Introduction
Cataracts represent about 42% of all causes of
blindness (1) and continue to be
the principal cause of blindness worldwide (2). Age-related cataracts are the most
common form in adults, and are associated with visual and cognitive
impairment as well as depression (3). Decreased visual function resulting
from cataracts may also be responsible for a high odds ratio of
nursing home placement (4) and a
higher risk of mortality (5).
There is a great deal of evidence suggesting that opacity of the
lens in cataracts is a direct result of oxidative stress (6); however, the exact molecular mechanism
of cataractogenesis remains unclear.
MicroRNAs (miRNAs) are small non-coding RNAs
consisting of 19–24 nucleotides, which post-transcriptionally
regulate the expression of target genes (7). Previous studies have shown that
miRNAs are essential for the development of the eye and ocular
homeostasis (8), and miRNA
dysregulation may play an important role in ocular diseases
including cataracts (9,10). In addition, a number of miRNAs have
been reported as potential diagnostic biomarkers or therapeutic
targets for cataracts (11,12).
MicroRNA-24 (miR-24) has diverse functions in cell
proliferation (13–15), and regulates key aspects of
age-related macular degeneration pathology (16,17).
The biological function of miR-24 in the progression of cataract
development is still unclear. In the present study, we found for
the first time that miR-24 is more highly expressed in age-related
cataracts, and enhances lens epithelial cell apoptosis by directly
targeting p53. The miR-24-p53 pathway may play a critical role in
cataractogenesis.
Materials and methods
Specimens
The present study was approved by the Ethical
Committee of the Fourth Affiliated Hospital of China Medical
University. Written informed consent was obtained from each
patient. Age-related cataract patients without other ocular
diseases undergoing cataract surgery (phacoemulsifcation) were
enrolled at the Fourth Affiliated Hospital of China Medical
University (Shenyang, China). Normal eyes were obtained from the
Eye Bank of the Fourth Affiliated Hospital of China Medical
University. Fresh anterior lens capsules isolated from age-related
cataract patients and normal eyes were immediately frozen in liquid
nitrogen at the time of surgery and stored at −80°C.
Cell culture
Human lens epithelial cell line cells (SRA01/04
cells, a kind gift of Dr. Yi-sin Liu, Doheny Eye Institute, Los
Angeles, CA, USA) were maintained in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml
streptomycin (Thermo Fisher Scientific, Inc.) in a humidifed
incubator at 37°C with 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells using
the Trizol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. For RT-qPCR analysis
of miR-24, the total RNA isolated from cells was subsequently
reverse transcribed to cDNA using a TaqMan MicroRNA Reverse
Transcription Kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The expression of miR-24 was determined using TaqMan
MicroRNA assays (Applied Biosystems; Thermo Fisher Scientific,
Inc.), and standardized to RNU6B expression. The upstream and
downstream primers of miR-24 and RNU6B were purchased from Thermo
Fisher Scientific, Inc., and their sequences can be found on their
website. For RT-qPCR analysis of p53, cDNAs were obtained using the
PrimerScript RT reagent kit (Takara Bio Inc., Tokyo, Japan),
RT-qPCR analysis of p53 was performed with TaqMan Universal Master
Mix II (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Primers for p53 were as follows: Forward
5′-CAGCAGTCAAGCACTGCCAAG-3′, reverse 5′-AGACAGGCATGGCACGGATAA-3′,
and β-actin was used for normalization, β-actin primer sequences
were: Forward: 5′-CATCCGTAAAGACCTCTATGCCAAC-3′, Reverse:
5′-ATGGAGCCACCGATCCACA-3′. The RT-qPCR analysis was performed on
ABI 7500 (Applied Biosystems; Thermo Fisher Scientific, Inc.). All
experiments were performed in triplicate. The relative expression
levels of mRNA or microRNA were calculated using the
2−ΔΔCt method.
Western blot analysis
Total protein was extracted using a RIPA lysis
buffer supplemented with a protease inhibitor cocktail (Pierce;
Thermo Fisher Scientific, Inc.). Equal amounts (40 µg) of proteins
were separated using NuPAGE 4–12% Bis-Tris Protein gels
(Invitrogen; Thermo Fisher Scientific, Inc.), then transferred to
PVDF membranes (EMD Millipore, Billerica, MA, USA). The membranes
were subsequently blocked with 5% fat-free milk at room temperature
for 2 h and incubated with the primary antibodies including rabbit
anti-p53 (1:1,000; Abcam, Cambridge, CA, USA) and rabbit anti-GAPDH
(1:2,000, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C
overnight, followed by incubation with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (H + L) secondary antibody
(1:2,500; Promega Corporation, Madison, WI, USA) at room
temperature for 2 h. The protein bands were visualized using the an
ECL Western Blotting Substrate kit (Pierce; Thermo Fisher
Scientific, Inc.) and quantified using Image J software (National
Institute of Health, USA).
Detection of reactive oxygen species
(ROS) level
A 2′,7′-dichloro-fluorescein diacetate (DCFH-DA)
probe was used to detect fluorescence derived from endogenous ROS
in hLECs. 1×104 cells were seeded into each well of a
96-well plate and were cultured for 16 h, until cells were observed
adhering to the sides of the well. The cells were then exposed to
different concentrations H2O2 (0, 100, 200,
400, 600, 800, 1,000 µM) for 1 h whereupon the culture medium was
aspirated and 10 uM fluorescent probe DCFH-DA was added to each
well. This mixture was then incubated in a 37°C incubator for 20
min. The cells were then washed three times with PBS and their DCF
fluorescence intensity value (i.e., the mean fluorescence intensity
of DCF, representing the level of intracellular ROS) was read using
a multifunctional microplate reader. The excitation wavelength used
was 485 nm and the emission wavelength was set at 530 nm.
Cell viability assay
Cell viability and proliferation were determined
using the CellTiter96AQueous One Solution Cell
Proliferation assay kit (Promega). The reagent contains a
tetrazolium compound
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt; MTS). After treatments, according to the manufacturer's
protocols, 20 µl MTS solution was added to each well of the 96-well
assay plate containing the cells in 100 µl of culture medium and
the cells were then incubated for 4 h at 37°C, 5% CO2.
The absorbance of each group was read using an absorbance plate
reader set to a 490 nm wavelength. The cell viability rates were
calculated according to the following formula: The cell viability
ratio (%)=[(As-Ab)/(Ac-Ab)]x100%, where As is the optical density
value at 490 nm (OD490) of H2O2 treatment
group, Ab is the OD490 of blank group, and Ac is the OD490 of
non-H2O2 control group. Each experiment was
repeated three times.
Caspase-3 activity assay
Caspase-3 activity was detected using a caspase-3
assay kit (Abcam). After treatments, in accordance with the
manufacturer instructions, these SRA01/04 cells were lysed in 50 µl
of chilled Cell Lysis buffer and incubated on ice for 10 min,
centrifuged, the supernatant protein concentration was determined
using the BCA method. 50 µl of Cell Lysis buffer containing 100 µg
protein were added to each well in a 96 well plate. Then, 50 µl 2×
Reaction buffer, 0.5 µl 10 mM DTT and 5 µl caspase-3 catalytic
substrate DEVD-p-NA substrate were added to each well. The samples
were incubated at 37°C for 2 h. The optical density (OD) value was
obtained using a microplate reader set at 405 nm wavelength. Each
experiment was repeated three times. In the caspase-3 activity
control group, OD was set to a value of 1 and the caspase-3
experimental group activity was standardized using the following
calculation: (OD value of experimental group-blank well OD)/(OD
value of control group-blank well OD) ×100%.
Transient transfection
The miR-24 mimic (miR-24), mimic negative control
(miR-Ctrl), miR-24 inhibitor (anti-miR-24), inhibitor negative
control (anti-miR-Ctrl), small interfering RNA for p53 (p53 siRNA)
and siRNA control were purchased from GenePharma, Inc., (Sunnyvale,
CA, USA). SRA01/04 cells were seeded in a 6-well plate, and
transfection was conducted after 24 h. Transfections were performed
according to the manufacturer's instructions with Lipofectamine
RNAiMAX Transfection Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). After 72 h, the cells were treated with 400 µM
H2O2 for 1 h after which the expression of
miR-24 was measured using RT-qPCR. The expression of p53 was
measured using RT-qPCR and western blotting, and the cell viability
was measured using MTS.
Luciferase reporter assay
We used human cDNA to generate wild-type and mutant
3′-UTR sequences for the p53 gene, including predicted miR-24
targeting regions. We then cloned these amplified fragments into
the pGL3-Promoter vector (Promega) at the same location. For
luciferase reporter assays, these reporters were cotransfected into
SRA01/04 cells together with miR-24 mimics and mimic controls.
Luciferase activity was then evaluated using a Dual-Luciferase
Reporter Assay System kit (Promega) at 72 h after transfection. The
Renilla luciferase plasmid was used as an endogenous control. The
experiments were performed in triplicate.
Statistical analysis
Each experiment was repeated independently at least
3 times with similar results. Measurement data were presented as
mean ± standard deviation (SD). Differences between two groups were
calculated using an independent sample t-test. Differences among
multiple groups were determined by one-way analysis of variance
followed by Dunnett's post hoc test. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was done using SPSS v16.0 (SPSS, Inc., Chicago, IL,
USA).
Results
Increased expression of miR-24 in the
anterior lens capsules of patients with age-related cataracts
One previously conducted microarray study observed
that miR-24 levels dramatically changed in human cataractous lenses
(18), but this finding has not
been verified. To explore the expression levels of miR-24 in the
lens epithelial cells (LECs) of age-related cataracts, RNAs
isolated from the 48 anterior lens capsules of age-related cataract
patients and the 32 normal anterior lens capsule specimens were
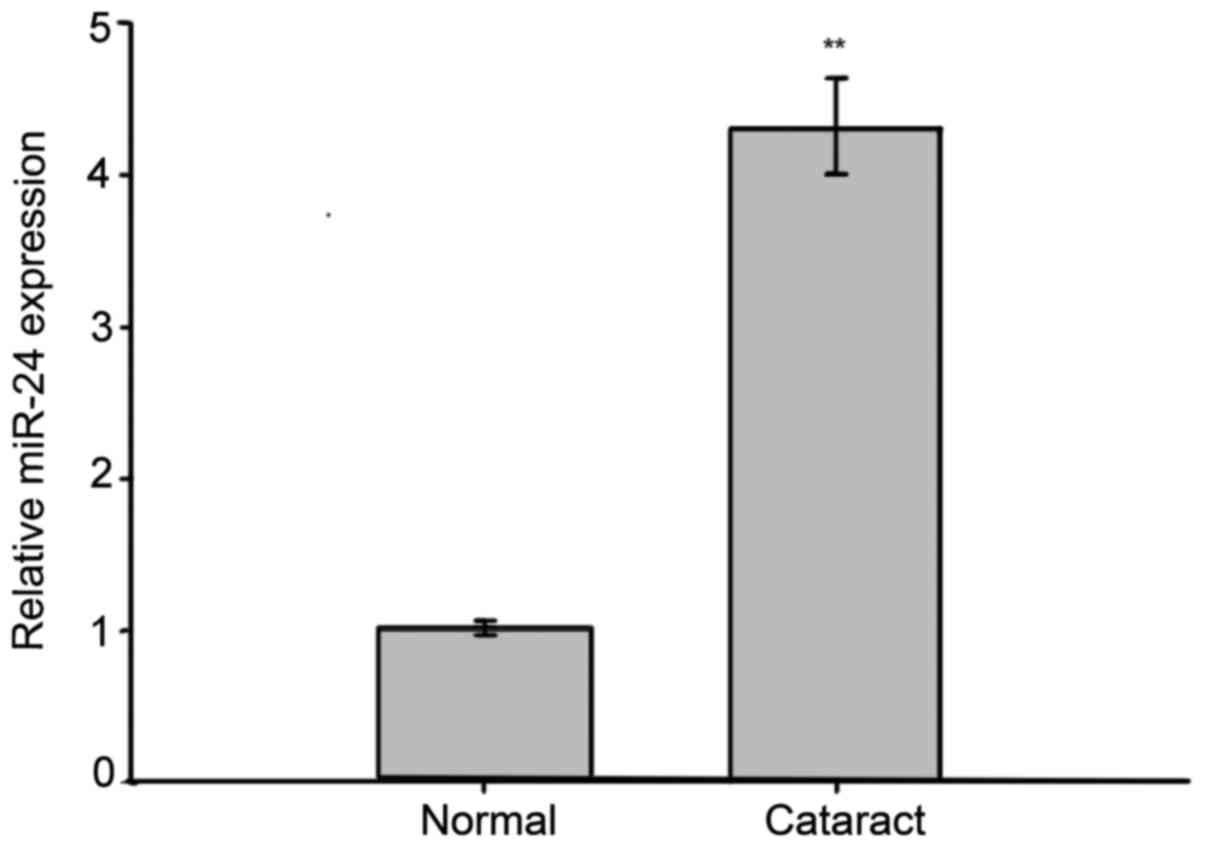
subjected to RT-qPCR analysis. The assays showed that the
expression of miR-24 was significantly increased in cataract
tissues as compared to normal tissues (Fig. 1). These results suggest that miR-24
may play a role in cataract development.
p53 is up-regulated in the anterior
lens capsules of patients with age-related cataracts
p53 has been implicated as an important protein in
cell proliferation and differentiation during embryonic lens
development (19). In the present
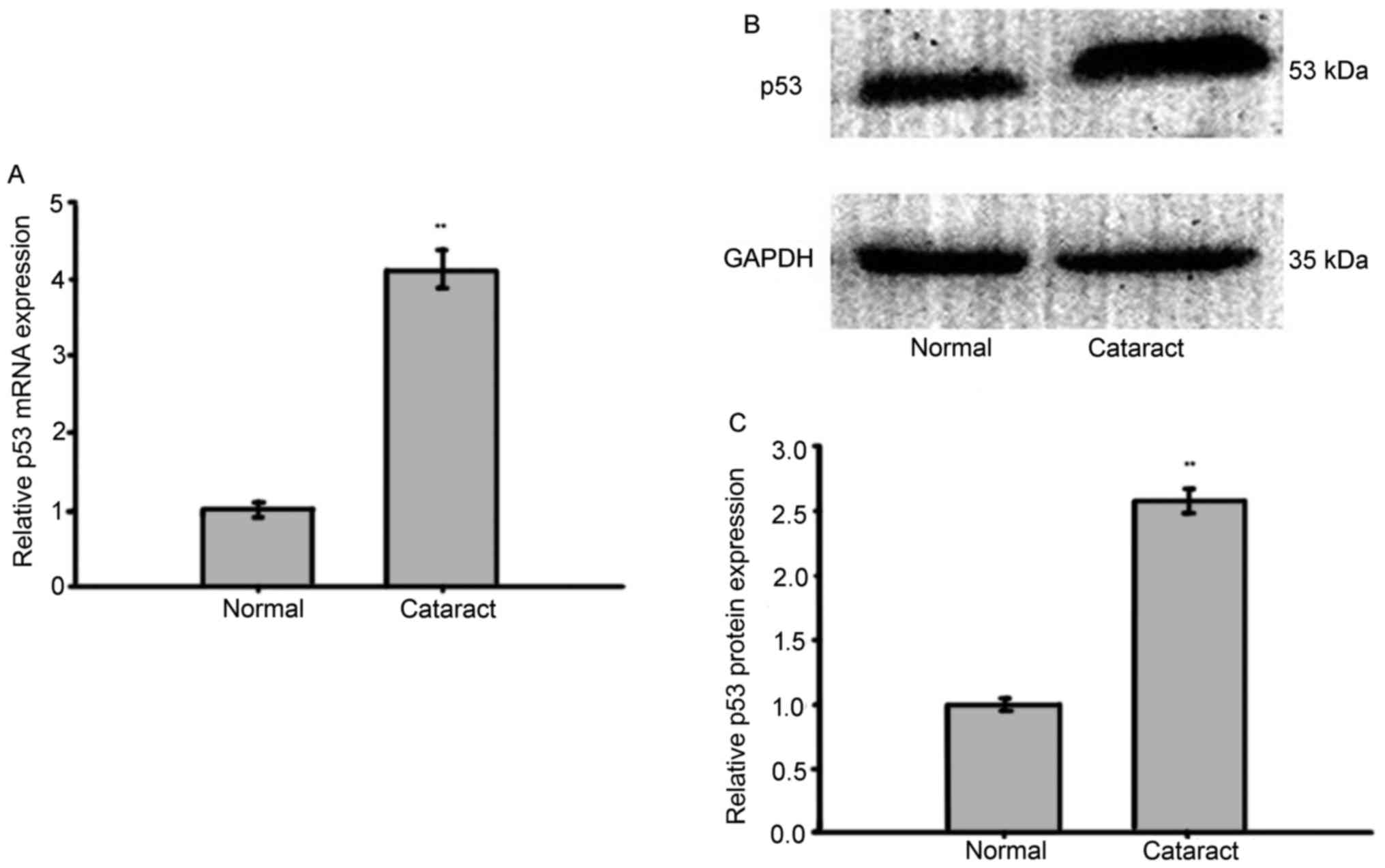
study, the expression of p53 was also examined by RT-qPCR and
western blotting in anterior lens capsules (control: n=44,
cataract: n=56). Both the expression of p53 protein and p53 mRNA
were found to be significantly up-regulated in the cataract tissues
when compared with the normal tissues (Fig. 2). This finding, combined with the
previous miR-24 experiment (Fig.
1) demonstrates a positive correlation between endogenous
miR-24 and p53 expression.
Increased levels of miR-24 and p53,
enhanced expression of ROS, and increased cell death and cell
apoptosis were detected in LECs exposed to oxidative stress induced
by H2O2
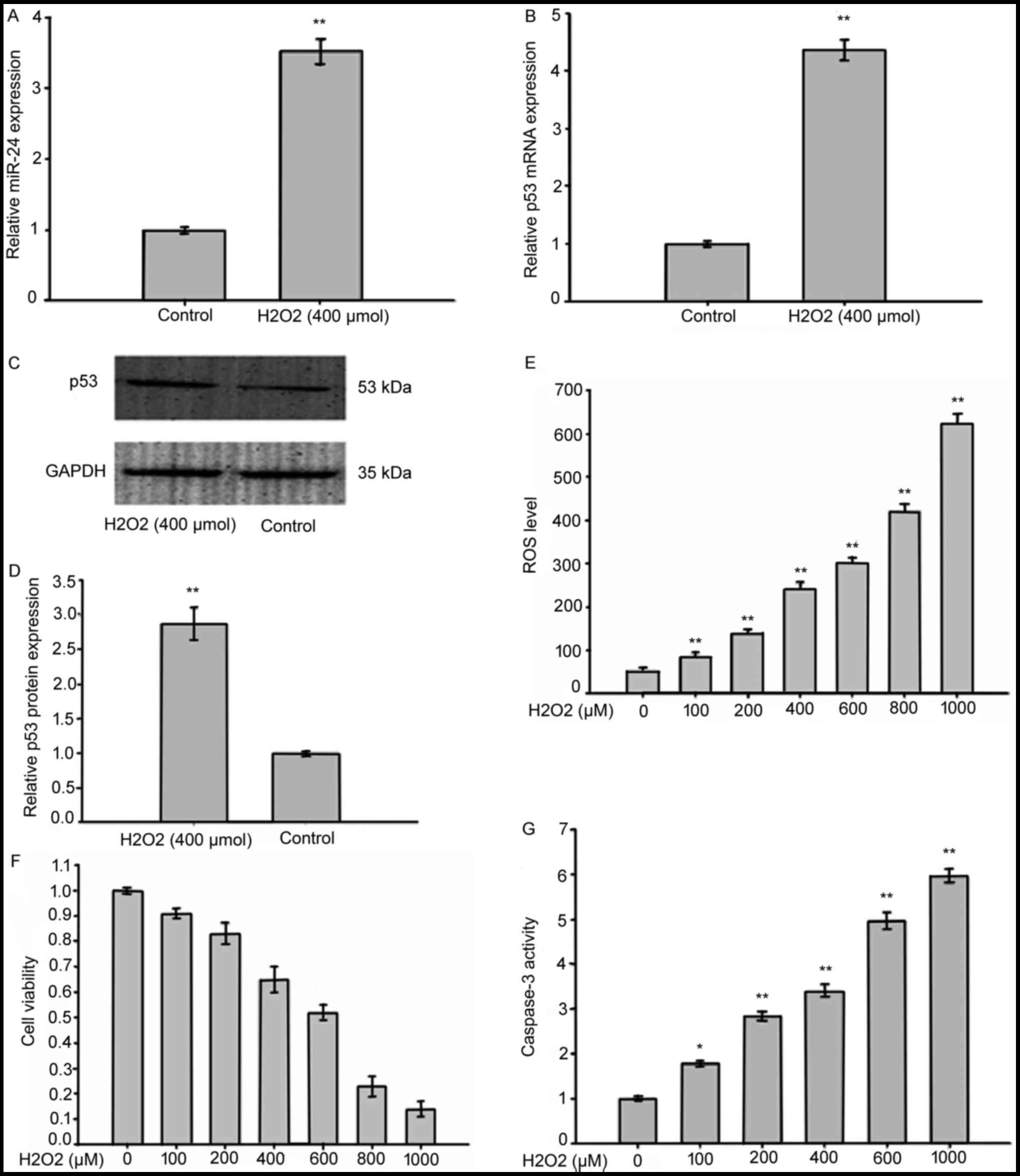
In order to establish the expression levels of
miR-24 and p53 in LECs exposed to oxidative stress, we treated
SRA01/04 cells with 400 µmol H2O2 for 1 h.
The expression of miR-24 was detected using RT-qPCR which showed a
great increase in SRA01/04 cells exposed to
H2O2 compared with controls (Fig. 3A). RT-qPCR (Fig. 3B) and western blotting analysis of
p53 (Fig. 3C and D) revealed a
significant increase of p53 in SRA01/04 cells treated with
H2O2. This increase was correlated with
higher expression of ROS (Fig.
3E), decreased cell viability (Fig. 3F) and increased caspase-3 activity
(Fig. 3G). This suggests that ROS
modulated both miR-24 and p53 in LECs.
miR-24 regulated the expression of p53
protein and mRNA in LECs exposed to oxidative stress
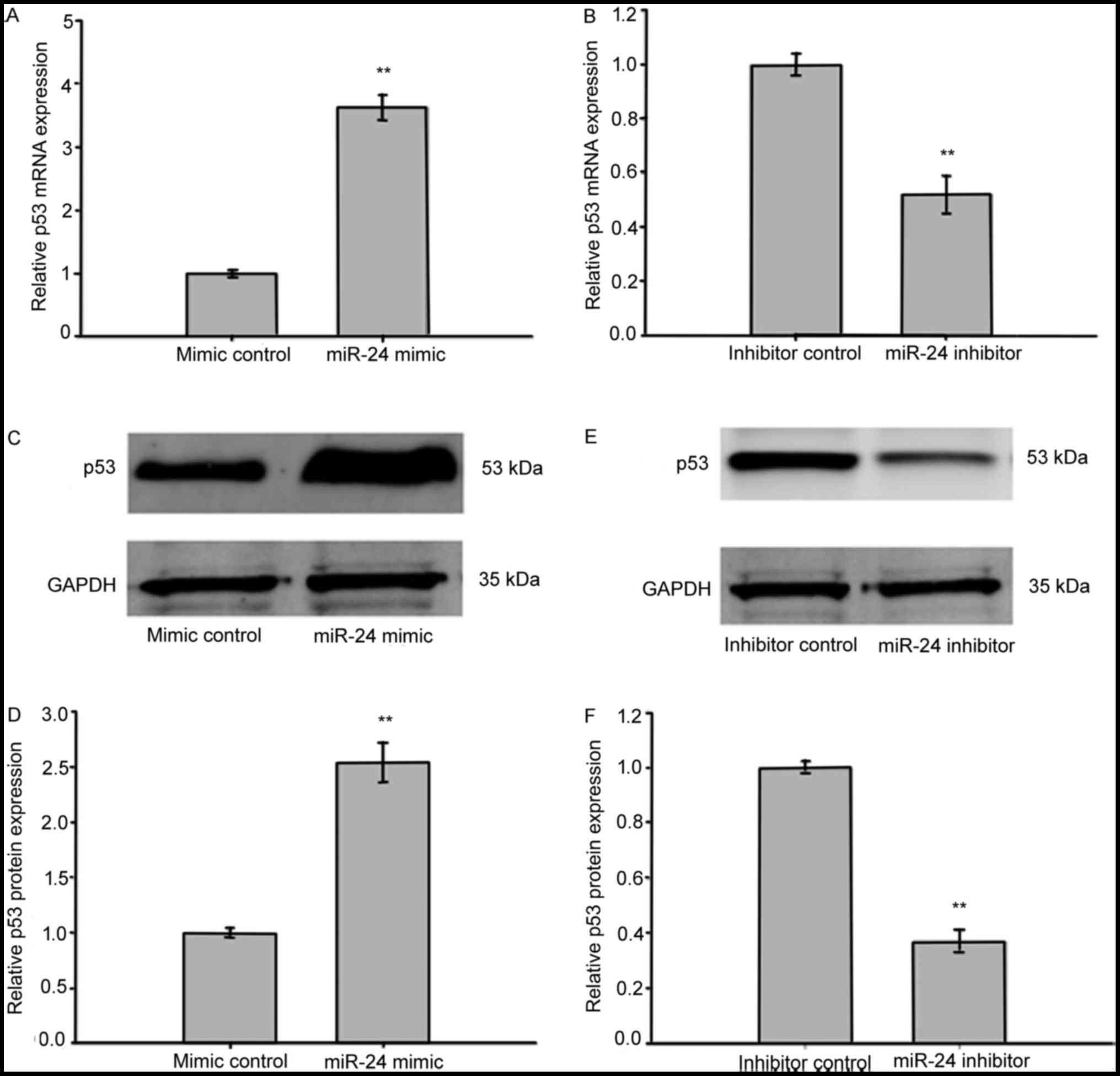
To investigate the correlation between miR-24 and
p53 expression in LECs exposed to oxidative stress, we transfected
SRA01/04 cells with either miR-24 mimics or inhibitors, then the
medium was removed and 400 µmol H2O2 was
added to induce oxidative stress. RT-qPCR was performed to assess
the level of p53-mRNA expression. As is shown in Fig. 4A and B, samples with overexpressed
miR-24 up-regulated p53 mRNA expression when compared with
controls, while inhibition of miR-24 down-regulated the expression
of p53 mRNA. Western blotting indicated that p53 protein levels
were enhanced in SRA01/04 cells transfected with miR-24 mimics and
reduced in cells exposed to miR-24 inhibitors (Fig. 4C-F). These results indicated that
the expression of p53 was regulated at both the mRNA and protein
level by miR-24 in LECs.
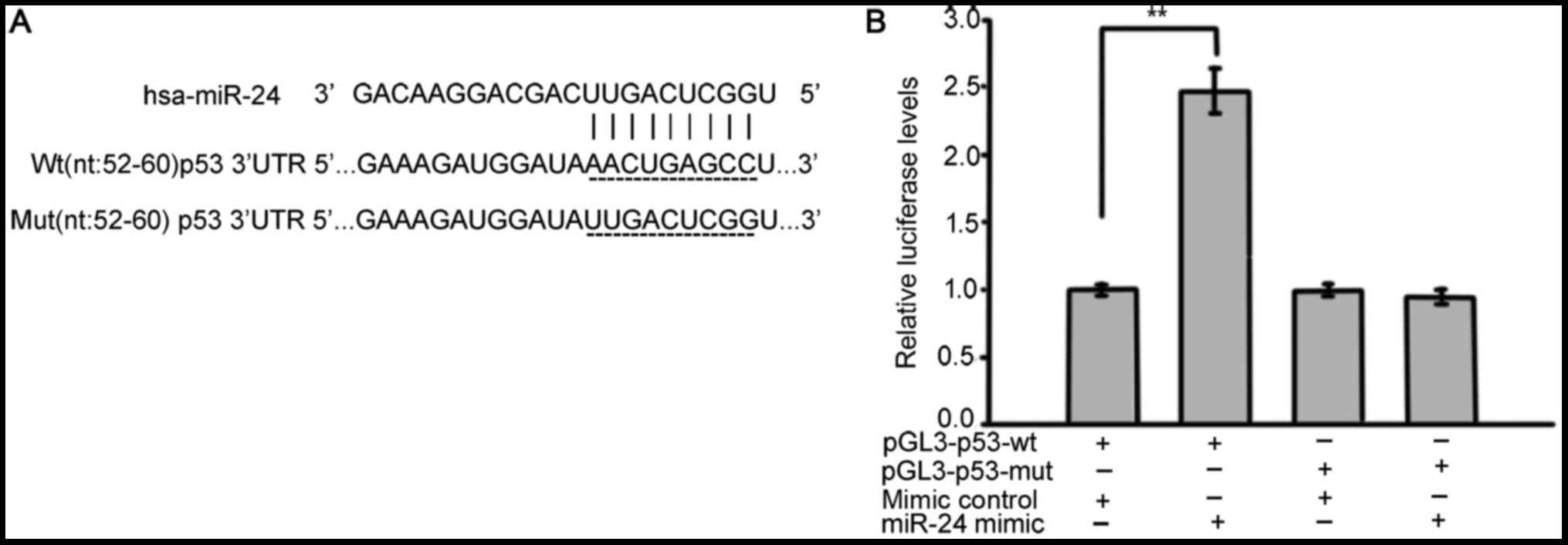
p53 was verified as a direct target of
miR-24
To more closely examine the mechanisms of miR-24 and
p53 in cataracts, we used bioinformatics with publicly available
databases (TargetScan, miRanda and miRBase) to determine whether
p53 may be the target of miR-24 (Fig.
5A). To confirm the targeting of p53 by miR-24, luciferase
activity assays were performed. SRA01/04 cells were co-transfected
the luciferase reporter construct pGL3-p53-wt or pGL3-p53-mut with
miR-24 mimics. As shown in Fig.
5B, SRA01/04 cells with pGL3-p53-wt and miR-24mimics had
significantly increased reporter activity when compared with the
controls, whereas no significant difference in reporter activity
was observed when the target site was mutated. Together, these
results indicate that 3′UTR of p53 carries a direct and functional
binding site for miR-24 in LECs.
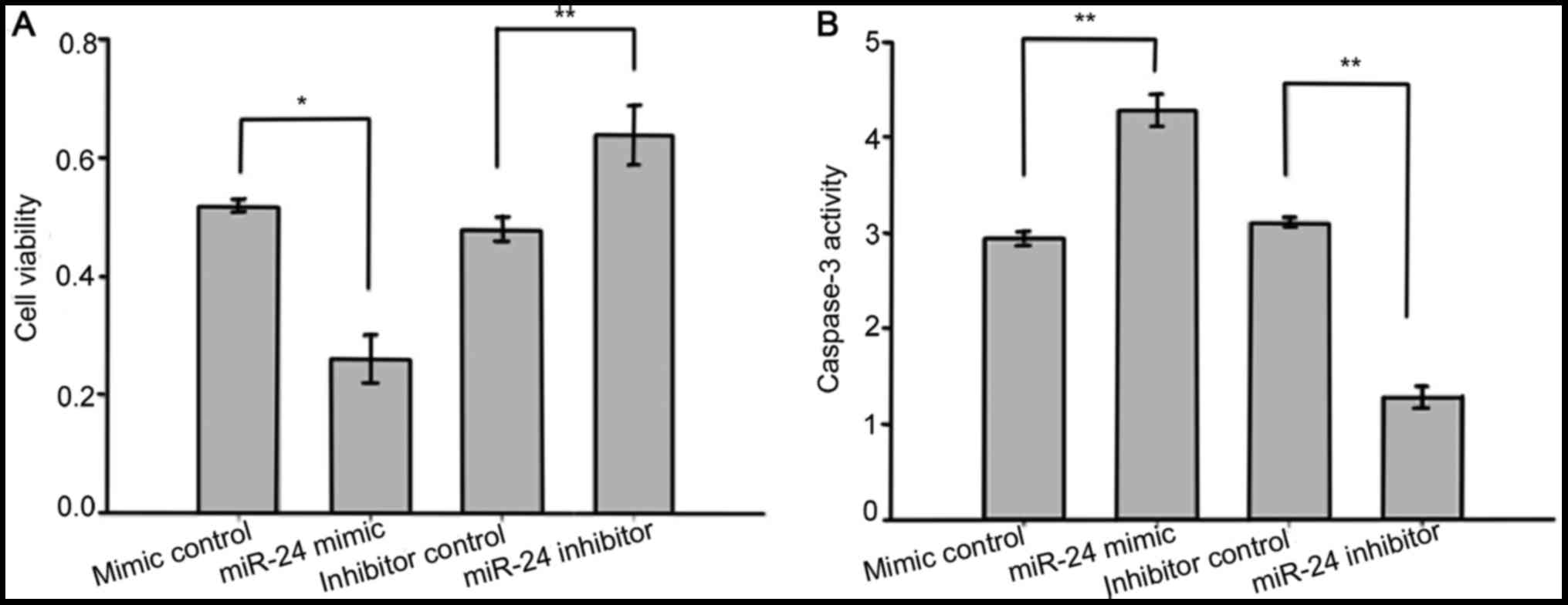
miR-24 enhanced LEC death and
apoptosis induced by oxidative stress
To identify the role of miR-24 in LEC viability and
apoptosis, we used MTS assays to measure the viability of LECs, and
caspase-3 activity was also assessed. The SRA01/04 cells were
transfected with miR-24 mimics, mimic controls, miR-24 inhibitors
and inhibitor controls before exposure to
H2O2 (400 µmol). As assayed by MTS, SRA01/04
cells transfected with miR-24 mimics displayed significantly
increased cell death compared to those transfected with mimic
controls, while transfection with miR-24 inhibitors significantly
suppressed H2O2-induced LEC death (Fig. 6A). Results of caspase-3 activity
shown that compared with the control group, the miR-24 mimic group
had significantly elevated caspase-3 activity while the caspase-3
activity of the miR-24 inhibitor group was markedly decreased
(Fig. 6B). These results suggest
that miR-24 promotes apoptosis and inhibits the proliferation of
human LECs exposed to oxidative stress.
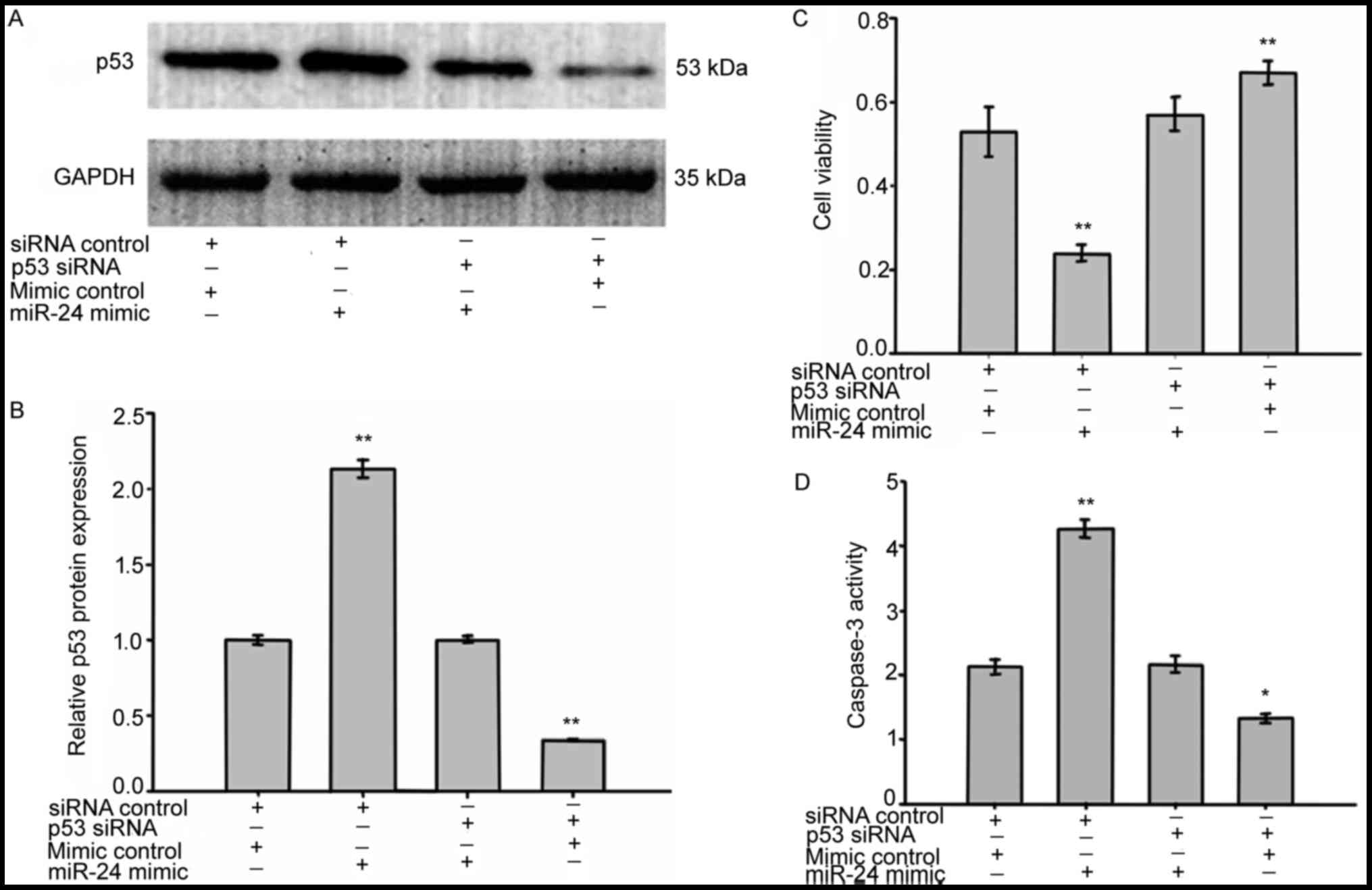
miR-24 enhanced LEC death by targeting
p53
The previous experiments clearly indicated that
miR-24 enhances LEC death. To explore whether p53 is involved in
miR-24 enhanced LEC death and apoptosis, we knocked down p53 using
p53 siRNA. Before exposure to H2O2, SRA01/04
cells were transfected with p53 siRNA in the absence or presence of
miR-24 mimics. 72 h after transfection, cell viability was assessed
by MTS assays, caspase-3 activity was assessed by caspase-3
activity assays and the protein expression of p53 was measured by
western blotting. Co-transfection of miR-24 mimics with p53 siRNA
significantly reversed the p53 protein expression induced by miR-24
mimics transfection (Fig. 7A and
B). As shown in Fig. 7C and D,
when compared with cells co-transfected with the mimic controls and
the siRNA controls, the viability and caspase-3 activity of LECs
co-transfected with miR-24 mimics and p53 siRNA showed little
change. In addition, knockdown of p53 in LECs resulted in an
increased cell survival rate and decreased caspase-3 activity.
These results indicate that miR-24 enhanced LEC death and apoptosis
by directly targeting p53.
Discussion
The major unique finding in this study is that the
oxidative stress induced upregulation of miR-24 enhances LEC
apoptosis and inhibits LEC proliferation by directly targeting p53.
Our experimental design elucidated the molecular mechanism of p53
regulation by miR-24 under oxidative stress. To the best of our
knowledge, this experiment is also the first to report that the
expression of miR-24 was significantly increased in human anterior
lens capsules affected by age-related cataracts as well as LECs
exposed to oxidative stress. Our data also showed that miR-24
expression was positively associated with p53 levels. These data
suggest that the miR-24-p53 pathway is involved in a novel
mechanism of age-related cataractogenesis.
There is a growing body of evidence that indicates
miRNAs play an important role in the development of the eye, ocular
homeostasis, and ocular diseases (20). In previously published research,
miRNA expression profiles in human lenses were identified through
the use of microarrays. The top eight miRNAs in cataractous lenses
were miR-184, miR-1826, let-7b/c, miR-24, miR-23b, miR-923, and
miR-23a (18). Our research
further determined the miR-24 levels in the anterior lens capsule
of patients with age-related cataracts, and for the first time
discovered that miR-24 expression was significantly upregulated in
lenses with age-related cataracts as compared to transparent
lenses. This fact suggests that the differential expression of
miR-24 may play a role in cataractogenesis.
It has been widely reported that the lens is
subjected to oxidative stress throughout its life and that
oxidative damage is a major cause of cataract formation (21). Current research also suggests that
with increasing age, the accumulation of oxidized lens components
and decreased capacity of repair mechanisms result in increased
levels of reactive oxidative species (22) and LEC death, promoting
cataractogenesis (21,23). In the present study, we used
H2O2 to induce LEC death and apoptosis as an
oxidative stress model.
The p53 signaling pathway plays an important role in
regulating the cell cycle and cell differentiation, promoting
apoptosis, and activating cell death (24–26).
It has been previously reported that LECs exposed to
H2O2-induced oxidative stress have increased
expression of p53 protein (27,28).
In addition, LECs with diabetic cataracts (29) and age-related cataracts (11) were also found to have higher levels
of p53 expression. Chen et al (13), found that miR-24 increases
hepatocellular carcinoma cell metastasis and invasion by targeting
p53. This study showed a positive correlation between endogenous
miR-24 and p53 expression in the anterior lens capsule of patients
with age-related cataracts. Consequently, it seems likely that
miR-24 enhances human LEC apoptosis through the activation of
p53.
By using a human lens epithelial cell line (SRA01/04
cells) as an in vitro model to study the effects of aging
and oxidative stress, we determined that the levels of both miR-24
and p53 were elevated and linked this heightened expression with
increased levels of ROS. We were then able to demonstrate that ROS
promote the miR-24-p53 pathway. The key novel observation of this
study is that miR-24 directly targeted p53 in human LECs, promoting
cell apoptosis and inhibiting cell proliferation. Taken together,
these findings indicate that the miR-24 evoked by oxidative stress
enhances LEC apoptosis and inhibits LEC proliferation by directly
targeting p53, also contributing to the development of
cataracts.
In recent years, miRNAs have emerged as one of the
most reliable diagnostic biomarkers and therapeutic targets in a
variety of diseases (29,30). miRNA-based therapeutics involve
modulating the functions of disease associated miRNAs by miRNA
antagonists or mimics (31–33).
For example, Miravirsen which is a β-D-oxy-locked nucleic
acid-modified phosphorothioate antisense oligonucleotide targeting
the liver-specific miR-122 has demonstrated broad antiviral
activity and a relatively high genetic barrier to resistance in
clinical trial study (34,35).
Although miRNA-based diagnostic tools and
therapeutics for ocular diseases are still on the horizon, there
have been several studies in recent years to suggest their
potential for clinical use. For example, Li et al (36) established miR-143 and miR-145 as
important regulators of intraocular pressure, which may have
important therapeutic implications in glaucoma. Additionally,
overexpressing miR-21, miR-31, miR-150, and miR-146a, or silencing
miR-23/27, have each been suggested as potential approaches for
treating choroidal neovascularization in wet age-related macular
degeneration (31,37–39).
Finally, miR-133b and miR-125b were shown to be downregulated in
age-related cataracts and appeared to inhibit lens epithelial cell
apoptosis (11,12).
Current investigations into the subject of our
study, miR-24, is mostly limited to cancer research. For instance,
Van Eijndhoven et al (40)
reported that purified extracellular vesicles fractions of
untreated classical Hodgkin lymphoma patients had enriched levels
of miR-24 and the concentration of miR-24 decreased during and
after therapy, suggesting miR-24 reflects the presence of vital
tumor tissue and is suitable for therapy response and relapse
monitoring in individual classical Hodgkin lymphoma patients. In
other studies, miR-24 was found to suppress cell migration,
invasion, and proliferation in breast cancer, osteosarcoma and
nasopharyngeal carcinoma (41–44),
indicating that miR-24 could be a potential target for the
diagnosis and therapy of cancer.
Ophthalmology research related to miR-24, by
contrast, has been less extensive. There is some evidence that
overexpression of miR-24 is effective in repressing choroidal
neovascularization in vivo, suggesting miR-24 may represent
an attractive therapeutic solution for wet age-related macular
degeneration (16,45). Unfortunately, data on miR-24 in
cataracts are still scarce.
In conclusion, miR-24 is up-regulated in age-related
cataracts. It appears to enhance lens epithelial cell apoptosis and
inhibit cell proliferation by directly targeting p53, suggesting
that the miR-24-p53 pathway may play a critical role in
cataractogenesis. These findings support the possibility of miR-24
as a desirable therapeutic target for age-related cataracts.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81170836,
81570838) and the Natural Science Foundation of Liaoning Province,
China (grant no. 2015020474).
References
|
1
|
Goutham G, Manikandan R, Beulaja M,
Thiagarajan R, Arulvasu C, Arumugam M, Setzer WN, Daglia M, Nabavi
SF and Nabavi SM: A focus on resveratrol and ocular problems,
especially cataract: From chemistry to medical uses and clinical
relevance. Biomed Pharmacother. 86:232–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee CM and Afshari NA: The global state of
cataract blindness. Curr Opin Ophthalmol. 28:98–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fukuoka H and Afshari NA: The impact of
age-related cataract on measures of frailty in an aging global
population. Curr Opin Ophthalmol. 28:93–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aditya BS, Sharma JC, Allen SC and
Vassallo M: Predictors of a nursing home placement from a non-acute
geriatric hospital. Clin Rehabil. 17:108–113. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khanna RC, Murthy GV, Giridhar P,
Krishnaiah S, Pant HB, Palamaner Subash Shantha G, Chakrabarti S,
Gilbert C and Rao GN: Cataract, visual impairment and long-term
mortality in a rural cohort in India: The Andhra Pradesh Eye
Disease Study. PLoS One. 8:e780022013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kubota M, Shui YB, Liu M, Bai F, Huang AJ,
Ma N, Beebe DC and Siegfried CJ: Mitochondrial oxygen metabolism in
primary human lens epithelial cells: Association with age, diabetes
and glaucoma. Free Radic Biol Med. 97:513–519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ebert MS and Sharp PA: Roles for microRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu S: microRNA expression in the eyes and
their significance in relation to functions. Prog Retin Eye Res.
28:87–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dunmire JJ, Lagouros E, Bouhenni RA, Jones
M and Edward DP: MicroRNA in aqueous humor from patients with
cataract. Exp Eye Res. 108:68–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szemraj M, Bielecka-Kowalska A, Oszajca K,
Krajewska M, Goś R, Jurowski P, Kowalski M and Szemraj J: Serum
MicroRNAs as potential biomarkers of AMD. Med Sci Monit.
21:2734–2742. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin Y, Zhao J, Min X, Wang M, Luo W, Wu D,
Yan Q, Li J, Wu X and Zhang J: MicroRNA-125b inhibits lens
epithelial cell apoptosis by targeting p53 in age-related cataract.
Biochim Biophys Acta. 1842:2439–2447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang F, Meng W and Tong B:
Down-regulation of MicroRNA-133b suppresses apoptosis of lens
epithelial cell by up-regulating BCL2L2 in age-related cataracts.
Med Sci Monit. 22:4139–4145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen L, Luo L, Chen W, Xu HX, Chen F, Chen
LZ, Zeng WT, Chen JS and Huang XH: MicroRNA-24 increases
hepatocellular carcinoma cell metastasis and invasion by targeting
p53: miR-24 targeted p53. Biomed Pharmacother. 84:1113–1118. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen W and Ou HS: Regulation of miR-24 on
vascular endothelial cell function and its role in the development
of cardiovascular disease. Sheng Li Xue Bao. 68:201–206. 2016.(In
Chinese). PubMed/NCBI
|
|
15
|
Yang J, Chen L, Ding J, Fan Z, Li S, Wu H,
Zhang J, Yang C, Wang H, Zeng P and Yang J: MicroRNA-24 inhibits
high glucose-induced vascular smooth muscle cell proliferation and
migration by targeting HMGB1. Gene. 586:268–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ertekin S, Yildirim O, Dinç E, Ayaz L,
Fidanci SB and Tamer L: Evaluation of circulating miRNAs in wet
age-related macular degeneration. Mol Vis. 20:1057–1066.
2014.PubMed/NCBI
|
|
17
|
Kutty RK, Samuel W, Jaworski C, Duncan T,
Nagineni CN, Raghavachari N, Wiggert B and Redmond TM: MicroRNA
expression in human retinal pigment epithelial (ARPE-19) cells:
Increased expression of microRNA-9 by
N-(4-hydroxyphenyl)retinamide. Mol Vis. 16:1475–1486.
2010.PubMed/NCBI
|
|
18
|
Wu C, Lin H, Wang Q, Chen W, Luo H, Chen W
and Zhang H: Discrepant expression of microRNAs in transparent and
cataractous human lenses. Invest Ophthalmol Vis Sci. 53:3906–3912.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi J and Donehower LA: p53 in embryonic
development: Maintaining a fine balance. Cell Mol Life Sci.
55:38–47. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lavker RM, Jia Yu and Ryan DG: The tiny
world of microRNAs in the cross hairs of the mammalian eye. Hum
Genomics. 3:332–348. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beebe DC, Holekamp NM and Shui YB:
Oxidative damage and the prevention of age-related cataracts.
Ophthalmic Res. 44:155–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brennan L, Khoury J and Kantorow M: Parkin
elimination of mitochondria is important for maintenance of lens
epithelial cell ROS levels and survival upon oxidative stress
exposure. Biochim Biophys Acta. 1863:21–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Acer S, Pekel G, Kucukatay V, Küçükatay V,
Karabulut A, Yağcı R, Çetin EN, Akyer ŞP and Şahin B: Oxidative
stress of crystalline lens in rat menopausal model. Arq Bras
Oftalmol. 79:222–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohamed MF, Samir N, Ali A, Ahmed N, Ali
Y, Aref S, Hossam O, Mohamed MS, Abdelmoniem AM and Abdelhamid IA:
Apoptotic induction mediated p53 mechanism and Caspase-3 activity
by novel promising cyanoacrylamide derivatives in breast carcinoma.
Bioorg Chem. 73:43–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
López-Luppo M, Catita J, Ramos D, Navarro
M, Carretero A, Mendes-Jorge L, Muñoz-Cánoves P, Rodriguez-Baeza A,
Nacher V and Ruberte J: Cellular senescence is associated with
human retinal microaneurysm formation during aging. Invest
Ophthalmol Vis Sci. 58:2832–2842. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moshrefi M, Spotin A, Kafil HS,
Mahami-Oskouei M, Baradaran B, Ahmadpour E and Mansoori B: Tumor
suppressor p53 induces apoptosis of host lymphocytes experimentally
infected by Leishmania major, by activation of Bax and caspase-3: A
possible survival mechanism for the parasite. Parasitol Res.
116:2159–2166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mok JW, Chang DJ and Joo CK: Antiapoptotic
effects of anthocyanin from the seed coat of black soybean against
oxidative damage of human lens epithelial cell induced by H2O2.
Curr Eye Res. 39:1090–1098. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng T and Lu Y: SIRT1 protects human
lens epithelial cells against oxidative stress by inhibiting
p53-dependent apoptosis. Curr Eye Res. 41:1068–1075. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Armand-Labit V and Pradines A: Circulating
cell-free microRNAs as clinical cancer biomarkers. Biomol Concepts.
8:61–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Yu T, Jiang S, Zhang Y, Li M, Tang
N, Ponnusamy M, Wang JX and Li PF: miRNAs as potential therapeutic
targets and diagnostic biomarkers for cardiovascular disease with a
particular focus on WO2010091204. Expert Opin Ther Pat.
27:1021–1029. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang S, Koster KM, He Y and Zhou Q: miRNAs
as potential therapeutic targets for age-related macular
degeneration. Future Med Chem. 4:277–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seto AG: The road toward microRNA
therapeutics. Int J Biochem Cell Biol. 42:1298–1305. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bruscella P, Bottini S, Baudesson C,
Pawlotsky JM, Feray C and Trabucchi M: Viruses and miRNAs: More
friends than foes. Front Microbiol. 8:8242017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ottosen S, Parsley TB, Yang L, Zeh K, van
Doorn LJ, van der Veer E, Raney AK, Hodges MR and Patick AK: In
vitro antiviral activity and preclinical and clinical resistance
profile of miravirsen, a novel anti-hepatitis C virus therapeutic
targeting the human factor miR-122. Antimicrob Agents Chemother.
59:599–608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gebert LF, Rebhan MA, Crivelli SE, Denzler
R, Stoffel M and Hall J: Miravirsen (SPC3649) can inhibit the
biogenesis of miR-122. Nucleic Acids Res. 42:609–621. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, Zhao F, Xin M, Li G, Luna C, Li G,
Zhou Q, He Y, Yu B, Olson E, et al: Regulation of intraocular
pressure by microRNA cluster miR-143/145. Sci Rep. 7:9152017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
SanGiovanni JP, SanGiovanni PM, Sapieha P
and De Guire V: miRNAs, single nucleotide polymorphisms (SNPs) and
age-related macular degeneration (AMD). Clin Chem Lab Med.
55:763–775. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu D, Sun X and Ye P: miR-31
overexpression exacerbates atherosclerosis by targeting NOX4 in
apoE(−/−) mice. Clin Lab. 61:1617–1624. 2015.PubMed/NCBI
|
|
39
|
Zhou Q, Gallagher R, Ufret-Vincenty R, Li
X, Olson EN and Wang S: Regulation of angiogenesis and choroidal
neovascularization by members of microRNA-23~27~24 clusters. Proc
Natl Acad Sci USA. 108:pp. 8287–8292. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
40
|
van Eijndhoven MA, Zijlstra JM,
Groenewegen NJ, Drees EE, van Niele S, Baglio SR, Koppers-Lalic D,
van der Voorn H, Libregts SF, Wauben MH, et al: Plasma vesicle
miRNAs for therapy response monitoring in Hodgkin lymphoma
patients. JCI Insight. 1:e896312016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kang H, Rho JG, Kim C, Tak H, Lee H, Ji E,
Ahn S, Shin AR, Cho HI, Huh YH, et al: The miR-24-3p/p130Cas: A
novel axis regulating the migration and invasion of cancer cells.
Sci Rep. 7:448472017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cui S, Liao X, Ye C, Yin X, Liu M, Hong Y,
Yu M, Liu Y, Liang H, Zhang CY and Chen X: ING5 suppresses breast
cancer progression and is regulated by miR-24. Mol Cancer.
16:892017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu Z, Liu Z, Zhang Y, Li Y, Liu B and
Zhang K: miR-24 represses metastasis of human osteosarcoma cells by
targeting Ack1 via AKT/MMPs pathway. Biochem Biophys Res Commun.
486:211–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li YQ, Lu JH, Bao XM, Wang XF, Wu JH and
Hong WQ: MiR-24 functions as a tumor suppressor in nasopharyngeal
carcinoma through targeting FSCN1. J Exp Clin Cancer Res.
34:1302015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou Q, Anderson C, Zhang H, Li X, Inglis
F, Jayagopal A and Wang S: Repression of choroidal
neovascularization through actin cytoskeleton pathways by
microRNA-24. Mol Ther. 22:378–389. 2014. View Article : Google Scholar : PubMed/NCBI
|