Introduction
Colorectal cancer (CRC) is a common malignant tumor
of the digestive system (1). In
recent years, with changes in lifestyle and dietary structure, the
incidence of CRC has increased annually. The symptoms of CRC are
typically not obvious at the early stages, and tumors often have
metastasized by the time the symptoms become noticeable. This is
the main reason for the high mortality rate. Therefore, it is
imperative to identify novel diagnostic markers, and to investigate
the underlying mechanisms of metastasis in CRC.
Collagen type I α 1 (COL1A1) encodes the pro-α 1
chains of type I collagen, which has a triple helix composed of two
α 1 chains and one α 2 chain (2).
COL1A1 contains three conservative domains, namely a von Willebrand
factor type C (vWFC) domain, a collagen triple-helix repeat and a
fibrillar collagen C-terminal domain (COLF) (3). It was recently found that COL1A1 is
associated with a variety of tumor types, and that the expression
of COL1A1 was high in tumor tissues and cells (4–14).
However, the function and mechanism of COL1A1 in CRC have not yet
been reported. Therefore, in this study we aimed to detect the
expression of COL1A1 in trios of tumor, normal and lymph node
tissue samples, as well as to analyze the function and molecular
mechanism of COL1A1 in the metastasis of CRC.
Materials and methods
Tissue microarrays and cell lines
Tissue chips, including 20 cases and 60 points, were
purchased from Outdo Biotech (Shanghai, China). A total of 20 trios
of CRC, adjacent normal, and lymph node tissues were included in
the tissue microarrays. The CRC SW480 and SW620 cell lines used in
this study were obtained from the ATCC and cultured in RPMI-1640
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 37°C and 5% CO2.
Immunohistochemistry (IHC)
The tissue microarrays were immunostained for COL1A1
as previously described (15). An
antibody against COL1A1 was purchased from Abclone (Cambridge, MA,
USA). The COL1A1 immunostaining score was calculated according to
the percentage of positively stained tumor cells and the staining
intensity. The percentage positivity was scored from 0 to 3, with 0
for <10%, 1 for 10–30%, 2 for 31–50%, and 3 for >50%. The
staining intensity was scored from 0 to 3, with 0 for no staining,
1 for weakly stained, 2 for moderately stained, and 3 for strongly
stained. Both the percentage positivity and the staining intensity
were scored in a double-blinded manner. The total score for COL1A1
expression was calculated as the percentage positivity score × the
staining intensity score, giving a value ranging from 0 to 9.
COL1A1 expression was defined as either ‘low’ (score 0–4) or ‘high’
(score 5–9) (16).
Construction of COL1A1-knockdown cell
lines and transfection
The siRNA used to inhibit COL1A1 expression was
purchased from GenePharma (Shanghai, China). The nucleotide
sequence of the siRNA against COL1A1 was TTGGTGTTGTGCGATGACGTG.
Cells were transfected with siRNA oligonucleotides and plasmids
using Lipofectamine® 2000 (Invitrogen Thermo Fisher
Scientific, Inc.).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from tissues or cells using
TRIzol® reagent (Takara Biotechnology Co., Ltd., Dalian,
China), according to the manufacturer's instructions. The reverse
transcription of RNA to cDNA was performed with a reverse
transcription kit (Takara). RT-qPCR analyses were conducted using
SYBR-Green® (Takara) in triplicate. Results were
normalized to the expression of GAPDH (17). The primer sequences used for
RT-qPCR were as follows: COL1A1 forward,
5′-GAGGGCCAAGACGAAGACATC-3′, and reverse,
5′-CAGATCACGTCATCGCACAAC-3′; GAPDH forward,
5′-GACTCATGACCACAGTCCATGC-3′, and reverse,
5′-AGAGGCAGGGATGATGTTCTG-3′.
Transwell assay
The migration of transfected CRC cells was
determined as previously described (18).
Western blot assay
Proteins were extracted using lysis buffer, and
quantified using a Bicinchoninic Acid (BCA) Protein Quantification
kit (KeyGen Biotech Co., Ltd., Nanjing, China). Protein lysates
were separated via 10% SDS-PAGE and transferred onto a PVDF
membrane (Roche, Basel, Switzerland). Subsequently, the membrane
was incubated with the specific primary antibodies, followed by the
appropriate second antibody. The bands were visualized using a
Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific,
Inc.). Antibodies against COL1A1, p-JNK and MMP9 were purchased
from Abclone. Antibodies against Rac1-GTP and RhoA-GTP were
purchased from NewEast Biosciences (Malvern, PA, USA).
Statistical analysis
Data was analyzed by SPSS 20.0 Statistical software.
Western blot bands were quantified by Image J 1.45 software.
Quantitative data was plotted by Graphpad prism 5 software and
presented as the mean ± SD of at least 3 independent experiments.
The differences between independent experimental groups were tested
by using a two-tailed paired Student's t-test. Differences were
considered significant if P<0.05: *P<0.05; **P<0.01;
***P<0.001.
Results
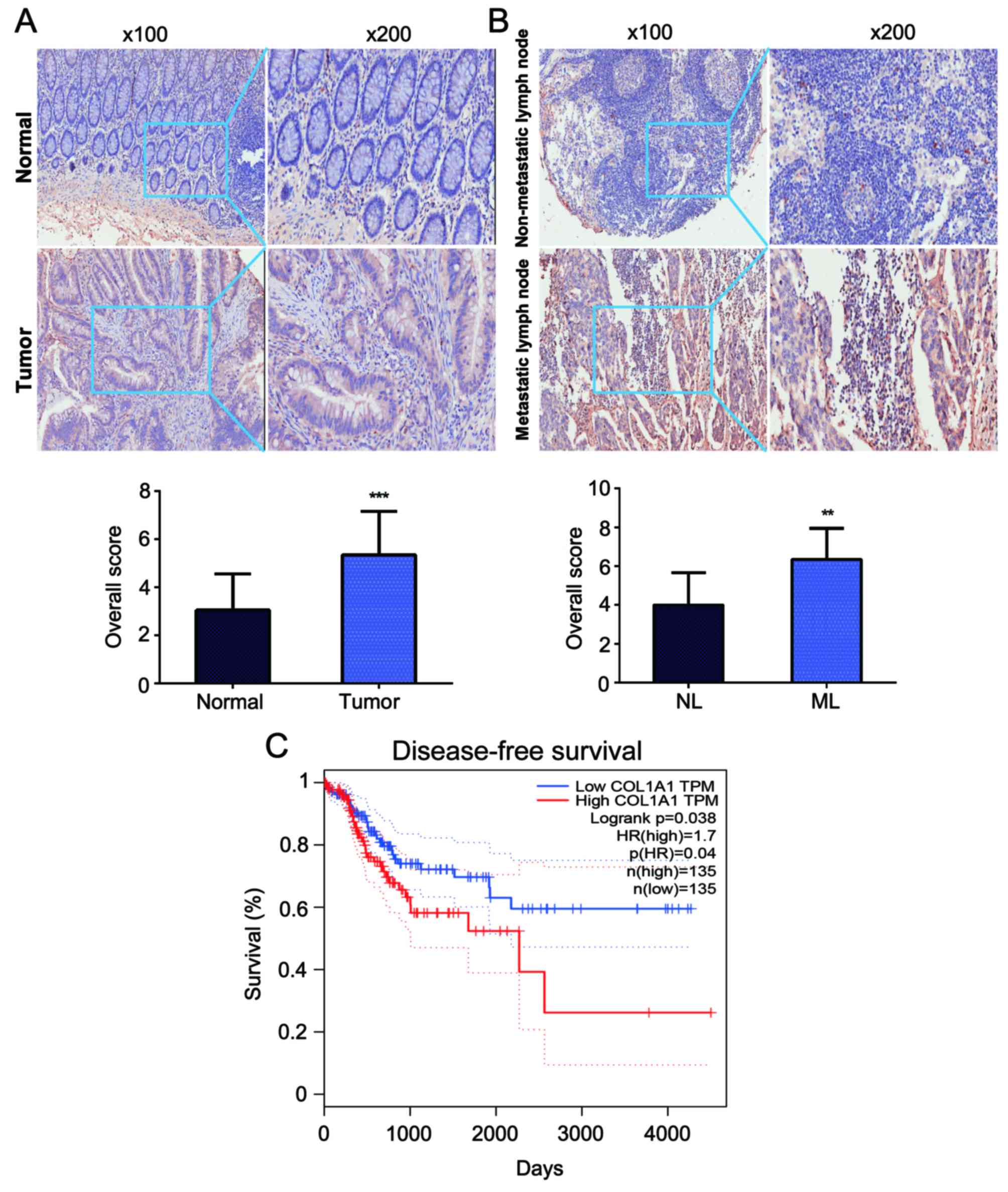
COL1A1 is upregulated in CRC and
metastatic lymph node tissues
To investigate the role of COL1A1 in CRC
tumorigenesis, the expression levels of COL1A1 protein were
detected in trios of CRC tissues, adjacent normal counterparts and
paired lymph node tissues from 20 patients using IHC analysis. We
observed that COL1A1 protein expression was increased in CRC tumor
tissues compared with in the adjacent normal mucosae (P<0.001)
(Fig. 1A). Furthermore, COL1A1
protein expression in metastatic lymph node tissues was higher than
that in non-metastatic lymph node tissues (P<0.01) (Fig. 1B). We further evaluated the
prognostic role of COL1A1 in CRC. The data from TCGA showed that
the disease-free survival (DFS) of patients with higher COL1A1
expression had worse outcomes than did patients with lower COL1A1
expression (Fig. 1C) (19).
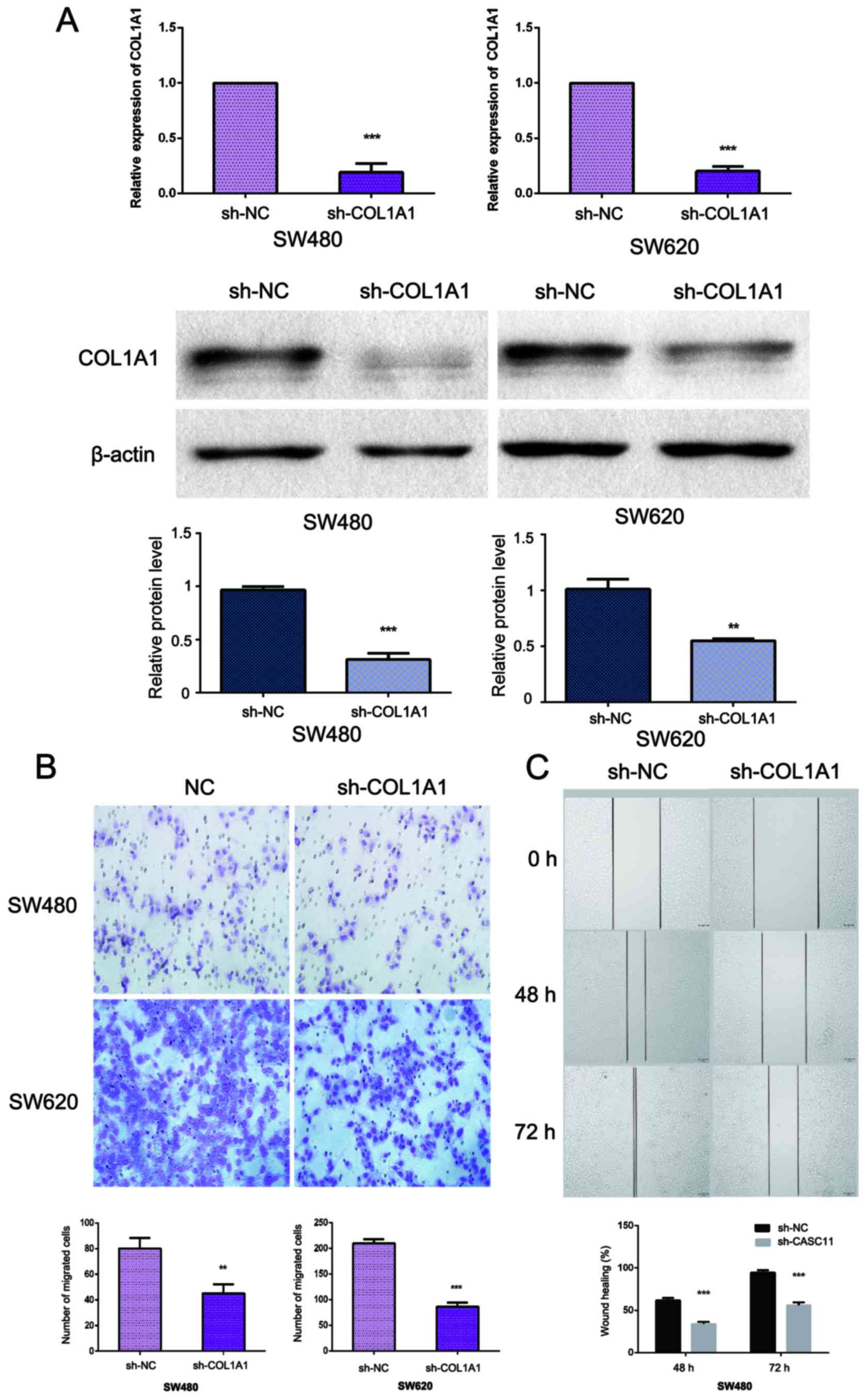
Knockdown of COL1A1 inhibits CRC cell
migration in vitro
As COL1A1 expression appeared to be associated with
metastasis, we evaluated the role of COL1A1 in cell migration. We
knocked down COL1A1 using siCOL1A1 in SW480 and SW620 cells
(Fig. 2A). Transwell and wound
healing assays were used to determine cell motility; the results
revealed that the suppression of COL1A1 could attenuate the
migration capabilities of SW480 and SW620 cells when compared with
cells transfected with a control vector (Fig. 2B and C).
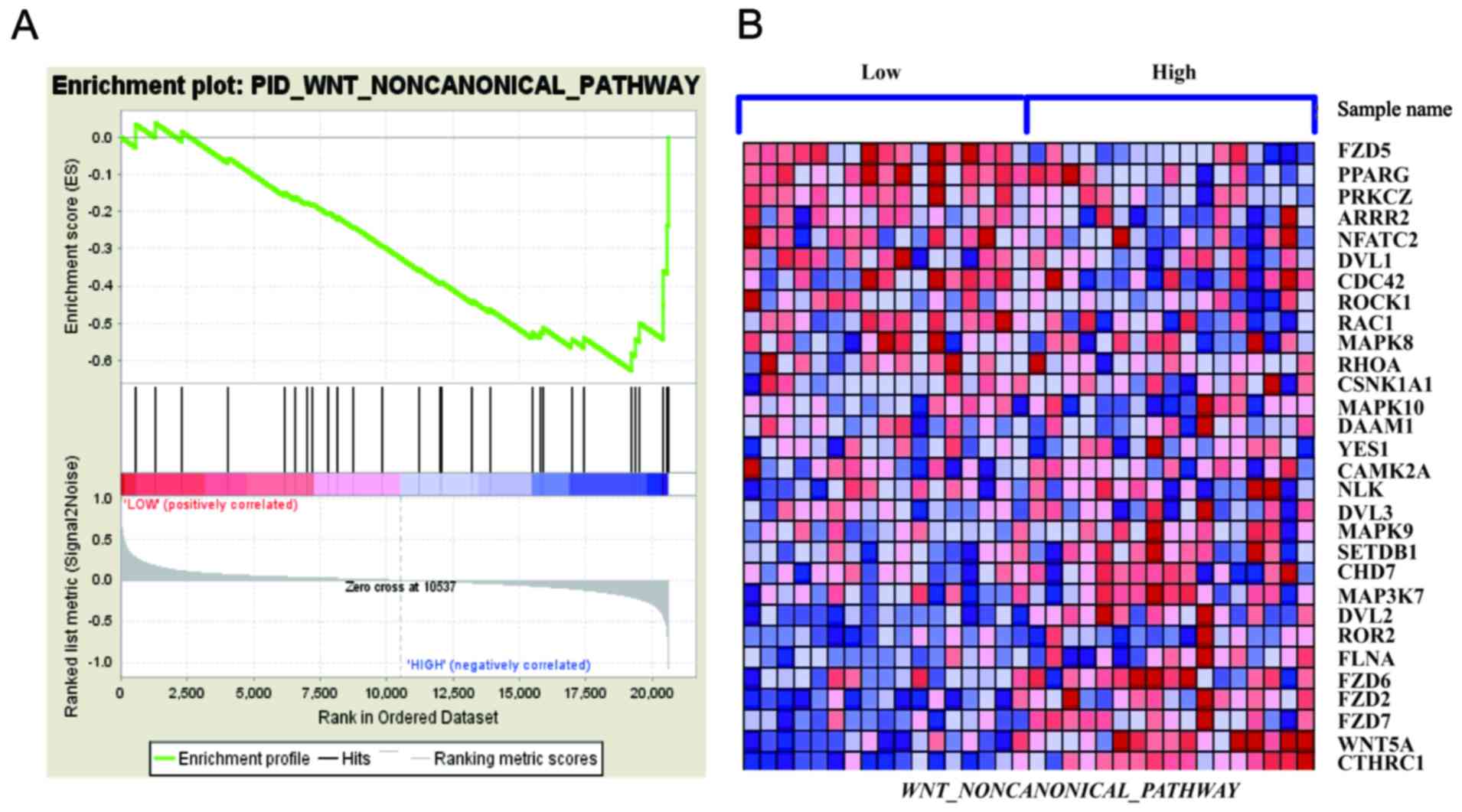
COL1A1 promotes WNT/planar cell
polarity (PCP) pathway activation
Through gene set enrichment analysis (GSEA), we
analyzed the GSE32323 data and observed that the WNT/PCP signaling
pathway was correlated with COL1A1 expression (Fig. 3) (20,21).
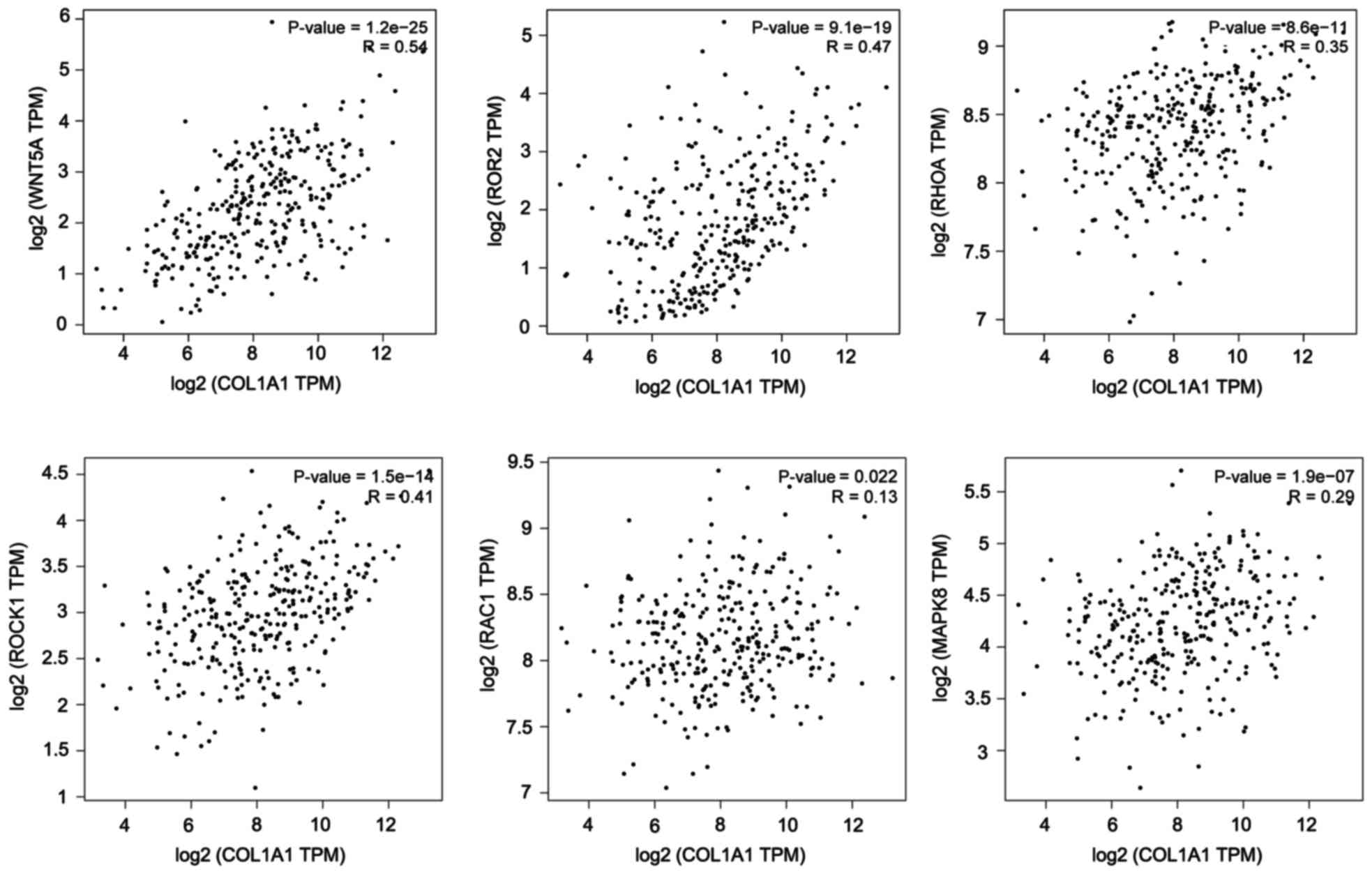
The results from the TCGA data analysis also showed that COL1A1is
correlated with key genes in the WNT/PCP pathway. The results were
calculated using the online web service GEPIA (http://gepia.cancer-pku.cn/index.html)
(Fig. 4) (19). We hypothesized that COL1A1 could
modulate Wnt/PCP signaling. To test the hypothesis that COL1A1
serves an important role in activating WNT/PCP signaling, we
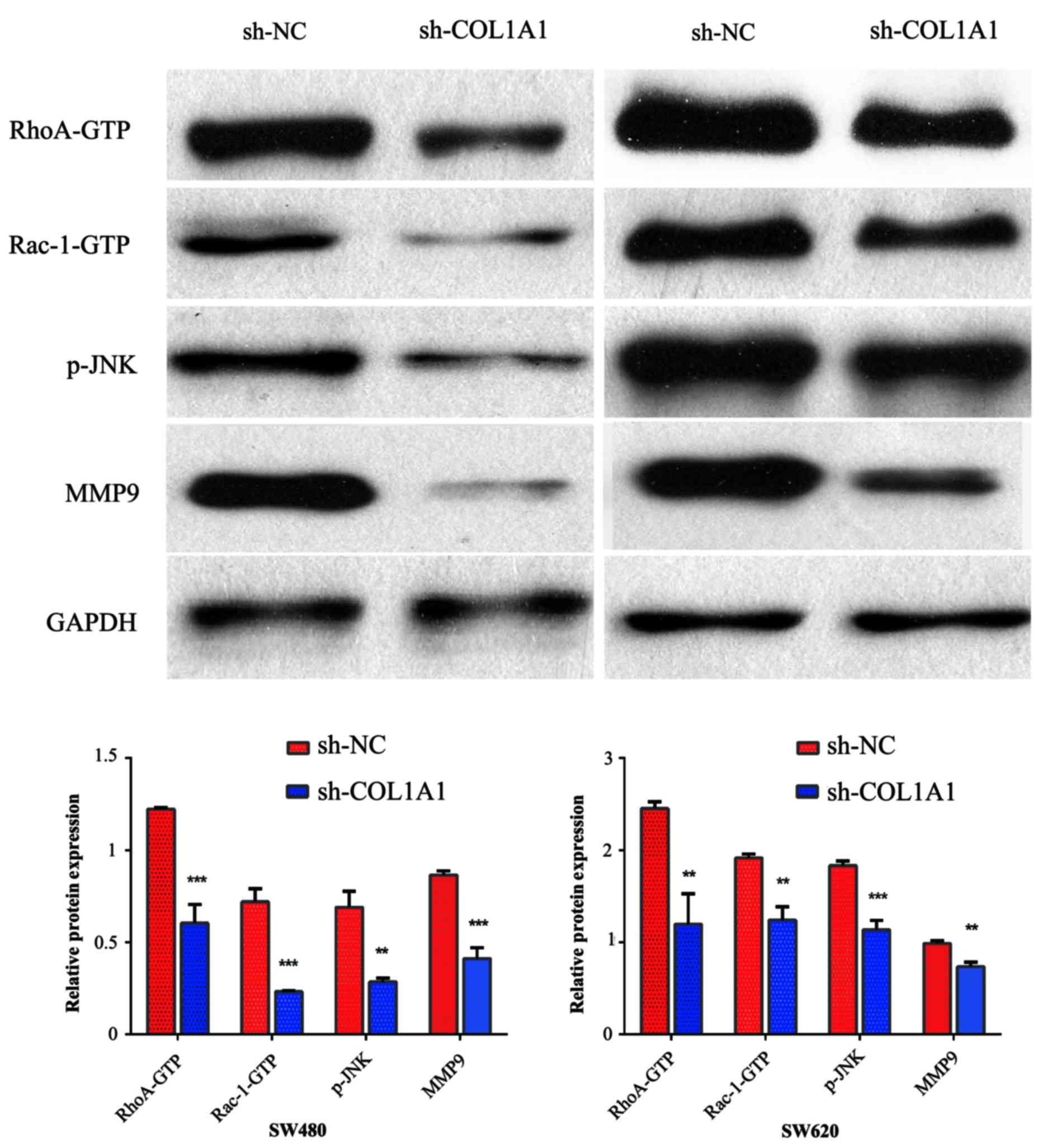
detected the expression of key mediators in the WNT/PCP pathway,
including Rac1-GTP, p-JNK, RhoA-GTP, and the target gene MMP9, all
of which are important contributors to tumor cell migration and
invasion. We found that the inhibition of COL1A1 decreased the
expression of Rac1-GTP, p-JNK, RhoA-GTP and MMP9 (Fig. 5).
Discussion
Metastasis remains the major cause of death in
patients with CRC, though the critical molecular mechanisms
underlying tumor metastasis are poorly understood. Prior studies
have shown that COL1A1 is upregulated in CRC tissues vs. normal
tissues (22). COL1A1 is a major
component of collagen type I. The available reports regarding
COL1A1 have mainly focused on osteogenesis, osteoporosis and bone
diseases (23). Recently, many
studies have shown COL1A1 to be associated with a variety of tumor
types, and that the expression of COL1A1 is increased in tumor
tissues and cells (4–14). However, little is known about the
function and mechanism of COL1A1 in CRC. In this study, we
investigated COL1A1 expression in CRC tumor tissues, adjacent
normal counterparts and paired lymph node tissues, and explored its
function and underlying mechanism in CRC. Compared with the normal
tissues, the expression of COL1A1 was increased in CRC tumor
tissues and paired lymph node tissues. Moreover, COL1A1
upregulation in patients with CRC indicated poorer outcomes and
DFS. These results indicated that COL1A1functions as an oncogene in
CRC progression and is associated with metastasis.
To further ascertain the roles of COL1A1 in CRC, we
determined the migration ability of cells with reducedCOL1A1
expression. The results showed that the suppression of COL1A1
decreased the migratory ability of CRC cells; therefore, COL1A1
appears to exert an oncogenic effect, promoting migration in
CRC.
The mechanism of COL1A1 in promoting CRC migration
is still uncertain. Through GSEA, we found that the WNT/PCP
signaling pathway was enriched when COL1A1 was expressed at higher
levels. Moreover, COL1A1 expression was correlated with key genes
in the WNT/PCP pathway. We further determined that COL1A1 could
regulate Rac1-GTP, p-JNK, and RhoA-GTP expression. These findings
suggest that COL1A1 may activate the WNT/PCP signaling pathway. The
Wnt signaling pathway consists of three branches: The canonical
Wnt/β-catenin signaling pathway, which activates gene transcription
through β-catenin nuclear localization; the Wnt/PCP pathway, which
regulates cytoskeletal rearrangements through the activation of JNK
by the small G protein; and the Wnt/Ca2+ pathway, which
affects cell adhesion and related gene expression by releasing
intracellular Ca2+. Among these, the Wnt/PCP pathway is
evolutionarily conserved, and carries signals from cell-surface
Frizzled and ROR2/RYK co-receptors to the nucleus via Rho GTPases
and JNK, processes that are essential for cell migration (24). Rho GTPases (e.g., Rac1 and RhoA)
and JNK are involved in cell morphology, adhesion and metastasis.
JNK can rearrange the actin cytoskeleton, thereby regulating the
planar polarity of the cell to promote invasion and metastasis of
the tumor. JNK can also increase the secretion of MMPs in CRC cells
to promote their metastasis (25–27).
Therefore, we speculated that COL1A1 may promote CRC cell migration
through the WNT/PCP pathway.
In summary, our results indicated that COL1A1
promotes tumor metastasis, and that its inhibition may suppress CRC
cell migration. In addition, the role of COL1A1 in CRC metastasis
seems to be associated with the regulation of the WNT/PCP pathway.
Our findings also indicated that COL1A1 may be a promising
therapeutic target for CRC.
Acknowledgements
The present study was supported by funding from the
Xinxiang Medical College (grant nos. XYBSKYZZ201632 and 2014ZD109),
Higher Education Institutions of Henan Province, China (no.
17A310023), the National Natural Science Foundation of China (no.
81702891), Taihang Young Scholar Foundation of Xinxiang Medical
University, Doctoral Scientific Research Foundation of Xinxiang
Medical University (no. 505079).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maasalu K, Nikopensius T, Kõks S, Nõukas
M, Kals M, Prans E, Zhytnik L, Metspalu A and Märtson A:
Whole-exome sequencing identifies de novo mutation in the COL1A1
gene to underlie the severe osteogenesis imperfecta. Hum Genomics.
9:62015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simon MP, Maire G and Pedeutour F: COL1A1
(collagen, type I, alpha 1). Atlas Genet Cytogenet Oncol Haematol.
5:78–82. 2001.
|
|
4
|
Tian ZQ, Li ZH, Wen SW, Zhang YF, Li Y,
Cheng JG and Wang GY: Identification of commonly dysregulated genes
in non-small-cell lung cancer by integrated analysis of microarray
data and qRT-PCR validation. Lung. 193:583–592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Ding Y and Li A: Identification of
COL1A1 and COL1A2 as candidate prognostic factors in gastric
cancer. World J Surg Oncol. 14:2972016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song Y, Kim SH, Kim KM, Choi EK, Kim J and
Seo HR: Activated hepatic stellate cells play pivotal roles in
hepatocellular carcinoma cell chemoresistance and migration in
multicellular tumor spheroids. Sci Rep. 6:367502016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang H, Liu B, Xu XF, Jiang TT, Zhang XQ,
Shi YL, Chen Y, Liu F, Gu J, Zhu LJ and Wu N: Pathophysiology of
chronic pancreatitis induced by dibutyltin dichloride joint ethanol
in mice. World J Gastroenterol. 22:2960–2970. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boguslawska J, Kedzierska H, Poplawski P,
Rybicka B, Tanski Z and Piekielko-Witkowska A: Expression of genes
involved in cellular adhesion and extracellular matrix remodeling
correlates with poor survival of patients with renal cancer. J
Urol. 195:1892–1902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Willis CM and Klüppel M: Chondroitin
sulfate-E is a negative regulator of a pro-tumorigenic
Wnt/beta-catenin-Collagen 1 axis in breast cancer cells. PLoS One.
9:e1039662014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brooks M, Mo Q, Krasnow R, Ho PL, Lee YC,
Xiao J, Kurtova A, Lerner S, Godoy G, Jian W, et al: Positive
association of collagen type I with non-muscle invasive bladder
cancer progression. Oncotarget. 7:82609–82619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hurst R, Elliott RM, Goldson AJ and
Fairweather-Tait SJ: Se-methylselenocysteine alters collagen gene
and protein expression in human prostate cells. Cancer Lett.
269:117–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poplawski P, Rybicka B, Boguslawska J,
Rodzik K, Visser TJ, Nauman A and Piekielko-Witkowska A: Induction
of type 1 iodothyronine deiodinase expression inhibits
proliferation and migration of renal cancer cells. Mol Cell
Endocrinol. 442:58–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu PN, Yan MD, Lai HC, Huang RL, Chou YC,
Lin WC, Yeh LT and Lin YW: Downregulation of miR-29 contributes to
cisplatin resistance of ovarian cancer cells. Int J Cancer.
134:542–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balbous A, Cortes U, Guilloteau K,
Villalva C, Flamant S, Gaillard A, Milin S, Wager M, Sorel N,
Guilhot J, et al: A mesenchymal glioma stem cell profile is related
to clinical outcome. Oncogenesis. 3:e912014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan L, Zhou C, Lu Y, Hong M, Zhang Z,
Zhang Z, Chang Y, Zhang C and Li X: IFN-γ-mediated IRF1/miR-29b
feedback loop suppresses colorectal cancer cell growth and
metastasis by repressing IGF1. Cancer Lett. 359:136–147. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gu Y, Wang Q, Guo K, Qin W, Liao W, Wang
S, Ding Y and Lin J: TUSC3 promotes colorectal cancer progression
and epithelial-mesenchymal transition (EMT) through WNT/β-catenin
and MAPK signalling. J Pathol. 239:60–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou C, Liu G, Wang L, Lu Y, Yuan L, Zheng
L, Chen F, Peng F and Li X: MiR-339-5p regulates the growth, colony
formation and metastasis of colorectal cancer cells by targeting
PRL-1. PLoS One. 8:e631422013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. Apr
12–2017.(Epub ahead of print). View Article : Google Scholar
|
|
20
|
Mootha VK, Lindgren CM, Eriksson KF,
Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E,
Ridderstråle M, Laurila E, et al: PGC-1alpha-responsive genes
involved in oxidative phosphorylation are coordinately
downregulated in human diabetes. Nat Genet. 34:267–273. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:pp. 15545–15550. 2005;
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zou X, Feng B, Dong T, Yan G, Tan B, Shen
H, Huang A, Zhang X, Zhang M, Yang P, et al: Up-regulation of type
I collagen during tumorigenesis of colorectal cancer revealed by
quantitative proteomic analysis. J Proteomics. 94:473–485. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Byers PH and Pyott SM: Recessively
inherited forms of osteogenesis imperfecta. Annu Rev Genet.
46:475–497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan Y, Guo X, Yang Z, Chen S, Lei Y, Lin
M, Wang L, Feng C and Ke Z: AEG-1 activates Wnt/PCP signaling to
promote metastasis in tongue squamous cell carcinoma. Oncotarget.
7:2093–2104. 2016.PubMed/NCBI
|
|
25
|
Zeke A, Misheva M, Reményi A and
Bogoyevitch MA: JNK signaling: Regulation and functions based on
complex protein-protein partnerships. Microbiol Mol Biol Rev.
80:793–835. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Monin MB, Krause P, Stelling R, Bocuk D,
Niebert S, Klemm F, Pukrop T and Koenig S: The anthelmintic
niclosamide inhibits colorectal cancer cell lines via modulation of
the canonical and noncanonical Wnt signaling pathway. J Surg Res.
203:193–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Lin L, Jin Y, Lin Y, Cao Y and
Zheng C: Overexpression of WNT5B promotes COLO 205 cell migration
and invasion through the JNK signaling pathway. Oncol Rep.
36:23–30. 2016. View Article : Google Scholar : PubMed/NCBI
|