Introduction
Obesity is a medical disorder characterized by
excessive fat accumulation that presents a risk to health, leading
to reduced life expectancy and increased morbidity (1). These risks most commonly present in
the following ways: Metabolic syndrome, hypertension, imprinting of
metabolic control in fetal life and early childhood, and physical
inactivity (1). Numerous studies
have demonstrated the association between a sedentary lifestyle and
weight gain; however, reliable direct measures of physical activity
are only now beginning to emerge (1). High fat-fed wild-type (WT) mice
demonstrated reduced exercise tolerance during an exercise stress
test, and attenuation in muscle glucose uptake and AMP-activated
protein kinase α-2 activity during a single bout of exercise
(2). However, recent work has
identified that older adults with obesity and systemic inflammation
have associated metabolic dysfunction (3), although they do not have associated
reduced muscular weight or strength. In 2013, the American Medical
Association classified obesity as a disease (3).
Obesity in pregnancy has become a global problem. In
2008, 64% of American women of child-bearing age were overweight or
obese (4). According to the
Chinese adult Body Mass Index classification, 11.9% of pregnant
females were overweight and 2.3% were obese at the first prenatal
visit, increasing from 6.9–17.5 and 1.0–4.0%, respectively between
2006 and 2009 (5). The traditional
idea that pregnant women require compensatory nutrition will
further drive the increase in obesity during pregnancy.
In 1992, Hales and Barker (6) published a hypothesis following
research at the University of Southampton (Southampton, UK), which
supported the idea that fetal development is modified in poor
nutritional conditions, resulting in a thrift phenotype. It was
concluded that infants whose birth weight fell on the low end of
normal were more likely to die of heart disease as adults.
Subsequently, Baker identified that the maternal environment
impacts fetal development in ways which remain to be completely
elucidated (7). In 2014,
researchers at Yale University (New Haven, CT, USA) demonstrated
that children of obese mothers who eat a high fat diet may be more
likely to have metabolic disorders, and be at an increased risk of
becoming obese and developing diabetes through hypothalamic
neuro-circuit formation (8).
Macrophage infiltration in the adipose tissue of obese animals, in
response to free fatty acids released by hypertrophied adipocytes
(9), contributes to inflammation
and insulin resistance (10).
Investigating the expression of transcripts encoding cluster of
differentiation CD68, a macrophage transmembrane protein, has led
to an improved understanding of the association between adipose
tissue infiltration and insulin resistance (11). There is increasing evidence that an
increase in the gene expression of tumor necrosis factor-α (TNF-α)
and interleukin-6 (IL-6) occurs in the hypothalamus in the
offspring of obese mothers, considered to be produced by
hypertrophied adipocytes as a marker of M1 polarization of adipose
tissue macrophages (9,10).
The controversy of alternative (M2) macrophage
polarization has previously been debated. Although the majority of
reports demonstrated a decline in M2 macrophages (10), researchers at Cornell University
(Ithaca, NY, USA) observed elevated M2 macrophage polarization in
adipose tissue with an acute high fat diet (HFD) challenge
(12). An increase in IL-10 caused
by the increase of M2 macrophages was reported in pregnant mice
(13).
Skeletal muscle is required for movement and to
sustain posture, and a loss of skeletal muscle occurs as
consequence of several chronic diseases as well as normal aging
(14). In addition, TNF-α
expression and systemic inflammation was demonstrated to be
associated with impaired angiogenesis in skeletal muscle through
von Hippel-Lindau disease tumor suppressor overexpression (15).
In the present study, the effects of moderate HFD
(MHFD) on the offspring of obese mice were investigated, including
blood pressure (BP), physical inactivity, glucose sensitivity,
macrophage infiltration, and the association between cytokine
fluctuations and physical inactivity.
Materials and methods
Animal care
The female C57BL/6J mice and their controls (n=64,
6-weeks-old, ~17±2.2 g) were purchased from the animal center of
Norman Bethune College of Medicine, Jilin University (Changchun,
China). All of the mice were maintained under a 12/12 h light-dark
cycle at a constant temperature (20±2°C) in the pathogen-free
facilities at the Biological Experimental Teaching Demonstration
Center of Jilin University, with food and water available ad
libitum. The experimental animal protocol used in the present
study was approved by the ethics committee of the School of Life
Sciences at Jilin University.
Obesity mouse model
Following a 3-day acclimation period, C57BL/6J
female mice at age 6 weeks were fed randomly with either normal
chow diet (NCD), with 9% fat, or MHFD, with 26% fat. At 14 weeks of
age, the female mice fed with NCD or MHFD were bred with C57BL/6J
male mice (n=8, 6-months-old, ~18±1.7 g). In order to improve the
rate of successful mating, the male mice and the female mice were
kept in one cage for 3 days. The female mice were examined every
morning for the presence of a vaginal plug; the day following
identification of a vaginal plug was designated day 0 of gestation.
The maternal mice were housed individually with free access to
their prior diet and water. Body weight and food intake were
monitored weekly between weeks 6 and 14, and on days 0, 7 and 14 of
gestation. Female mice with <7 or >10 offspring were excluded
from the present study, as described previously (9). Offspring were fed with NCD and MHFD
separately, and separated from the maternal mice at the 4th week
following birth.
Sampling
In the 8, 16 and 24th weeks, following an overnight
fast, the mice were sacrificed by cervical dislocation for the
collection of blood and tissue samples. When blood was visible in
the eyes, the abdomen was rapidly opened and single samples of
subcutaneous adipose tissue, parametrial or epididymal adipose
tissue, perirenal adipose tissue and mesenteric adipose tissue were
dissected and weighed to determine visceral fat content. The
subcutaneous adipose tissue samples were either fixed with 4%
formalin for 48 h at room temperature and embedded in paraffin, or
frozen by dry ice-isopentane and optimal cutting temperature
compound (OCT; Sakura Finetek USA, Inc., Torrance, CA,
USA)-embedded for at ~69°C for histological analyses. The heart,
liver, spleen, lungs and kidneys were also rapidly removed,
weighed, frozen in liquid nitrogen and stored at −80°C. The
subtraction method was used to measure The wet weight of organs and
fat tissues from the sacrificed mice were measured by the
subtraction method: Total weight of containers and tissues minus
the weight of the container.
Treadmill test
The exhaustion treadmill test was conducted to
measure the physical performance of the skeletal muscle of the
fetal mice. Prior to the exhaustion test, each mouse was placed on
the belt of a six-lane motorized treadmill (FT-100 Animal
Treadmill) at an incline of 0° and speed of 12.28 m/min for 5 min.
As physical condition declined with age, the optimum speed for
older mice was selected to analyze mice of all groups. Subsequent
to three repeats for acclimation, the exhaustion test was carried
out until mice stayed on the shaker plate for more than 10 sec
without attempting to run. The exhaustion time and distance were
recorded for each subject (16,17).
Rotarod test
In order to assess motor coordination and motor
learning, the fetal mice were trained three times on the rotarod
(ZB-200 Rotarod; Timen Co.), as described by Jung et al
(18,19). The apparatus consisted of a
polyvinyl chloride rotating rod with 6 opaque Plexiglas barriers
dividing the rod into sections, which exhibited individual holding
chambers located 39 cm below the rod. Each mouse was put on the rod
individually, facing away from the experimenter, and the rod was
programmed to accelerate to 16 rpm. The time at which the mice fell
from the rod was recorded. Following ≥10 min rest, the mice were
placed back on the rod. Each mouse was tested 3 times and the mean
latency to fall, across the three trials, was analyzed.
BP measurement
BP was measured by tail-cuff plethysmography, as
described previously by Xu et al (20). The measurements were conducted in a
heated room (30°C) in order to get optimal BP readings, and at the
same time of day. Once the animals were restrained properly, heart
rate (HR), systolic BP (SBP), mean BP (MBP) and diastolic BP (DBP)
were automatically measured by BP2010 (Softron Corp., Tokyo,
Japan). A total of ≥5 readings were taken from each animal/session
and averaged to obtain a single session value. BP was measured at
8, 16 and 24 weeks post-weaning. At each time-point, the average BP
values were taken from 6–12 offspring with equal numbers of each
gender in each treatment group.
Glucose tolerance test
The glucose tolerance test was carried out on the
pregnant mice at 14 weeks, and on the offspring at each time-point
(8, 16 and 24 weeks). The mice fasted overnight and were
administered a glucose solution (1 g/kg body weight) by
subcutaneous injection. Blood samples were collected from a tail
vein, and the glucose concentration measurement was performed
during the light phase and determined using a blood glucose meter
(SanoCare, Inc., FL, USA) (9).
Histopathological examination
A section of offspring subcutaneous abdominal
adipose tissue was fixed in 4% formalin in PBS for 48 h at room
temperature and dehydrated in gradient ethanol (50, 70, 80, 90, 95
and 100%). Samples were embedded in paraffin, and cut into serial
sections at 5 µm thickness using a microtome (Leica Microsystems
GmbH, Wetzlar, Germany). The sections were stained with hematoxylin
at room temperature for 10 min and images were captured using an
upright microscope (magnification, ×10 and ×40; Eclipse ci; Nikon
Corp., Tokyo, Japan). Further sections of offspring subcutaneous
adipose tissue, embedded in OCT, were cut into 20 µm sections and
mounted on glass slides, and stained with Oil Red O at room
temperature for 10 min. The three fields of vision were selected at
random. The diameter of each adipocyte in the field was measured
manually, and the diameters of 20 adipocytes were measured
microscopically by a single observer, as described previously
(13).
RNA isolation and reverse
transcription (RT)
A total of 6 offspring were randomly selected from
each litter for each of the 3 time-points. Total RNA isolation from
subcutaneous adipose tissue was conducted by the Trizol method as
described previously by Campbell with minor modification (20). RT of total RNA was carried out with
the PrimeScript RT reagent kit with genomic DNA Eraser (cat. no.
RR047A; Takara Biotechnology Co., Ltd., Dalian, China). DNA
contamination was removed from the total RNA before its reverse
transcription to cDNA according to the manufacturer's protocol.
Quantitative polymerase chain reaction
(qPCR)
qPCR was performed using TaqMan gene expression to
measure the expression of cluster of differentiation (CD)68 in
subcutaneous adipose tissue. TaqMan probes and primers were
purchased from Takara Biotechnology Co., Ltd. as follows: Mouse
CD68, forward, 5′-GCTACTAGTCCAAGATCC-3′ and reverse,
5′-CCTGAATTGGGTATAGGA-3′ and probe, 5′-(FAM)
CACTGTTGGCCCTCACCCTG(Eclipse)-3′; and mouse GAPDH, forward,
5′-CAATGTGTCCGTCGTGGATCT-3′ and reverse,
5′-GTCCTCAGTGTAGCCCAAGATG-3′ and probe,
CGTGCCGCCTGGAGAAACCTGCC(Eclipse)-3′.
qPCR analysis was performed in a 20 µl volume using
Premix Ex Taq™ (cat. no. RR390A; Takara Biotechnology Co., Ltd.)
and Applied Biosystems 7500 (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and the three-step RT-qPCR was performed under
the following conditions: Denaturation at 95°C for 30 sec followed
by 40 cycles of denaturing at 95°C for 5 sec, annealing at 55°C for
10 sec and extension at 72°C for 30 sec. Each reaction was repeated
three times. Quantification was conducted using the
2−ΔΔCq method (21).
Serum TNF-α and IL-10 protein
levels
An inflammatory cytokine, TNF-α, and an
anti-inflammatory cytokine, IL-10, were detected in the plasma of
the offspring using mouse TNF-α and mouse IL-10 ELISA (MTA00B and
DY417-05, R&D Systems, Inc., Minneapolis, MN, USA), which were
conducted according to the manufacturers' protocol.
Statistical analyses
Results were expressed as the mean ± standard error.
Comparisons of body weight and food intake were made using the
paired-samples t-test. Other multi-group comparisons of biochemical
and biophysical parameters in the offspring and maternal mice, were
made using independent-samples t-tests using SPSS software (version
19.0; IBM SPSS, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Maternal body weight and food intake,
fetal body weight and prime body weight
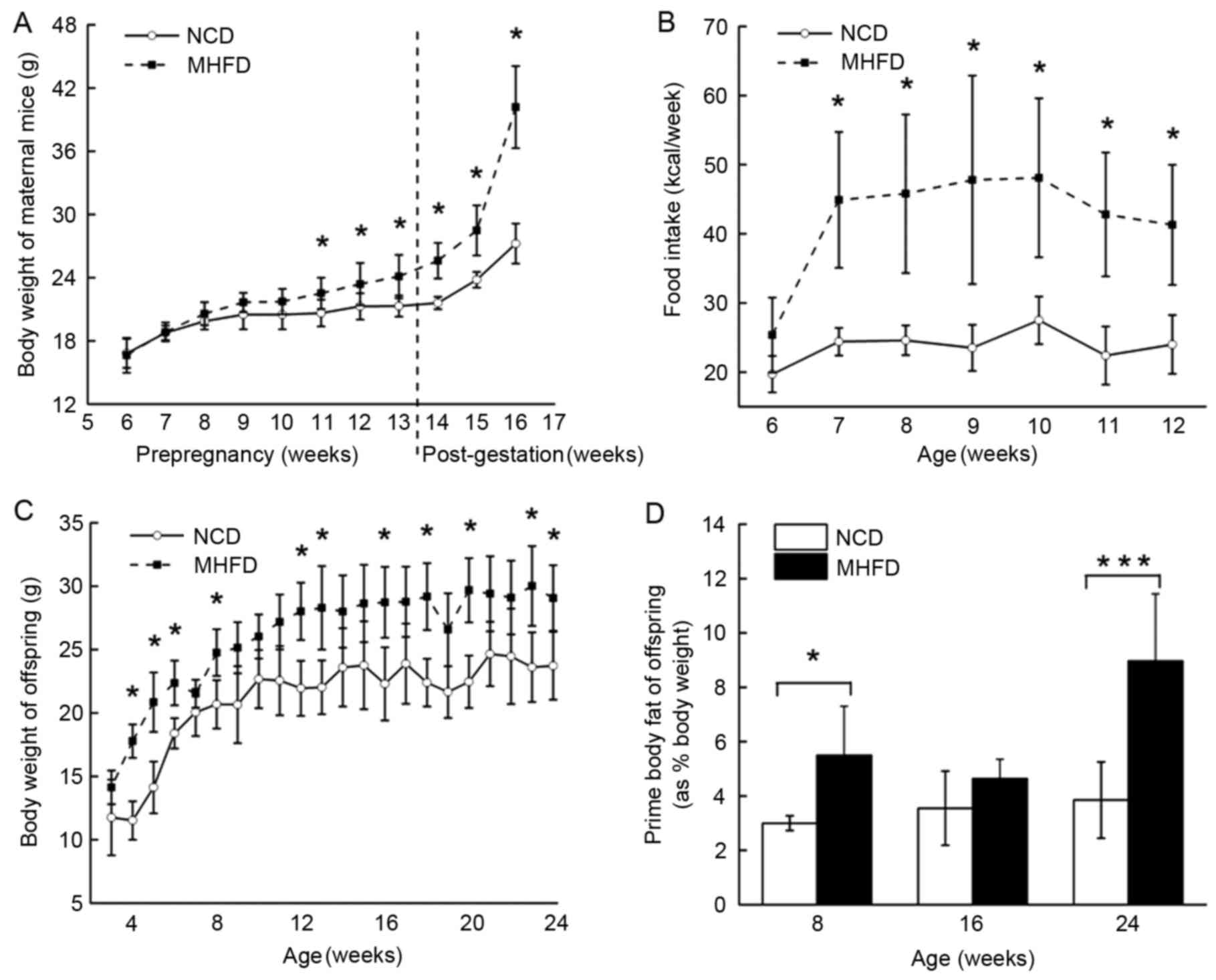
Following 8 weeks of treatment with NCD or MHFD, the
female MHFD mice were significantly heavier compared with the
female NCD mice with an increase in body weight of 38.45%
(P<0.05), which gave rise to a continued weight gain (Fig. 1A) and increased food intake
(Fig. 1B) following pregnancy. In
the MHFD offspring group, body weight increased following the
offspring being separated from the maternal mice, and plateaued at
the age of 12 weeks. The offspring of MHFD mice were heavier
compared with those of NCD mice throughout the experimental period
(Fig. 1C). Correspondingly, a
marked increase in prime body fat was observed in the MHFD
offspring group at 8 and 24 weeks old (Fig. 1D).
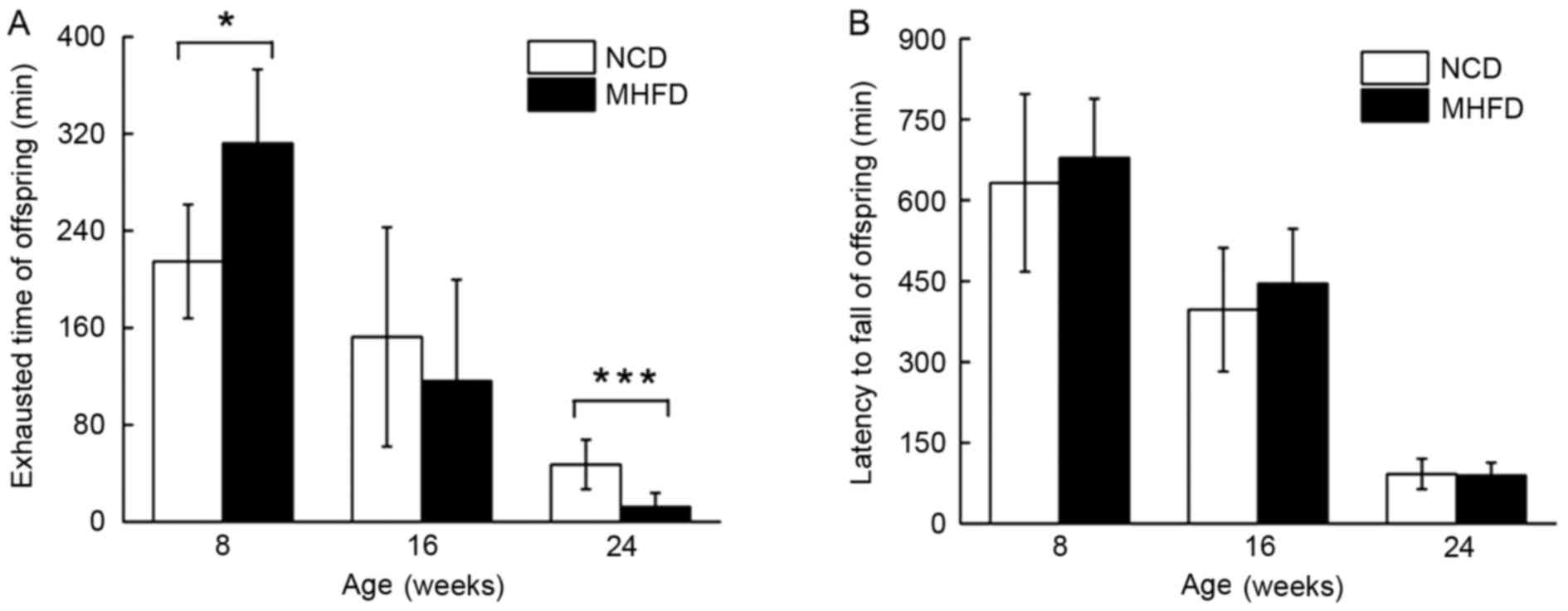
Treadmill test and rotarod test
In the treadmill test, 8-week old MHFD mice with
larger mass exhibited an extended running time until exhaustion of
47%; at the age of 16 weeks, the endurance capacity of MHFD mice
decreased by 37%, and there was a 28% decline compared with the
control group. The two groups of 24-week old mice underachieved;
the MHFD mice ran 29% of the running distance of the NCD mice
(Fig. 2A). Despite a difference in
the performance of the rotarod test between the two 8-week old
groups, no significant differences were observed between the MHFD
and NCD mice of any age group (Fig.
2B).
Maternal and fetal BP
SBP was elevated among the 14-week old MHFD maternal
mice compared with their controls (Table I). A trend was exhibited in the
offspring of the MHFD and NCD groups, with increased BP observed
among the MHFD offspring (Table
I). It is notable that the group with the highest SBP
measurements was not the oldest mice of 24 weeks, rather the
16-week groups (Table I). The data
for MBP and DBP were consistent with that for SBP between the
experimental groups (Table I). In
addition, although the MHFD mice exhibited an increase in HR in the
maternal mice and the offspring, no significant difference between
groups was observed (Table I).
 | Table I.Comparisons of maternal and offspring
levels of SBP, MBP, DBP and HR between mice fed NCD and MHFD. |
Table I.
Comparisons of maternal and offspring
levels of SBP, MBP, DBP and HR between mice fed NCD and MHFD.
|
| Group |
|---|
|
|
|
|---|
|
| Mother | 8-week old
offspring | 16-week old
offspring | 24-week old
offspring |
|---|
|
|
|
|
|
|
|---|
| Characteristic | NCD (n=8) | MHFD (n=8) | NCD (n=8) | MHFD (n=8) | NCD (n=8) | MHFD (n=8) | NCD (n=12) | MHFD (n=12) |
|---|
| SBP | 98.76±7.93 |
123.48±16.70b | 90.78±11.67 |
111.03±11.15b | 105.49±18.37 |
130.95±9.43a | 96.62±8.64 |
114.40±7.48c |
| MBP | 77.76±6.78 |
93.21±12.56a | 69.64±12.69 |
85.33±9.19a | 81.04±14.94 |
94.26±12.64a | 70.80±10.90 |
83.32±7.52b |
| DBP | 67.33±6.74 |
78.10±11.79a | 58.83±13.36 | 70.79±10.46 | 65.90±14.56 | 75.75±15.06 | 57.59±13.42 |
67.72±8.72a |
| HR | 669.95±65.77 | 683.83±58.52 | 490.83±98.48 | 518.84±101.84 | 512.68±78.74 | 519.31±125.33 | 501.47±82.1 | 527.32±76.60 |
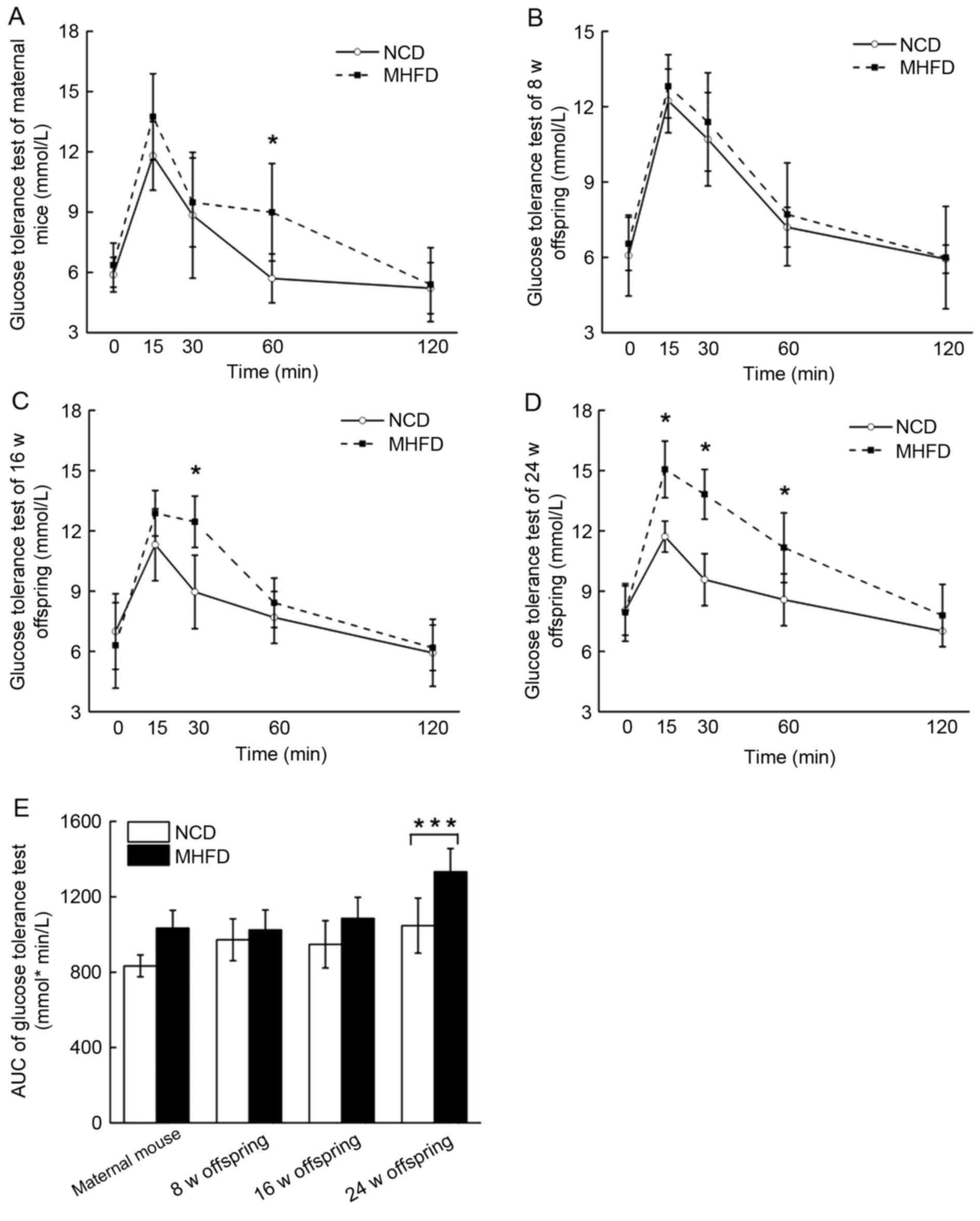
Maternal and fetal glucose
metabolism
The maternal mice were glucose intolerant (Fig. 3A). The glucose tolerance of the
8-week old MHFD offspring was comparable with that of the control
group (Fig. 3B). A 14% and a 24%
rise in the area under the curve of the glucose sensitivity
measurements occurred in the 16-week and 24-week old MHFD offspring
groups, respectively (Fig.
3C-E).
Major organs and issue of
offspring
The subtraction method was used to measure the wet
weight of organs and fat tissues from the sacrificed mice. As
presented in Table II, the
subcutaneous adipose tissue samples of the 8-week old MHFD
offspring exhibited a ~1.5-fold increased mass compared with those
of the 8-week old NCD offspring; this difference was increased
between the two 16-week old groups, and ended with a 3-fold
difference between 24-week old MHFD offspring and 24-week old NCD
offspring. The kidneys of 24-week old MHFD mice also exhibited an
increase in mass compared with the control group (Table II).
 | Table II.Comparisons of the wet mass of
adipose tissue and major organs between mice fed NCD and mice fed
MHFD in offspring of 8, 16 and 24 weeks of age. |
Table II.
Comparisons of the wet mass of
adipose tissue and major organs between mice fed NCD and mice fed
MHFD in offspring of 8, 16 and 24 weeks of age.
|
| Group |
|---|
|
|
|
|---|
|
| 8 weeks | 16 weeks | 24 weeks |
|---|
|
|
|
|
|
|---|
| Characteristic | NCD | MHFD | NCD | MHFD | NCD | MHFD |
|---|
| Body weight
(g) | 20.012±2.711 | 21.703±2.312 | 21.315±1.621 | 23.213±1.794 | 22.720±2.501 | 27.401±3.579 |
| Kidney (g) | 0.140±0.021 | 0.133±0.051 | 0.142±0.020 | 0.133±0.015 | 0.148±0.030 |
0.177±0.034a |
| Subcutaneous fat
(g) | 0.097±0.015 |
0.147±0.043a | 0.107±0.050 | 0.148±0.019 | 0.122±0.050 |
0.308±0.118c |
| Epididymal or
parametrial fat (g) | 0.122±0.04 | 0.295±0.252 | 0.156±0.051 | 0.204±0.061 | 0.174±0.071 |
0.499±0.190c |
| Mesenteric fat
(g) | 0.118±0.058 | 0.175±0.041 | 0.140±0.044 |
0.228±0.037c | 0.150±0.047 |
0.344±0.157b |
| Perirenal fat
(g) | 0.022±0.012 |
0.067±0.035a | 0.045±0.039 | 0.072±0.038 | 0.066±0.045 |
0.250±0.132c |
| Spleen (g) | 0.076±0.011 | 0.074±0.011 | 0.080±0.022 | 0.066±0.014 | 0.072±0.010 | 0.086±0.028 |
| Pancreas (g) | 0.088±0.021 | 0.108±0.033 | 0.124±0.024 | 0.111±0.019 | 0.116±0.027 | 0.119±0.032 |
| Liver (g) | 0.878±0.117 | 0.979±0.146 | 0.963±0.216 | 0.977±0.051 | 1.092±0.197 | 1.077±0.133 |
| Heart (g) | 0.149±0.040 | 0.135±0.015 | 0.141±0.025 | 0.127±0.018 | 0.142±0.029 | 0.144±0.036 |
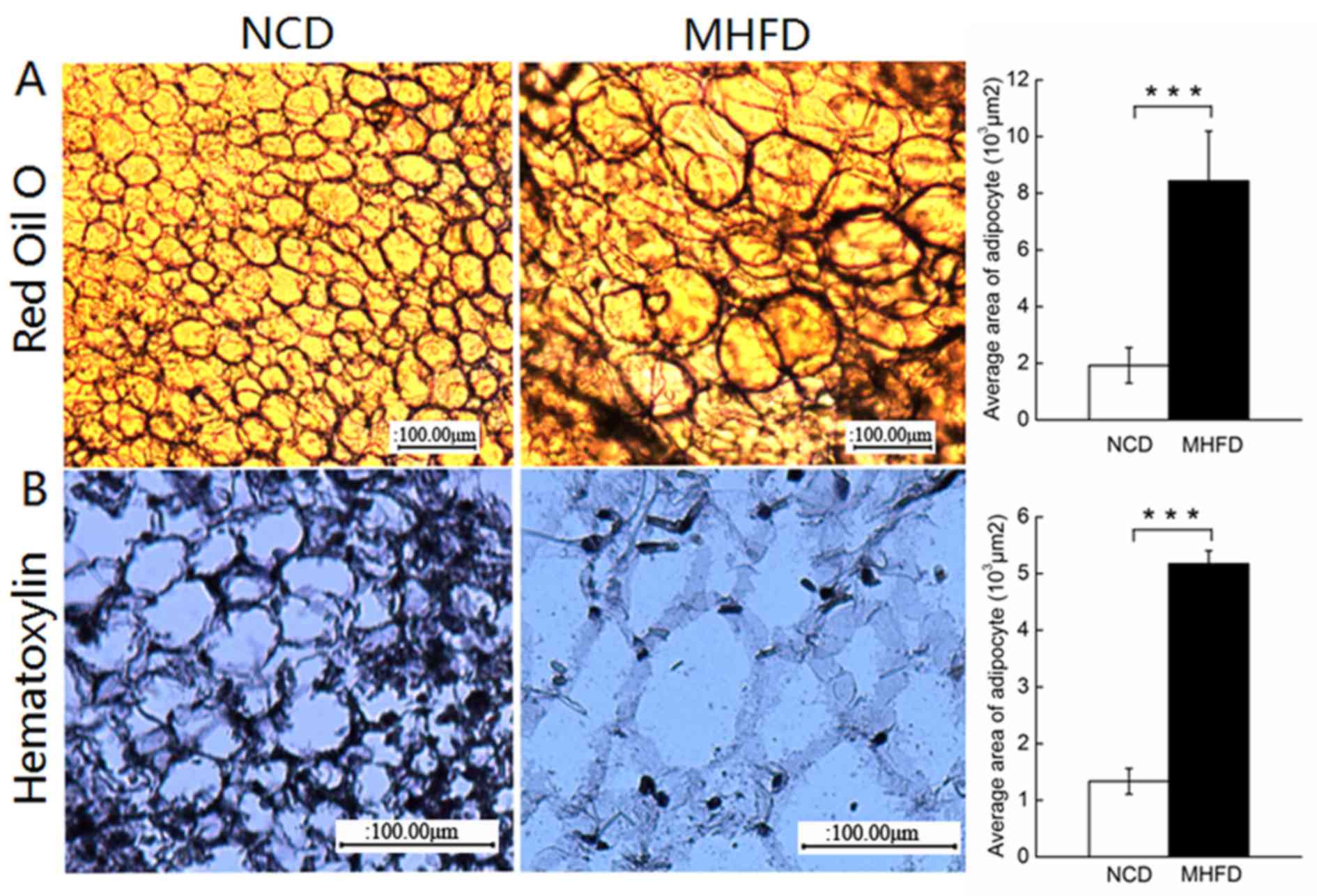
Morphological analysis of fetal
adipose tissue
Consistent with previous studies, MHFD nutrition for
24 weeks caused significant adipocyte hypertrophy of ~4-fold, as
demonstrated in paraffin sections stained with hematoxylin or
frozen sections stained with Oil Red O (Fig. 4). The 24-week old MHFD mice
exhibited fewer nuclei (Fig. 4A)
and the cells were swollen with fat deposits (Fig. 4B), specifically stained using Oil
Red O.
Inflammatory and anti-inflammatory
alterations
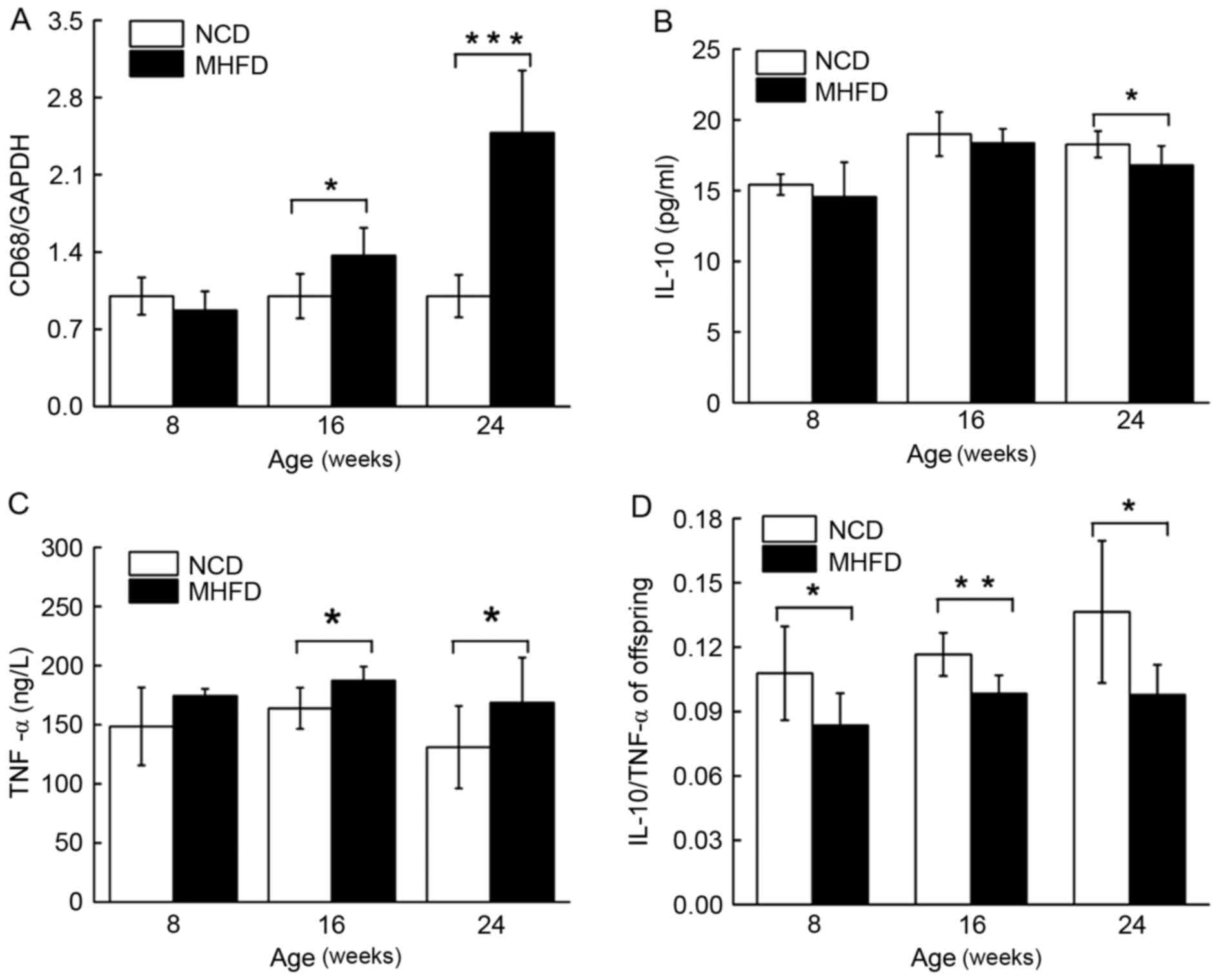
Although no distinction was observed between the
groups of young mice, the expression of transcripts encoding CD68
increased by 2.48-fold among the MHFD offspring compared with those
of NCD mice (Fig. 5A). A ~10%
decrease in serum IL-10 expression was exhibited among the young
and the aged MHFD mice; however, the difference between the two
young groups was not statistically significant (Fig. 5B). By contrast, the level of plasma
TNF-α of the MHFD offspring was increased by 17% compared with the
controls in the 8-week old groups, and peaked at 36.42% in the
24-week old groups (Fig. 5C). The
serum ratio of IL-10/ TNF-α among the NCD mice increased
continuously, while remaining constant in the MHFD mice.
Consequently, the IL-10/ TNF-α ratio was reduced in MHFD group
compared with their controls throughout the experiment (Fig. 5D).
Discussion
The present study is, to the best of our knowledge,
the first to investigate the physical endurance of obese C57BL/6
offspring throughout their life cycle. Obesity increases the
likelihood of chronic diseases (1). Despite evidence that obesity is
associated with hypertension, glucose sensitive, macrophage
infiltration and adipokine changes (1,22)
these associations require further investigation.
As previously reported, BMI is markedly associated
with SBP and DBP (23). Obesity
and salt intake are the most modifiable risk factors for high BP
(24). The present study
demonstrated that the SBP, MBP and DBP of middle-aged offspring are
increased compared with those of other age groups, including more
aged mice. The present results suggest that MHFD induces obesity
and hypertension among maternal mice and their offspring, and that
a MHFD will increase the probability of offspring hypertension by
≤25% if other factors remain unaltered.
Feeding MHFD to WT mice has been demonstrated to
cause hyperglycemia and glucose intolerance (10). The oral glucose tolerance test is a
method with which to exclude the subsequent manifestation of
gestational diabetes mellitus in pregnant women at high risk
(25). In the present study,
adolescent offspring demonstrate little difference in glucose
tolerance. After 16 weeks, a significant increase in glucose
tolerance was exhibited. The present results demonstrated that the
accumulation of body fat contributes to the formation of insulin
resistance in a time-dependent manner. The blood glucose graph of
the maternal mice exhibited a similar trend to that described by
Murabayashi et al (9).
Compared with the 10–15% macrophage content observed
in lean animals (26), adipose
tissue macrophages comprise 45–60% of stromal cells in obese
animals (27). Adipose tissue is
the principal site for the long-term storage of nutrients and also
regulates systemic metabolism through the release of hormones
termed adipokines (28). Obesity
increases tissue infiltration by macrophages and polarization to
the pro-inflammatory M1 state (10). TNF-α is an M1 marker cytokine,
while IL10 expression is associated with M2 polarization (10). In the present study, the increasing
expression of CD68 in the adipose tissue of MHFD offspring
demonstrates that obesity elevates tissue macrophage levels. The
increased serum TNF-α of MHFD offspring may be due to the
increasing proportion of M1 macrophages in adipose tissues; this
trophic effect of alternatively activated macrophages is partly
mediated by IL-10 secretion, which potentiates insulin action in
adipocytes (26). There exists a
controversy in previous research around the association between
obesity and M2 macrophage content. Following treatment with acute
high fat diet, alternative macrophage polarization was promoted in
adipose tissue (12). In other
studies, the decline of local IL-10 has been reported (10). In the present study, the serum
IL-10 protein levels of the young and aged MHFD offspring were
significantly decreased compared with those exhibited by NCD
offspring, while no significant difference was demonstrated between
the middle-aged groups.
Although exercising is among one of the primary
treatments for obesity (2), little
has been reported about the association between diet-induced
obesity and skeletal muscle function. The present study
demonstrated that in aged offspring groups, fat accumulation
significantly reduced physical endurance capacity by 71% compared
with healthy control mice. Previous research demonstrated that
imbalanced local expression of TNF-α and IL-10 leads to
inflammation-induced myopathy, including heart failure (14). Therefore, the physical decline of
aged offspring may be due, in part, to the long-term imbalance
between IL-10 and TNF-α.
It was observed that MHFD increased running time by
47% in the early age MHFD group, compared with the control group.
However, the leanness may be the reason for the poor performance of
the normal control offspring compared with the MHFD offspring.
Prolonged moderate-level aerobic exercise at 65% maximum aerobic
capacity results in the maximum contribution of fat to the total
energy expenditure; at this level, fat may contribute 40–60% of the
total energy expenditure, depending on the duration of the exercise
(29). An alteration in body
movement and coordination was not demonstrated in the present
study. In conclusion, obesity causes metabolic disorders and
hypertension, and alterations in physical endurance capacity, one
of the underlying reasons for which may be the long-term altered
IL-10/TNF-α ratio.
Acknowledgements
The authors of the present study would like to
acknowledge Dr Zhihong Wang of Changchun University of Chinese
Medicine (Changchun, China), for the efforts and support by
providing the BP2010; Miss Yang Liu of the Biological Experimental
Teaching Demonstration Center of Jilin University (Changchun,
China), for assistance and help with mouse breeding; and Dr Liming
Hao of the Department of Histology and Embryology, College of Life
Science, Jilin University Changchun, China), for the suggestions
about histology. The present study was supported by the Natural
Science Fund Program of China (grant nos. 31070309 and 81200454),
Jilin Provincial Bureau of Science and Technology (grant no.
20140307014NY) and Project 985 of Jilin University.
References
|
1
|
Haslam DW and James WP: Obesity. Lancet.
366:1197–1209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee-Young RS, Ayala JE, Fueger PT, Mayes
WH, Kang L and Wasserman DH: Obesity impairs skeletal muscle AMPK
signaling during exercise: Role of AMPK alpha 2 in the regulation
of exercise capacity in vivo. Int J Obesity (Lond). 35:982–989.
2011. View Article : Google Scholar
|
|
3
|
Church TS: Why obesity should be treated
as a disease. Curr Sports Med Rep. 13:205–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vahratian A: Prevalence of overweight and
obesity among women of childbearing age: Results from the 2002
national survey of family growth. Matern Child Health J.
13:268–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi P, Yang W, Yu Q, Zhao Q, Li C, Ma X,
Jin L, Han X, Zhang Y and Yan W: Overweight, gestational weight
gain and elevated fasting plasma glucose and their association with
macrosomia in chinese pregnant women. Matern Child Health J.
18:10–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hales CN and Barker DJP: Type 2
(non-insulin-dependent) diabetes mellitus: The thrifty phenotype
hypothesis. Diabetologia. 35:595–601. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barker DJ: Maternal nutrition, fetal
nutrition, and disease in later life. Nutrition. 13:807–813. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vogt MC, Paeger L, Hess S, Steculorum SM,
Awazawa M, Hampel B, Neupert S, Nicholls HT, Mauer J, Hausen AC, et
al: Neonatal insulin action impairs hypothalamic neurocircuit
formation in response to maternal high-fat feeding. Cell.
156:495–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murabayashi N, Sugiyama T, Zhang L,
Kamimoto Y, Umekawa T, Ma N and Sagawa N: Maternal high-fat diets
cause insulin resistance through inflammatory changes in fetal
adipose tissue. Eur J Obstet Gynecol Reprod Biol. 169:39–44. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han MS, Jung DY, Morel C, Lakhani SA, Kim
JK, Flavell RA and Davis RJ: JNK expression by macrophages promotes
obesity-induced insulin resistance and inflammation. Science.
339:218–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Gregorio GB, Yao-Borengasser A, Rasouli
N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE
and Kern PA: Expression of CD68 and macrophage chemoattractant
protein-1 genes in human adipose and muscle tissues: Association
with cytokine expression, insulin resistance, and reduction by
pioglitazone. Diabetes. 54:2305–2313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji Y, Sun S, Xia S, Yang L, Li X and Qi L:
Short term high fat diet challenge promotes alternative macrophage
polarization in adipose tissue via natural killer T cells and
interleukin-4. J Biol Chem. 287:24378–24386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang LY, Sugiyama T, Murabayashi N,
Umekawa T, Ma N, Kamimoto Y, Ogawa Y and Sagawa N: The inflammatory
changes of adipose tissue in late pregnant mice. J Mol Endocrinol.
47:157–165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shreeram S, Ramesh S, Puthan JK,
Balakrishnan G, Subramanian R, Reddy MT and Pereira SL: Age
associated decline in the conversion of leucine to
β-hydroxy-β-methylbutyrate in rats. Exp Gerontol. 80:6–11. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Batista ML Jr, Rosa JC, Lopes RD, Lira FS,
Martins E Jr, Yamashita AS, Brum PC, Lancha AH Jr, Lopes AC and
Seelaender M: Exercise training changes IL-10/TNF-alpha ratio in
the skeletal muscle of post-MI rats. Cytokine. 49:102–108. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Basic VT, Jacobsen A, Sirsjö A and
Abdel-Halim SM: TNF stimulation induces VHL overexpression and
impairs angiogenic potential in skeletal muscle myocytes. Int J Mol
Med. 34:228–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim DS, Cha HN, Jo HJ, Song IH, Baek SH,
Dan JM, Kim YW, Kim JY, Lee IK, Seo JS and Park SY: TLR2 deficiency
attenuates skeletal muscle atrophy in mice. Biochem Biophys Res
Commun. 459:534–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung SY, Kim DY, Yune TY, Shin DH, Baek SB
and Kim CJ: Treadmill exercise reduces spinal cord injury-induced
apoptosis by activating the PI3K/Akt pathway in rats. Exp Ther Med.
7:587–593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stover KR, Campbell MA, Van Winssen CM and
Brown RE: Analysis of motor function in 6-month-old male and female
3×Tg-AD mice. Behav Brain Res. 281:16–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu L and Liu Y: Administration of
telmisartan reduced systolic blood pressure and oxidative stress
probably through the activation of PI3K/Akt/eNOS pathway and NO
release in spontaneously hypertensive rats. Physiol Res.
62:351–359. 2013.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Preston Campbell J, Mulcrone P, Masood SK,
Karolak M, Merkel A, Hebron K, Zijlstra A, Sterling J and
Elefteriou F: TRIzol and Alu qPCR-based quantification of
metastatic seeding within the skeleton. Sci Rep. 5:126352015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grundy SM: Obesity, metabolic syndrome,
and cardiovascular disease. J Clin Endocr Metab. 89:2595–2600.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozturk C, Aparci M, Karaduman M, Balta S,
Çelik T and Iyisoy A: Relationship of systolic blood pressure and
body mass index with left ventricular mass and mass index in
adolescents. Angiology. 67:58–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Correia-Costa L, Cosme D, Nogueira-Silva
L, Morato M, Sousa T, Moura C, Mota C, Guerra A, Albino-Teixeira A,
Areias JC, et al: Gender and obesity modify the impact of salt
intake on blood pressure in children. Pediatr Nephrol. 31:279–88.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bitó T, Nyári T, Kovács L and Pál A: Oral
glucose tolerance testing at gestational weeks ≤16 could predict or
exclude subsequent gestational diabetes mellitus during the current
pregnancy in high risk group. Eur J Obstet Gynecol Reprod Biol.
121:51–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lumeng CN, Bodzin JL and Saltiel AR:
Obesity induces a phenotypic switch in adipose tissue macrophage
polarization. J Clin Invest. 117:175–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weisberg SP, McCann D, Desai M, Rosenbaum
M, Leibel RL and Ferrante AW: Obesity is associated with macrophage
accumulation in adipose tissue. J Clin Invest. 112:1796–1808. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|