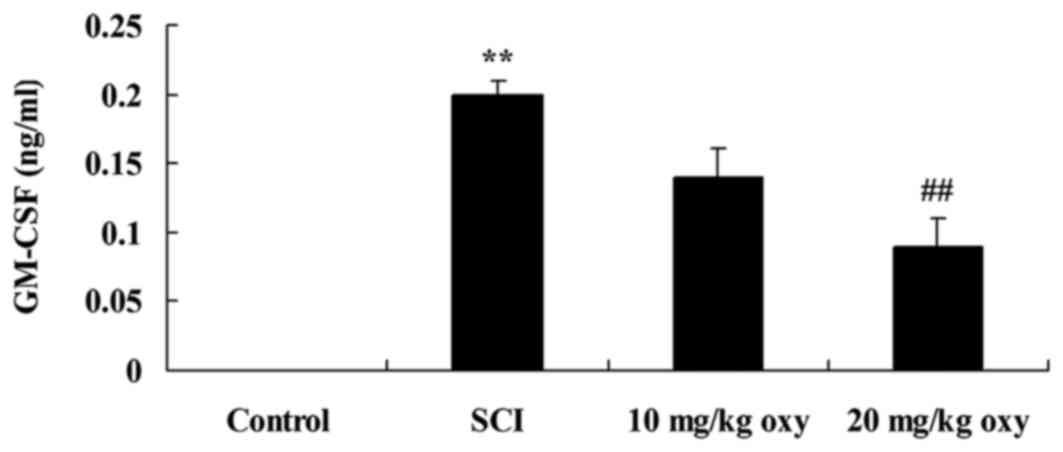
Oxyresveratrol reverses the SCI-induced increase in
GM-CSF levels. In order to determine whether oxyresveratrol
affected GM-CSF expression in a rat model of SCI, the authors
measured GM-CSF protein expression levels in all experimental
groups by western blotting. The results demonstrated that the level
of GM-CSF was significantly higher in the SCI group when compared
with the control group (Fig. 8).
By contrast, treatment with 20 mg/kg oxyresveratrol significantly
reduced the SCI-mediated increase in GM-CSF expression levels
(Fig. 8).
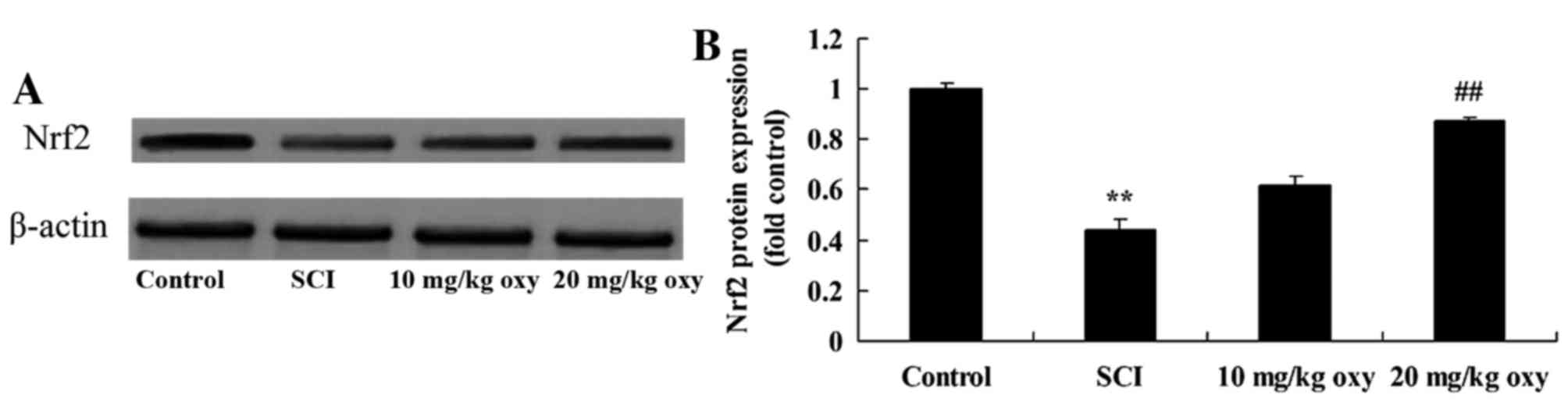
Oxyresveratrol reverses the SCI-mediated reduction
in Nrf2 expression. In order to investigate the effects of
oxyresveratrol on Nrf2 expression in SCI rats, Nrf2 protein
expression levels were detected by western blot analysis. The
results demonstrated that Nrf2 protein expression levels were
significantly reduced in the SCI model group when compared with
control group (Fig. 9). However
treatment with 20 mg/kg oxyresveratrol significantly reversed the
SCI-induced inhibition of Nrf2 protein expression (Fig. 9).
Discussion
Inflammatory chemokine factors and cytokines enhance
the activity of and activate immune cells and neurons. They serve
key roles in promoting and maintaining inflammation (18). Chemokine factors and cytokines
possess a number of functions, including the transformation of
cells from a proinflammatory to an anti-inflammatory state
(19). If the inflammatory
response at the site of injury were not inhibited during an immune
response, it may lead to a number of additional adverse effects
(20). As the capabilities of
injured neurons are reduced and axonal regeneration is restricted,
the adverse effects of inflammation may be more obvious in the
central nervous system when compared with other systems (21). The apoptosis of neurons and
oligodendroglia, as well as scarring may be triggered. The
excessive expression of COX-2 is associated with microvessel
density and angiogenesis induced by inflammatory cytokines
(22). The present study
demonstrated that oxyresveratrol significantly increased the
SCI-mediated activities. Andrabi et al (15) demonstrated that oxyresveratrol
inhibits apoptotic cell death during transient cerebral ischemia,
whereas Ashraf et al (23)
indicated that the compound ameliorates inflammation in the airways
following an allergic response.
As a cytokine involved in hemocytogenesis, GM-CSF
promotes long-term recovery following SCI through inhibiting glial
scar formation and increasing the structural integrity of axons
(24). Previous studies have
demonstrated that the use of TGF-β to treat primary spongiocytes in
an in vitro system, may inhibit the expression of
chondroitin sulfate proteoglycans in astrocytes, which would
further inhibit the formation of glial scars and provide an
effective treatment for neuronal injury (25). In addition, GM-CSF may regulate the
ability of macrophages to produce brain-derived neurotrophic factor
(26). GM-CSF improves tactile
sense and psychroesthesia in SCI rats, indicating that it may
promote the recovery functional of SCI (26). In the present study, oxyresveratrol
significantly reduced the SCI-induced increase in GM-CSF levels in
rats. Oxyresveratrol suppresses lipopolysaccharide-induced
inflammatory responses in murine macrophages.
Following SCI, a number of factors may be involved
in mediating nerve cell apoptosis (6). An increasing number of studies
investigating NO have addressed its functional role in secondary
SCI (6,7). NO is produced during the catalyzation
of L-arginine to citrulline by NOS. Under normal physiological
conditions, organisms produce NO of base quantity, and inhibit
platelet aggregation and accumulation, as well as maintain
conditions of vasodilatation (7).
During the early stages of acute ischemic injury, small increases
in NO prevent vasoconstriction induced by trauma and ischemia. With
the infiltration of inflammatory cells and the production of
inflammatory factors, NF-κB is activated, which induces an increase
in iNOS expression. The activity of iNOS is not calcium-dependent
(22). Once synthesized, it
continuously catalyzes the formation of NO (22). The results from the present study
demonstrated that oxyresveratrol significantly inhibited
SCI-induced iNOS protein expression in rats. Lee et al
(14) indicated that
oxyresveratrol suppresses lipopolysaccharide-induced inflammatory
responses via iNOS, COX-2 and GM-CSF in murine macrophages.
It is generally thought that, under pathological
conditions, including inflammation, trauma, cellular injuries and
tumors, COX-2 exerts harmful functions (22). However, a study discovered that
COX-2 serves a role in mediating normal physiological functions
(22). For instance, COX-2 serves
a functional role in glutamatergic neurons, which are
constitutively expressed in the hippocampus and cortex, and
demonstrates effects on long-term synaptic plasticity and the
coupling of neurovascular structures during hyperemia (27). Previous studies hypothesize that
the expression of COX-2 is associated with anoxia following SCI,
peroxidation and neuronal death induced by excitatory amino acids
(27,28). In the current study, oxyresveratrol
significantly inhibited the SCI-induced increase in COX-2 protein
expression in rats.
SCI is a severe traumatic disease of the central
nervous system. Despite current medical strategies, functional
rehabilitation is not satisfactory, even with the observed decrease
in death rates (29). As a
consequence, the prevention, treatment and rehabilitation of SCI
have become an essential issue. In addition, secondary damage of
the spinal cord following the primary damage may aggravate the
degree of injury (30). Therefore,
the reduction and reversion of secondary injury has become the
focus of current studies. Oxidative stress serves a fundamental
role in subsequent pathological alterations in SCI (18). As a key regulator of transcription
in cytophylaxis and antioxidative stress pathways, Nrf2 regulates
cytoprotective genes, such as those with antioxidative and
anti-inflammatory functions, as well as associated proteins in
order to enhance antioxidative capacities (31). The present study demonstrated that
oxyresveratrol significantly activated the SCI-mediated inhibition
of Nrf2 protein expression in rats. Choi et al (32) confirmed that oxyresveratrol
abrogates oxidative stress in the liver via activating the ERK-Nrf2
signaling pathway (32).
In conclusion, the results of the present study
demonstrated that oxyresveratrol significantly increased the
SCI-mediated reduction in BBB scores, inhibited the SCI-induced
increase in spinal cord water content, suppressed SCI-induced
NF-κB/p65, TNF-α, IL-1β and IL-6 activities and reversed the MDA,
SOD, GSH and GSH-PX activities in SCI rats potentially via the
iNOS, COX-2 and GM-CSF/Nrf2 signaling pathways. The results suggest
that oxyresveratrol may present a novel therapeutic agent for the
treatment of SCI.
References
|
1
|
DePaul MA, Palmer M, Lang BT, Cutrone R,
Tran AP, Madalena KM, Bogaerts A, Hamilton JA, Deans RJ and Mays
RW: Intravenous multipotent adult progenitor cell treatment
decreases inflammation leading to functional recovery following
spinal cord injury. Sci Rep. 5:167952015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amemori T, Ruzicka J, Romanyuk N,
Jhanwar-Uniyal M, Sykova E and Jendelova P: Comparison of
intraspinal and intrathecal implantation of induced pluripotent
stem cell-derived neural precursors for the treatment of spinal
cord injury in rats. Stem Cell Res Ther. 6:2572015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khayrullina G, Bermudez S and Byrnes KR:
Inhibition of NOX2 reduces locomotor impairment, inflammation, and
oxidative stress after spinal cord injury. J Neuroinflammation.
12:1722015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen G, Park CK, Xie RG and Ji RR:
Intrathecal bone marrow stromal cells inhibit neuropathic pain via
TGF-β secretion. J Clin Invest. 125:3226–3240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jahan N and Hannila SS: Transforming
growth factor β-induced expression of chondroitin sulfate
proteoglycans is mediated through non-Smad signaling pathways. Exp
Neurol. 263:372–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heise RL, Parekh A, Joyce EM, Chancellor
MB and Sacks MS: Strain history and TGF-β1 induce urinary bladder
wall smooth muscle remodeling and elastogenesis. Biomech Model
Mechanobiol. 11:131–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quinlan JF, Watson RW, Kelly G, Kelly PM,
O'Byrne JM and Fitzpatrick JM: Transforming growth factor-beta
(TGF-beta) in acute injuries of the spinal cord. J Bone Joint Surg
Br. 88:406–410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mao L, Wang HD, Wang XL, Tian L and Xu JY:
Disruption of Nrf2 exacerbated the damage after spinal cord injury
in mice. J Trauma Acute Care Surg. 72:189–198. 2012.PubMed/NCBI
|
|
9
|
Wang C, Wang P, Zeng W and Li W:
Tetramethylpyrazine improves the recovery of spinal cord injury via
Akt/Nrf2/HO-1 pathway. Bioorg Med Chem Lett. 26:1287–1291. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirakawa A, Shimizu K, Fukumitsu H and
Furukawa S: Pyrroloquinoline quinone attenuates iNOS gene
expression in the injured spinal cord. Biochem Biophys Res Commun.
378:308–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song Y, Liu J, Zhang F, Zhang J, Shi T and
Zeng Z: Antioxidant effect of quercetin against acute spinal cord
injury in rats and its correlation with the p38MAPK/iNOS signaling
pathway. Life Sci. 92:1215–1221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin CC, Chiang TH, Chen WJ, Sun YY, Lee YH
and Lin MS: CISD2 serves a novel role as a suppressor of nitric
oxide signalling and curcumin increases CISD2 expression in spinal
cord injuries. Injury. 46:2341–2350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lorenz P, Roychowdhury S, Engelmann M,
Wolf G and Horn TF: Oxyresveratrol and resveratrol are potent
antioxidants and free radical scavengers: Effect on nitrosative and
oxidative stress derived from microglial cells. Nitric Oxide.
9:64–76. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HS, Kim DH, Hong JE, Lee JY and Kim
EJ: Oxyresveratrol suppresses lipopolysaccharide-induced
inflammatory responses in murine macrophages. Hum Exp Toxicol.
34:808–818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andrabi SA, Spina MG, Lorenz P, Ebmeyer U,
Wolf G and Horn TF: Oxyresveratrol
(trans-2,3′,4,5′-tetrahydroxystilbene) is neuroprotective and
inhibits the apoptotic cell death in transient cerebral ischemia.
Brain Res. 1017:98–107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weber JT, Lamont M, Chibrikova L, Fekkes
D, Vlug AS, Lorenz P, Kreutzmann P and Slemmer JE: Potential
neuroprotective effects of oxyresveratrol against traumatic injury.
Eur J Pharmacol. 680:55–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sasivimolphan P, Lipipun V, Ritthidej G,
Chitphet K, Yoshida Y, Daikoku T, Sritularak B, Likhitwitayawuid K,
Pramyothin P, Hattori M and Shiraki K: Microemulsion-based
oxyresveratrol for topical treatment of herpes simplex virus (HSV)
infection: Physicochemical properties and efficacy in cutaneous
HSV-1 infection in mice. AAPS Pharm Sci Tech. 13:1266–1275. 2012.
View Article : Google Scholar
|
|
18
|
Wang W, Shen H, Xie JJ, Ling J and Lu H:
Neuroprotective effect of ginseng against spinal cord injury
induced oxidative stress and inflammatory responses. Int J Clin Exp
Med. 8:3514–3521. 2015.PubMed/NCBI
|
|
19
|
Min SH, Soh JS, Park JY, Choi SU, Lee HW,
Lee JJ and Kim JH: Epidural dexamethasone decreased inflammatory
hyperalgesia and spinal cPLA2 expression in a rat formalin test.
Yonsei Med J. 55:1631–1639. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stammers AT, Liu J and Kwon BK: Expression
of inflammatory cytokines following acute spinal cord injury in a
rodent model. J Neurosci Res. 90:782–790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han D, Wu C, Xiong Q, Zhou L and Tian Y:
Anti-inflammatory mechanism of bone marrow mesenchymal stem cell
transplantation in rat model of spinal cord injury. Cell Biochem
Biophys. 71:1341–1347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
López-Vales R, García-Alías G,
Guzmán-Lenis MS, Forés J, Casas C, Navarro X and Verdú E: Effects
of COX-2 and iNOS inhibitors alone or in combination with olfactory
ensheathing cell grafts after spinal cord injury. Spine (Phila Pa
1976). 31:1100–1106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ashraf MI, Shahzad M and Shabbir A:
Oxyresveratrol ameliorates allergic airway inflammation via
attenuation of IL-4, IL-5, and IL-13 expression levels. Cytokine.
76:375–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hayashi K, Ohta S, Kawakami Y and Toda M:
Activation of dendritic-like cells and neural stem/progenitor cells
in injured spinal cord by GM-CSF. Neurosci Res. 64:96–103. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watanabe S, Uchida K, Nakajima H, Matsuo
H, Sugita D, Yoshida A, Honjoh K, Johnson WE and Baba H: Early
transplantation of mesenchymal stem cells after spinal cord injury
relieves pain hypersensitivity through suppression of pain-related
signaling cascades and reduced inflammatory cell recruitment. Stem
Cells. 33:1902–1914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung J, Kim MH, Yoon YJ, Kim KH, Park SR
and Choi BH: Effects of granulocyte colony-stimulating factor and
granulocyte-macrophage colony-stimulating factor on glial scar
formation after spinal cord injury in rats. J Neurosurg Spine.
21:966–973. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Quan HH, Kang KS, Sohn YK and Li M: Tempol
reduces injury area in rat model of spinal cord contusion injury
through suppression of iNOS and COX-2 expression. Neurol Sci.
34:1621–1628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song HX, Scarpatetti M, Kreil W, Shen HL,
Bodo K, Ebner B, Schröttner H and Mokry M: Quantitative analysis of
cyclooxygenase 2 in the posterior longitudinal ligament of cervical
spondylotic myelopathy. Chin Med J (Engl). 124:2480–2484.
2011.PubMed/NCBI
|
|
29
|
Kanninen KM, Pomeshchik Y, Leinonen H,
Malm T, Koistinaho J and Levonen AL: Applications of the Keap1-Nrf2
system for gene and cell therapy. Free Radic Biol Med. 88:350–361.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Campos CR, Peart JC, Smith LK,
Boni JL, Cannon RE and Miller DS: Nrf2 upregulates ATP binding
cassette transporter expression and activity at the blood-brain and
blood-spinal cord barriers. J Neurosci. 34:8585–8593. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miller DM, Singh IN, Wang JA and Hall ED:
Nrf2-ARE activator carnosic acid decreases mitochondrial
dysfunction, oxidative damage and neuronal cytoskeletal degradation
following traumatic brain injury in mice. Exp Neurol. 264:103–110.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi HY, Lee JH, Jegal KH, Cho IJ, Kim YW
and Kim SC: Oxyresveratrol abrogates oxidative stress by activating
ERK-Nrf2 pathway in the liver. Chem Biol Interact. 245:110–121.
2016. View Article : Google Scholar : PubMed/NCBI
|