Introduction
Stroke is currently the world's second greatest
contributor to death rate and morbidity, among which 75% patients
suffered from ischemic stroke, suggesting that ischemic stroke is
type of stroke most likely to severely endanger people's health and
safety (1–4). In China, around 2 million new cases
of stroke were reported every year, and approximately 1.5 million
people died of cerebrovascular disease, which places a heavy burden
on both families and society (5).
It has become a hot topic in recent years to find effective
protective mechanism after cerebral ischemia to reduce neuronal
death, to improve neurological function, and to delay the
progression of the disease (6–8).
Inflammatory response is considered the main
pathophysiological mechanism underlying cerebral ischemia (9). Cerebral ischemia produces endogenous
damage associated molecular patterns (DAMPs), activating the
corresponding receptors leading to ischemic brain damage (10). High-mobility group box 1 (HMGB1) is
an important endogenous DAMPs that significantly elevates early in
the early stage of cerebral ischemia, promoting the expression of
inflammatory factor (TNF-α, IL-1, IL-6), which causes pathological
inflammatory response (11–14).
Currently, the mechanism by which HMGB1 overexpression is activated
after cerebral ischemia has not been sufficiently studied (15,16).
The JAK2/STAT3 signaling pathway is involved in a variety of
inflammatory and anti-inflammatory signaling pathways and multiple
physiological and pathological regulation processes (17). Research showed that the JAK2/STAT3
signaling pathway can be activated after cerebral ischemia,
mediating the post-ischemic inflammatory response (18,19).
Studies of peripheral macrophages showed that activation of the
JAK2/STAT3 signaling pathway activation can induce increased
expression of HMGB1, which promotes the release of cytokines such
as TNF-α, thus inducing the inflammatory reaction (20). However, whether or not the
activation of JAK2/STAT3 signaling pathway is associated with an
increase in HMGB1 expression during cerebral ischemia, so inducing
the inflammatory reaction, have not yet been reported.
In the present study, a rat model of middle cerebral
artery occlusion (MCAO) was established and confirmed with
detection of changes in the expression of p-JAK2, p-STAT3, HMGB1,
and inflammatory factor using ELISA and western blot analysis. The
effects of JAK2/STAT3 inhibitor and curcumin on the expression of
p-JAK2, p-STAT3, HMGB1 and inflammatory factors after cerebral
ischemia were observed to assess the mechanism underlying the
inflammatory reaction as mediated by the JAK2/STAT3 signaling
pathway after cerebral ischemia and the efficacy of clinical
intervention.
Materials and methods
Experimental animals and reagents
Male Sprague-Dawley rats (Shanghai Laboratory Animal
Center, China) aged 7–8 weeks and weighing 250–300 g were used in
the experiments. The rats were housed under standard laboratory
conditions at a temperature of 20–22°C and 12 h light-dark cycle
and given access to food and water. All procedures performed in
studies involving animals were in accordance with the ethical
standards of the institution or practice at which the studies were
conducted. The study protocol was approved by the local Ethics
Committee of the 117th Hospital of PLA. Rapamycin, AG490, and
curcumin were purchased from Sigma-Aldrich (St. Louis, MO, USA). A
TNF-α ELISA Kit, IL-1β ELISA Kit, and IL-6 ELISA Kit were purchased
from Beyotime Biotechnology (Haimen, China). Antibodies against
HMGB1, JAK2, p-JAK2, STAT3, p-STAT3, and the internal standard
GAPDH (all from Affinity Biosciences, Cincinnati, OH, USA) were
used for western blot analysis and immunohistochemical (IHC)
analysis. The secondary antibody was a ready-to-use goat-anti
rabbit (or goat-anti-mouse, or donkey-anti-goat) HRP-IgG dilution
purchased from Beyotime Biotechnology (Haimen, China).
Preparation of focal cerebral ischemia
model in rats
Rats were fasted overnight before the operation with
free access to drinking water. The rats were anesthetized with
intraperitoneal injection of anesthetic and fixed in supine
position. The hair in the median position of the neck was removed
and disinfected, and the skin was covered with dressing. The middle
part of the neck was incised to expose the right common carotid
artery, internal carotid artery, and extracranial branch
(pterygopalatine artery), and the external carotid artery and its
branches (occipital artery and its superior thyroid artery). The
occipital artery, superior thyroid artery, and pterygopalatine
artery were clipped and the occipital artery and superior thyroid
artery were sliced off. The distal segment of the external carotid
artery was ligated, and with a slipknot at the proximal end. A
small hemostatic clamp was used to clamp down the common carotid
artery and the internal carotid artery. This was followed by a
slight incision with micro scissor on the distal end of external
carotid artery (proximal end of ligature). A single-head vein
indwelling needle sealed up with heparin was inserted along the
small cut all the way to the bifurcation of the common carotid
artery. The stylet was removed and the micro hemostatic clamp was
removed from the internal carotid artery. After completely drilling
the blood and gas, the prepared embolus was slowly injected into
the internal carotid artery (preparation of embolus: Collect blood
samples and centrifuged at 3,000 rpm for 10 min, followed by
addition of CaCl2 and thrombin. The mixture was injected
into an anesthesia catheter and the tube was placed in a 37°C water
bath for 15 min. Then, the gel was sliced into 0.8–1.5 mm-long
segments. Then, 4–6 segments were selected and placed in 1 ml PBS
solution). After complete injection of the embolus, the micro
hemostatic clamp was removed from the internal carotid artery and
the blood flow was restored for 1 min. Then, the venous indwelling
needle was withdrawn, the external carotid artery was ligated, and
the neck skin was stitched. The mice in the sham group were given
the same treatment, except no embolus was injected. Twenty-four
hours after surgery, the rat was incised in the chest under
anesthesia to expose the thoracic cavity. The right atrial
appendage was cut open and perfused with physiological saline from
the left ventricle until clear fluid flowed out. Then, the ischemic
tissue of the right cerebrum was collected to prepare tissue
homogenate or paraffin sections with 4°C physiological saline in
the proportion of 1:10 by weight.
ELISA
The rat brain tissue homogenate was prepared and
centrifuged at 12,000 × g and 4°C for 15 min. The supernatant was
collected. Standard control and tissue homogenate was added
separately into the well of coated plate. The plate was covered
with plate sealing film and incubated at 37°C for 30 min. Then, the
plate was washed 5 times, followed by addition of 50 µl of ELISA
reagents. Repeat the incubation and washing steps, then add
coloring agent 50 µl, coloring agent B 50 µl and finally the
termination solution 50 µl to stop the reaction. The absorbance was
measured in a microplate reader and a histogram was drawn.
Western blotting
Every 20 mg of rat brain tissue homogenate was mixed
with 200 µl lysate. The mixture was treated with homogenizer until
complete lysis. Then, the sample was centrifuged at 4°C, 12,000 g
for 15 min. The supernatant was collected to measure the protein
concentration with BCA kits (Bio-Rad, Hercules, CA, USA). The
denatured protein sample was mixed into the sample buffer and added
to the sample lanes. After 70 min electrophoresis, the protein was
transferred to PVDF membrane (Millipore, Bedford, MA, USA) and
blocked with 1X TBST solution containing 5% skim milk for 1 h. This
was followed by overnight incubation at 4°C with primary antibody,
the membranes were washed 4 times with 1X TBST for 10 min each
time. Then, the membrane was incubated with secondary antibody at
room temperature for 1 h, then washed 4 times with 1X TBST for 10
min each. Finally the fluorescence was developed, enhanced and
fixed with enhanced chemiluminescence ECL kit (Pierce, Rockford,
IL, USA).
Immunohistochemistry
Paraffin sections were dewaxed and hydrated and
placed in EDTA-containing antigen repair buffer (pH 9.0). Antigen
retrieval was performed in a microwave oven. The slices were placed
in 3% hydrogen peroxide solution to block endogenous peroxidase and
blocked with 5% BSA solution at room temperature for 30 min. Then,
the slices were incubated with primary antibody at 4°C overnight,
followed by incubation with secondary antibody for 50 min at room
temperature. Then, the slices were washed with PBS for 15 min.
Finally, DAB color developing solution was added dropwise. The
development time was controlled by observing the slides under a
microscope. Positive staining was indicated with brown spots. The
development was terminated by washing with tap water. The nuclei
were stained with Harris hematoxylin, and each slice was dehydrated
and sealed.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean (SEM) of at least three independent experiments. Standard
error bars were included for all data points. Statistical analysis
was performed using Student's t-test when only two groups were
present or a one-way analysis of variance followed by the
Student-Newman-Keuls test when more than two groups were compared.
Statistical analyses were conducted with SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered significant.
Results
Changes of TNF-α expression in rat
brain after cerebral ischemia
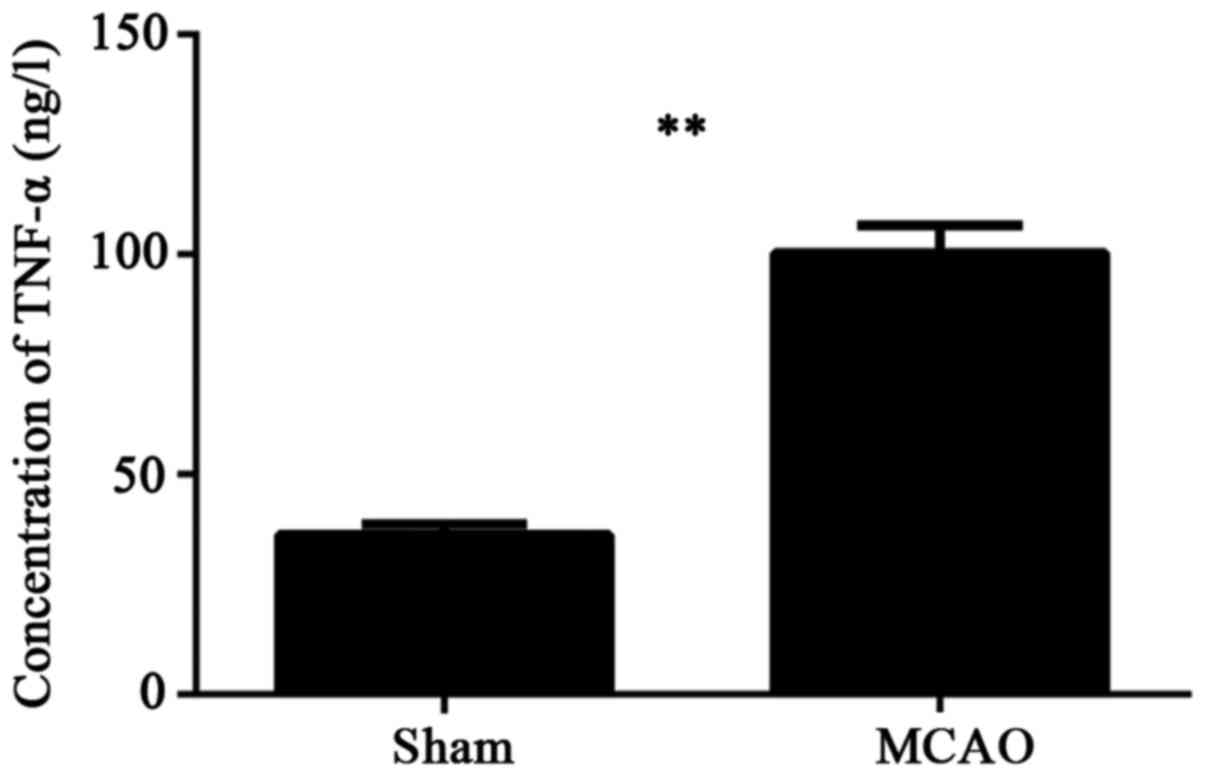
Rats were allocated into two groups, a sham group
and a MCAO group, with 5 rats in each group. ELISA was performed to
detect the TNF-α change in cerebral tissue in each group. As
indicated in Fig. 1. The amount of
TNF-α in the brain tissue homogenate of MCAO group was
significantly higher than in the sham group (P<0.01).
Changes in HMGB1, JAK2, p-JAK2, STAT3,
and p-STAT3 expression in brain tissue of rats after cerebral
ischemia
Rats were allocated into two groups, a sham group
and MCAO group, with 3 rats in each group. The expression of HMGB1,
JAK2, p-JAK2, STAT3, and p-STAT3 were assessed by western blot
analysis in the brain tissue of the rats in both groups. As
indicated in Fig. 2, the
concentration of HMGB1 protein in MCAO group was significantly
higher than in the sham group (P<0.01). The relative levels of
p-JAK2/JAK2 and p-STAT3/STAT3 in brain tissue homogenate of MCAO
group were significantly higher than in the sham group (P<0.01),
suggesting that cerebral ischemia activates the JAK2/STAT3
signaling pathway and promotes HMGB1 overexpression. There remains
some question regarding whether the JAK2/STAT3 signaling pathway is
involved in HMGB1 induction and its induced inflammatory
response.
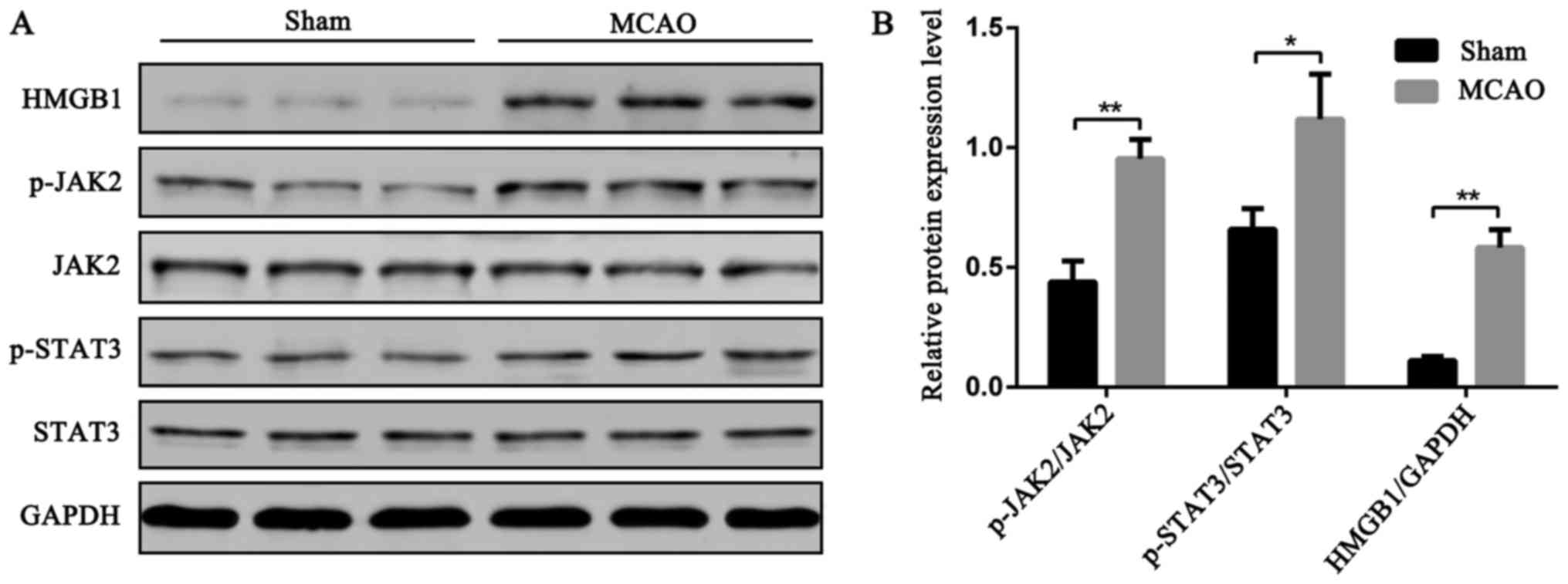 | Figure 2.Changes in HMGB1, JAK2, p-JAK2, STAT3,
and p-STAT3 expression in brain tissue of rats after cerebral
ischemia. Sham group and MCAO group, 3 rats in each group. The
brain tissues were harvested 24 h after operation and change of
HMGB1, JAK2, p-JAK2, STAT3, and p-STAT3 contents in the cerebral
tissue homogenate was measured using western blotting. (A) Protein
bands of cerebral tissue homogenate; (B) Relative expression of the
target protein in the homogenate of brain tissue was calculated
using the ImageJ image analysis software (Bethesda, MD, USA)
(**P<0.01, *P<0.05). |
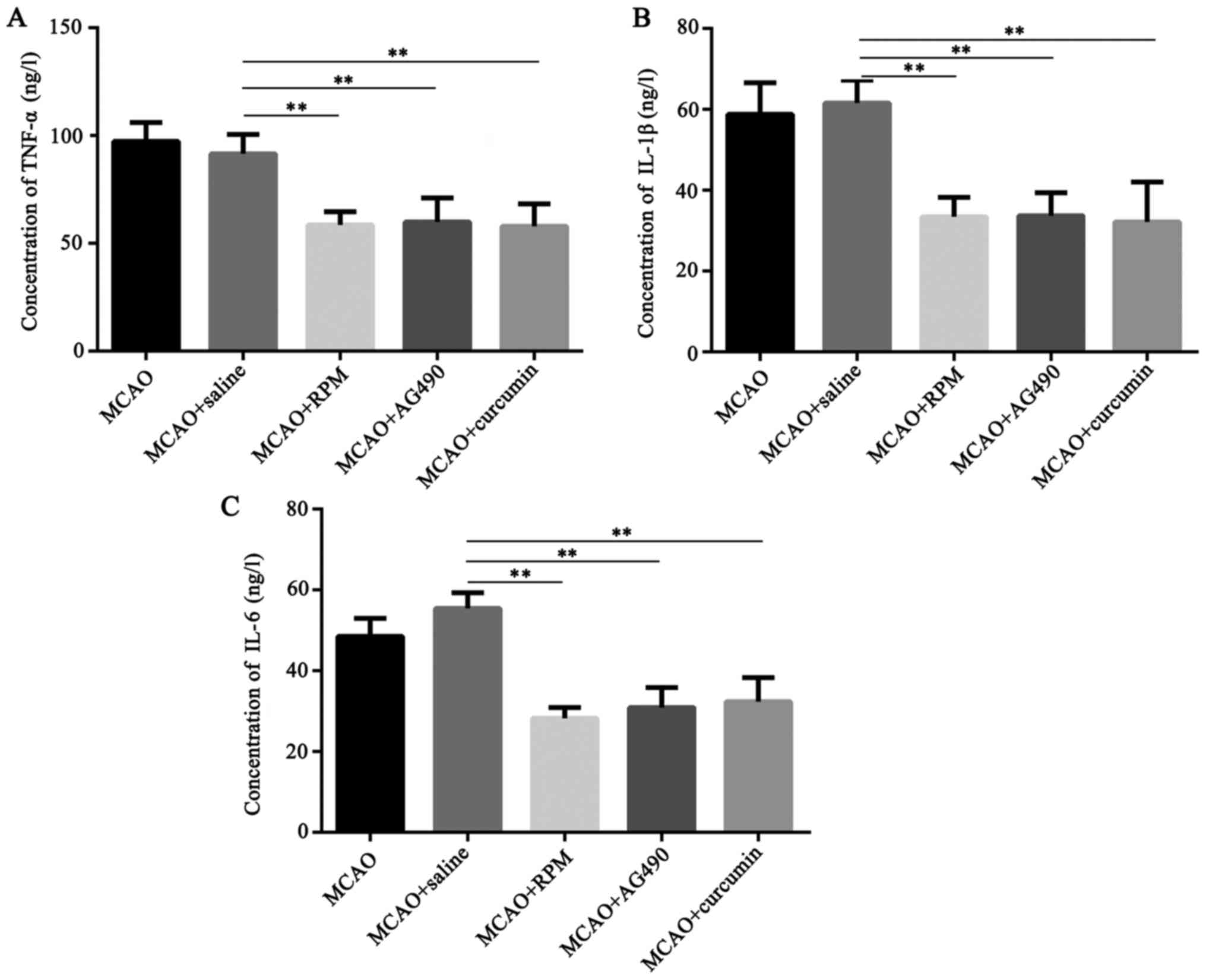
Effects of rapamycin, AG490, and
curcumin on expression of TNF-α, IL-1β, and IL-6 in brain tissue of
rats with cerebral ischemia
Rats were allocated into 5 groups of 3 rats each: a
cerebral ischemia group (MCAO), inhibitor-treated group (MCAO +
saline), STAT3 inhibitor-treated group (MCAO + RPM), JAK2
inhibitor-treated group (MCAO + AG490), and curcumin-treated group
(MCAO + curcumin). The expression of TNF-α, IL-1β, and IL-6 in
brain tissue of four groups were detected by ELISA. As indicated in
Fig. 3, the concentrations of
TNF-α, IL-1β, and IL-6 in cerebral tissue homogenate of MCAO group
and MCAO + saline group were comparable (P>0.05). Compared with
MCAO + saline group, the contents of TNF-α, IL-1β, IL-6 in MCAO+RPM
group, MCAO + AG490 group, and MCAO + curcumin group were
significantly lower than in the MCAO + saline group (P<0.01),
suggesting that inhibition of JAK2/STAT3 signaling pathway such as
curcumin reduces the release of inflammatory factors after cerebral
ischemia.
Effects of rapamycin, AG490, and curcumin on the
expression of HMGB1, JAK2, p-JAK2, STAT3, and p-STAT3 in brain
tissue of rats with cerebral ischemia. The expression levels of
HMGB1, JAK2, p-JAK2, STAT3, and p-STAT3 were detected by Western
Blot and immunohistochemistry. As indicated in Fig. 4, the expression of HMGB1, JAK2,
p-JAK2, STAT3, and p-STAT3 in the brain tissue homogenate of the
MCAO + saline group was comparable to that of the MCAO group
(P>0.05). The concentrations of p-JAK2/JAK2 and p-STAT3/STAT3 in
MCAO + RPM group, MCAO + AG490 group, and MCAO + curcumin group
were significantly lower than in the MCAO + saline group
(P<0.01), suggesting that inhibition of JAK2/STAT3 signaling
pathway such as curcumin can reduce the expression of HMGB1 in
ischemic brain tissue.
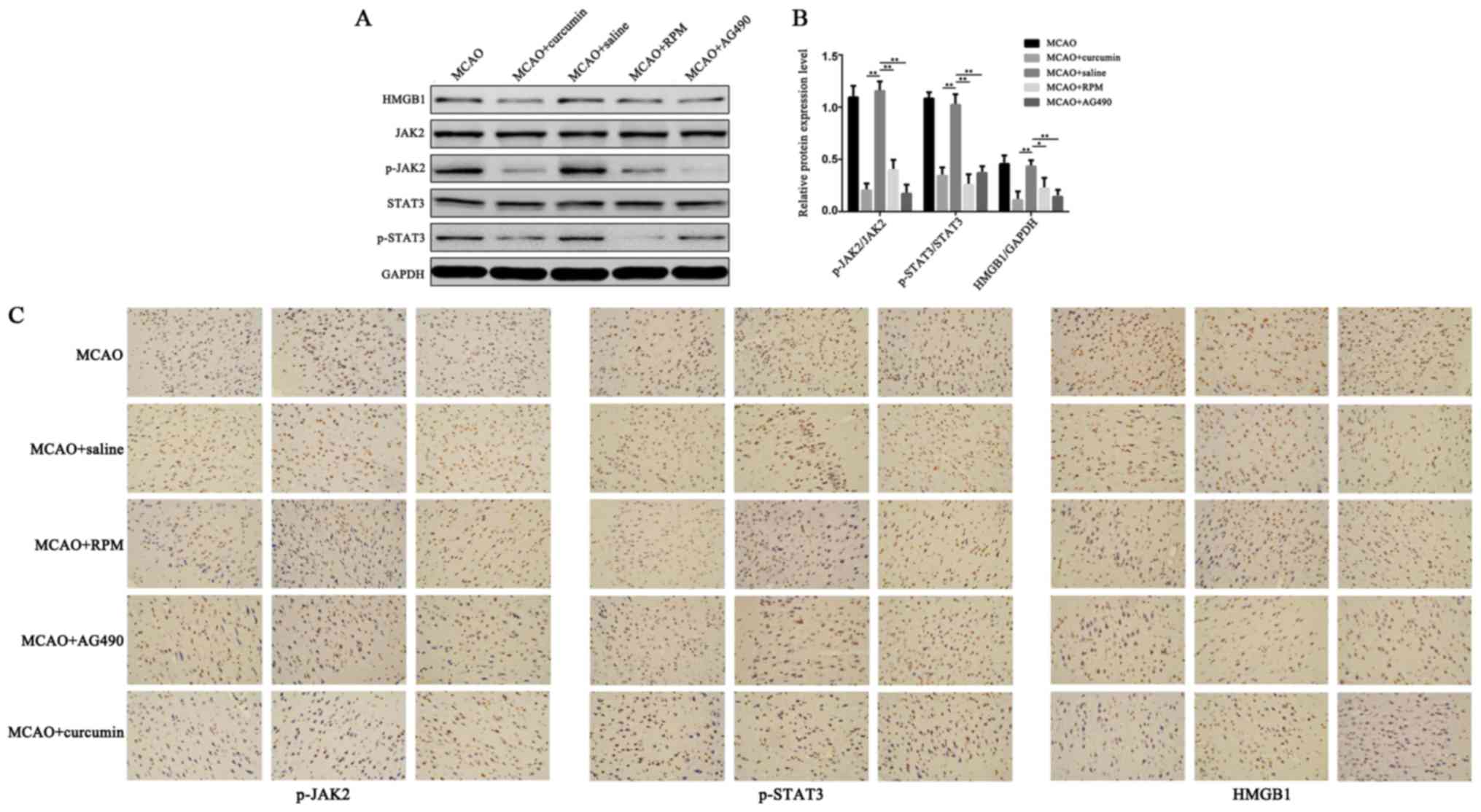 | Figure 4.Effects of rapamycin and AG490 on the
expression of HMGB1, JAK2, p-JAK2, STAT3, and p-STAT3 in brain
tissue of rats with cerebral ischemia. Expression of HMGB1, JAK2,
p-JAK2, STAT3, and p-STAT3 in the rat brain were detected by (A, B)
Western blot analysis and (C) immunohistochemistry (magnification,
×200) (**P<0.01, *P<0.05). |
Discussion
The pathogenesis of ischemic stroke is very complex.
Evidence has shown that inflammation is the most important
pathophysiology of cerebral ischemia (21,22).
In case of cerebral ischemia, the generation of mediators of
inflammation, breakdown of blood-brain barrier, inflammatory cell
activation and infiltrate (23,24)
can provoke and exacerbate the inflammatory response and lead to
brain damage after a series of complex pathological and
physiological reactions. HMGB1 plays an important role in the
inflammatory cascade after cerebral ischemia and promotes the
expression of inflammatory factors (TNF-α, IL-1, IL-6), leading to
nerve injury and dysfunction (11–14).
This study also showed that, in rats with cerebral ischemia, the
brain can show high expression of HMGB1 and various inflammatory
factors. HMGB1 is essentially a nuclear protein. It is combined
with DNA and stored in the nucleus, and it affects the structure of
the chromosome to regulate transcription, repair and recombination,
and other functions (25). In
normal brain tissue, most of the brain cells do not express or
express only low levels of HMGB1, only under pathological
conditions (ischemia, trauma, etc.) that HMGB1 expression increased
by transferring cytoplasm and out to the extracellular area. HMGB1
generates important pro-inflammatory mediators by binding to
receptors on the membrane (26).
Previous studies have shown that HMGB1 can be activated and
significantly increased during the early stage of cerebral ischemia
and induce leukocyte infiltration and glial cell activation after
ischemia, leading to pathological inflammatory response; while
activated inflammatory cells can continue to secrete HMGB1
aggravate inflammatory injury (27). HMGB1 also activates astrocytes in
the cerebral ischemic region to secrete MMP-9 to disrupt brain
tissue and blood-brain barrier and aggravate the inflammatory
response (28). HMGB1 shRNA
injection into the striatum can reduce the expression of
inflammatory cells in brain tissue, reducing neuronal death.
Injection of HMGB1 into the brain tissue of normal mice can induce
the expression of inflammatory factors in the tissues (29). The use of anti-HMGB1 neutralizing
antibody could significantly reduce the inflammatory reaction and
ischemic brain injury (30). In
this way, the development of HMGB1 as the target of neuroprotective
agents for the clinical treatment of cerebral ischemic injury may
provide a new means of intervention.
The mechanism by which HMGB1 becomes activated in
patients after ischemic stroke is still not clearly described.
JAKs/STATs are important intracellular signal transduction pathways
mediated by cytokines, oxidative stress, etc. The JAK protein
family includes JAK1, JAK2, JAK3, and TYK2. The STAT protein family
includes STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6
(31–34), among which JAK2/STAT3 is closely
related to cerebral ischemia and it can be activated during an
early stage of cerebral infraction, thus inducing enhanced
expression of pro-inflammatory factors (18,19,35–37).
Liu et al reported in a study of peripheral macrophages that
activation of the JAK2/STAT3 pathway induced higher expression of
HMGB1, HMGB1 further promoting the release of cytokines such as
TNF-α (20). Zhang et al
found that rapamycin inhibited JAK2/STAT3 signaling pathway to
reduce the expression of HMGB1 after acute liver injury, thereby
reducing inflammation caused by liver damage (38). Li et al in a study of the
intestine, found that activation of JAK2/STAT3 signaling pathway
could induce inflammatory reaction, which could be reduced by
rapamycin and AG490. However, HMGB1 was not evaluated in the
present study (39). In the
present research, we found that cerebral expression of HMGB1 and
TNF-α would be significantly increased after cerebral ischemia,
thus inhibiting the JAK2/STAT3 pathway. Meanwhile, the expression
of HMGB1 and inflammatory factors in brain tissue was significantly
decreased. We here concluded that inhibition of the JAK2/STAT3
signaling pathway can reduce the expression of HMGB1, thereby
significantly reducing the inflammatory response after cerebral
ischemia.
Curcumin is a naturally occurring yellow acidic
phenol widely found in the rhizoma of turmeric plants such as
curcuma longa and curcuma zedoary (40), and has become a research focus
given its effects including anti-inflammatory, anti-oxidation,
anti-tumor, anti-virus, anti-atherosclerosis and lipid-lowering
effects and retarding brain degeneration (41). JAK2/STAT3 signaling pathway is
activated after cerebral ischemia, leading to increased expression
of HMGB1 and aggravating the postischemic inflammatory responses.
In tumor cells and microglia, curcumin has been found to inhibit
the activation of JAK2/STAT3 signaling pathway (42,43).
Therefore this study also involved curcumin intervention in rats
with cerebral ischemia, noting that curcumin could inhibit
JAK2/STAT3 signaling pathway and reduce expression of HMGB1 and
inflammatory factors. The above findings suggest that curcumin has
a protective effect on cerebral ischemic injury, potentially by the
mechanism of inhibiting JAK2/STAT3 signaling pathway to reduce
HMGB1 expression and alleviate the inflammatory responses.
In conclusion, the JAK2/STAT3 signaling inhibitors
such as curcumin have protective effects on cerebral ischemic
injury, potentially by the mechanism of reducing HMGB1 expression,
thus reducing expression of the inflammatory factors and
alleviating the inflammatory responses.
Acknowledgements
This study was supported by Science and Technology
Project of Zhejiang Province (no. 2015C33179) and Medical
Scientific Research Fund Project of Nanjing Military Region (no.
14MS147).
References
|
1
|
Balami JS, Chen RL and Buchan AM: Stroke
syndromes and clinical management. QJM. 106:607–615. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Norrving B and Kissela B: The global
burden of stroke and need for a continuum of care. Neurology. 80 3
Suppl 2:S5–S12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meschia JF and Brott T: Ischemic stroke.
Eur J Neurol. 2017.(Epub ahead of print). PubMed/NCBI
|
|
4
|
Zevallos J, Santiago F, Gonzalez J,
Rodriguez A, Pericchi L, Rodriguez-Mercado R and Nobo U: Burden of
stroke in Puerto Rico. Int J Stroke. 10:117–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu M, Wu B, Wang WZ, Lee LM, Zhang SH and
Kong LZ: Stroke in China: Epidemiology, prevention and management
strategies. Lancet Neural. 6:456–458. 2007. View Article : Google Scholar
|
|
6
|
Lo EH, Dalkara T and Moskowitz MA:
Mechanisms, challenges and opportunities in stroke. Nat Rev
Neurosci. 4:399–415. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler
RA, Lawton MT and Yang GY: Vascular remodeling after ischemic
stroke: mechanisms and therapeutic potentials. Prog Neurobiol.
115:138–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hachinski V, Donnan GA, Gorelick PB, Hacke
W, Cramer SC, Kaste M, Fisher M, Brainin M, Buchan AM, Lo EH, et
al: Stroke: Working toward a prioritized world agenda. Stroke.
41:1084–1099. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dirnagl U, Iadecola C and Moskowitz MA:
Pathobiology of ischaemic stroke: An integrated view. Trends
Neurosci. 22:391–397. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khoshnam SE, Winlow W, Farzaneh M, Farbood
Y and Moghaddam HF: Pathogenic mechanisms following ischemic
stroke. Neurol Sci. 38:1167–1186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu M, Zhou GM, Wang LH, Zhu L, Liu JM,
Wang XD, Li HT and Chen L: Inhibiting high-mobility group box 1
(HMGB1) attenuates inflammatory cytokine expression and
neurological deficit in ischemic brain injury following cardiac
arrest in rats. Inflammation. 39:1594–1602. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang C, Jiang J, Zhang X, Song L, Sun K
and Xu R: Inhibiting HMGB1 reduces cerebral ischemia reperfusion
injury in diabetic mice. Inflammation. 39:1862–1870. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ooboshi H and Shichita T: DAMPs
(damage-associated molecular patterns) and inflammation. Nihon
Rinsho. 74:573–578. 2016.(In Japanese). PubMed/NCBI
|
|
14
|
Singh V, Roth S, Veltkamp R and Liesz A:
HMGB1 as a key mediator of immune mechanisms in ischemic stroke.
Antioxid Redox Signal. 24:635–651. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murphy J: Pharmacological treatment of
acute ischemic stroke. Crit Care Nurs Q. 26:276–282. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun K, Fan J and Han J: Ameliorating
effects of traditional Chinese medicine preparation, Chinese
materia medica and active compounds on ischemia/reperfusion-induced
cerebral microcirculatory disturbances and neuron damage. Acta
Pharm Sin B. 5:8–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rawlings JS, Rosler KM and Harrison DA:
The JAK/STAT signaling pathway. J Cell Sci. 117:1281–1283. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Satriotomo I, Bowen KK and Vemuganti R:
JAK2 and STAT3 activation contributes to neuronal damage following
transient focal cerebral ischemia. J Neurochem. 98:1353–1368. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie HF, Xu RX, Wei JP, Jiang XD and Liu
ZH: P-JAK2 and P-STAT3 protein expression and cell apoptosis
following focal cerebral ischemia-reperfusion injury in rats. Nan
Fang Yi Ke Da Xue Xue Bao. 27:208–211. 2007.(In Chinese).
PubMed/NCBI
|
|
20
|
Liu H, Yao YM, Yu Y, Dong N, Yin HN and
Sheng ZY: Role of Janus kinase/signal transducer and activator of
transcription pathway in regulation of expression and
inflammation-promoting activity of high mobility group box protein
1 in rat peritoneal macrophages. Shock. 27:55–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vidale S, Consoli A, Arnaboldi M and
Consoli D: Postischemic inflammation in acute stroke. J Clin
Neurol. 13:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tuttolomondo A, Pecoraro R, Casuccio A, Di
Raimondo D, Buttà C, Clemente G, Della Corte V, Guggino G, Arnao V,
Maida C, et al: Peripheral frequency of CD4+ CD28-cells
in acute ischemic stroke: Relationship with stroke subtype and
severity markers. Medicine. 94:e8132015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tuttolomondo A, Pedone C, Pinto A, Di
Raimondo D, Fernandez P, Di Sciacca R and Licata G; Gruppo Italiano
di Farmacoepidemiologia dell'Anziano (GIFA) researchers, :
Predictors of outcome in acute ischemic cerebrovascular syndromes:
The GIFA study. Int J Cardiol. 125:391–396. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ueda T and Yoshida M: HMGB proteins and
transcriptional regulation. Biochim Biophys Acta. 1799:114–118.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harris HE, Andersson U and Pisetsky DS:
HMGB1: A multifunctional alarm in driving autoimmune and
inflammatory disease. Nat Rev Rheumatol. 8:195–202. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Muhammad S, Barakat W, Stoyanov S,
Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth
PP, Bierhaus A and Schwaninger M: The HMGB1 receptor RAGE mediates
ischemic brain damage. J Neurosci. 28:12023–12031. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Svedin P, Hagberg H, Sävman K, Zhu C and
Mallard C: Matrix metalloproteimse-gene knock-out protects
theimrnatare train alter cerebral hypoxia-ischema. J Neurosci.
27:1511–1518. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS,
Kim SW, Lee MH, Han PL, Park JS and Lee JK: HMGB1, a novel
cytokine-like mediator linking acute neuronal death and delayed
neuroinflammation in the postischemic brain. J Neurosci.
26:6413–6421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu K, Mori S, Takahashi HK, Tomono Y,
Wake H, Kanke T, Sato Y, Hiraga N, Adachi N, Yoshino T and
Nishibori M: Anti-high mobility group box 1 monoclonal antibody
ameliorates brain infarction induced by transient ischemia in rats.
FASEB J. 21:3904–3916. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Babon JJ, Lucet IS, Murphy JM, Nicola NA
and Varghese LN: The molecular regulation of Janus kinase (JAK)
activation. Biochem J. 462:1–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chai HT, Yip HK, Sun CK, Hsu SY and Leu S:
AG490 suppresses EPO-mediated activation of JAK2-STAT but enhances
blood flow recovery in rats with critical limb ischemia. J Inflamm.
13:182016. View Article : Google Scholar
|
|
33
|
Tao Z, Cheng M, Wang SC, Lv W, Hu HQ, Li
CF and Cao BZ: JAK2/STAT3 pathway mediating inflammatory responses
in heatstroke-induced rats. Int J Clin Exp Pathol. 8:6732–6739.
2015.PubMed/NCBI
|
|
34
|
Ghoreschi K, Laurence A and O'Shea JJ:
Janus kinases in immune cell signaling. Immunol Rev. 228:273–287.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du W, Hong J, Wang YC, Zhang YJ, Wang P,
Su WY, Lin YW, Lu R, Zou WP, Xiong H and Fang JY: Inhibition of
JAK2/STAT3 signalling induces colorectal cancer cell apoptosis via
mitochondrial pathway. J Cell Mol Med. 16:1878–1888. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang HH, Kuang S, Wang Y, Sun XX, Gu Y,
Hu LH and Yu Q: Bigelovin inhibits STAT3 signaling by inactivating
JAK2 and induces apoptosis in human cancer cells. Acta Pharmacol
Sin. 36:507–516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Planas AM, Gorina R and Chamorro A:
Signalling pathways mediating inflammatory responses in brain
ischemia. Biochem Soc Trans. 34:1267–1270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, Zhao ZF, Han DW, Wang F, Xu RL
and Liu MS: Rapamycin-induced inhibition of Janus Kinase/signal
transducer and activator of transcription pathway affects
expression of high-mobility group box 1 in rats with acute liver
injury. World Chin J Dig. 14:1916–1920. 2006. View Article : Google Scholar
|
|
39
|
Li Y, Li KH, Wen SH, Li C, Li YS, Liu Y,
Zhang XY, Yao X and Liu KX: Role of JAK/STAT in intestinal injury
induced by intestinal ischemia/reperfusion in rats. Chin J
Pathophysiol. 27:2338–2344. 2011.
|
|
40
|
Sharma RA, Gescher AJ and Steward WP:
Curcumin: The story so far. Eur J Cancer. 41:1955–1968. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Esatbeyoglu T, Huebbe P, Ernst IM, Chin D,
Wagner AE and Rimbach G: Curcumin-from molecule to biological
function. Angew Chem Int Ed Engl. 51:5308–5332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Weissenberger J, Priester M, Bernreuther
C, Rakel S, Glatzel M, Seifert V and Kögel D: Dietary curcumin
attenuates glioma growth in a syngeneic mouse model by inhibition
of the JAK1,2/STAT3 signaling pathway. Clin Cancer Res.
16:5781–5795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saydmohammed M, Joseph D and Syed V:
Curcumin suppresses constitutive activation of STAT-3 by
up-regulating protein inhibitor of activated STAT-3 (PIAS-3) in
ovarian and endometrial cancer cells. J Cell Biochem. 110:447–456.
2010.PubMed/NCBI
|