Introduction
Out of the total estimated new cases of cancer, the
occurrence of renal carcinomas ranked 7th in men and 10th in women
in 2017 (1). Clear cell renal cell
carcinoma (ccRCC) is the most common type of renal carcinomas. Over
the past few decades, the incidence of renal cell carcinoma has
increased by 2–3% per decade, particularly in developed countries
(2,3). In 2012, an estimated 144,000 patients
died from renal cancer, which was 1.7% of the estimated number of
total cancer-associated mortalities worldwide (3). If diagnosed at an early stage, 60% of
patients with renal cell carcinoma are estimated to have a low risk
of cancer-specific mortality. However, following therapy, ~30% of
patients exhibit recurrence with a poor prognosis (4). Therefore, it is important to
investigate the factors associated with ccRCC, in order to improve
its management.
Hypoxic conditions have been identified in several
types of solid tumors. Hypoxia promotes anaerobic metabolism,
angiogenesis and relevant genes expression, such as connective
tissue growth factor (CTGF), osteopontin (OPN) and interleukin-6
(IL-6) in solid tumors, which are typically associated with poor
prognosis (5). Acute hypoxia may
inhibit cellular proliferation and induce apoptosis in tumor cells.
However, in a chronic hypoxic environment, tumor cells may adapt
through alterations to an intricate regulatory network of
transcription factors and associated proteins, leading to cellular
multiplication, migration and bioactivity (6,7).
Overexpression of hypoxia inducible factor-1α
(HIF-1α) has been identified to be a common characteristic of
several types of solid tumors, and numerous studies have linked its
elevated levels to poor prognosis, angiogenesis and tumor
metastasis (8,9). HIF-1α overexpression is thought to be
an adaptive response to hypoxic stimulation in the tumor
microenvironment and is associated with the activation of a number
of survival pathways (10).
Chronic obstructive pulmonary disease (COPD) is a chronic hypoxic
condition of the respiratory system that is characterized by
persistent and progressive airflow limitation. Low levels of
hemoglobin have been linked with elevated HIF-1α expression in COPD
(11). Similar events may occur in
ccRCC and contribute to its poor prognosis.
Materials and methods
Patients
A total of 128 patients aged 35–85 (mean age, 61.5)
years, diagnosed with ccRCC by pathological examination between
November, 2006 and December, 2015 at The Second Xiangya Hospital
(Changsha, China), were enrolled. Of the participants, 96 patients
were male and 32 were female, with 69 patients undergoing a full
nephrectomy, and 59 patients undergoing a partial nephrectomy. The
basic clinicopathological characteristics were collected for
further analysis, such as R.E.N.A.L scores (Radius,
Exophytic/endophytic, Nearness to collecting system or sinus,
Anterior/posterior, Location relative to polar lines) (12), TNM stages (tumor size, lymph node
status and metastasis) (13),
Fuhrman grades (Fuhrman 1 and Fuhrman 2 were merged as G1, Fuhrman
3 was considered as G2, and Fuhrman 4 was classified as G3)
(14), and so on. All enrolled
patients were followed-up according to their individual
characteristics. Follow-up typically consisted of a urine cytology
assessment and abdominal ultrasound every 3 months in the first 2
years, every 6 months in the following 2 years and yearly
thereafter. A chest and abdominal computed tomography scan was
performed every year in the first 5 years following surgery.
Informed consent was obtained from all participants and approval
for the present study was given by the ethics committee of The
Second Xiangya Hospital of Central South University.
Assessment of COPD and the
preoperative hemoglobin
Patients with COPD were diagnosed by a chest
physician. The diagnosis was based on respiratory symptoms and
spirometric assessment of airflow obstruction, with or without a
history of risk factor exposure. Hemoglobin levels were determined
by routine venous blood examinations prior to surgery and patients
were split into three groups: >120, 100–120 and <100 g/l.
Immunohistochemistry
Postoperative renal carcinoma tissues were fixed in
10% neutral buffered formalin for 24 h at room temperature, and
washed thoroughly with PBS. Following conventional dehydration,
tissues were embedded in molten paraffin and kept at 4°C. The
paraffin-embedded tissues were cut into 5-µm thick sections on
poly-L-lysine coated glass slides using a microtome. Following 2 h
of heat-drying (55°C), serial sections were deparaffinized in
xylene and a descending ethanol series (100, 90, 80 and 70%
ethanol). Circles were drawn on the slides around the tissues using
a hydrophobic barrier pen iprior to placing the sections in
moderate sodium citrate buffer (pH 6.0) in a microwave oven. The
slides were boiled for 3 min in microwave (SHARP) at full power and
subsequently maintained at a sub-boiling temperature for 5 min with
30% power. Slides were washed with PBS and subsequently blocked
with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 30 min at room temperature. Sections were incubated
overnight at 4°C with anti-HIF-1α monoclonal antibody (Mab H1α67,
IgG2b isotype; cat. no. MS-1164-P0; diluted 1:100; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Next day, following three
washes with PBS, the paraffin-embedded sections were stained using
an Elivision Super HRP Immunohistochemistry Kit (cat. no. KIT-9931,
Maixin Bio, Inc., Fujian, China), and the second antibody was
included in this kit (reagent B, high sensitive enzyme labelled
anti-mouse), 50 µl per slide at room temperature for 30 min, with
1% diaminobenzidine and counterstaining with hematoxylin for 5 min
at room temperature.
Expression of HIF-1α was semi-quantitatively
assessed by two blinded independent reviewers. HIF-1α expression
was interpreted and scored in the tumor region. A total of six
randomly-selected fields throughout the entire section at ×400
magnification were analyzed, and 200 cells were counted by two
different operators in each field. The expression of HIF-1α was
graded as follows: -, <1% positive cells; 1+, 1–10% positive
cells; 2+, 10–50% positive cells; and 3+, >50% positive
cells.
Statistical analysis
Data analysis was performed using SPSS version 19.0
(IBM Corp., Armonk, NY, USA). Kaplan-Meier survival curve is a
non-parametric statistic method used to estimate the survival
influence with lifetime data in different patients, and
Kaplan-Meier survival curves were constructed and analyzed using
GraphPad prism 6 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference. All P-values were two-sided. The significance of the
results was assessed using Student's t-tests, one-way analysis of
variance, followed by Tukey post hoc, and χ2 tests. The
hazard ratio (HR) and 95% confidence interval of the HR was
calculated using the Cox proportional hazard model.
Results
Patient characteristics
Table I presents
the detailed characteristics of the patients. A total of 92
patients were classified as stage T1-T2 and 36 patients were
classed as stage T3-T4. The number of patients classified as
Fuhrman grade G1was 69; G2, 40 and G3, 19. A total of 30 patients
were diagnosed with ccRCC and COPD. The clinicopathological
characteristics of patients with or without COPD are presented in
Table II. There was no
significant difference detected in age, pathological stages,
R.E.N.A.L Scores, types of surgery or comorbidity between patients
with or without COPD. However, Fuhrman grades, pulmonary function,
smoking history, C-reactive protein levels, serum hemoglobin levels
and HIF-1α expression were significantly different between patients
with or without COPD.
 | Table I.Patient characteristics (n=128). |
Table I.
Patient characteristics (n=128).
| Mean age, years | 61.6 (35–85) |
|---|
| Sex |
|
| Male | 96 (75.0%) |
|
Female | 32 (25.0%) |
| Affected kidney |
|
|
Right | 68 (53.1%) |
| Left | 60 (46.9%) |
| Furhman grade |
|
| Good | 69 (53.9% |
|
Moderate | 40 (31.3%) |
| Poor | 19 (14.8%) |
| Pathological
stage |
|
|
T1-T2 | 92 (71.9%) |
|
T3-T4 | 36 (28.9%) |
| Smoking history |
|
| Smoking
index ≤400 | 86 (67.2%) |
| Smoking
index >400 | 42 (32.8%) |
| COPD | 30 (23.4%) |
| Other
comorbidities |
|
|
Hypertension | 32 (25.0%) |
| Diabetes
mellitus | 18 (14.1%) |
| Coronary
disease | 12 (9.4%) |
|
Tuberculosis | 7 (5.5%) |
| Mean serum
haemoglobin, g/l | 129 (75–165) |
 | Table II.Clinicopathological characteristics
and COPD incidence. |
Table II.
Clinicopathological characteristics
and COPD incidence.
|
| No COPD | COPD |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | No. | % | No. | % | P-value |
|---|
| Age, years |
|
|
|
| 0.694 |
|
<60 | 28 | 28.6 | 7 | 23.3 |
|
|
60–70 | 39 | 39.8 | 11 | 36.7 |
|
|
>70 | 31 | 31.6 | 12 | 40.0 |
|
| Pathological
stage |
|
|
|
| 0.169 |
|
T1-T2 | 73 | 74.5 | 19 | 63.3 |
|
|
T3-T4 | 25 | 25.5 | 11 | 36.7 |
|
| Fuhrman grade |
|
|
|
| 0.008 |
| G1 | 60 | 61.2 | 9 | 30.0 |
|
| G2 | 27 | 27.6 | 13 | 43.3 |
|
| G3 | 11 | 11.2 | 8 | 26.7 |
|
| R.E.N.A.L
score |
|
|
|
| 0.329 |
| ≤8 | 62 | 63.3 | 17 | 56.7 |
|
| ≥9 | 36 | 36.7 | 13 | 43.3 |
|
| Surgical means |
|
|
|
| 0.290 |
|
Nephrectomy | 51 | 52.0 | 18 | 60.0 |
|
| Partial
nephrectomy | 47 | 47.96 | 12 | 40.0 |
|
| Pulmonary function
test |
|
|
|
| 0.002 |
|
Normal | 75 | 76.5 | 8 | 26.7 |
|
|
Abnormal | 23 | 23.5 | 22 | 73.3 |
|
| Smoking
history |
|
|
|
| <0.001 |
| Smoking
index ≤400 | 76 | 77.6 | 10 | 33.3 |
|
| Smoking
index >400 | 22 | 22.4 | 20 | 67.7 |
|
| C-reactive protein,
mg/l |
|
|
|
| <0.001 |
|
<4.0 | 56 | 57.1 | 7 | 23.3 |
|
|
4.1–10.0 | 34 | 34.7 | 12 | 40.0 |
|
|
>10.0 | 8 | 8.2 | 11 | 36.7 |
|
| Serum haemoglobin,
g/l |
|
|
|
| 0.044 |
|
>120 | 68 | 69.4 | 14 | 46.7 |
|
|
100–120 | 21 | 21.4 | 9 | 30.0 |
|
|
<100 | 9 | 9.2 | 7 | 23.3 |
|
| Other
comorbidities |
|
|
|
| 0.793 |
|
Hypertension | 23 | 23.5 | 6 | 20.0 |
|
|
Diabetes mellitus | 13 | 13.3 | 5 | 16.7 |
|
|
Coronary disease | 8 | 8.1 | 4 | 13.3 |
|
|
Tuberculosis | 5 | 5.1 | 1 | 3.3 |
|
| Expression of
HIF-1α |
|
|
|
| <0.001 |
| − | 26 | 26.5 | 2 | 6.7 |
|
| + | 42 | 42.9 | 4 | 13.3 |
|
| ++ | 18 | 19.4 | 17 | 56.7 |
|
|
+++ | 12 | 12.2 | 7 | 23.3 |
|
As presented in Table
III, a significant difference was detected between HIF-1α
expression levels and pathological stages or Fuhrman grades of
ccRCC (P<0.001 and P=0.002, respectively), indicating that
elevated HIF-1α expression levels were associated with higher TNM
stages and Fuhrman grades in ccRCC.
 | Table III.Chi-square (χ2) test
analysis of HIF-1α expression and its association with pathological
stage and histological grade of tumours. |
Table III.
Chi-square (χ2) test
analysis of HIF-1α expression and its association with pathological
stage and histological grade of tumours.
|
| Pathological
stage | Fuhrman grade |
|---|
|
|
|
|
|---|
| Expression of
HIF-1α |
T1-T2 |
T3-T4 | χ2 | P-value | G1 | G2 | G3 | χ2 | P-value |
|---|
| − | 25 | 3 | 21.97 | <0.001 | 22 | 5 | 1 | 20.38 | 0.002 |
| + | 39 | 7 |
|
| 26 | 14 | 6 |
|
|
| ++ | 21 | 14 |
|
| 18 | 12 | 5 |
|
|
| +++ | 7 | 12 |
|
| 3 | 9 | 7 |
|
|
Survival analysis
The median overall survival (OS) was 50.2 months for
the COPD group and 62.5 months for the no COPD group (Table IV). Progression free survival
(PFS) was 22.2 months for the COPD group and 45.0 months for the no
COPD group. This difference was statistically significant
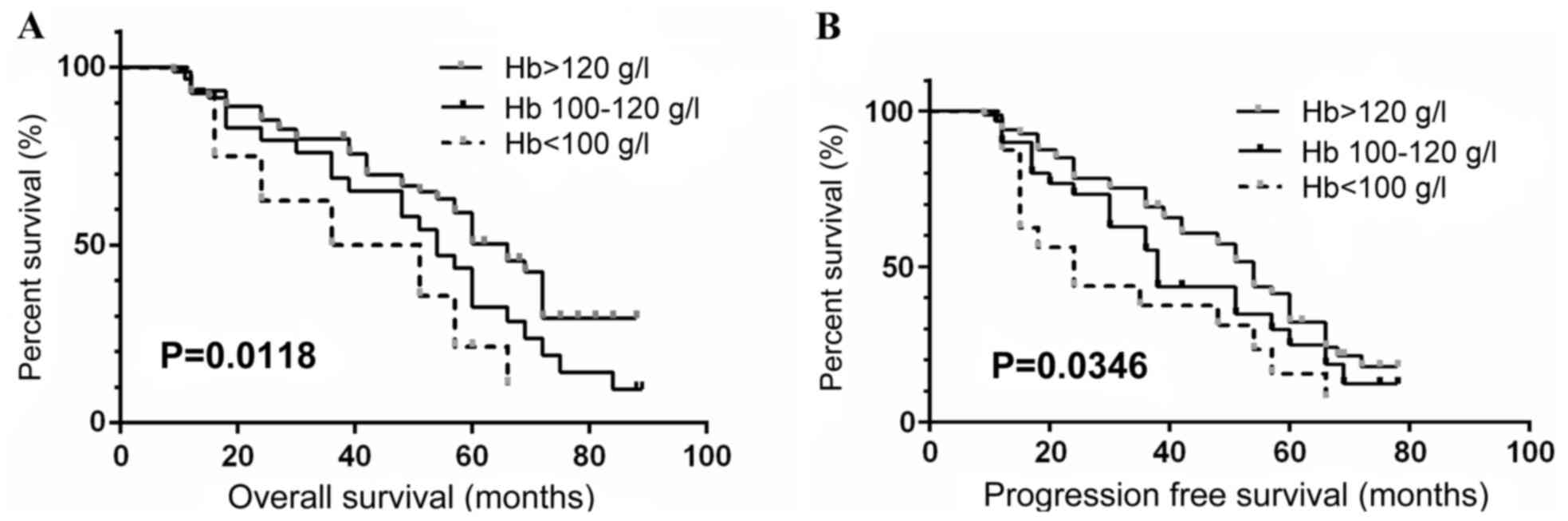
(P<0.001). The median OS for the patients whose preoperative
hemoglobin counts were >120, 100–120 and <100 g/l was 66.4,
54.1 and 43.5 months, respectively. The median PFS for the patients
grouped as above was 53.6, 38.0 and 24.2 months, respectively.
Analysis between the groups indicated a statistically significant
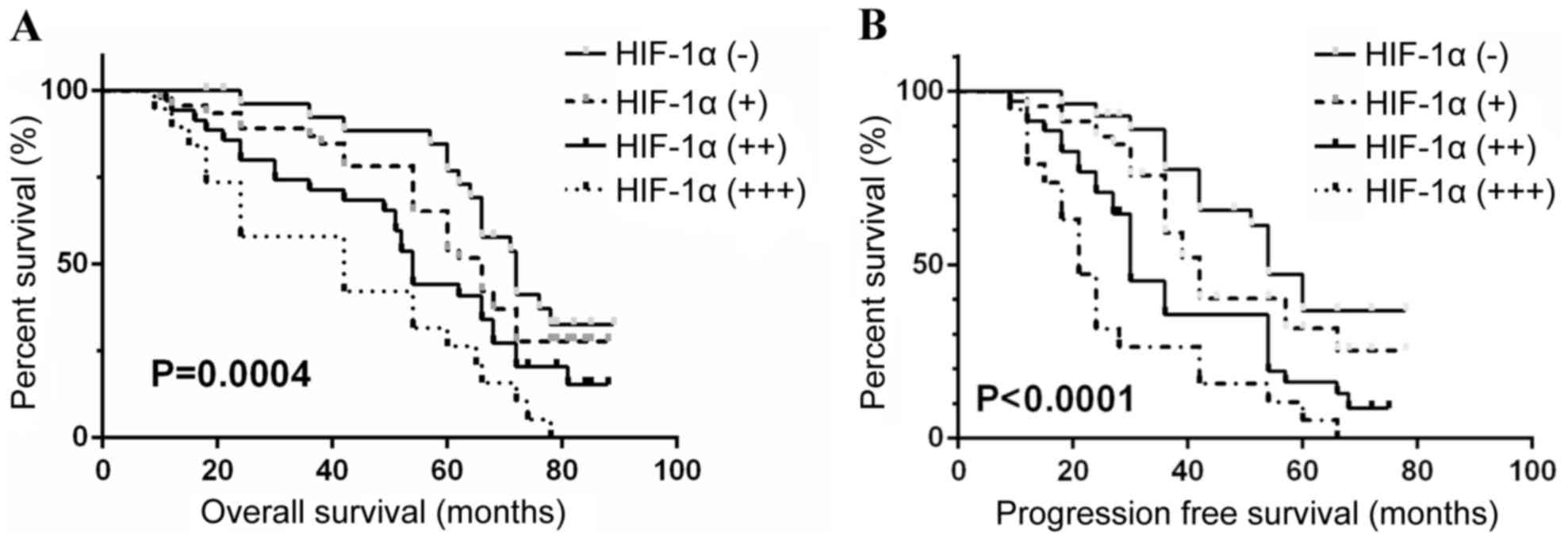
result (P<0.05). The median OS for the patients with HIF-1α
expression levels defined as ‘−’, ‘+’, ‘++’ and ‘+++’ was 71.2,
62.5, 54.2 and 42.1 months, respectively. The median PFS for the
patients grouped as above was 51.0, 42.5, 30.2 and 20.5 months,
respectively. Analysis between these groups additionally indicated
a statistically significant result (P<0.05). As presented in
Table IV, the median OS and PFS
were significantly different depending on pathological stage,
histological grade and R.E.N.A.L Score (Table IV). The multivariate Cox
proportional hazard model of variables is presented in Table V. These results indicated that
pathological stage, R.E.N.A.L score, COPD diagnosis and HIF-1α
expression were independent prognostic variables for OS and PFS.
The preoperative levels of hemoglobin were an independent
prognostic variable for PFS only. The OS and PFS for patients
calculated with respect to hemoglobin, COPD and HIF-1α levels were
depicted by Kaplan-Meier survival curves (Figs. 1–3), which indicated low preoperative
hemoglobin (<100 g/l), COPD status and high levels of HIF-1α
expression in tumor (+++) were significantly associated with poor
OS and PFS in ccRCC patients (all P<0.05).
 | Table IV.Significant prognostic variables on
survival by univariate analysis. |
Table IV.
Significant prognostic variables on
survival by univariate analysis.
| Variables | No. | Median OS,
months | P-value | Median PFS,
months | P-value |
|---|
| Age, years |
|
| 0.076 |
| 0.091 |
|
<60 | 35 | 56.2 |
| 36.0 |
|
|
60–70 | 50 | 60.4 |
| 39.0 |
|
|
>70 | 43 | 49.5 |
| 32.0 |
|
| Pathological
stage |
|
| <0.001 |
| 0.002 |
|
T1-T2 | 92 | 65.1 |
| 41.0 |
|
|
T3-T4 | 36 | 46.2 |
| 26.7 |
|
| Histological
grade |
|
| 0.011 |
| 0.001 |
|
Good | 69 | 70.6 |
| 51.0 |
|
|
Moderate | 40 | 60.4 |
| 40.2 |
|
|
Poor | 19 | 44.6 |
| 21.5 |
|
| R.E.N.A.L
score |
|
| <0.001 |
| <0.001 |
| ≤8 | 79 | 69.5 |
| 39.8 |
|
| ≥9 | 49 | 43.4 |
| 29.4 |
|
| COPD |
|
| 0.009 |
| 0.006 |
|
Presence | 30 | 50.2 |
| 22.2 |
|
|
Absence | 98 | 62.5 |
| 45.0 |
|
| Hemoglobin,
g/l |
|
| 0.012 |
| 0.035 |
| >120
g/l | 82 | 66.4 |
| 53.6 |
|
| 100-120
g/l | 30 | 54.1 |
| 38.0 |
|
| <100
g/l | 16 | 43.5 |
| 24.2 |
|
| C-reactive
protein |
|
| 0.011 |
| <0.001 |
| <4.0
mg/l | 63 | 68.2 |
| 49.1 |
|
|
4.1–10.0 mg/l | 46 | 57.6 |
| 34.0 |
|
|
>10.0 mg/l | 19 | 46.1 |
| 26.4 |
|
| Expression of
HIF-1α |
|
| 0.001 |
| <0.001 |
| − | 28 | 71.2 |
| 51.0 |
|
| + | 46 | 62.5 |
| 42.5 |
|
| ++ | 35 | 54.2 |
| 30.2 |
|
|
+++ | 19 | 42.1 |
| 20.5 |
|
 | Table V.Multivariate Cox proportional hazard
model of variables associated with prognosis. |
Table V.
Multivariate Cox proportional hazard
model of variables associated with prognosis.
| Variables | Hazard ratio | 95% CI | P-value |
|---|
| Pathological
stage |
|
|
|
| OS | 4.306 | 2.316–8.003 | <0.001 |
|
PFS | 3.061 | 1.703–5.502 | 0.002 |
| R.E.N.A.L
score |
|
|
|
| OS | 6.204 | 3.556–10.830 | <0.001 |
|
PFS | 2.651 | 1.623–4.330 | 0.001 |
| COPD |
|
|
|
| OS | 2.106 | 1.197–3.706 | 0.015 |
|
PFS | 2.230 | 1.255–3.965 | 0.001 |
| Serum haemoglobin,
g/l |
|
|
|
| OS | 1.517 | 0.812–2.837 | 0.005 |
|
PFS | 2.250 | 1.39–4.087 | 0.013 |
| Expression of
HIF-1α |
|
|
|
| OS | 1.775 | 1.109–2.841 | 0.018 |
|
PFS | 1.974 | 1.226–3.179 | 0.002 |
Discussion
Hypoxia is commonly observed in various tumor types
and is typically associated with poor prognosis. A number of
studies have demonstrated that hypoxic conditions are associated
with tumor glycolysis, angiogenesis and prognosis, and hypoxic
conditions can induce and regulate tumor metastasis (6,15).
Therefore, interfering with hypoxia may be an effective approach to
preventing or reducing the growth and metastasis of tumours, as
well as improving patient prognosis (5,16).
Certain diseases, including anemia and COPD, may contribute to the
hypoxic environment present in tumours. Lower levels of
preoperative hemoglobin, higher HIF-1α expression and COPD
diagnosis were identified in the present study to indicate poor
prognosis in patients with ccRCC.
Anemia is defined as a lack of sufficient red blood
cells to maintain adequate tissue oxygenation, and is a common
comorbidity of solid tumours. Renal cancer-associated anemia is
associated with multiple mechanisms of causation, including
iatrogenic or intrinsic blood loss, cancer-associated nutritional
deficiencies and bone marrow failure due to tumor encroachment
(17). The levels of peripheral
serum hemoglobin indicate the degree of anemia in patients with
ccRCC. Patients with a lower preoperative level of serum hemoglobin
are associated with a higher stage of cancer at diagnosis, and a
poor prognosis even following surgery (18,19).
A meta-analysis including 949,445 patients concluded that
preoperative anemia was associated with increased mortality,
infection and acute kidney injury in patients following surgery
(20). The retrospective results
of the present study additionally indicated that lower preoperative
hemoglobin levels were associated with shorter OS and PFS, and may
be an independent prognostic variable in ccRCC patients.
COPD is a common public health problem, and it is
estimated that it will rank 3rd in terms of mortality and 5th in
terms of disease burden worldwide by 2020 (21). COPD is a preventable and manageable
disease, characterized by persistent airflow limitation (22). Cigarette smoking is one of the
best-studied risk factors for COPD, which in turn contributes to
tumor invasion ability and poor prognosis in ccRCC (23). Previous studies have indicated that
COPD is an independent poor prognostic factor, and a high-risk
factor for postoperative recurrence in patients with lung tumors
(24,25). However, the underlying mechanisms
are yet to be elucidated. A possible hypothesis is that COPD alters
the immunological status of patients, leading to systemic disease
including cachexia, cardiovascular disease, osteoporosis and
diabetes, and such comorbidities may lead to poor patient prognosis
(26). Furthermore, previous
studies have showed that COPD status was associated with high
HIF-1α expression, which was a critical factor responsible for
maintaining oxygen homeostasis in hypoxic conditions and a
potential marker for the prognosis of various types of cancer
(27,28). The present study demonstrated that
smoking history and COPD diagnosis have a significant contribution
to the OS and PFS of patients with ccRCC.
The heterodimer transcription factor HIF-1 was first
detected in 1991 as a regulator of renal erythropoietin production
(29). HIF-1α is a subunit of HIF,
which is expressed at significantly higher levels in cancer
anabolism. In hypoxic conditions, HIF-1α has a central function in
cellular and systemic oxygen homeostasis, inducing the
transcription of >60 proteins (30). The biological functions of HIF-1α
are regulated by post-translational modifications, including
phosphorylation, ubiquitination, hydroxylation and acetylation. In
normoxic conditions, HIF-1α is degraded through the
ubiquitin-proteasome pathway, due to the hydroxylation of proline
and lysine residues in the oxygen-dependent degradation domain.
When HIF-1α becomes stable and active, it is essential for the
regulation of tumor-associated gene expression (30). Dimerization of HIF-1α and HIF-1β in
hypoxic conditions results in the formation of the HIF-1
transcription factor, which translocates to the nucleus to bind to
hypoxia response elements and activate the transcription of
oxygen-dependent genes (31).
In hypoxic conditions, the rapid proliferation of
cancer cells and tumor vasculature abnormalities lead to an
imbalance between oxygen delivery and consumption. Activation of
HIF-1α is essential in regulating the adaptive responses of tumor
cells. HIF-1α aids in tumor survival and progression by shifting
the metabolism towards glycolysis, inducing angiogenesis,
regulating apoptosis and inducing tumor migration (32).
The importance of HIF-1α in ccRCC remains
controversial, although numerous studies have demonstrated that
HIF-1α promotes tumorigenesis, metastasis, and chemotherapy and
radiotherapy resistance in ccRCC (33,34).
HIF-1α may be associated with the functioning of the Von
Hippel-Lindau (VHL) gene in ccRCC, and VHL function, in turn, is
closely associated with ccRCC incidence. In normoxic conditions,
HIF-1α and −2α are degraded by the VHL-associated protein, a member
of the E3 ubiquitin ligase complex (33). VHL-associated proteolysis of HIF-1α
and 2α is disrupted in patients with an inhibited VHL gene or
congenital defect, resulting in elevated HIF-1α and 2α expression,
contributing to the progression and poor prognosis of ccRCC.
Razorenova et al (35)
demonstrated that HIF-1α expression was associated with ccRCC
tumorigenesis and proliferation, whereas Gudas et al
(36) demonstrated the function of
HIF-1α as a tumor suppressor in ccRCC. Therefore, the involvement
of HIF-1α in ccRCC is complex and the precise function of HIF-1α in
ccRCC is not completely understood (37). In the present study, elevated
HIF-1α expression in ccRCC was associated with higher pathological
stage or histological grade, in addition to a reduction in OS and
PFS.
ccRCC is the most common type of RCC. Diagnosed
patients may have hypoxic tumor conditions, anemia and/or COPD, all
contributing to a poor prognosis. Elevated HIF-1α levels indicate a
more advanced cancer stage and reduced survival in ccRCC patients.
Thus, HIF-1α may be a potential ccRCC biomarker, and hypoxic
conditions may be corrected in order to prevent and treat
ccRCC.
Acknowledgements
The present study was supported by the grant from
Science and Technology Agency of Hunan Province (no. 2016JJ3178)
and Finance Department of Hunan Province (2016–129). The funders
had no role in the study design, data collection and analysis,
decision to publish, or preparation of the manuscript.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thakur A and Jain SK: Kidney cancer:
Current progress in treatment. World J Oncol. 2:158–165.
2011.PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu WS, Liu YD, Fu Q, Zhang WJ, Xu L,
Chang Y and Xu JJ: Prognostic significance of ubiquinol cytochrome
c reductase hinge protein expression in patients with clear cell
renal cell carcinoma. Am J Cancer Res. 6:797–805. 2016.PubMed/NCBI
|
|
5
|
Lu X and Kang Y: Hypoxia and
hypoxia-inducible factors: Master regulators of metastasis. Clin
Cancer Res. 16:5928–5935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nallamshetty S, Chan SY and Loscalzo J.
Hypoxia: A master regulator of microRNA biogenesis and activity.
Free Radic Biol Med. 64:20–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang Z, Xu R, Lv C, Zhong Z, Zhang L, Zhu
L, Tang Y and Zhao X: A chronic obstructive pulmonary disease
negatively influences the prognosis of patients with bladder
urothelial carcinoma via hypoxia inducible factor-1α. Int J Clin
Exp Med. 7:3344–3353. 2014.PubMed/NCBI
|
|
8
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ke QD and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Powis G and Kirkpatrick L: Hypoxia
inducible factor-1alpha as a cancer drug target. Mol Cancer Ther.
3:647–654. 2004.PubMed/NCBI
|
|
11
|
Kong CC and Dai AG: Expression of
mitogen-actived protein kinase, phosphatidylinositol 3-kinase and
hypoxia-inducible factor-1alpha in pulmonary arteries of patients
with chronic obstructive pulmonary disease. Zhonghua Jie He He Hu
Xi Za Zhi. 29:372–375. 2006.(In Chinese). PubMed/NCBI
|
|
12
|
Spaliviero M, Poon BY, Aras O, Di Paolo
PL, Guglielmetti GB, Coleman CZ, Karlo CA, Bernstein ML, Sjoberg
DD, Russo P, et al: Interobserver variability of R.E.N.A.L., PADUA,
and centrality index nephrometry score systems. World J Urol.
33:853–858. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bellmunt J, Puente J, Garcia de Muro J,
Lainez N, Rodríguez C and Duran I; Spanish Society for Medical
Oncology, : SEOM clinical guidelines for the treatment of renal
cell carcinoma. Clin Transl Oncol. 16:1043–1050. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang C, Li X, Hao H, Yu W, He Z and Zhou
L: The correlation between size of renal cell carcinoma and its
histopathological characteristics: A single center study of 1867
renal cell carcinoma cases. BJU Int. 110:E481–E485. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
16
|
Harris AL: Hypoxia-a key regulatory factor
in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spivak JL: Cancer-related anemia: Its
causes and characteristics. Semin Oncol. 21 2 Suppl 3:S3–S8.
1994.
|
|
18
|
Dunne JR, Gannon CJ, Osborn TM, Taylor MD,
Malone DL and Napolitano LM: Preoperative anemia in colon cancer:
Assessment of risk factors. Am Surg. 68:582–587. 2002.PubMed/NCBI
|
|
19
|
Richards T, Musallam KM, Nassif J,
Ghazeeri G, Seoud M, Gurusamy KS and Jamali FR: Impact of
preoperative anaemia and blood transfusion on postoperative
outcomes in gynaecological surgery. PLoS One. 10:e01308612015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fowler AJ, Ahmad T, Phull MK, Allard S,
Gillies MA and Pearse RM: Meta-analysis of the association between
preoperative anaemia and mortality after surgery. Br J Surg.
102:1314–1324. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vestbo J, Hurd SS, Agustí AG, Jones PW,
Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ,
Nishimura M, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 187:347–365. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bettoncelli G, Blasi F, Brusasco V,
Centanni S, Corrado A, De Benedetto F, De Michele F, Di Maria GU,
Donner CF, Falcone F, et al: The clinical and integrated management
of COPD. Sarcoidosis Vasc Diffuse Lung Dis. 31 Suppl 1:S3–S21.
2014.
|
|
23
|
Ishida M, Mikami S, Shinojima T, Kosaka T,
Mizuno R, Kikuchi E, Miyajima A, Okada Y and Oya M: Activation of
aryl hydrocarbon receptor promotes invasion of clear cell renal
cell carcinoma and is associated with poor prognosis and cigarette
smoke. Int J Cancer. 137:299–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiang G, Liang C, Xiao F, Yu Q, Wen H,
Song Z, Tian Y, Shi B, Guo Y and Liu D: Impact of chronic
obstructive pulmonary disease on postoperative recurrence in
patients with resected non-small-cell lung cancer. Int J Chron
Obstruct Pulmon Dis. 11:43–49. 2015.PubMed/NCBI
|
|
25
|
Yoshida Y, Kage H, Murakawa T, Sato Y, Ota
S, Fukayama M and Nakajima J: Worse prognosis for stage IA lung
cancer patients with smoking history and more severe chronic
obstructive pulmonary disease. Ann Thorac Cardiovasc Surg.
21:194–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sin DD, Anthonisen NR, Soriano JB and
Agusti AG: Mortality in COPD: Role of comorbidities. Eur Respir J.
28:1245–1257. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schultz K, Fanburg BL and Beasley D:
Hypoxia and hypoxia-inducible factor-1alpha promote growth
factor-induced proliferation of human vascular smooth muscle cells.
Am J Physiol Heart Circ Physiol. 290:H2528–H2534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei WT, Li B, Chen M, Jia HR and Zhang HX:
Associations between HIF-1α polymorphisms C1772T and G1790A and
susceptibility to chronic obstructive pulmonary disease. Genet Mol
Res. 14:17341–17347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Biol. 12:5447–5454. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1) alpha: Its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zimna A and Kurpisz M: Hypoxia-inducible
factor-1 in physiological and pathophysiological angiogenesis:
Applications and therapies. Biomed Res Int. 2015:5494122015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ajduković J: HIF-1-α big chapter in the
cancer tale. Exp Oncol. 38:9–12. 2016.PubMed/NCBI
|
|
33
|
Gudas LJ, Fu L, Minton DR, Mongan NP and
Nanus DM: The role of HIF1α in renal cell carcinoma tumorigenesis.
J Mol Med (Berl). 92:825–836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rankin EB and Giaccia AJ: Hypoxic control
of metastasis. Science. 352:175–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Razorenova OV, Castellini L, Colavitti R,
Edgington LE, Nicolau M, Huang X, Bedogni B, Mills EM, Bogyo M and
Giaccia AJ: The apoptosis repressor with a CARD domain (ARC) gene
is a direct hypoxia-inducible factor 1 target gene and promotes
survival and proliferation of VHL-deficient renal cancer cells. Mol
Cell Biol. 34:739–751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gudas LJ, Fu L, Minton DR, Mongan NP and
Nanus DM: The role of HIF1α in renal cell carcinoma tumorigenesis.
J Mol Med (Berl). 92:825–836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nanus DM and Gudas LJ: The tale of two
hypoxia-inducible factors in renal cell carcinoma. Eur Urol.
69:658–659. 2016. View Article : Google Scholar : PubMed/NCBI
|