Introduction
Cardiovascular diseases are a major public health
burden in numerous countries, and have high rates of morbidity and
mortality. Coronary artery disease, such as acute myocardial
infarction, has an important role in cardiovascular diseases
(1). Acute myocardial infarction
(AMI) is an acute coronary syndrome that is characterized by
ischemic necrosis of the cardiac muscle, whose pathogeny is the
absence of blood flow to the myocardium caused by the blocking of
the coronary artery (2,3). At present, improved methods to
prevent, diagnose and treat patients with AMI, which have lowered
the mortality associated with AMI, have been developed. However,
the morbidity of AMI remains high. The most effective method for
treating AMI is myocardial reperfusion (4), however, myocardial reperfusion is
associated with various side effects, including cardiomyocyte death
and dysfunction, which are collectively termed myocardial
reperfusion injury (5).
The MYB proto-oncogene like 2 (MYBL2) gene, also
termed B-MYB, is a member of the MYB family that also includes MYB
proto-oncogene like 1 (also termed A-MYB) and MYB proto-oncogene,
transcription factor (also termed C-MYB). A-MYB is expressed in the
testis and is rarely observed in the ovaries, spleen and brain.
C-MYB, which was identified earlier than A-MYB, is primarily
expressed in hematopoietic stem cells (6), the colon (7) and the brain (8). MYBL2, which is essential to the
regulation of proliferation and differentiation, is vital to the
generation of embryonic stem cells and the formation of inner cell
mass (9). MYBL2 is predominantly
expressed in proliferative cells and has an important role in
guiding cell cycle progression (10). A previous study has reported that
downregulation of MYBL2 leads to the inhibition of cell cycle
progression (11). Other reports
have demonstrated that MYBL2 is overexpressed in lung cancer
(12), hepatocellular carcinoma
(13) and breast cancer (14), and may promote cell proliferation
and metastasis.
H9c2 embryonic rat cardiac cells are a subclone of
the original clonal cell line that are derived from the embryonic
hearts of rats and have previously been used as in vitro
models for investigating the mechanisms of myocardial apoptosis
induced by hypoxia (15). In the
current study, H9c2 myocardial cells were subjected to hypoxia to
simulate the process of myocardial ischemia in vitro.
Downregulation of MYBL2 was observed in H9c2 cells following
hypoxia, indicating that MYBL2 may participate in the
hypoxia-induced apoptosis of H9c2 cells.
Materials and methods
Cell culture and hypoxia
treatment
The myoblast cell line H9c2 (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was cultured in Dulbecco's modified
Eagle's Medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum, 1%
penicillin/streptomycin (100 U/ml and 100 mg/ml, respectively) and
1% GlutaMAX (200 mM) (All purchased from Thermo Fisher Scientific,
Inc.) in a 5% CO2 containing atmosphere at a relative
humidity of 95% at 37°C. Metabolic ischemia was induced by a buffer
exchange to an ischemia-mimetic solution (in mM: 125 NaCl, 8 KCl,
1.2 KH2PO4, 1.25 MgSO4, 1.2 CaCl2, 6.25 NaHCO3, 5 sodium lactate,
20 HEPES, pH 6.6) and by placing the dishes in hypoxic pouches with
an indicator strip in a GasPak EZ Anaerobe Gas Generating Pouch
System (BD Biosciences, Franklin Lakes, NJ, USA) for 2 h at 37°C.
As certified by the manufacturer, the Anaerobe Gas Generating Pouch
System produces an atmosphere containing 10% CO2 and 1%
O2. Non-hypoxia cells exposed to normoxic conditions
(95% O2, 5% CO2) were included as the control
for hypoxia.
Plasmids and small interfering RNA
(siRNA) transfection
An MYBL2 expression vector (pc-MYBL2) was
constructed by subcloning the full-length wild-type MYBL2 coding
sequence into pcDNA3.1 (+) vector (Thermo Fisher Scientific, Inc.),
and confirmed by Sanger sequencing. The empty construct pcDNA3.1
was transfected as a control. The target sequence for
MYBL2-specific siRNA was 5′GCAGAGGACAGUAUCAACA(dT)(dT)′3, and both
MYBL2 siNRA and control siRNA were synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China). Cells were seeded into
6-well plates at a concentration of 105 cells/well and
incubated at 37°C in a humidified atmosphere containing 5%
CO2 for 24 h. Then plated cells were transfected with
MYBL2 siRNA or siNC at a final concentration of 50 nM using
Lipofectamine 3000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Approximately 4
weeks later, stable MYBL2 transfection was generated under G418
(Gibco; Thermo Fisher Scientific, Inc.) selection, as previously
described (16).
Cell proliferation assay
H9c2 cells were seeded in 96-well plates at a
density of 5×103 cells/well. Cell proliferation was
assessed by a Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Rockville, MD, USA). Briefly, following
stimulation of hypoxia, CCK-8 solution was added to the culture
medium and the cultures were incubated for 1 h at 37°C in
humidified 95% air and 5% CO2. The absorbance was
measured at 450 nm using a microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Apoptosis analysis
Apoptosis analysis was performed to identify and
quantify the apoptotic cells by using an Annexin V-Fluorescein
Isothiocyanate/Propidium Iodide (FITC/PI) Apoptosis Detection kit
(Beijing Biosea Biotechnology, Beijing, China). The cells
(1×105 cells/well) were seeded into 6 well-plates.
Treated cells were washed twice with cold PBS and resuspended into
single cell solution of 1×106 cell/ml in 500 µl binding
buffer of Annexin V-Fluorescein Isothiocyanate/Propidium Iodide
(FITC/PI) Apoptosis Detection kit (Beyotime Institute of
Biotechnology, Haimen, China). Then, cells were stained with 5 µl
Annexin V-FITC and 5 µl PI for 30 min at room temperature in the
dark. Samples were measured with a flow cytometer (Beckman Coulter,
Inc., Brea, CA, USA) to differentiate apoptotic cells (Annexin V
positive and PI-negative) from necrotic cells (Annexin V positive
and PI-positive). Flow cytometry results were analyzed using FlowJo
Software (version 7.6, TreeStar, Inc., Ashland, OR, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from transfected cells
(1×106) by using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), which was followed by treatment with
DNase I (Promega Corporation, Madison, WI, USA). RT was performed
by using the TaqMan™ Reverse Transcription Reagents kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The RT conditions were
10 min at 25°C, 30 min at 48°C and a final step of 5 min at 95°C.
qRT-PCR was performed using a SYBR Premix Ex Taq kit (TaKaRa
Biotech, Kyoto, Japan) in a 7500 Real-Time PCR System (ABI; Applied
Biosystems, Carlsbad, CA, USA). The PCR primers were obtained from
Invitrogen, and the reaction conditions included the following
steps: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec
and 60°C for 1 min. The PCR primers were obtained from Invitrogen
(Thermo Fisher Scientific, Inc.), and the sequences of the primers
used for PCR were as follows: MYBL2, 5′AAAACAGTGAGGAGGAAC′3
(forward) and 5′CAGGGAGGTCAAATTTAC′3 (reverse); GAPDH,
5′GCACCGTCAAGGCTGAGAAC′3 (forward) and 5′TGGTGAAGACGCCAGTGGA3′
(reverse). mRNA expression of MYBL2 was normalized to GAPDH
expression which was used as an internal control and relative
expression changes of MYBL2 were calculated using the
2−ΔΔCq method as previous described (17). The experiments were repeated three
times.
Western blot analysis
Protein from 2×106 cells was extracted
using radio immune precipitation assay lysis buffer (Beyotime
Institute of Biotechnology) supplemented with protease inhibitors
(Roche Applied Science, Penzberg, Germany). Proteins were
quantified using the BCA Protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Equal amounts of protein samples (30 µg) were
separated by 12.5% sodium dodecyl sulfate-polyacrylamide gel
(SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF)
membranes (EMD Millipore, Billerica, MA, USA). Subsequently,
membranes were blocked with 5% bovine serum albumin (BSA,
Sigma-Aldrich; Merck KGaA) for 2 h at room temperature. Primary
antibodies were prepared in 5% BSA at a dilution of 1:1,000.
The western blotting system was established using a
Bis-Tris Gel system (Bio-Rad Laboratories, Inc.), according to the
manufacturer's protocol. MYBL2 antibody (cat. no. ab76009) was
purchased from Abcam (Cambridge, UK) and GAPDH antibody (cat. no.
G9545) was purchased from Sigma-Aldrich (Merck KGaA). Antibodies:
t-AKT (cat. no. 4685), p-AKT (p308; cat. no. 13038), p-AKT (p473;
cat. no. 4060), t-IκBα (cat. no. 4812), p-IκBα (cat. no. 2859),
t-p65 (cat. no. 8242) and p-p65 (cat. no. 3033) were purchased from
Cell Signaling Technology (Beverly, MA, USA). Bcl-3 antibody (cat.
no. ab27780) was purchased from Abcam (Cambridge, UK). Primary
antibodies were incubated with the corresponding membranes at 4°C
overnight, followed by washing and incubation with a goat
anti-rabbit IgG secondary antibody horseradish peroxidase (HRP)
conjugated (cat. no. sc-2004; 1:5,000 in 5% BSA) (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature.
After rinsing, membranes were transferred into a ChemiDoc XRS
system (Bio-Rad Laboratories, Inc.) and 200 µl Immobilon Western
Chemiluminescent HRP Substrate (EMD Millipore, Billerica, MA, USA)
was added to cover the membrane surface. The signals were captured
and the intensity of the bands was quantified using Image Lab™
Software version 4.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were repeated three times. Data are
presented as the mean ± standard deviation. Statistical analyses
were performed using GraphPad Prism 6.0 (GraphPad Software, Inc.,
La Jolla, CA, USA). P-values were calculated using one-way analysis
of variance followed by Tukey's multiple comparison test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Hypoxia induces H9c2 cell injury and
downregulates the expression of MYBL2
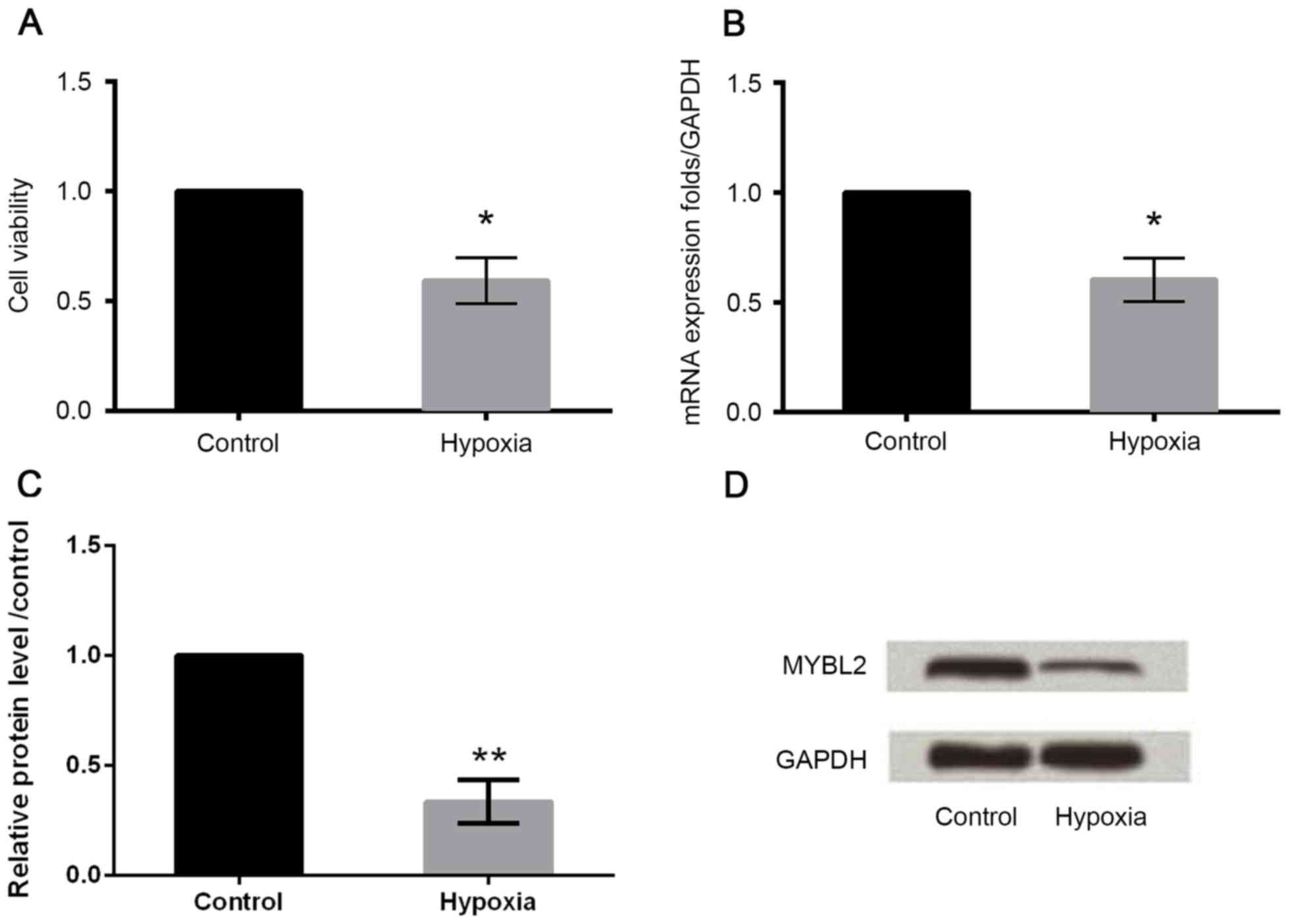
As demonstrated in Fig.
1A, the treatment of H9c2 cells with hypoxia reduced cell
viability compared with the control group. In addition, following
hypoxia treatment, the mRNA and protein expression of MYBL2 in H9c2
cells was downregulated compared with the control group (Fig. 1B-D), which indicated that hypoxia
may induce injury in H9c2 cells.
Upregulation and downregulation of
MYBL2 in H9c2 cells
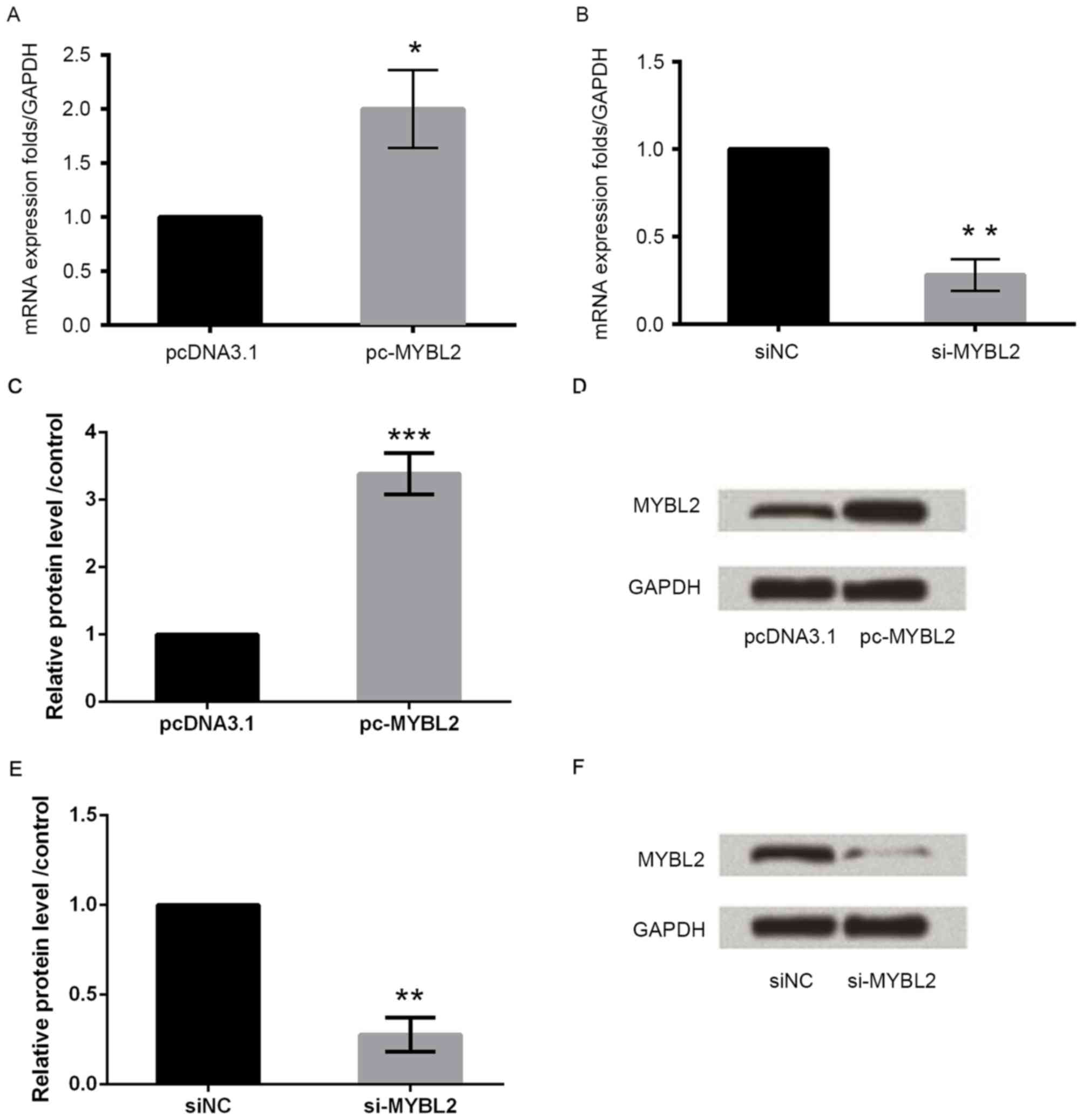
To investigate the function of MYBL2, myocardial
cells were transfected with MYBL2-specific siRNA or an
overexpression plasmid. Overexpression of MYBL2 upregulated MYBL2
mRNA and protein expression in these cells (Fig. 2A, C and D, respectively), while
knockdown of MYBL2 downregulated MYBL2 mRNA and protein expression,
compared with control cells (Fig. 2B,
E and F, respectively).
MYBL2 gene enhances cell proliferation
of H9c2 cells
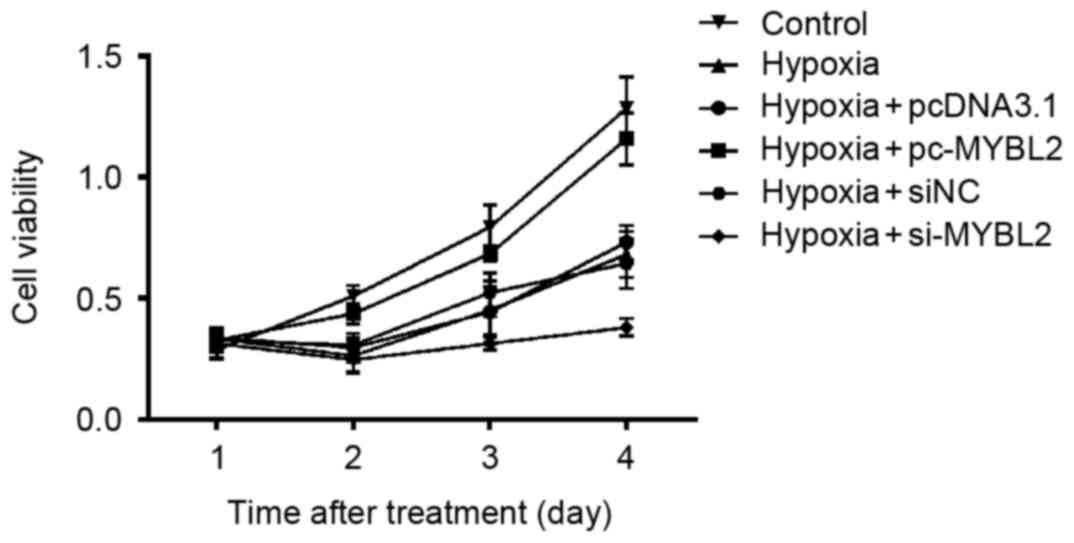
The results indicated that following hypoxia
treatment, the cell viability of H9c2 cells increased over time.
The growth of the MYBL2-specific siRNA-transfected cells was slower
compared with the negative control cells, which indicated that
downregulation of MYBL2 may suppress cell proliferation.
Furthermore. overexpression of MYBL2 alleviated cellular damage
caused by hypoxia, as cell viability was increased compared with
hypoxia-treated cells that were transfected with pcDNA3.1
vector-only (Fig. 3).
Overexpression of MYBL2 inhibits H9c2
apoptosis and downregulation of MYBL2 promotes H9c2 apoptosis
To determine whether MYBL2 has an association with
apoptosis, the percentage of apoptotic cells was assessed using
Annexin V-FITC/PI staining. Overexpression of MYBL2 resulted in a
significant decline in the percentage of apoptotic cells compared
with cells transfected with pcDNA3.1 vector-only, which indicates
that overexpression of MYBL2 inhibited H9c2 apoptosis. However, the
transfection of H9c2 cells with MYBL2-specific siRNA resulted in a
significant increase in apoptotic cells compared with cells
transfected with negative control siRNA (Fig. 4), which indicates that
downregulation of MYBL2 induced apoptosis in H9c2 cells.
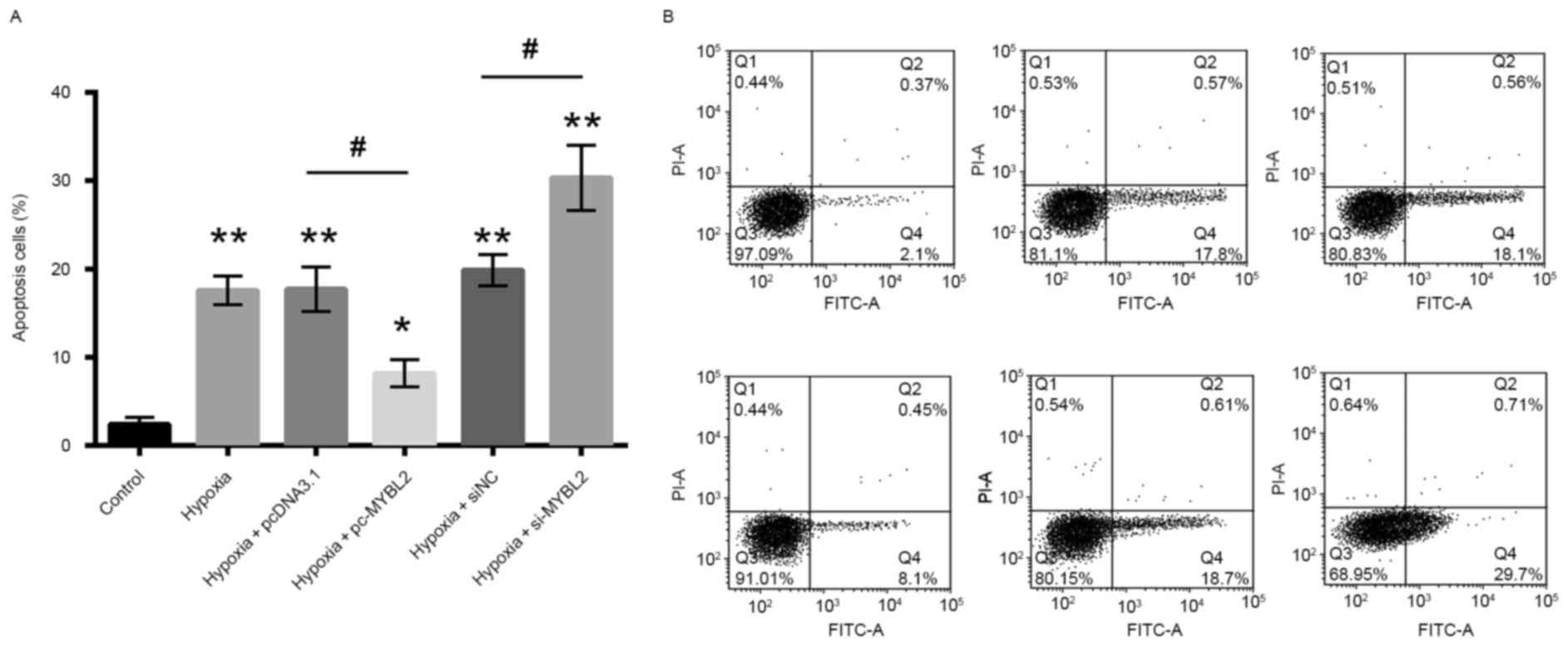 | Figure 4.The percentage of apoptotic cells was
determined by Annexin V-FITC/PI staining and flow cytometry. (A)
Percentage of apoptotic cells as determined by flow cytometry. (B)
Representative flow cytometry scatter plots for each treatment
group. The percentage of apoptotic cells was reduced following
transfection with pc-MYBL2, and was increased following
transfection with si-MYBL2. Q4 was considered to indicate apoptotic
cells. *P<0.05 and **P<0.01 vs. control group;
#P<0.05, as indicated FITC, fluorescein
isothiocyanate; PI, propidium iodide; MYBL2, MYB proto-oncogene
like 2; pc-MYBL2, MYBL2 overexpression plasmid; si, small
interfering RNA; pcDNA3.1, control vector; NC, negative control; Q,
quadrant. |
Overexpression of MYBL2 suppresses AKT
and NF-κB pathways, while downregulation of MYBL2 activates AKT and
NF-κB pathways
To investigate the protection mechanism of MYBL2 on
H9c2 injury induced by hypoxia, the present study analyzed the
expression of AKT and NF-κB by western blotting. The protein levels
of p-AKT, p-IκBα, p-p65 and Bcl-3 were markedly increased in the
hypoxia-treated group compared with the control group, while
overexpression of MYBL2 suppressed the expression of p-AKT, p-IκBα,
p-p65 and Bcl-3, which indicates that overexpression of MYBL2
suppressed AKT and NF-κB pathways (Fig. 5). By contrast, the expression of
p-AKT, p-IκBα, p-p65 and Bcl-3 in the siRNA-MYBL2 group was
enhanced to a certain extent compared with the group transfected
with negative control siRNA, indicating that downregulation of
MYBL2 may activate AKT and NF-κB pathways.
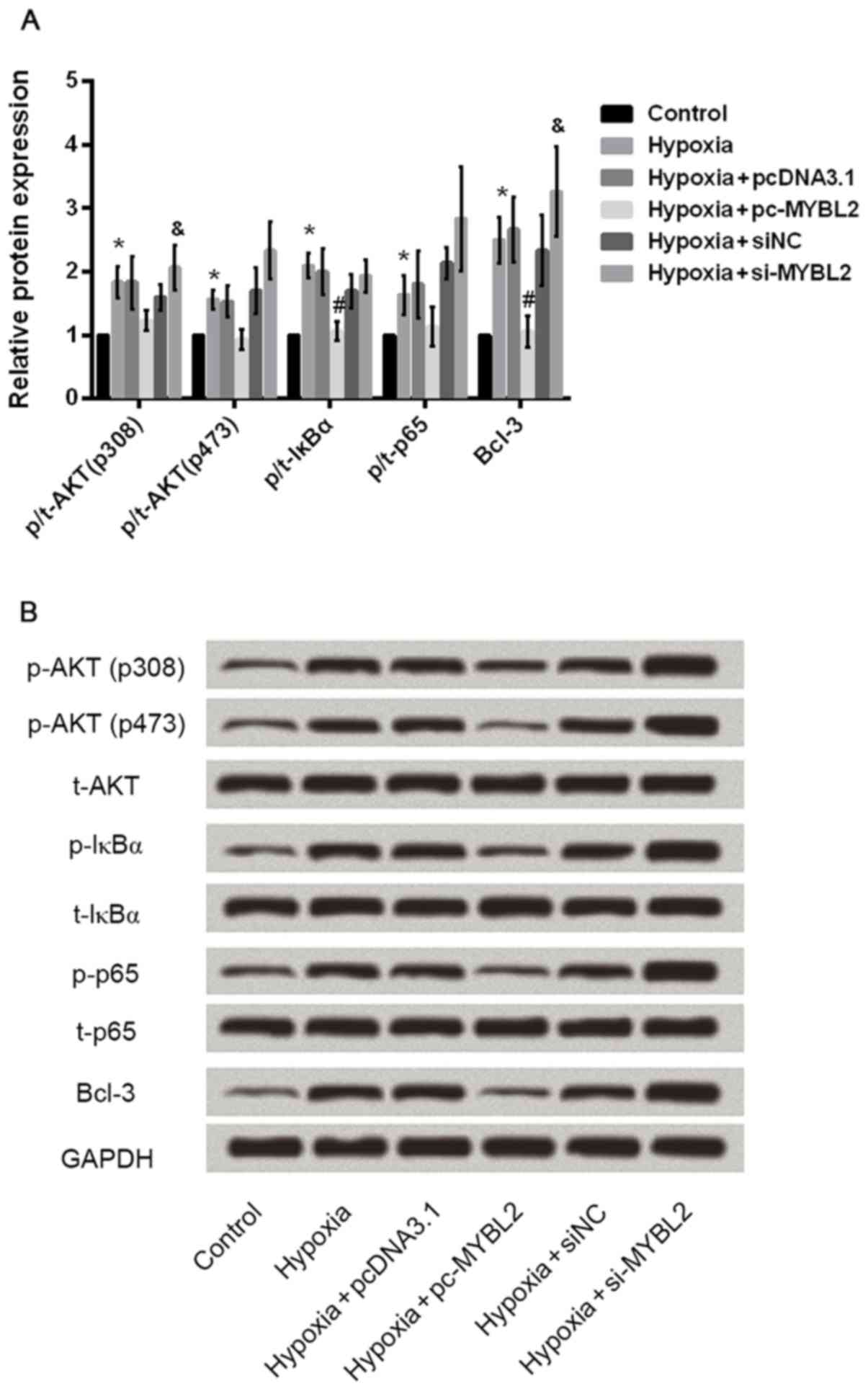 | Figure 5.AKT and NF-κB pathways were
investigated by RT-qPCR and western blotting. (A) The quantified
results for protein expression of AKT and NF-κB pathways-associated
factors. (B) Representative western blots of proteins associated
with AKT and NF-κB pathways. GAPDH was used as the loading control.
*P<0.05 vs. control group, #P<0.05 vs. hypoxia +
pcDNA3.1, &P<0.05 vs. hypoxia + siNC. NF-κB,
nuclear factor-κB; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; p-, phosphorylated-; t-, total-; IκBα,
NF-κB inhibitor α; Bcl-3, B-cell CLL/lymphoma 3; pcDNA3.1, control
vector; MYBL2, MYB proto-oncogene like 2; pc-MYBL2, MYBL2
overexpression plasmid; si, small interfering RNA. |
Discussion
AMI is associated with various suppressor genes,
such as MYBL2. MYBL2 is a positive growth control gene, which
participates in cell apoptosis and cell cycle progression. Previous
studies have reported that MYBL2 is overexpressed in various types
of cancer, including hepatocellular carcinoma (18) and colorectal cancer, amongst others
(19). Furthermore, overexpression
of MYBL2 indicates poor prognosis in patients with acute myeloid
leukemia (20) and breast cancer
(21). The present study, to the
best of our knowledge, was the first to investigate the effect of
MYBL2 on H9c2 injury induced by hypoxia. The current study
investigated the effect of hypoxia on H9c2 cells and discovered
that hypoxia induced H9c2 cell injury and downregulated the
expression of MYBL2.
The MYBL2 gene participates in cell growth, the cell
cycle and cell apoptosis. To understand the function of MYBL2 in
H9c2 injury, MYBL2-specific siRNA was used to downregulate MYBL2
expression in H9c2 cells and cell proliferation was analyzed using
the CCK-8 assay. The growth of cells transfected with siRNA was
slower compared with the negative control cells, which indicates
that the MYBL2 gene may enhance cell proliferation. Similar results
have previously been reported in neuroblastoma cell lines (22) and hepatocellular carcinoma
(23). We hypothesized that the
inhibition of apoptosis by MYBL2 may explain the cell proliferation
results in H9c2 cells. In the present study, Annexin V-FITC/PI
apoptosis staining was used to assess apoptotic cells. The results
demonstrated that upregulation of MYBL2 resulted in a significant
decline in the percentage of apoptotic cells, indicating that the
MYBL2 gene may exhibit anti-apoptotic functions. A previous study
reported similar results for hepatocellular carcinoma (13), which demonstrated that
overexpression of MYBL2 led to the downregulation of proapoptotic
genes.
Previous studies have reported that MYBL2 was
contained in a segment of the Bcl-2 promoter (24), and stimulated the activity of the
promoter at the 5′ end of Bcl-2 (25). NF-κB is a transcription factor that
has important roles in inflammation (26). Activation of NF-κB due to
inflammatory agents leads to the induction of a large number of
genes, including inflammatory cytokines and adhesion molecules
(27). The activation of NF-κB
requires the phosphorylation of NF-κB proteins, such as p65
(28). To further understand the
mechanism responsible for the induction of proliferation, the
current study analyzed the expression of p-AKT, p-IκBα, p-p65 and
Bcl-3 by western blotting. The results demonstrated that
upregulation of MYBL2 suppressed the expression of p-p65 and
p-IκBα, indicating that the protective effect of MYBL2 on H9c2 cell
injury may be mediated by the inhibition of the phosphorylation of
p65 and IκBα. In terms of Bcl-3, it has been reported that Bcl3 is
an IκB-related protein with ankyrin repeat motifs (29). Bcl-3 tightly associates with the
NF-kB p50 or p52 subunits and strongly enhances cell proliferation
and oncogenesis in various cancers (30–32).
However, the role of Bcl-3 in cardiomyocytes is rarely reported.
The present results indicate that MYBL2 might have a regulatory
effect on BCL-3 in hypoxia-injured H9c2 cells.
The phosphoinositide 3-kinase (PI3K)/AKT pathway has
a key role in various biological responses, which include cellular
proliferation and survival. Previous studies have reported that the
PI3K/AKT pathway may be involved in NF-κB activation (33,34).
In the present study, upregulation of MYBL2 suppressed the
expression of p-AKT, and the downregulation of MYBL2 promoted the
expression of p-AKT, indicating that MYBL2 may inhibit NF-κB
activation via the PI3K/AKT pathway. Based on the results of the
present study, MYBL2 may inhibit apoptosis and promote cell
proliferation by suppressing AKT and NF-κB pathways.
The present study investigated the effect of MYBL2
on H9c2 injury induced by hypoxia. The expression of MYBL2 was
demonstrated to be reduced in H9c2 cells exposed to hypoxia. The
results indicated that MYBL2 may inhibit apoptosis and promote cell
proliferation by suppressing AKT and NF-κB pathways, and that MYBL2
may protect against H9c2 injury induced by hypoxia via AKT and
NF-κB pathways. Myocardial infarction is determined by numerous
mediators and signaling pathways, leading to the irreparable loss
of cardiomyocytes due to oxidative stress, along with insufficient
oxygen and blood supply to the heart (35). It is critical to maintain efficient
cardiomyocytes for the preservation of cardiac structural integrity
and function. Therefore, MYBL2 is a potential therapeutic target
for the treatment of myocardial infarction.
References
|
1
|
Writing Group Members, ; Mozaffarian D,
Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de
Ferranti S, Després JP, et al: Heart disease and stroke
statistics-2016 update: A report from the american heart
association. Circulation. 133:e38–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Silva H, Freitas J, Moreira S, Santos A
and Almeida V: Alexithymia and psychopathology in patients with
acute myocardial infarction. Acta Cardiol. 71:213–220. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taniyama Y, Katsuragi N, Sanada F, Azuma
J, Iekushi K, Koibuchi N, Okayama K, Ikeda-Iwabu Y, Muratsu J, Otsu
R, et al: Selective blockade of periostin exon 17 preserves cardiac
performance in acute myocardial infarction. Hypertension.
67:356–361. 2016.PubMed/NCBI
|
|
4
|
Sun G, Ye N, Dai D, Chen Y, Li C and Sun
Y: The protective role of the TOPK/PBK pathway in myocardial
ischemia/reperfusion and H2O2-induced injury
in H9C2 cardiomyocytes. Int J Mol Sci. 17:2672016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hearse DJ and Bolli R: Reperfusion induced
injury: Manifestations, mechanisms, and clinical relevance.
Cardiovasc Res. 26:101–108. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sandberg ML, Sutton SE, Pletcher MT,
Wiltshire T, Tarantino LM, Hogenesch JB and Cooke MP: c-Myb and
p300 regulate hematopoietic stem cell proliferation and
differentiation. Dev Cell. 8:153–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malaterre J, Carpinelli M, Ernst M,
Alexander W, Cooke M, Sutton S, Dworkin S, Heath JK, Frampton J,
McArthur G, et al: c-Myb is required for progenitor cell
homeostasis in colonic crypts. Proc Natl Acad Sci USA. 104:pp.
3829–3834. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Malaterre J, Mantamadiotis T, Dworkin S,
Lightowler S, Yang Q, Ransome MI, Turnley AM, Nichols NR, Emambokus
NR, Frampton J and Ramsay RG: c-Myb is required for neural
progenitor cell proliferation and maintenance of the neural stem
cell niche in adult brain. Stem Cells. 26:173–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanaka Y, Patestos NP, Maekawa T and Ishii
S: B-myb is required for inner cell mass formation at an early
stage of development. J Biol Chem. 274:28067–28070. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhan M, Riordon DR, Yan B, Tarasova YS,
Bruweleit S, Tarasov KV, Li RA, Wersto RP and Boheler KR: The B-MYB
transcriptional network guides cell cycle progression and fate
decisions to sustain self-renewal and the identity of pluripotent
stem cells. PloS One. 7:e423502012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Papetti M and Augenlicht LH: MYBL2, a link
between proliferation and differentiation in maturing colon
epithelial cells. J Cell Physiol. 226:785–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hibi K, Liu Q, Beaudry GA, Madden SL,
Westra WH, Wehage SL, Yang SC, Heitmiller RF, Bertelsen AH,
Sidransky D and Jen J: Serial analysis of gene expression in
non-small cell lung cancer. Cancer Res. 58:5690–5694.
1998.PubMed/NCBI
|
|
13
|
Calvisi DF, Simile MM, Ladu S, Frau M,
Evert M, Tomasi ML, Demartis MI, Daino L, Seddaiu MA, Brozzetti S,
et al: Activation of v-Myb avian myeloblastosis viral oncogene
homolog-like2 (MYBL2)-LIN9 complex contributes to human
hepatocarcinogenesis and identifies a subset of hepatocellular
carcinoma with mutant p53. Hepatology. 53:1226–1236. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Forozan F, Mahlamäki EH, Monni O, Chen Y,
Veldman R, Jiang Y, Gooden GC, Ethier SP, Kallioniemi A and
Kallioniemi OP: Comparative genomic hybridization analysis of 38
breast cancer cell lines: A basis for interpreting complementary
DNA microarray data. Cancer Res. 60:4519–4525. 2000.PubMed/NCBI
|
|
15
|
Ekhterae D, Lin Z, Lundberg MS, Crow MT,
Brosius FC III and Nunez G: ARC inhibits cytochrome c release from
mitochondria and protects against hypoxia-induced apoptosis in
heart-derived H9c2 cells. Circ Res. 85:e70–e77. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hirst M: Gene targeting: A practical
approach. J Med Genet. 31:8211994. View Article : Google Scholar :
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakajima T, Yasui K, Zen K, Inagaki Y,
Fujii H, Minami M, Tanaka S, Taniwaki M, Itoh Y, Arii S, et al:
Activation of B-Myb by E2F1 in hepatocellular carcinoma. Hepatology
Res. 38:886–895. 2008.
|
|
19
|
Parikh N, Hilsenbeck S, Creighton CJ,
Dayaram T, Shuck R, Shinbrot E, Xi L, Gibbs RA, Wheeler DA and
Donehower LA: Effects of TP53 mutational status on gene expression
patterns across 10 human cancer types. J Pathol. 232:522–533. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuster O, Llop M, Dolz S, García P, Such
E, Ibáñez M, Luna I, Gómez I, López M, Cervera J, et al: Adverse
prognostic value of MYBL2 overexpression and association with
microRNA-30 family in acute myeloid leukemia patients. Leuk Res.
37:1690–1696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thorner AR, Hoadley KA, Parker JS, Winkel
S, Millikan RC and Perou CM: In vitro and in vivo analysis of B-Myb
in basal-like breast cancer. Oncogene. 28:742–751. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gualdrini F, Corvetta D, Cantilena S,
Chayka O, Tanno B, Raschellà G and Sala A: Addiction of MYCN
amplified tumours to B-MYB underscores a reciprocal regulatory
loop. Oncotarget. 1:278–288. 2010.PubMed/NCBI
|
|
23
|
Frau M, Ladu S, Calvisi DF, Simile MM,
Bonelli P, Daino L, Tomasi ML, Seddaiu MA, Feo F and Pascale RM:
Mybl2 expression is under genetic control and contributes to
determine a hepatocellular carcinoma susceptible phenotype. J
Hepatol. 55:111–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi H, Bevier M, Johansson R,
Enquist-Olsson K, Henriksson R, Hemminki K, Lenner P and Försti A:
Prognostic impact of polymorphisms in the MYBL2 interacting genes
in breast cancer. Breast Cancer Res Treat. 131:1039–1047. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grassilli E, Salomoni P, Perrotti D,
Franceschi C and Calabretta B: Resistance to apoptosis in CTLL-2
cells overexpressing B-Myb is associated with B-Myb-dependent bcl-2
induction. Cancer Res. 59:2451–2456. 1999.PubMed/NCBI
|
|
26
|
Viatour P, Merville MP, Bours V and
Chariot A: Phosphorylation of NF-kappaB and IkappaB proteins:
Implications in cancer and inflammation. Trends Biochem Sci.
30:43–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Checker R, Patwardhan RS, Sharma D, Menon
J, Thoh M, Bhilwade HN, Konishi T and Sandur SK: Schisandrin B
exhibits anti-inflammatory activity through modulation of the
redox-sensitive transcription factors Nrf2 and NF-κB. Free Radic
Biol Med. 53:1421–1430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu J, Nakano H, Sakurai H and Colburn NH:
Insufficient p65 phosphorylation at S536 specifically contributes
to the lack of NF-kappaB activation and transformation in resistant
JB6 cells. Carcinogenesis. 25:1991–2003. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bours V, Franzoso G, Azarenko V, Park S,
Kanno T, Brown K and Siebenlist U: The oncoprotein Bcl-3 directly
transactivates through kappa B motifs via association with
DNA-binding p50B homodimers. Cell. 72:729–739. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Massoumi R, Chmielarska K, Hennecke K,
Pfeifer A and Fässler R: Cyld inhibits tumor cell proliferation by
blocking Bcl-3-Dependent NF-kappaB signaling. Cell. 125:665–677.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rocha S, Martin AM, Meek DW and Perkins
ND: p53 represses cyclin D1 transcription through down regulation
of Bcl-3 and inducing increased association of the p52 NF-kappaB
subunit with histone deacetylase 1. Mol Cell Biol. 23:4713–4727.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Westerheide SD, Mayo MW, Anest V, Hanson
JL and Baldwin AS Jr: The putative oncoprotein Bcl-3 induces cyclin
D1 to stimulate G(1) transition. Mol Cell Biol. 21:8428–8436. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hwang HJ, Jung TW, Hong HC, Choi HY, Seo
JA, Kim SG, Kim NH, Choi KM, Choi DS, Baik SH and Yoo HJ:
Progranulin protects vascular endothelium against atherosclerotic
inflammatory reaction via Akt/eNOS and nuclear factor-κB pathways.
PloS One. 8:e766792013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guha M and Mackman N: The
phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide
activation of signaling pathways and expression of inflammatory
mediators in human monocytic cells. J Biol Chem. 277:32124–32132.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Timmers L, Henriques JP, de Kleijn DP,
Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek
JJ, Doevendans PA, et al: Exenatide reduces infarct size and
improves cardiac function in a porcine model of ischemia and
reperfusion injury. J Am Coll Cardiol. 53:501–510. 2009. View Article : Google Scholar : PubMed/NCBI
|