Introduction
In China, as the levels of air pollution have grown
worse, the incidence and mortality rates of environment-associated
diseases, such as cardiovascular disease, and the prevalence of
adverse health conditions during early human life, such as
decreased heart rate variability have increased (1). Coronary heart disease (CHD) is one of
the most common environment-associated diseases in the world, with
the highest rate of mortality (2).
It has been reported that in China, ~100 million people succumb to
CHD each year, and approximately half of these deaths are
associated with acute myocardial infarction (AMI); thus, AMI is a
cardiovascular disease that greatly threatens human health
(2). Therefore, further research
is required to strengthen the previous basic research performed,
and to improve the current prevention and treatment strategies for
AMI, in order to protect China's sustainable economic development
and improve mortality rates (3).
The cause of AMI is complex as a result of the interactions between
genes and the environment (3).
Heart rate variability reflects the regulation of cardiac rhythm by
the autonomic nervous system (4).
Epidemiological studies have demonstrated that air pollution can
generate a decline in the levels of heart rate variability, which
in turn significantly affects the prediction of acute
cardiovascular events, such as AMI (1,2).
A previous study revealed that, in several diseases,
although the sequences of certain genes are not altered, the
modification processes and gene expression are, however, abnormal:
This is the focus of epigenetic research (5). Epigenetics is the link between
external environmental factors and internal genetic factors
(6). Epigenetic dysregulation may
result in a variety of diseases, such as cardiovascular disease;
microRNAs (miRNAs/miRs) are one of the most important aspects of
the epigenetic regulatory mechanism, serving an important role in
the occurrence and development of cardiovascular disease (5). A recent study demonstrated that miRNA
is involved in the regulation of numerous physiological processes,
including the growth and development of the cardiovascular system
and angiogenesis (7). At the same
time, miRNAs can stably exist in the peripheral blood circulation
system (including plasma and serum); circulating miRNAs with
disease-characteristic expression profiles are expected to become
biomarkers of the disease, with potential clinical value (8). However, the association between
plasma miRNA and the risk of AMI requires further epidemiological
research (8,9).
Insulin-like growth factor-1 (IGF-1) serves an
important role in maintaining homeostatic processes; for example,
it has a positive role in the development of myocardial cell
growth, primarily regulating protein metabolism and protein
synthesis, as well as promoting cell growth and preventing cell
death (10). IGF-1 is also
involved in a number of physiological and pathophysiological
processes, including tissue remodeling, glucose and lipid
metabolism, and insulin sensitivity (11). In addition, IGF-1 promotes
myocardial contraction, improves hemodynamics and energy
metabolism, and protects the heart against myocardial apoptosis
induced by ischemia or oxidative stress; cardiac-specific
overexpression has also been demonstrated (12). IGF-1 in transgenic mice is able to
reduce myocardial apoptosis and ventricular pressure, and suppress
the expansion of the heart chamber associated with myocardial
infarction or increasing age; thus, it serves a vital role in the
maintenance of cardiac morphology and function (12). The regulation of IGF-1 levels may
improve the overall survival rate in patients with sepsis, which
may be realized by strengthening the hepatic clearance of bacterial
toxins and improvements to immunity (13). A lack of IGF-1 leads to alterations
in body composition, and cytokine and neuroendocrine
factor-associated activities. A recent study revealed that IGF-1
deficiency in the heart is able to cause cardiac atrophy and
dysfunction (14). Saddic et
al (15) reported that
miR-483-3p targets may be associated with novel mechanisms
mitigating damage caused by ischemic insults on the human heart
(15). The aim of the present
study was to investigate the functional association between the
expression of miR-483-3p and AMI, in patients and in
vitro.
Materials and methods
Study design and patients
Patients with AMI (n=6, 3 males and 3 females) and
normal volunteers (n=6, 3 males and 3 females) were recruited from
The Heart Center, Beijing Chaoyang Hospital, Capital Medical
University (Beijing, China) during August 2015 to September 2015.
Normal volunteers did not have a history of heart disease.
Peripheral blood samples (5 ml) were collected and centrifuged at
2,000 × g for 5 min at room temperature and stored at 80°C. The
present study was approved by the Heart Center Ethics Committee,
Beijing Chaoyang Hospital, Capital Medical University, and written
informed consent was obtained from each individual.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total plasma RNA was isolated from blood and H9c2
cell samples using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Total RNA (1–2 µg) was
reverse-transcribed using the TaqMan microRNA Reverse Transcription
kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocols. RT-qPCR was performed using the
FastStart Universal SYBR Green Master Mix (Roche Diagnostics,
Basel, Switzerland) in a 7500 fast Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). U6, forward
ATTGGAACGATACAGAGAAGATT and reverse GGAACGCTTCACGAATTTG;
miR-483-3p, forward GCTGACTCACTCCTCCCCTC and reverse
TATGGTTGTTCACGACTCCTTCAC. The thermocycling conditions were as
follows: 95°C for 10 min, followed by 40 cycles of 95°C for 20 sec
and 60°C for 30 sec. The results were normalized to those of GAPDH
and were quantified using the 2−∆∆Cq method (16). Experiments were performed in
triplicate.
H9c2 cell culture and
transfection
H9c2 cells (70–80% confluency) were acquired from
The Cell Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China) and cultured in Dulbecco's modified
Eagle's medium (DMEM; Life Technologies; Thermo Fisher Scientific,
Inc.) and 10% (v/v) fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), under an atmosphere of 5% (v/v) CO2
at 37°C. The miR-483-3p plasmid and negative plasmid were
structured and purchased from MyGenostics, Inc. (Beijing, China).
H9c2 cells (1×106) were seeded into 6-well plates and
transfected with the miR-483-3p or negative plasmid (50 nM) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Following 24 h after transfection, H9c2 cells
were incubated for 2 h in 5% CO2, 5% H2 and
90% N2, generated by a vacuum; his generated the in
vitro AMI model. Following 4 h after transfection, H9c2 cells
were incubated with 0.5 nM of picropodophyllotoxin for 20 h at
37°C, and incubated for 2 h at 37°C in 5% CO2, 5%
H2 and 90% N2, generated by a vacuum; this
generated the in vitro AMI model. Control group constituted
H9c2 cells that were incubated without picropodophyllotoxin for 2 h
at 37°C in 5% CO2, 5% H2 and 90%
N2, generated by a vacuum.
Flow cytometry analysis
H9c2 cells (1×106 cell/well) were washed
with PBS and resuspended with cell apoptosis buffer (Ruisai, Inc.,
Shanghai, China). H9c2 cells were incubated using the Annexin
V-FITC Apoptosis Detection kit (Ruisai, Inc., Shanghai, China)
according to the manufacturer's protocols. H9c2 cells
(1×104/well) were analyzed by flow cytometric analysis
using a BD Accuri C6 flow cytometer and C-Flow Plus v1.0 software
(BD Biosciences, Franklin Lakes, NJ, USA) and analyzed uisng
Image-ProPlus 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
ELISA analysis
Total protein was extracted from H9c2 cells
(1×103 cell/well) using radioimmunoprecipitation (RIPA)
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
on ice for 20–30 min. Protein content was measured using
bicinchoninic acid (BCA) lysis buffer (Beyotime Institute of
Biotechnology), and 10 µg protein was incubated with the caspase-3
(C1116) and caspase-9 (C1158) activity kits (Beyotime Institute of
Biotechnology) according to the manufacturer's protocols at 37°C
for 1 h for ELISA analysis.
Western blot analysis
Total protein was extracted from H9c2 cells
(1×106 cell/well) using RIPA lysis buffer (Beyotime
Institute of Biotechnology) on ice for 20–30 min. Protein content
was then measured using BCA lysis buffer (Beyotime Institute of
Biotechnology). Protein (50 µg) was subjected to electrophoresis on
8–10% polyacrylamide SDS gels and transferred onto polyvinylidene
fluoride membranes. Membranes were blocked with 5% non-fat milk in
TBST for 1 h at 37°C and preincubated in 5% non-fat milk prior to
incubation with anti-B-cell lymphoma 2 (Bcl-2; 1:1,000, sc-783,
Santa Cruz Biotechnology), anti-Bcl-2-associated X protein (Bax;
1:1,000, sc-493, Santa Cruz Biotechnology), anti-IGF (1:2,000,
sc-5622, Santa Cruz Biotechnology) and GAPDH (1:2,000, sc-25778,
Santa Cruz Biotechnology) overnight at 4°C, followed by incubation
with the peroxidase-conjugated anti-rabbit secondary antibody
(1:5,000, cat. no. 14708, Cell Signaling Technology, Inc., Danvers,
MA, USA). Image J software (National Institutes of Health,
Bethesda, MA, USA) was used to quantify protein bands by optical
density and visualizated with a BeyoECL Star kit (Beyotime
Institute of Biotechnology).
Statistical analysis
The results were expressed as the mean ± standard
error of the mean. The differences between the groups were analyzed
using one-way analysis of variance and Tukey's post test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of miR-483-3p in patients
with AMI
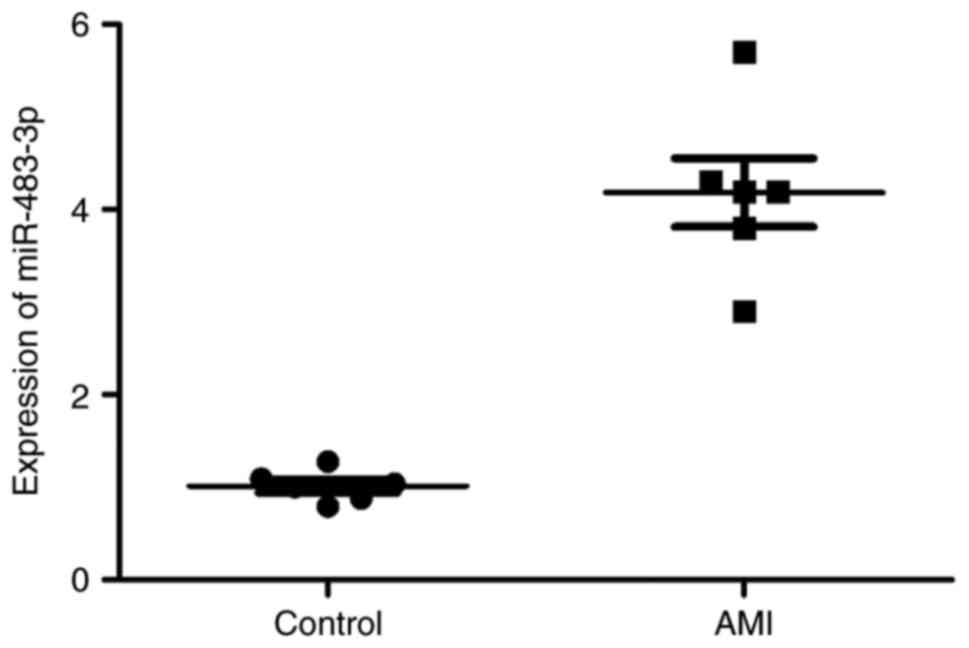
To identify the expression levels of miR-483-3p in
patients with AMI, blood samples were collected from the patients
with AMI and the normal volunteers. In patients with AMI,
miR-483-3p expression was markedly enhanced when compared with the
normal control group (Fig. 1).
Overexpression of miR-483-3p promotes
apoptosis in vitro
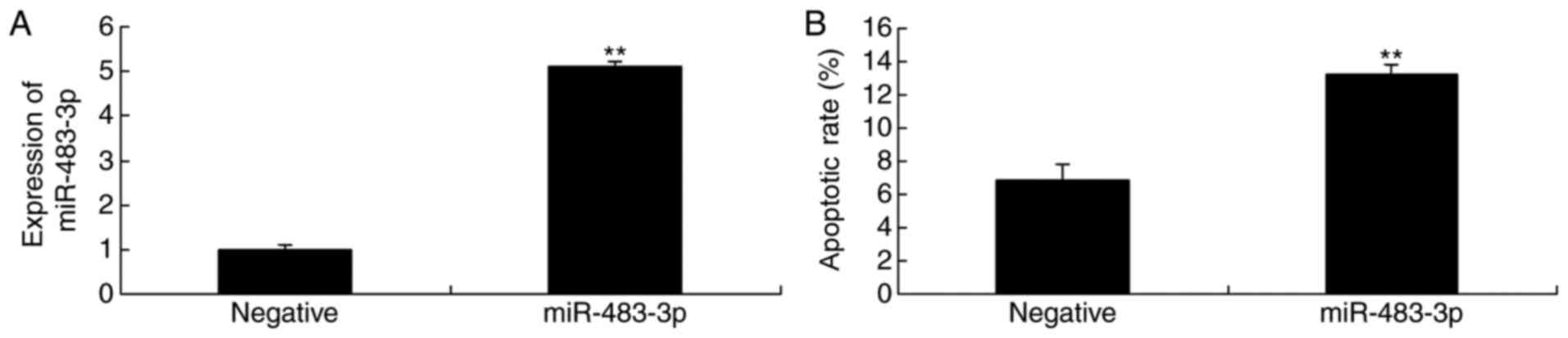
The miR-483-3p and negative plasmids were
transfected in H9c2 cells, which were then incubated without oxygen
to produce the AMI model. As shown in Fig. 2A, presence of the miR-483-3p
plasmid significantly increased miR-483-3p expression in the in
vitro AMI model. In addition, the miR-483-3p plasmid
significantly promoted the rate of apoptosis in the AMI model H9c2
cells (Fig. 2B).
Overexpression of miR-483-3p increases
caspase-3 and caspase-9 activity in vitro
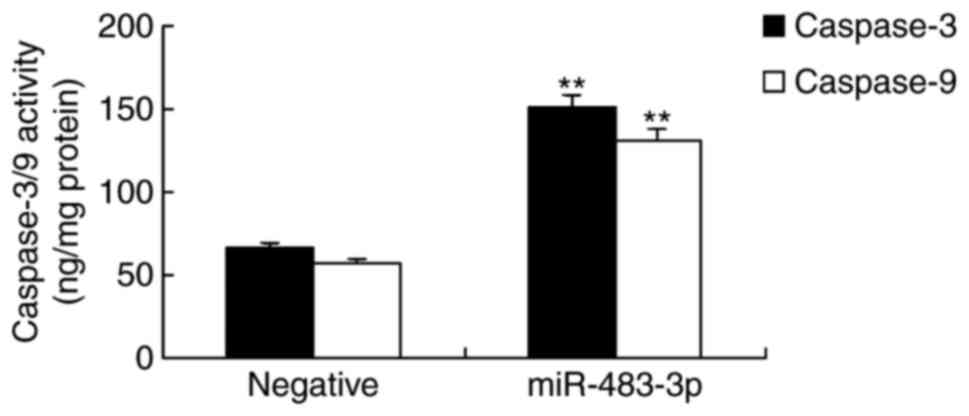
In vitro, H9c2 cells overexpressing
miR-483-3p were incubated without oxygen, in order to determine the
associated apoptotic mechanism of miR-483-3p in AMI. As shown in
Fig. 3, caspase-3 and caspase-9
activities were significantly increased in H9c2 cells transfected
with miR-483-3p, when compared with the negative control group.
Overexpression of miR-483-3p increases
Bax/Bcl-2 and IGF-1 protein expression in vitro
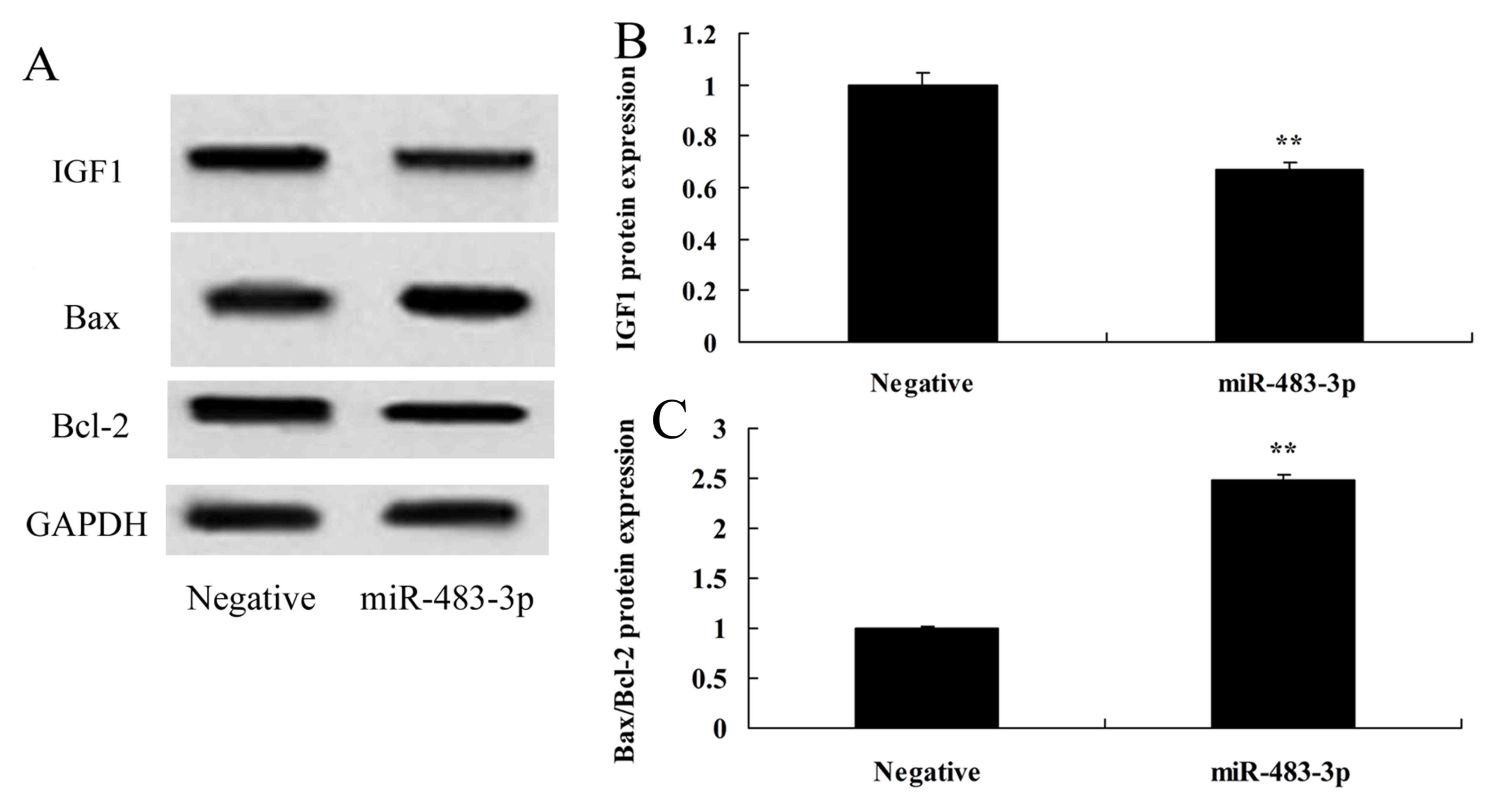
To confirm the roles of miR-483-3p in apoptosis, the
expression of IGF-1, Bax and Bcl-2 were investigated in the
established in vitro AMI model. Overexpression of miR-483-3p
increased Bax/Bcl-2 and decreased IGF-1 protein expression in
vitro, when compared with the negative control group (Fig. 4).
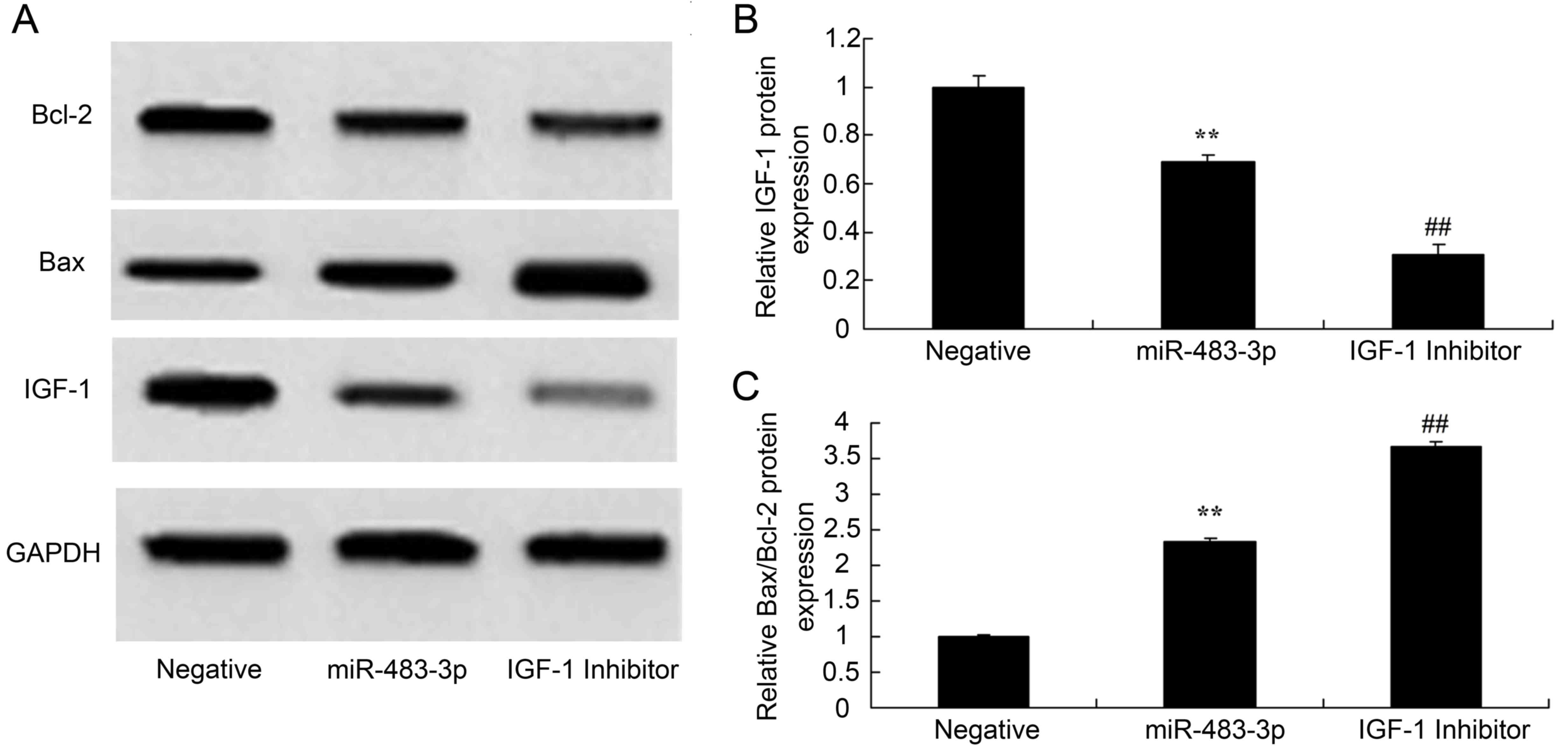
IGF-1 inhibitor increases IGF-1
protein expression in H9c2 cells following overexpression of
miR-483-3p
To confirm whether a direct association exists
between miR-483-3p and IGF-1 protein expression in AMI, the IGF-1
inhibitor, picropodophyllotoxin, was applied to inhibit IGF-1
expression in the in vitro model of AMI. As shown in
Fig. 5, IGF-1 inhibitor
significantly suppressed IGF-1 and increased Bax protein expression
(and, hence, the Bax/Bcl-2 ratio) in H9c2 cells overexpressing
miR-483-3p, when compared with H9c2 cells overexpressing miR-483-3p
only.
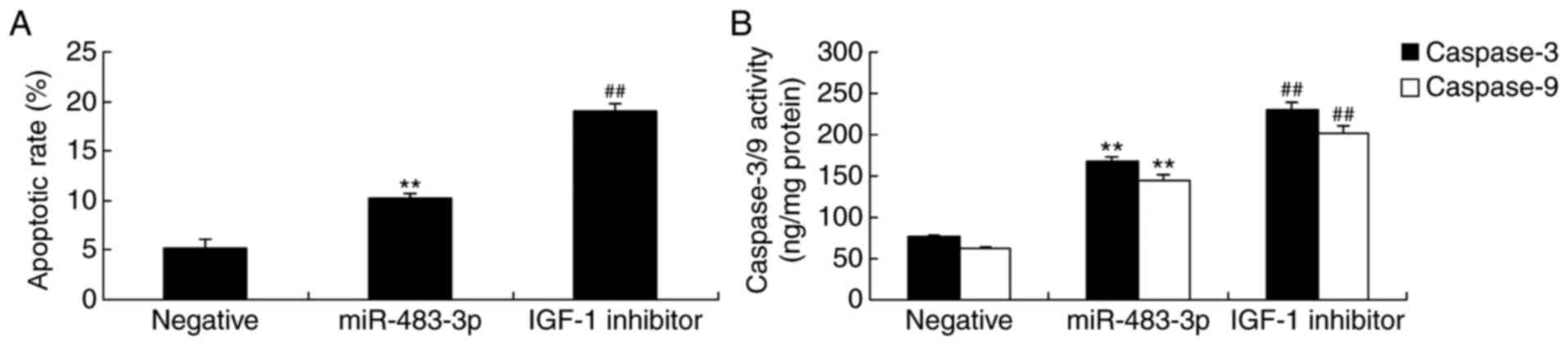
IGF-1 inhibitor promotes apoptosis in
H9c2 cells following the overexpression of miR-483-3p
The present study then investigated the effect of
the IGF-1 inhibitor and miR-483-3p overexpression on the regulation
of the apoptotic rate in the H9c2 cell model of AMI. As expected,
inhibiting IGF-1 expression significantly increased the rate of
apoptosis in AMI model H9c2 cells overexpressing miR-483-3p
(Fig. 6A). The suppression of
IGF-1 expression also significantly increased caspase-3 and −9
activity in H9c2 cells overexpressing miR-483-3p when compared with
miR-483-3p transfection alone (Fig.
6B).
Discussion
In recent years, environmental pollution has become
a serious public health concern, as the rising levels of air
pollution pose a serious threat to health, increasing the levels of
morbidity and mortality associated with environment-related
diseases (9,17). It has been predicted that between
the years 2000–2030, the number of the mortalities associated with
cardiovascular disease will grow from 5 million to 60 million in
developed countries; while in developing countries, it is
considered that this fig. will increase from 10 million to 19
million (9). CHD is a chronic,
non-communicable disease with the highest mortality rate in the
world (18). The World Health
Organization has estimated that ~1 million people succumb to CHD
every year, of which AMI accounts for half the mortalities; thus,
AMI is a cardiovascular disease considered to be a serious threat
to public health, as it is associated with some of the highest
recorded rates of morbidity and mortality (19). The present study revealed that
miR-483-3p expression was enhanced in patients with AMI, which
indicated that miR-483-3p may participate in AMI-induced
apoptosis.
The main cause of AMI is associated with coronary
artery disease, which is characterized by serious and lasting acute
ischemia, or necrosis of the corresponding myocardia induced by the
drastic reduction or interruption of coronary blood supply
(20). The etiology of AMI is
complicated, and this is generally considered to be as a result of
the interactions between external environmental factors and genetic
factors (21). The sequences of
several genes are not altered in the disease state; instead, only
modifications to genetic processing and gene expression are
abnormal: These alternations are known as epigenetic modifications
(21). Epigenetics has been an
important discovery in the field of medical biology in recent
years, and it has enriched our ability to control the phenotypic
effect of genes; epigenetics also connects external environmental
factors and internal genetic factors, enabling the body to adapt to
changes in the environment (22).
In recent years, miRNA has become the focus of epigenetic research
in the field of cardiovascular disease as an important mechanism of
genetic regulation (6). Using an
AMI cell model, the present study revealed that the overexpression
of miR-483-3p promoted apoptosis and increased the levels of
caspase-3 and −9 activity; therefore, the underlying molecular
mechanism of apoptosis in AMI was further investigated.
Apoptosis is an important physiological process that
supports biological life and normal activities (23). If the signal transmission pathways
that control and regulate apoptosis are severely disrupted, this
causes a number of human diseases, including cancer and infectious
diseases (24). Cellular factors,
including proto-oncogenes, cytokines and Bcl-2 family members, may
directly stimulate apoptotic signaling, and important membrane
proteins are also associated with apoptosis, controlling apoptosis
via different signals inside and outside of the cell (25). A previous study has demonstrated
that certain genes, including Bax, p53 and Fas, can promote
apoptosis, and other genes, such as Bcl-2 and Bax, are able to
inhibit apoptosis (26). The ratio
of apoptosis-inducing and apoptosis-inhibiting proteins in
vivo is important for determining whether or not apoptosis will
proceed; for example, if Bcl-2 protein expression is higher than
that of Bax, cell survival is promoted; when Bax expression is
higher than that of Bcl-2, apoptosis is accelerated (24). There are notable differences
between apoptosis and necrosis in terms of the morphological
changes induced, biochemical metabolism and the molecular
mechanisms involved. Necrotic cells typically accumulate together
to induce cell death, whereas apoptosis is induced by an
apoptosis-associated mechanism inside the cell, which is stimulated
by specific factors (25).
Therefore, it is important to distinguish between the two different
cell death phenomena. A significant increase in Bax/Bcl-2
expression in H9c2 cells overexpressing miR-483-3p was observed in
the present study, which indicated that miR-483-3p may serve a key
role in promoting apoptosis in AMI.
IGF-1 is a single-chain polypeptide growth factor
regulated by hormones that has a high homology with insulin, which
is involved in cell differentiation, proliferation and insulin-like
metabolism (10). The IGF-1
receptor and its binding protein are widely expressed in various
tissues, including those of the cardiovascular system (13). It has been established that IGF-1
may participate in a variety of physiological and pathological
processes in the heart via endocrine, autocrine and
paracrine-associated mechanisms (27). It stimulates the growth of cardiac
myocytes, affects cardiac ion channels and enhances myocardial
contractility, thereby increasing cardiac output and improving
cardiac ejection function (28).
The important role of IGF-1 receptors and binding proteins in the
developmental process of certain cardiovascular diseases has
received increasing interest from researchers. It has been observed
that, in cardiac cells overexpressing IGF-1, the sensitivity of
filaments to Ca2+ is weakened, the shrinking rate is
increased and their compliance is enhanced (29). The decreased sensitivity of
filaments to Ca2+ has a negative inotropic effect on
damaged cardiac muscle; however, it reduces the energy demand of
cardiac decompensation and improves pumping function following
heart failure (30). In animal
models of myocardial infarction induced by coronary artery
ligation, IGF-1 overexpression reduces myocardial cell death and
ventricular expansion (31). In a
dog model of tachycardia-induced heart failure, IGF-1 reduces the
level of cardiomyocyte apoptosis, and enhances cardiac contractile
function (31). In the present
study, overexpression of miR-483-3p suppressed IGF-1 protein
expression, which further promoted the effect of miR-483-3p
overexpression on apoptosis in AMI H9c2 cells via the caspase and
Bax/Bcl-2 signaling pathways.
In conclusion, the significant results of the
present study demonstrated that the overexpression of miR-483-3p
promoted apoptosis, increased caspase-3 and −9 activity levels, and
induced the protein expression of Bax/Bcl-2 in the AMI model. These
results may contribute towards the identification of a potential
therapeutic target of IGF-1 in AMI-induced apoptosis.
References
|
1
|
Thiele H, Desch S, Piek JJ, Stepinska J,
Oldroyd K, Serpytis P, Montalescot G, Noc M, Huber K, Fuernau G, et
al: Multivessel versus culprit lesion only percutaneous
revascularization plus potential staged revascularization in
patients with acute myocardial infarction complicated by
cardiogenic shock: Design and rationale of CULPRIT-SHOCK trial. Am
Heart J. 172:160–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cerisano G, Buonamici P, Valenti R, Moschi
G, Taddeucci E, Giurlani L, Migliorini A, Vergara R, Parodi G,
Sciagrà R, et al: Effects of a timely therapy with doxycycline on
the left ventricular remodeling according to the pre-procedural
TIMI flow grade in patients with ST-elevation acute myocardial
infarction. Basic Res Cardiol. 109:4122014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang HL, Xing SY, Dong PS, Han YH, Zhu JH,
Lai LH and Zhao JF: Safety and efficacy of intracoronary tirofiban
administration in patients with serious thrombus burden and
ST-elevation myocardial infarction undergoing percutaneous coronary
intervention. Eur Rev Med Pharmacol Sci. 18:3690–3695.
2014.PubMed/NCBI
|
|
4
|
Dohi T, Maehara A, Brener SJ, Généreux P,
Gershlick AH, Mehran R, Gibson CM, Mintz GS and Stone GW: Utility
of peak creatine kinase-MB measurements in predicting myocardial
infarct size, left ventricular dysfunction, and outcome after first
anterior wall acute myocardial infarction (from the INFUSE-AMI
trial). Am J Cardiol. 115:563–570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsumoto S, Sakata Y, Suna S, Nakatani D,
Usami M, Hara M, Kitamura T, Hamasaki T, Nanto S, Kawahara Y and
Komuro I: Circulating p53-responsive microRNAs are predictive
indicators of heart failure after acute myocardial infarction. Circ
Res. 113:322–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Biasucci LM and Cardillo MT: MicroRNA and
myocardial infarction: A mystery turning into glory? J Am Coll
Cardiol. 62:999–1001. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang W, Tian SS, Hang PZ, Sun C, Guo J
and Du ZM: Combination of microRNA-21 and microRNA-146a attenuates
cardiac dysfunction and apoptosis during acute myocardial
infarction in mice. Mol Ther Nucleic Acids. 5:e2962016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eryilmaz U, Akgüllü Ç, Beşer N, Yıldız Ö,
Kurt Ömürlü İ and Bozdoğan B: Circulating microRNAs in patients
with ST-elevation myocardial infarction. Anatol J Cardiol.
16:392–396. 2016.PubMed/NCBI
|
|
9
|
Marfella R, Sasso FC, Siniscalchi M,
Paolisso P, Rizzo MR, Ferraro F, Stabile E, Sorropago G, Calabrò P,
Carbonara O, et al: Peri-procedural tight glycemic control during
early percutaneous coronary intervention is associated with a lower
rate of in-stent restenosis in patients with acute ST-elevation
myocardial infarction. J Clin Endocrinol Metab. 97:2862–2871. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ketha H and Singh RJ: Clinical assays for
quantitation of insulin-like-growth-factor-1 (IGF1). Methods.
81:93–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jackson R, Tilokee EL, Latham N, Mount S,
Rafatian G, Strydhorst J, Ye B, Boodhwani M, Chan V, Ruel M, et al:
Paracrine engineering of human cardiac stem cells with insulin-like
growth factor 1 enhances myocardial repair. J Am Heart Assoc.
4:e0021042015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nelson DM, Hashizume R, Yoshizumi T,
Blakney AK, Ma Z and Wagner WR: Intramyocardial injection of a
synthetic hydrogel with delivery of bFGF and IGF1 in a rat model of
ischemic cardiomyopathy. Biomacromolecules. 15:1–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ge RT, Mo LH, Wu R, Liu JQ, Zhang HP, Liu
Z, Liu Z and Yang PC: Insulin-like growth factor-1 endues monocytes
with immune suppressive ability to inhibit inflammation in the
intestine. Sci Rep. 5:77352015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krieger F, Elflein N, Saenger S, Wirthgen
E, Rak K, Frantz S, Hoeflich A, Toyka KV, Metzger F and Jablonka S:
Polyethylene glycol-coupled IGF1 delays motor function defects in a
mouse model of spinal muscular atrophy with respiratory distress
type 1. Brain. 137:1374–1393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saddic LA, Chang TW, Sigurdsson MI,
Heydarpour M, Raby BA, Shernan SK, Aranki SF, Body SC and
Muehlschlegel JD: Integrated microRNA and mRNA responses to acute
human left ventricular ischemia. Physiol Genomics. 47:455–462.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao LR, Chen Y, Zhang NK, Yang XL, Liu HL,
Wang ZG, Yan XY, Wang Y, Zhu ZM, Li TC, et al: Intracoronary
infusion of Wharton's jelly-derived mesenchymal stem cells in acute
myocardial infarction: Double-blind, randomized controlled trial.
BMC Med. 13:1622015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tan NS, Goodman SG, Cantor WJ, Tan MK, Yan
RT, Bagnall AJ, Mehta SR, Fitchett D, Strauss BH and Yan AT:
TRANSFER-AMI Investigators: Comparison of the efficacy of
pharmacoinvasive management for ST-segment elevation myocardial
infarction in smokers versus non-smokers (from the Trial of Routine
Angioplasty and Stenting After Fibrinolysis to Enhance Reperfusion
in Acute Myocardial Infarction). Am J Cardiol. 114:955–961. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Minamisawa M, Izawa A, Motoki H, Kashima
Y, Hioki H, Abe N, Miura T, Ebisawa S, Miyashita Y, Koyama J and
Ikeda U: Prognostic significance of neuroadrenergic dysfunction for
cardiovascular events in patients with acute myocardial infarction.
Circ J. 79:2238–2245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weinreuter M, Kreusser MM, Beckendorf J,
Schreiter FC, Leuschner F, Lehmann LH, Hofmann KP, Rostosky JS,
Diemert N, Xu C, et al: CaM Kinase II mediates maladaptive
post-infarct remodeling and pro-inflammatory chemoattractant
signaling but not acute myocardial ischemia/reperfusion injury.
EMBO Mol Med. 6:1231–1245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rios E, Mancio J, Rodrigues-Pereira P,
Magalhães D and Bartosch C: Large myocardial infarction with
myocardium calcium deposits associated with reperfusion injury.
Cardiovasc Pathol. 23:379–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rayner K, Dimmeler S, Calin GA, Thum T,
Raizman JE and Diamandis EP: Novel biomarkers for acute myocardial
infarction: Is microRNA the new kid on the block? Clin Chem.
60:812–817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma LN, Li LD, Li SC, Hao XM, Zhang JY, He
P and Li YK: Allicin improves the cardiac function by protecting
against apoptosis in rat model of myocardial infarction. Chin J
Integr Med. 23:589–597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ueda S, Yamagishi S, Matsui T, Jinnouchi Y
and Imaizumi T: Administration of pigment epithelium-derived factor
inhibits left ventricular remodeling and improves cardiac function
in rats with acute myocardial infarction. Am J Pathol. 178:591–598.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Zhang H, Chai F, Liu X and Berk M:
The effects of escitalopram on myocardial apoptosis and the
expression of Bax and Bcl-2 during myocardial ischemia/reperfusion
in a model of rats with depression. BMC Psychiatry. 14:3492014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Malick M, Gilbert K, Barry M, Godbout R
and Rousseau G: Desvenlafaxine reduces apoptosis in amygdala after
myocardial infarction. Brain Res Bull. 109:158–163. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Y, Harrison MR, Osorio A, Kim J,
Baugh A, Duan C, Sucov HM and Lien CL: Igf signaling is required
for cardiomyocyte proliferation during zebrafish heart development
and regeneration. PLoS One. 8:e672662013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sengupta A, Kalinichenko VV and Yutzey KE:
FoxO1 and FoxM1 transcription factors have antagonistic functions
in neonatal cardiomyocyte cell-cycle withdrawal and IGF1 gene
regulation. Circ Res. 112:267–277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anversa P, Reiss K, Kajstura J, Cheng W,
Li P, Sonnenblick EH and Olivetti G: Myocardial infarction and the
myocyte IGF1 autocrine system. Eur Heart J. 16 Suppl N:S37–S45.
1995. View Article : Google Scholar
|
|
30
|
Andreassen M, Raymond I, Kistorp C,
Hildebrandt P, Faber J and Kristensen LØ: IGF1 as predictor of all
cause mortality and cardiovascular disease in an elderly
population. Eur J Endocrinol. 160:25–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lai CH, Ho TJ, Kuo WW, Day CH, Pai PY,
Chung LC, Liao PH, Lin FH, Wu ET and Huang CY: Exercise training
enhanced SIRT1 longevity signaling replaces the IGF1 survival
pathway to attenuate aging-induced rat heart apoptosis. Age
(Dordr). 36:97062014. View Article : Google Scholar : PubMed/NCBI
|