Introduction
Osteosarcoma, the most common primary malignant bone
tumor in children and adolescents, is considered to be a
significant potential threat to the health of teenagers (1). Osteosarcoma originates from
mesenchymal tissue, and frequently occurs in the metaphyseal region
of growing bones. With its characteristics of rapid growth and
marked invasiveness, osteosarcoma is highly malignant and prone to
lung metastases. Pulmonary micrometastasis may be observed in ~80%
of patients at the time of diagnosis, which may be the cause of the
low survival rate of patients with osteosarcoma (2,3). At
present, the treatment for osteosarcoma is primarily surgery
combined with preoperative chemotherapy (2). Although progress has been made in the
treatment of osteosarcoma, the 5-year survival rate of patients
with osteosarcoma is only ~30% (2). It is very important to identify novel
therapeutic targets and develop effective drugs for the treatment
of osteosarcoma.
L-mimosine, a plant amino acid which is extracted
from Leucaena leucocephala or Mimosa pudica, is a
type of iron chelator and prolyl hydroxylase inhibitor (4,5).
L-mimosine has been reported to exhibit anti-tumor activity in a
number of types of tumor, including pancreatic cancer, prostate
cancer, breast cancer and cervical cancer (4–6);
however, the effect of L-mimosine in osteosarcoma has not been
reported, and the underlying mechanisms remain to be clarified. In
the present study, two osteosarcoma cell lines, MG63 and U2OS, were
used to examine the antitumor activity of L-mimosine in
osteosarcoma. In addition, the associated mechanisms were further
investigated.
Materials and methods
Reagents
The following reagents were used in the present
study: L-mimosine and Z-LEHD-FMK (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany); SCH772984 [specific inhibitor of extracellular
signal-regulated kinase (ERK)] (MedChem Express, Monmouth Junction,
NJ, USA); fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA); RPMI-1640 medium (HyClone; GE Healthcare
Life Sciences, Logan, UT, USA); Dulbecco's modified Eagle's medium
(DMEM; HyClone; GE Healthcare Life Sciences); DMEM/F-12 (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA); Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies Inc., Kumamoto,
Japan); Annexin V/propidium iodide (PI) apoptosis kit [Hangzhou
Multi Sciences (Lianke) Biotech Co., Ltd., Hangzhou, China];
Hoechst staining kit (Beyotime Institute of Biotechnology, Haimen,
China); cleaved caspase-9 (cat. no. 9929), cleaved caspase-3 (cat.
no. 9929), cleaved poly(ADP-ribose) polymerase (PARP) (cat. no.
9929), apoptosis regulator Bcl-2 (Bcl-2) (cat. no. 9941), apoptosis
regulator BAX (BAX) (cat. no. 9942), ERK (cat. no. 9902),
phosphorylated (p)-ERK (cat. no. 9910) and GAPDH (cat. no. 5174)
antibodies (Cell Signaling Technology, Inc., Danvers, MA, USA); and
cleaved caspase-8 antibody (Novus Biologicals, LLC, Littleton, CO,
USA). The secondary antibody was a donkey anti-rabbit IgG (cat. no.
925–32213; LI-COR Biosciences, Lincoln, NE, USA).
Cell culture
Human osteosarcoma cell lines MG63 and U2OS, which
were originally purchased from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China), were conserved in
the laboratory, and were respectively cultured in DMEM and
RPMI-1640, supplemented with 10% fetal bovine serum and 20 µg/ml
antibiotics (ampicilin and kanamycin), at 37°C and 5%
CO2. Human normal osteoblast cells hFOB 1.19 were
obtained from the Type Culture Collection of the Chinese Academy of
Sciences, and was cultured in DMEM/F-12, supplemented with 10%
fetal bovine serum and 20 µg/ml antibiotics (ampicilin and
kanamycin), at 33.5°C and 5% CO2.
Cell proliferation assay
Cells were harvested and adjusted to
2×104 cells/ml, and seeded in 96-well plates. A total of
three replicates were performed in every group, and a blank control
was additionally set up. A concentration gradient of L-mimosine (0,
200, 400 and 800 µM) was used for treatment. Following 24, 48 and
72 h of culture, 10 µl CCK-8 was added to each well, and the plate
was incubated at 37°C for 1 h, and the absorbance value was
measured at 450 nm. The experiment was repeated three times
independently.
Flow cytometry
A concentration gradient of L-mimosine (0, 200, 400
and 800 µM) was used for treatment for 24 h. Cells in 6-well plate
at a density of 1×104/well were collected and washed
twice with cold PBS. An Annexin V/PI apoptosis kit was used for
detection. The cells were resuspended in Annexin-V binding buffer,
and stained with 5 µl Annexin-V-fluorescein isothiocyanate (FITC)
and 10 µl PI in the dark for 15 min at room temperature.
Fluorescence was analyzed on a FACSCanto™ II
spectrometer (BD Biosciences, Franklin Lakes, NJ, USA), and the
software used for the analysis was CellQuest Pro (BD Biosciences).
Cells stained with FITC/PI were counted as apoptotic cells. The
experiment was repeated three times independently.
Hoechst staining
Cells were harvested and seeded into 6-well plates
at a density of 1×104 cells/well, a concentration
gradient of L-mimosine (0, 200, 400 and 800 µM) was used for
treatment for 24 h. Cells were washed with PBS once, 1 ml/well
Hoechst was added, and the plate was placed in the dark for a 30
min incubation. Subsequently, the Hoechst was removed and the cells
were washed with PBS twice, and observed with a fluorescence
microscope at a magnification of ×40. The staining results were
quantified using Image Studio v3.1 software (LI-COR Biosciences)
and the experiment was repeated three times independently.
Transmission electron microscopy
(TEM)
Cells were harvested and seeded into 6-well plates
at a density of 1×104 cells/well, and a concentration
gradient of L-mimosine (0, 200, 400 and 800 µM) was used for
treatment for 24 h. Cells were placed in 4°C pre-cooled 2.5%
glutaraldehyde and fixed for 2 h. Cells were washed 3 times with
PBS buffer, fixed in 1% osmium tetroxide for 2 h at 4°C, and washed
with buffer three times. The cells were soaked with gradient
ethanol, acetone dehydrated and embedded with Epon 812 at 60°C for
24 h. Double staining was performed with uranyl acetate and lead
citrate at room temperature for 20 min. Sections were observed
under TEM and images were captured.
Western blotting
A concentration gradient of L-mimosine (0, 200, 400
and 800 µM) was used for treatment for 24 h. Z-LEHD-FMK (40 µM) was
used for caspase-9 inhibtion; and 5 nM SCH772984 was used for ERK
inhibition. Cells were collected and seeded into 6-well plates, and
a concentration gradient of L-mimosine (0, 200, 400 and 800 µM) was
used for treatment for 24 h. Cells were harvested and lysed in a
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) containing a protease inhibitor cocktail and 2 mM
dithiothreitol. A biconchoninic acid assay (Thermo Fisher
Scientific, Inc.) was used to determine the protein concentration
in each sample. The loading quantity of samples per lane was 30 µg.
Lysates were resolved by SDS-PAGE on a 10% gel, transferred to
polyvinylidene fluoride (PVDF) membranes. The PVDF membrane was
blocked with the blocking solution (5% milk) at room temperature
for 2 h. And then immunoblotted with primary antibodies (1:1,000).
Following immunoblotting with secondary antibodies (1:10,000), the
membranes were scanned with the Odyssey CLx Infrared Imaging System
(LI-COR Biosciences). The western blot bands were quantified using
Image Studio v3.1 software, and the experiment was repeated three
times independently.
Statistical analysis
All values are expressed as the mean ± standard
deviation. Statistical analyses were performed using one-way
analysis of variance followed by Tukey's post hoc test with SPSS
13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of L-mimosine on the
proliferation of the osteosarcoma cell lines MG63 and U2OS
In order to evaluate the in vitro effect of
L-mimosine on the proliferation of the osteosarcoma cell lines MG63
and U2OS, the CCK-8 method was used to assess cell viability
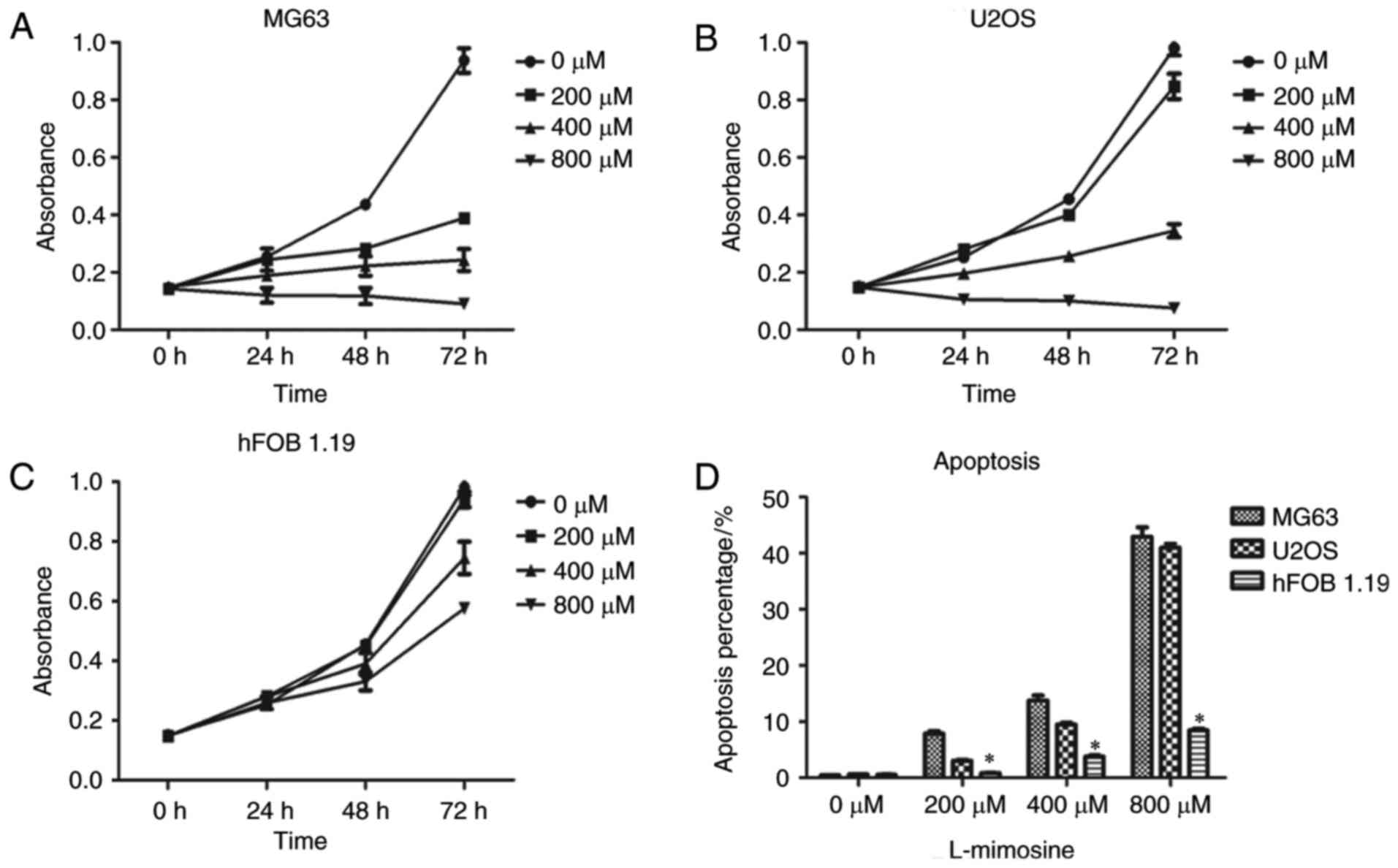
(Fig. 1). A concentration gradient
of L-mimosine (0, 200, 400 and 800 µM) was used to treat the
osteosarcoma cell lines MG63 and U2OS for 24, 48 and 72 h. The
results demonstrated that L-mimosine exhibited anti-proliferative
effects in human osteosarcoma cells, and the effects were observed
to be concentration-dependent (Fig. 1A
and B). The results indicated that the cell line MG63 was more
sensitive to L-mimosine compared with U2OS. In addition, the
toxicity of L-mimosine on human normal osteoblasts was assessed in
the present study. The human normal osteoblast cell line hFOB 1.19
was chosen as the normal control in the CCK-8 assay to evaluate the
toxicity of L-mimosine. The results demonstrated that L-mimosine
was less toxic to normal human osteoblasts, exerting a weak
inhibitory effect on proliferation (Fig. 1C).
Effect of L-mimosine on the apoptosis
of osteosarcoma cell lines MG63 and U2OS
To test the in vitro effect of L-mimosine on
the apoptosis of osteosarcoma cell lines MG63 and U2OS, a flow
cytometry experiment was performed with gradient concentrations of
L-mimosine (0, 200, 400 and 800 µM) incubated for 24 h. The results
demonstrated that the apoptosis rate of the cells increased with
the increase in the concentration of L-mimosine, and that the
effect was therefore concentration dependent (Fig. 1). The results demonstrated that
L-mimosine exerted a pro-apoptotic effect on human osteosarcoma
cells, and that MG63 cells were more sensitive to L-mimosine
(Fig. 1). In addition, human
normal osteoblast hFOB 1.19 cells were selected as the normal
control in the flow cytometry assay to evaluate the toxicity of
L-mimosine. The results demonstrated that L-mimosine was less toxic
to normal human osteoblasts, exerting a weak effect on apoptosis
(Fig. 1D).
Nuclear damage in MG63 cells increases
with the increase in L-mimosine concentration
The flow cytometry assay illustrated marked
apoptosis following treatment with gradient concentrations of
L-mimosine; the effect was most notable in the MG63 cell line. In
order to further confirm this result, the nuclear damage induced by
L-mimosine was examined in the more sensitive MG63 cell line using
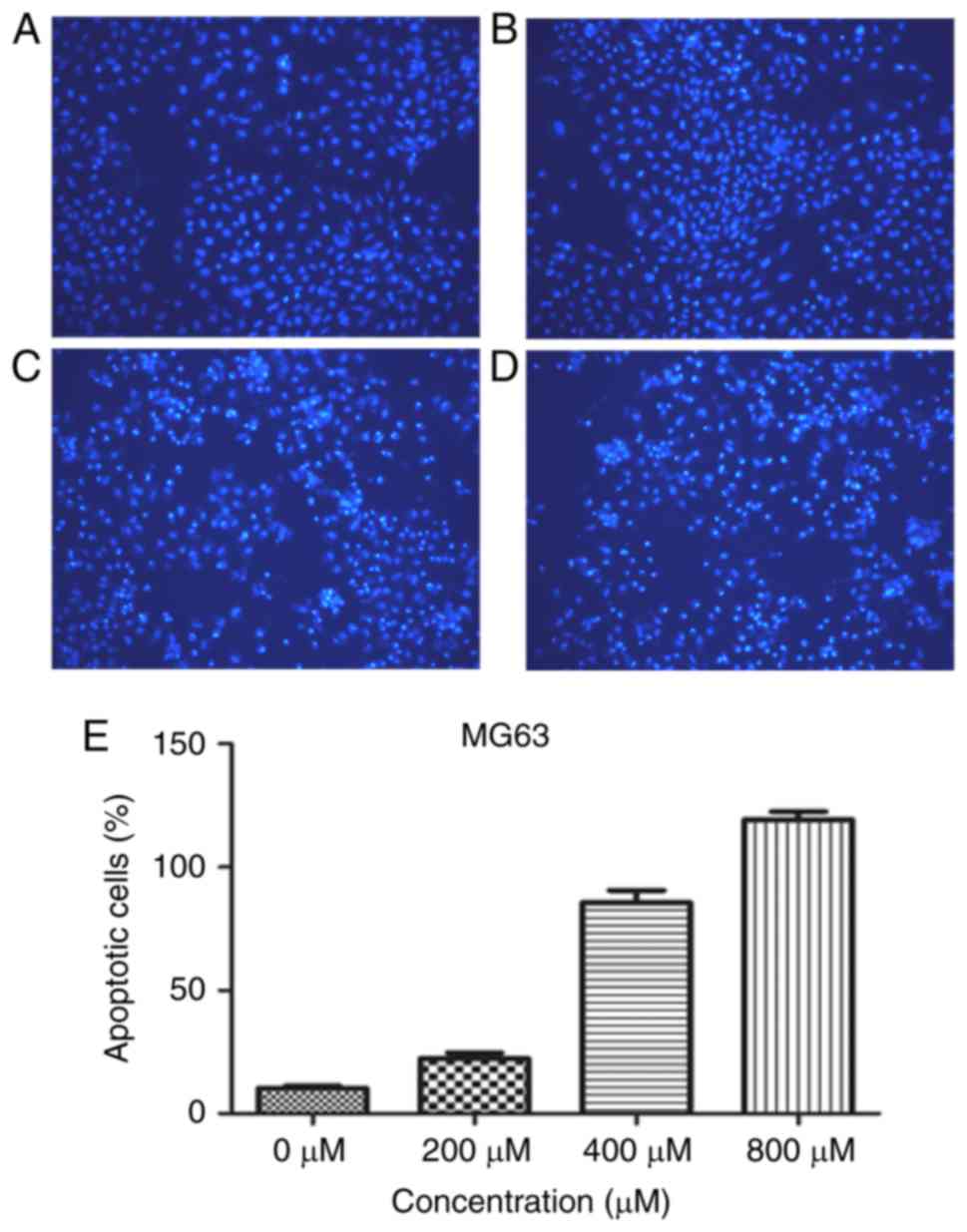
Hoechst staining (Fig. 2). The
cells in the control group exhibited weak blue fluorescence, and
the apoptotic cells exhibited membrane permeability, which was
observed as bright blue fluorescence. The experimental results
demonstrated that with the increased concentration of L-mimosine
(0, 200, 400, 800 µM), the number of nuclei appearing with bright
blue fluorescence increased, indicating that the number of
apoptotic cells increased.
Ultrastructural alterations in MG63
cells treated with L-mimosine
Hoechst staining in the previous experiment
demonstrated that the apoptosis of MG63 cells was markedly induced
by L-mimosine. In order to understand the alterations in the cell
during apoptosis, TEM was used for the observation of the
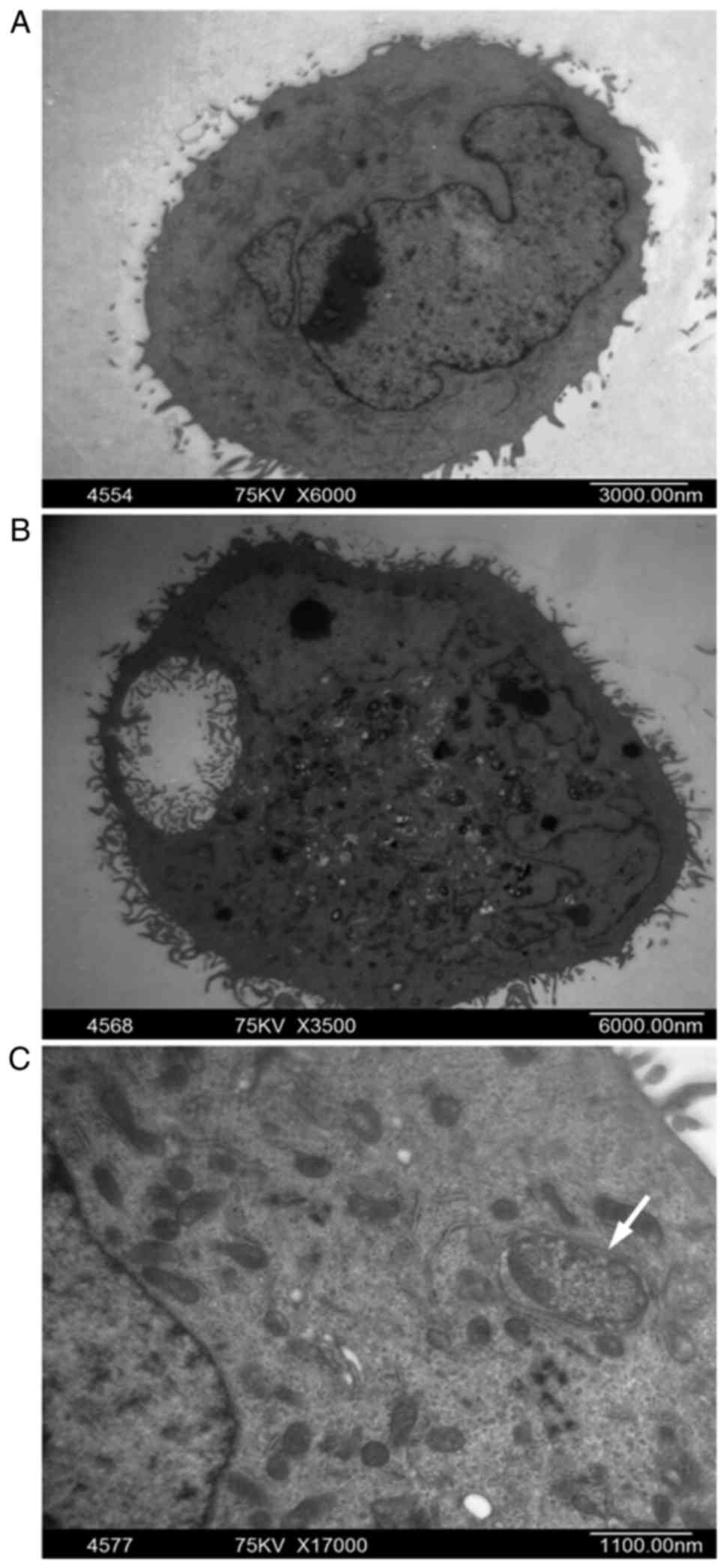
ultrastructural alterations in MG63 treated with L-mimosine. Under
TEM observation (Fig. 3), the
control group of MG63 displayed varied forms, a large nucleus and
an imbalance in the nucleus-cytoplasm ratio, with an intact nuclear
membrane, prominent nucleoli and evenly distributed nuclear
chromatin (Fig. 3A). By contrast,
the L-mimosine-treated group appeared with typical apoptosis
morphological features, including cell shrinkage, cytoplasm
condensation, pyknotic nuclei and a lack of nucleoli (Fig. 3B), and apoptotic bodies were
observed (Fig. 3C; white
arrow).
L-mimosine regulated apoptosis related
proteins in MG63 cells
To investigate the apoptotic effect of L-mimosine,
western blotting was performed (Fig.
4). As presented in Fig. 4A,
cleaved PARP, cleaved caspase-9 and cleaved caspase-3 exhibited
increased expression as the concentration of L-mimosine increased,
while the expression of cleaved caspase-8 did not notably alter.
Attenuated expression of Bcl-2 and increased expression of BAX were
observed in MG63 cells treated with gradient concentrations of
L-mimosine. Additionally, it is known that xenobiotics may alter
cellular functions, including proliferation, the cell cycle and
apoptosis, by affecting cell survival pathways; consequently, the
present study further examined the signaling pathways associated
with L-mimosine. As presented in Fig.
4A, L-mimosine reduced the levels of ERK and p-ERK in a
concentration-dependent manner. The role of L-mimosine in this
signaling pathway was further confirmed by the ERK signaling
specific inhibitor SCH772984. As presented in Fig. 4C, the suppressed ERK signaling
pathway following treatment with L-mimosine suggested this pathway
be an additional mechanism for apoptosis induction.
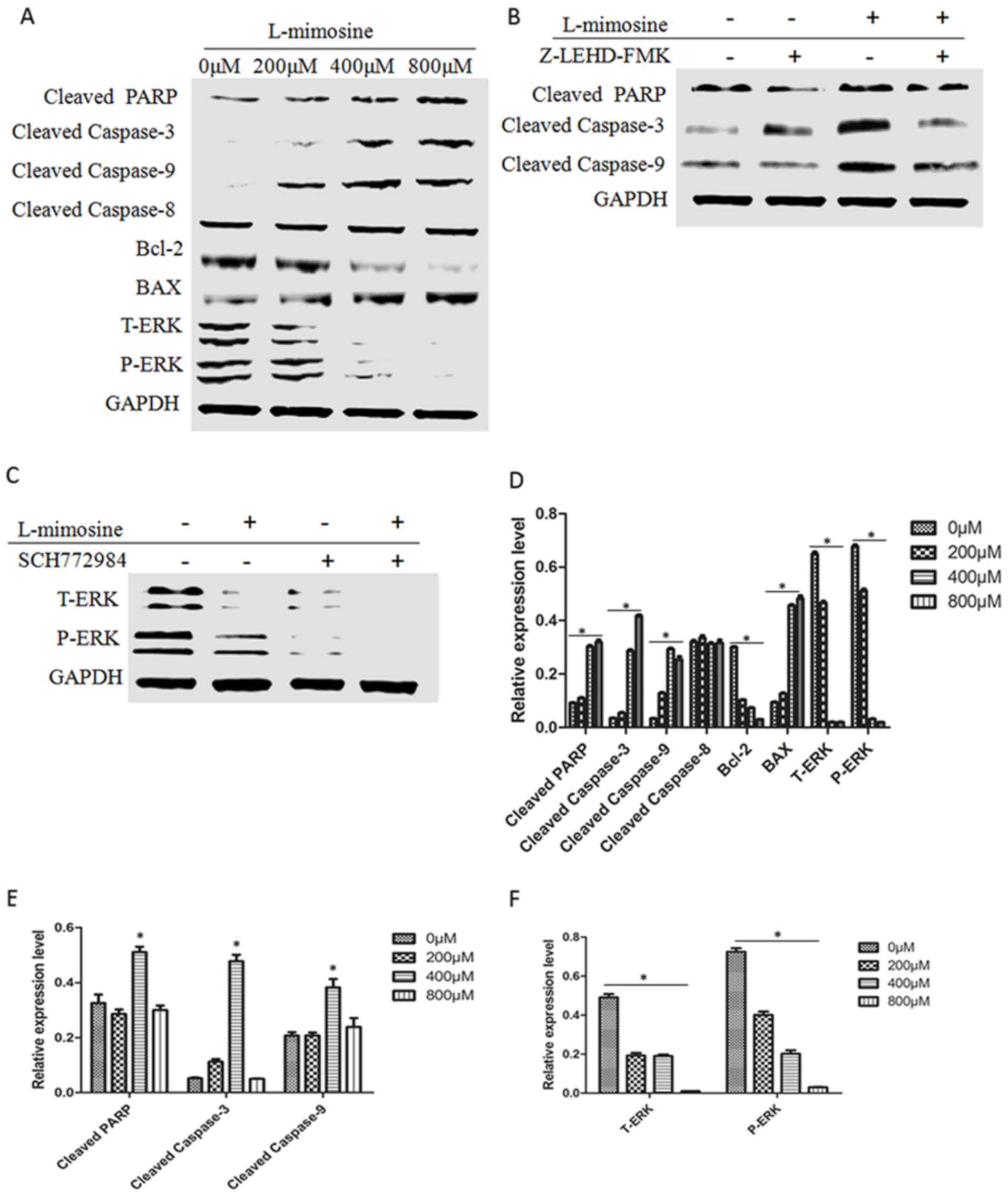 | Figure 4.Effect of L-mimosine on the expression
of apoptosis-associated proteins and the ERK signaling pathway in
MG63 cells. The results of the western blot analysis demonstrated
that (A) as L-mimosine concentration increases, cleaved PARP,
cleaved caspase-9 and cleaved caspase-3 exhibited increased
expression levels, whereas the expression of cleaved caspase-8 did
not change significantly. Attenuated expression of Bcl-2 and
increased expression of BAX was observed as the L-mimosine
concentration increased. L-mimosine reduced the levels of t-ERK and
p-ERK in a concentration-dependent manner. (B) L-mimosine-induced
apoptosis was inhibited by the caspase-9 inhibitor Z-LEHD-FMK. (C)
The role of L-mimosine in the ERK signaling pathway was confirmed
by the ERK signaling specific inhibitor SCH772984. (D-F) The
statistical graphs of the data shown in parts A-C, respectively.
*P<0.05. PARP, poly(ADP ribose) polymerase; Bcl-2, apoptosis
regulator Bcl-2; BAX, apoptosis regulator BAX; ERK, extracellular
signal-regulated kinase; p, phosphorylated; t, total. |
According to the results of the western blot
analysis, it was hypothesized that the treatment of cells with
L-mimosine was accompanied by an increase in cleaved caspase-9
expression. The present study assessed whether L-mimosine-induced
apoptosis was inhibited by the caspase-9 inhibitor Z-LEHD-FMK. As
presented in Fig. 4B, the results
suggested that L-mimosine induced apoptosis through the
mitochondrial apoptotic pathway.
Discussion
L-mimosine is a rare plant amino acid extracted from
Mimosa or Leucaena spp. The molecular formula of
L-mimosine is α-amino-β-N-[3-hydroxy-4-pyridone]-propionic acid,
C8H10N2O4. The molecular weight is 198.2. L-mimosine is
structurally different from other commonly used anti-cancer drugs,
and has a high degree of similarity to the structure of thymine
(7,8). Due to the particular chemical
structure of L-mimosine and its inhibitory effects on mammalian DNA
replication, L-mimosine is used as a type of cell cycle
synchronization drug in experiments, in addition to the study of
the induction of tumor cell death. Previous studies have
demonstrated the cytotoxicity of L-mimosine against a number of
types of of tumor cell line. The sensitivity of L-mimosine in
numerous human tumor cell lines was detected and the possible
mechanisms were examined. Studies have reported that L-mimosine is
a reversible cell cycle inhibitor in mammalian cells, which acts on
the G1/S phase of the cell cycle (9,10).
In addition, L-mimosine may interfere with the initiation of DNA
replication and the extension of the replication chain (7,11,12).
However, the exact mechanism of action of L-mimosine remains
unclear. Mechanisms which have previously been reported include:
Effectively preventing DNA synthesis by blocking the late G1 phase
(13); interfering with the
synthesis of histone H1 kinase (14,15);
and upregulating cyclin-dependent kinase inhibitor p27 protein
expression (8,16–18).
The question of whether L-mimosine is a reversible
cell cycle inhibitor has remained controversial due to a number of
reasons, including different research methods or experimental
conditions, and differences among different species and different
cells in previous studies. Cell type is one of the factors which
determines whether cells are prone to apoptosis (19); for example, cell lines which are
sensitive to chemotherapeutic agents are prone to apoptosis
(20), while apoptosis is
difficult to induce in certain cell lines following treatment with
chemotherapeutic agents (21).
Previously, researchers have reported the pro-apoptotic effect of
L-mimosine in a number of types of cancer (4,6,17,22),
including pancreatic cancer, prostate cancer, breast cancer and
cervical cancer. However, when induced by L-mimosine, the effects
on these tumors are different. Therefore, the present study sought
to investigate whether L-mimosine may have a pro-apoptotic effect
on osteosarcoma cells. The present study used two different types
of osteosarcoma cell line, MG63 and U2OS.
The present study tested the effect of L-mimosine on
osteosarcoma cell proliferation. The results of the CCK-8 assay
indicated that L-mimosine inhibited osteosarcoma cell
proliferation, and that the inhibitory effect was dose-dependent.
Subsequently, apoptosis was assessed in osteosarcoma cells induced
by L-mimosine. The Annexin V-FITC/PI double staining assay
demonstrated that L-mimosine induced osteosarcoma cell apoptosis,
and that the induction effect was dose-dependent. Previous studies
reported that L-mimosine inhibited tumor cell proliferation by
inducing tumor cell cycle arrest (17,23).
In addition, it is reported L-mimosine inhibited tumor cell
proliferation by altering the expression of
proliferation-associated genes (4). In the present study, it was
hypothesized that L-mimosine inhibited osteosarcoma cell
proliferation through the induction of cellular apoptosis.
Apoptosis is characterized by membrane blebbing,
cell shrinkage, chromatin condensation, DNA damage and
fragmentation of the cell into membrane-bound apoptotic bodies
(24). Subsequently, it was
observed that the apoptosis of osteosarcoma cells was caused by DNA
damage, via a Hoechst assay and TEM. In the Hoechst assay, the
damaged nuclei were increased with the increase in L-mimosine
concentration. When observed under TEM, the L-mimosine treated
group exhibited typical morphological features of apoptosis: Cell
shrinkage, cytoplasm condensation, nuclear pyknosis and a lack of
nucleoli, in addition to apoptotic body formation.
Cellular apoptosis induced by L-mimosine was
confirmed by western blot analysis of apoptosis-associated
proteins. The caspases belong to a family of highly conserved
aspartate-specific cysteine proteases, and they constitute
important components of the apoptotic pathway (25,26).
PARP, a type of DNA repair enzyme, is recognized to be the cleavage
substrate of caspase. PARP is thought to be an important indicator
of apoptosis, and is generally considered to be an indicator of
caspase-3 activation. Bcl-2, encoded by the BCL2 gene, is the key
member of the Bcl-2 protein family and negatively regulates
cellular apoptosis (27). Bax
protein, another apoptosis regulator belonging to the Bcl-2 protein
family, promotes apoptosis by binding to the Bcl-2 protein
(28). The expression of the
proteins mentioned above was detected when cells were treated with
gradient concentrations of L-mimosine. The expression of cleaved
PARP and cleaved caspase-3 was increased as the concentration of
L-mimosine increased. Attenuated expression of Bcl-2 and increased
expression of BAX were observed as the concentration of L-mimosine
increased.
It is known that cellular apoptosis is mediated
through two principal pathways: The extrinsic (death
receptor-mediated) and intrinsic (mitochondrial-mediated) pathways
(29). Caspase-8 is important for
the initiation of apoptosis via death receptors, as its recruitment
to and activation at the death-inducing signaling complex is the
decisive step for the initiation of the caspase cascade, leading to
apoptosis (30). Caspase-9 is the
apoptotic initiator protease of the intrinsic, or mitochondrial,
apoptotic pathway (31). In the
present study, with the increase in the concentration of
L-mimosine, cleaved caspase-9 exhibited increased expression, while
the expression alteration of cleaved caspase-8 was not apparent,
indicating that the intrinsic (mitochondrial) apoptotic pathway was
induced by L-mimosine in osteosarcoma cells. This hypothesis was
additionally confirmed by treatment with the caspase-9 inhibitor
Z-LEHD-FMK.
Xenobiotics may alter cell survival pathways and
cause alterations in cell proliferation, the cell cycle and
apoptosis (32), and the results
of the present study further demonstrated that the ERK signaling
pathway was associated with L-mimosine. L-mimosine reduced the
levels of ERK and p-ERK in a concentration-dependent manner, and
the ERK signaling specific inhibitor SCH772984 was used for
verification. The results suggested ERK signaling to be an
additional mechanism for apoptosis induction.
In conclusion, the present study confirmed that
L-mimosine was able to effectively inhibit the proliferation of
osteosarcoma cells, and concluded that L-mimosine induces
caspase-9-mediated apoptosis in osteosarcoma cells. In the future,
further studies are required to detect the inhibitory effects of
L-mimosine in more types of tumor, or to compare the toxic effects
of chemotherapeutic drugs with clear antitumor mechanisms and
L-mimosine. The present study may provide the basis for a more
comprehensive understanding and evaluation of this type of plant
amino acid. Further research is required to assess the potential of
L-mimosine as an antitumor drug. Different types of antitumor drugs
act via different mechanisms, and the same type of drug may have
different modes of action according to cell cycle specificity.
Combinations of currently-used drugs and L-mimosine may provide a
broader options for the treatment of cancer.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81571811).
References
|
1
|
Picci P: Osteosarcoma (osteogenic
sarcoma). Orphanet J Rare Dis. 2:62007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrari S and Palmerini E: Adjuvant and
neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr
Opin Oncol. 19:341–346. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chung LC, Tsui KH, Feng TH, Lee SL, Chang
PL and Juang HH: L-Mimosine blocks cell proliferation via
upregulation of B-cell translocation gene 2 and N-myc downstream
regulated gene 1 in prostate carcinoma cells. Am J Physiol Cell
Physiol. 302:C676–C685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zalatnai A and Bocsi J: Mimosine, a
plant-derived amino acid induces apoptosis in human pancreatic
cancer xenografts. Anticancer Res. 23:4007–4009. 2003.PubMed/NCBI
|
|
6
|
Kulp KS and Vulliet PR: Mimosine blocks
cell cycle progression by chelating iron in asynchronous human
breast cancer cells. Toxicol Appl Pharmacol. 139:356–364. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hughes TA and Cook PR: Mimosine arrests
the cell cycle after cells enter S-phase. Exp Cell Res.
222:275–280. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwang HS, Davis TW, Houghton JA and
Kinsella TJ: Radiosensitivity of thymidylate synthase-deficient
human tumor cells is affected by progression through the G1
restriction point into S-phase: Implications for fluoropyrimidine
radiosensitization. Cancer Res. 60:92–100. 2000.PubMed/NCBI
|
|
9
|
Lalande M: A reversible arrest point in
the late G1 phase of the mammalian cell cycle. Exp Cell Res.
186:332–339. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mosca PJ, Dijkwel PA and Hamlin JL: The
plant amino acid mimosine may inhibit initiation at origins of
replication in Chinese hamster cells. Mol Cell Biol. 12:4375–4383.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gilbert DM, Neilson A, Miyazawa H,
DePamphilis ML and Burhans WC: Mimosine arrests DNA synthesis at
replication forks by inhibiting deoxyribonucleotide metabolism. J
Biol Chem. 270:9597–9606. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalejta RF and Hamlin JL: The dual effect
of mimosine on DNA replication. Exp Cell Res. 231:173–183. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krude T: Mimosine arrests proliferating
human cells before onset of DNA replication in a dose-dependent
manner. Exp Cell Res. 247:148–159. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang HC, Lee TH, Chuang LY, Yen MH and
Hung WC: Inhibitory effect of mimosine on proliferation of human
lung cancer cells is mediated by multiple mechanisms. Cancer Lett.
145:1–8. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oppenheim EW, Nasrallah IM, Mastri MG and
Stover PJ: Mimosine is a cell-specific antagonist of folate
metabolism. J Biol Chem. 275:19268–19274. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang HC, Weng CF, Yen MH, Chuang LY and
Hung WC: Modulation of cell cycle regulatory protein expression and
suppression of tumor growth by mimosine in nude mice. Int J Oncol.
17:659–665. 2000.PubMed/NCBI
|
|
17
|
Dong Z and Zhang JT: EIF3 p170, a mediator
of mimosine effect on protein synthesis and cell cycle progression.
Mol Biol Cell. 14:3942–3951. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen J, Yin JY, Li XP, Liu ZQ, Wang Y,
Chen J, Qu J, Xu XJ, McLeod HL, He YJ, et al: The prognostic value
of altered eIF3a and its association with p27 in non-small cell
lung cancers. PLoS One. 9:e960082014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Staunton MJ and Gaffney EF: Tumor type is
a determinant of susceptibility to apoptosis. Am J Clin Pathol.
103:300–307. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fisher DE: Apoptosis in cancer therapy:
Crossing the threshold. Cell. 78:539–542. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan S, Smith ML, Rivet DJ II, Duba D, Zhan
Q, Kohn KW, Fornace AJ Jr and O'Connor PM: Disruption of p53
function sensitizes breast cancer MCF-7 cells to cisplatin and
pentoxifylline. Cancer Res. 55:1649–1654. 1995.PubMed/NCBI
|
|
22
|
Le NT and Richardson DR: Iron chelators
with high antiproliferative activity up-regulate the expression of
a growth inhibitory and metastasis suppressor gene: A link between
iron metabolism and proliferation. Blood. 104:2967–2975. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zalatnai A: P-glycoprotein expression is
induced in human pancreatic cancer xenografts during treatment with
a cell cycle regulator, mimosine. Pathol Oncol Res. 11:164–169.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lavrik IN, Golks A and Krammer PH:
Caspases: Pharmacological manipulation of cell death. J Clin
Invest. 115:2665–2672. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grütter MG: Caspases: Key players in
programmed cell death. Curr Opin Struct Biol. 10:649–655. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hata AN, Engelman JA and Faber AC: The
BCL2 family: Key mediators of the apoptotic response to targeted
anticancer therapeutics. Cancer Discov. 5:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei MC, Zong WX, Cheng EH, Lindsten T,
Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and
Korsmeyer SJ: Proapoptotic BAX and BAK: A requisite gateway to
mitochondrial dysfunction and death. Science. 292:727–730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parsons MJ and Green DR: Mitochondria in
cell death. Essays Biochem. 47:99–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kantari C and Walczak H: Caspase-8 and
bid: Caught in the act between death receptors and mitochondria.
Biochim Biophys Acta. 1813:558–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim B, Srivastava SK and Kim SH: Caspase-9
as a therapeutic target for treating cancer. Expert Opin Ther
Targets. 19:113–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gu X and Manautou JE: Molecular mechanisms
underlying chemical liver injury. Expert Rev Mol Med. 14:e42012.
View Article : Google Scholar : PubMed/NCBI
|