Introduction
Gastric cancer (GC) is the fourth most prevalent
type of malignancy and the second leading cause of
cancer-associated mortality worldwide; GC exhibits its highest
incidence rates in East Asia, Eastern Europe, and South America
(1). Over 70% of new cases and
mortalities occur in developing countries (1). The 5-year survival rate for patients
with GC is <20% due to the frequently late diagnosis; when the
tumor is diagnosed and treated at an early stage, the 5-year
survival rate may reach 90% (2).
The majority of cases of GC are asymptomatic or cause nonspecific
symptoms, which may lead to a delayed diagnosis. Approximately 80%
of patients are diagnosed at the advanced stages in the majority of
countries (3). The conventional
treatments for advanced GC, including chemotherapy, radiotherapy
and surgery, are associated with poor outcomes (4). Therefore, it is necessary to develop
novel noninvasive biomarkers to improve early prognostic
prediction, and to develop more effective treatment strategies for
GC.
MicroRNAs (miRNAs/miRs) are 19–22-base small
non-coding RNA molecules that regulate protein-coding gene
expression via the degradation of target mRNAs, or repress
translation by binding to the 3′-untranslated regions (3′-UTRs) of
the target mRNAs (5). Circulating
miRNAs are stable under extreme conditions, including boiling, low
or high pH, extended storage and freeze-thaw cycles (6), indicating that they may serve as
noninvasive biomarkers for the diagnosis of cancer and other
diseases. Accumulating evidence has demonstrated that miRNAs are
involved in various cellular processes, including proliferation,
apoptosis, development, differentiation and metabolism (7). miRNAs function as oncogenes or tumor
suppressors and are aberrantly expressed in various types of
malignancy, including pancreatic cancer (8) and GC (9).
Previous studies have demonstrated that miR-23b/27b
clustered miRNAs were markedly reduced in numerous types of cancer
(10–12). However, the potential role of
miR-27b in GC remains unclear. Therefore, the aim of the present
study was to determine the functional roles served by miR-27b in
the development of GC and to elucidate the molecular mechanisms
underlying the pathogenesis of GC.
Materials and methods
Patients and plasma samples
Plasma samples from 46 patients (31 males and 15
females; age, 31–76 years) with GC who had not received surgery,
chemotherapy or radiotherapy prior to blood sample collection, and
40 healthy controls (22 males and 18 females; age, 38–70 years),
were obtained between January and October 2015 at Guangzhou First
People's Hospital of Guangzhou Medical University (Guangzhou,
China). The present study was approved by the ethics committee of
Guangzhou First People's Hospital of Guangzhou Medical University
and informed consent was obtained from each patient.
miRNA microarray analysis
Plasma from 5 random patients with GC and 3 healthy
controls was used for miRNA microarray analysis (KangChen Bio-tech,
Inc., Shanghai, China). Total RNA was isolated using an miRNeasy
mini kit (Qiagen GmbH, Hilden, Germany), according to the
manufacturer's protocol. The samples were labeled using the
miRCURY™ Hy3™/Hy5™ Power labeling kit and hybridized on the
miRCURY™ LNA microRNA Array (version 18.0; Exiqon, Inc., Vedbaek,
Denmark). Following salt buffer and detergent (included in the
miRCURY™ Hy3™/Hy5™ Power labeling kit; Exiqon, Inc.) washing steps,
and in situ hybridization, the slides were scanned using the Axon
GenePix 4000B microarray scanner (Molecular Devices LLC, Sunnyvale,
CA, USA). Scanned images were then imported into GenePix Pro 6.0
software (Molecular Devices LLC) for grid alignment and data
extraction.
Cell culture and transfection
The human GC cell line SGC7901 was purchased from
Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). The cells were
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere
with 5% CO2. SGC7901 cells (5×104 cells/ml)
were transfected with the miR-27b mimic (miR-27b-3p mimic sequence
5′-UUCACAGUGGCUAAGUUCUGC-3′) or its negative control
(5′-UUUGUACUACACAAAAGUACUG-3′; both Guangzhou RiboBio Co., Ltd.,
Guangzhou, China) using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol,
at a final concentration of 50 nM. Following 24 h culture,
transfection efficiency was monitored using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis.
RNA isolation and RT-qPCR
analysis
Total RNA was extracted from SGC7901 cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Reverse transcription was
performed with a reaction mixture of: 5× reaction buffer (2.0 µl),
nuclease-free water (5.0 µl), enzyme mix Template (1.0 µl) and
total RNA (2.0 µl). The reaction mixture was incubated for 60 min
at 42°C, heat-inactivated for 5 min at 95°C prior to immediate
cooling at 4°C. The expression level of miR-27b-3p [mature
sequence, 5′-UUCACAGUGGCUAAGUUCUGC-3′ (primer sequence
unavailable); Takara Biotechnology Co., Ltd., Dalian, China] was
assessed using miRCURY LNA™ SYBR® Green master mix
(Exiqon, Inc.). miR-16 [mature sequence,
5′-UAGCAGCACGUAAAUAUUGGCG-3′ (primer sequence unavailable); Takara
Biotechnology Co., Ltd.] was used for normalization. The qPCR
reaction system contained: SYBR-Green master mix (4.8 µl), PCR
primer mix (1.0 µl), diluted cDNA templates (4.0 µl), ROX Reference
Dye II (0.2 µl). PCR was performed with the following thermocycling
conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 10
sec and 60°C for 60 sec.
For quantitative analysis of vascular endothelial
growth factor C (VEGFC) mRNA expression, a RT-PCR kit (Takara
Biotechnology Co., Ltd.) was used to amplify the target genes,
according to the manufacturer's protocol. The expression level of
VEGFC was normalized using β-actin mRNA levels. β-actin and VEGFC
for RT-qPCR were synthesized by Takara Biotechnology Co., Ltd. The
primer sequences were as follows: β-actin, forward (5′-3′)
GTAAAGACCTCTATGCCAACA and reverse (5′-3′) GGACTCATCGTACTCCTGCT;
VEGFC, forward (5′-3′) AGCACGAGCTACCTCAGCAAGAC and reverse (5′-3′)
TTTAGACATGCATCGGCAGGAA. The relative expression levels were
calculated using the comparative 2−ΔΔCq method (13).
Cell proliferation assays
SGC7901 cells were seeded in 96-well plates
(5×103 cells/well). Following culture at 37°C for 24, 48
and 72 h, respectively, an MTS assay was performed. A total of 20
µl reagent (CellTiter 96 AQueous One Solution Reagent; Promega
Corporation, Madison, WI, USA) was added into each well and
incubated for 4 h at 37°C in a humidified atmosphere with 5%
CO2. The absorbance was recorded at 490 nm using a
96-well plate reader. The proliferation analysis experiments were
performed in triplicate.
Cellular apoptosis analysis
SGC7901 cells were seeded in 6-well plates at a
density of 105 cells/well. A total of 24 h subsequent to
transfection, SGC7901 cells were stained with Annexin V-fluorescein
isothiocyanate and propidium iodide (Bestbio, Shanghai, China),
according to the manufacturer's protocol. Apoptosis rates were
analyzed using Guava EasyCyte Mini System with Cytosoft version 1.2
(EMD Millipore, Billerica, MA, USA). The experiments were repeated
three times.
Statistical analysis
All statistical analyses were performed using SPSS
13.0 software (SPSS, Inc., Chicago, IL, USA). The Mann-Whitney U
test was used to compare the differences in plasma miR-27b level
between the patients with GC and the healthy controls. A receiver
operating characteristic (ROC) curve and the area under the ROC
curve was used to assess the utility of miR-27b expression levels
in distinguishing patients with GC from the healthy controls. All
values were presented as the mean ± standard deviation. Statistical
analysis was performed using one-way analysis of variance,
differences among specific means were assessed by the least
significant difference post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
miRNAs are expressed differentially
between patients with GC and healthy controls
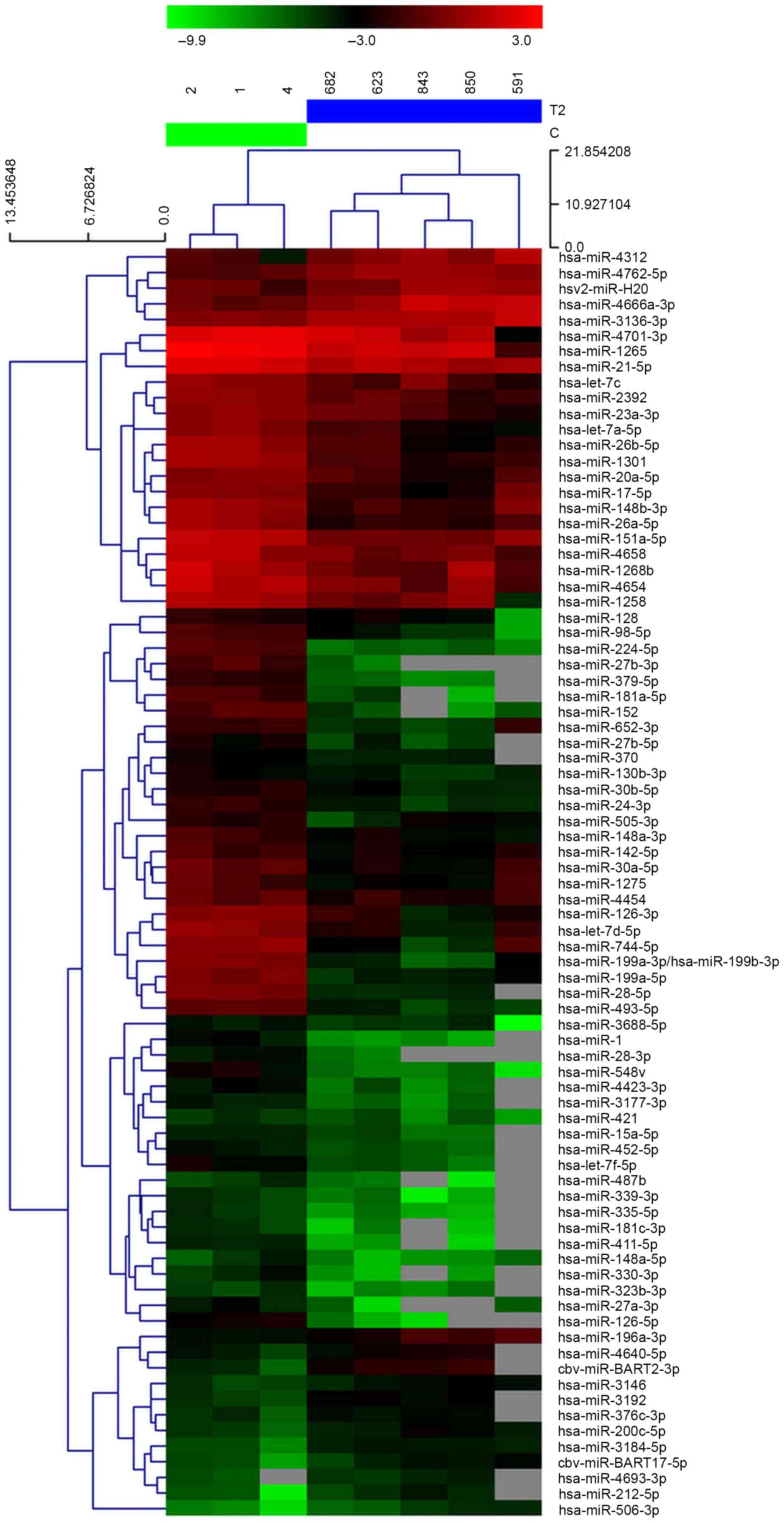
miRNA microarray profiling was performed in 5 GC and
3 healthy control plasma samples. Hierarchical clustering analysis
based on the miRNA expression pattern indicated a significant
difference between GC plasma and the matched healthy controls. It
was observed that 17 miRNAs were significantly upregulated in GC
plasma and 64 miRNAs were downregulated compared with the healthy
controls (Fig. 1).
Expression of miR-27b is downregulated
in GC plasma
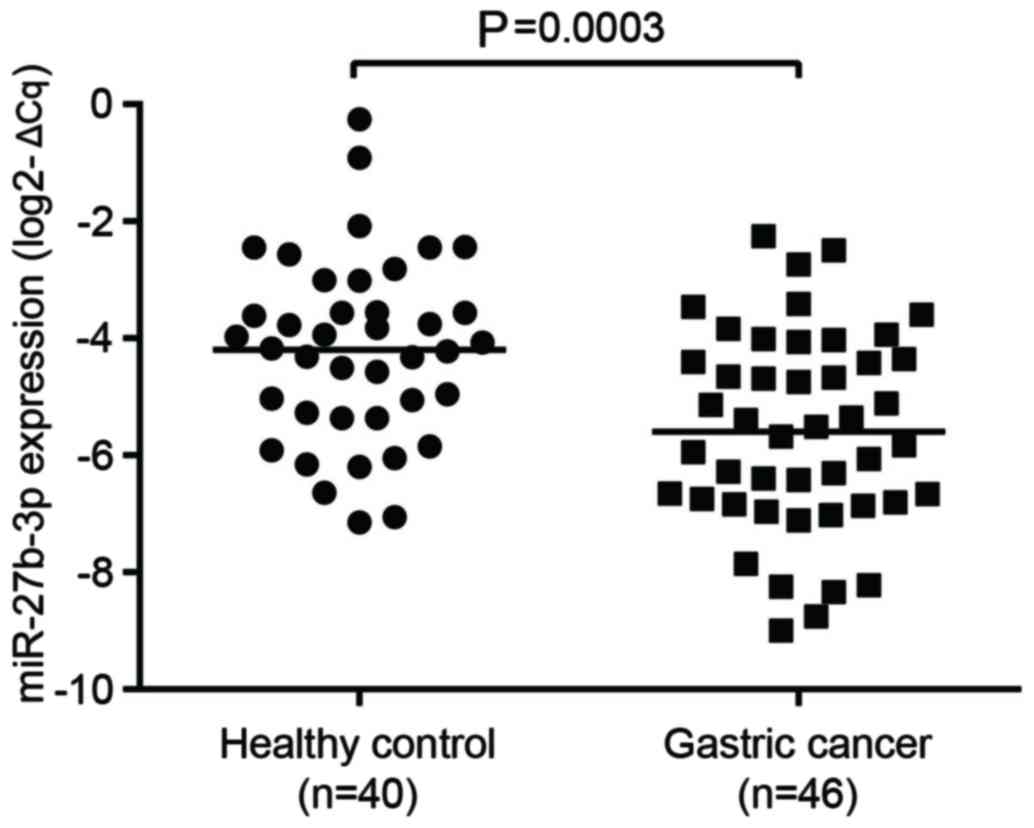
To verify the microarray data, RT-qPCR was used to
compare the expression of miR-27b between 46 samples of GC plasma
and 40 samples of healthy control plasma. miR-16 expression was
used as an internal reference to normalize the RT-qPCR data
(14). As presented in Fig. 2, it was observed that the
expression level of miR-27b was significantly decreased in GC
compared with healthy controls. The results of the microarray
analysis were consistent with those from RT-qPCR.
Plasma miR-27b correlates with
clinicopathological features in GC
The association between circulating miR-27b
expression and clinicopathological features of GC is summarized in
Table I. The results demonstrated
that the level of circulating miR-27b was significantly correlated
with differentiation (P=0.029). However, there was no correlation
of miR-27b expression with other clinical features, including age,
gender, tumor size and invasion (all P>0.05).
 | Table I.The association between plasma
miR-27b-3p expression and clinicopathological characteristics in
patients with gastric cancer (n=46). |
Table I.
The association between plasma
miR-27b-3p expression and clinicopathological characteristics in
patients with gastric cancer (n=46).
| Clinicopathological
features | No. cases (%) | miR-27b-3p level
(2−ΔΔCq) | U | P-value |
|---|
| Gender |
|
| 167.0 | 0.125 |
| Male | 31 (67.4) | 1.620×10−2
(8.900×10−3, 3.960×10−2) |
|
|
|
Female | 15 (32.6) | 3.880×10−2
(8.000×10−3, 9.390×10−2) |
|
|
| Tumor location |
|
| 260.0 | 0.930 |
| Body +
cardia | 22 (47.8) | 2.060×10−2
(9.700×10−3, 4.710×10−2) |
|
|
|
Antrum | 24 (52.2) | 2.070×10−2
(7.900×10−3, 6.180×10−2) |
|
|
| TNM stage |
|
| 249.0 | 0.733 |
| I+II | 23 (50.0) | 1.490×10−2
(8.800×10−3, 6.000×10−2) |
|
|
|
III+IV | 23 (50.0) | 2.450×10−2
(8.900×10−3, 4.730×10−2) |
|
|
| Differentiation |
|
| 155.0 | 0.029a |
|
Poor | 28 (60.9) |
1.460×10−2
(7.800×10−3, 3.670×10−2) |
|
|
| Well
and moderate | 18 (39.1) |
3.920×10−2
(1.200×10−2, 7.600×10−2) |
|
|
| Regional lymph node
metastasis |
|
| 255.0 | 0.869 |
|
Yes | 25 (54.3) |
2.370×10−2
(9.100×10−3, 4.350×10−2) |
|
|
| No | 21 (45.7) |
1.760×10−2
(7.500×10−3, 6.060×10−2) |
|
|
| Metastasis |
|
| 235.0 | 0.559 |
|
Yes | 11 (63.4) |
1.760×10−2
(8.900×10−3, 6.120×10−2) |
|
|
| No | 35 (36.6) |
2.370×10−2
(3.300×10−3, 3.930×10−2) |
|
|
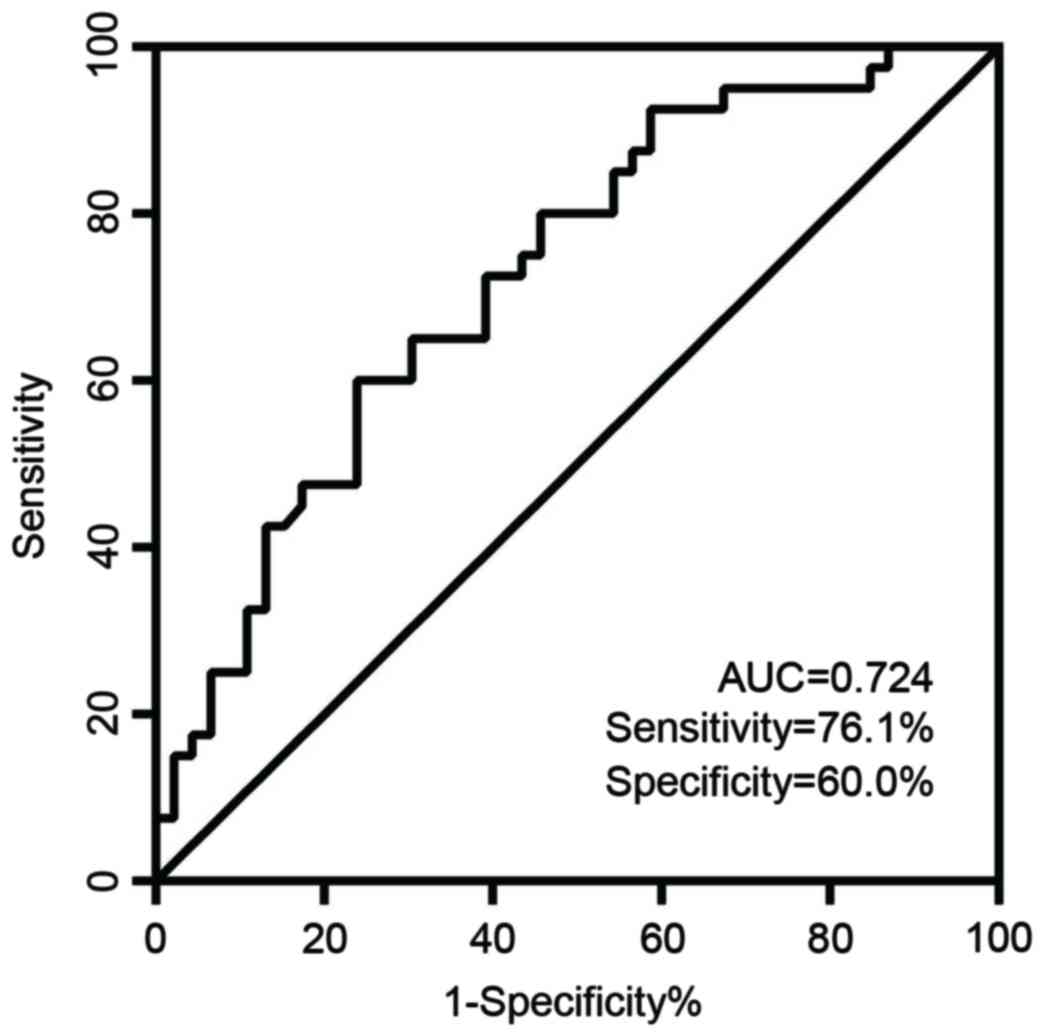
Diagnostic value of circulating
miR-27b
ROC curve analysis indicated that the plasma level
of miR-27b is a potential biomarker for differentiating patients
with GC from healthy controls, with a ROC curve area of 0.724 (95%
confidence interval=0.618–0.831; P=0.0004) (Fig. 3). When the threshold value for
miR-27b was 0.0495, the sensitivity was 76.1% with a specificity of
60%.
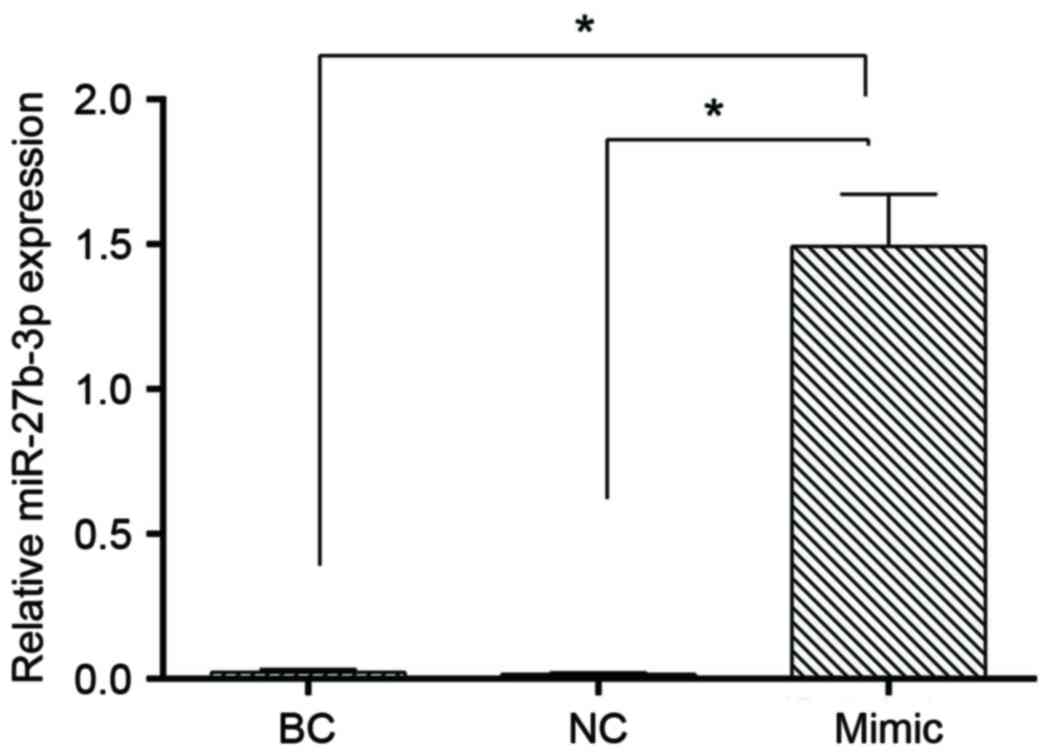
Overexpression of miR-27b was examined
by RT-qPCR analysis
In order to investigate the role of miR-27b in GC
cells, miR-27b was overexpressed using miR-27b mimics, and the
expression of miR-27b was determined by RT-qPCR analysis. The
results of the present study demonstrated that the expression of
miR-27b in the blank control, negative control and miR-27b mimics
groups were 0.022±0.104, 0.016±0.005 and 1.493±0.179, respectively.
There were ~67.87-fold and ~91.60-fold increases in miR-27b levels
in SGC7901 cells transfected with miR-27b mimics compared with the
blank control and the negative control, respectively (P<0.05;
Fig. 4).
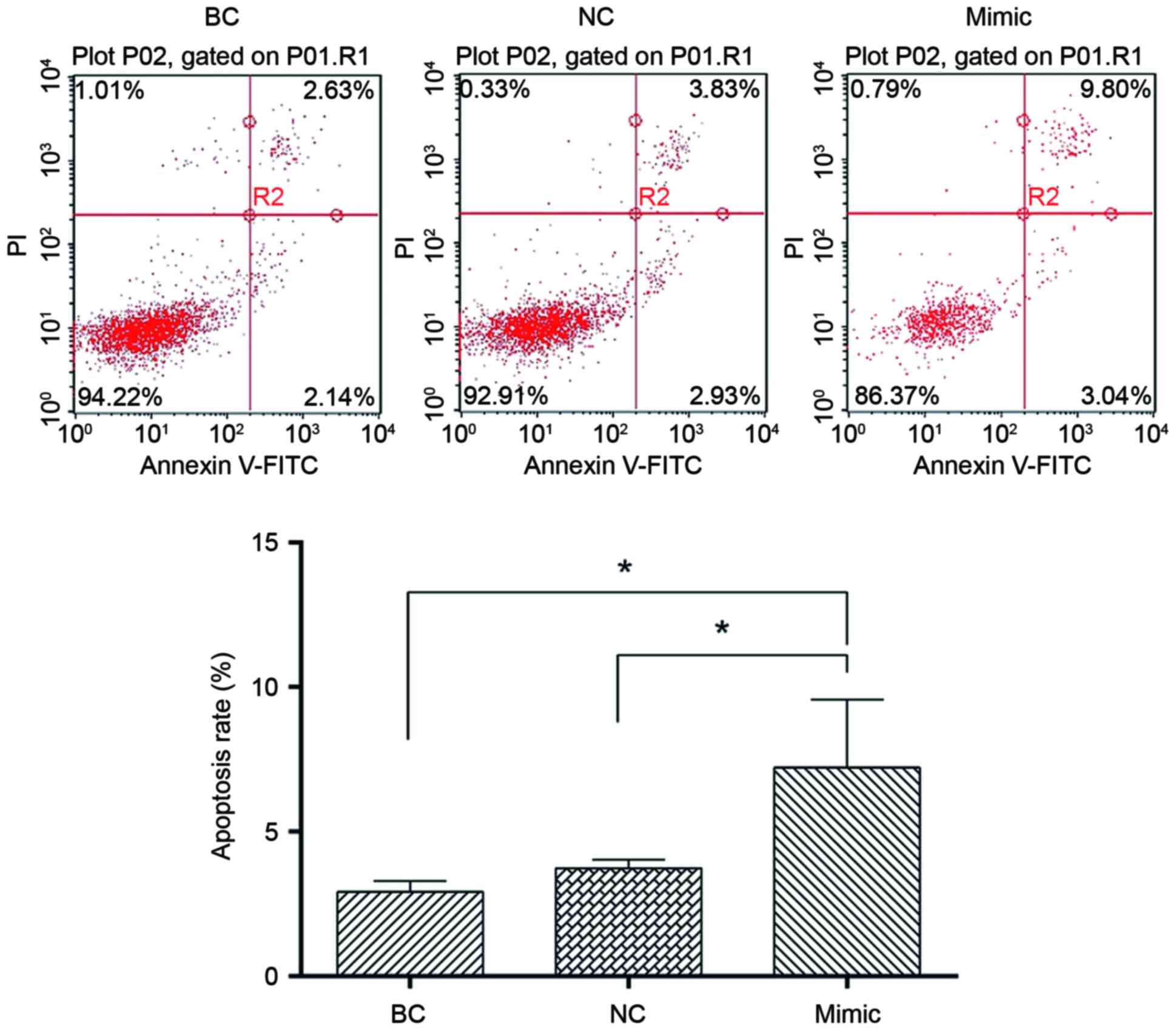
miR-27b overexpression induces GC
cellular apoptosis
In order to evaluate the tumor-suppressing potential
of miR-27b, SGC7901 cells were transiently transfected with miR-27b
mimics or negative control miRNA. Flow cytometric analysis
demonstrated that apoptosis in SGC7901 cells transfected with
miR-27b mimics was significantly increased compared with cells
transfected with the negative control or untreated cells (Fig. 5), suggesting that miR-27b may
induce cellular apoptosis. These results indicated that miR-27b may
function as a tumor suppressor in human GC development by inducing
apoptosis.
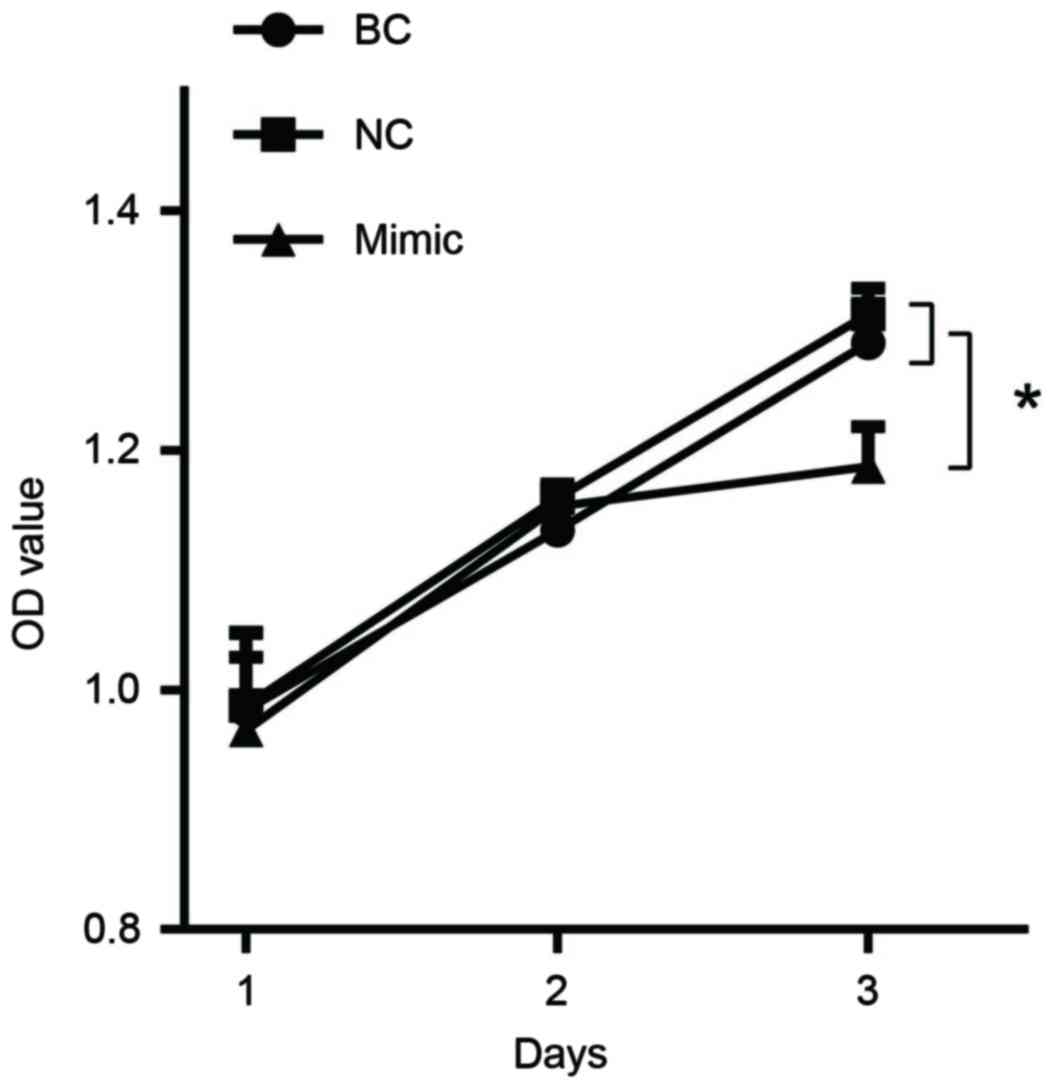
miR-27b overexpression suppresses GC
cell proliferation
In order to evaluate the effects of miR-27b
overexpression on cell proliferation, miR-27b mimics or negative
control were transfected into SGC7901 cells. An MTS assay
demonstrated that miR-27b mimics induced a significant inhibition
of cell proliferation compared with the negative control and blank
control on the 3rd day (Fig. 6;
P<0.05). Furthermore, a cell growth curve assay additionally
demonstrated that miR-27b mimics inhibited cell growth (Fig. 6).
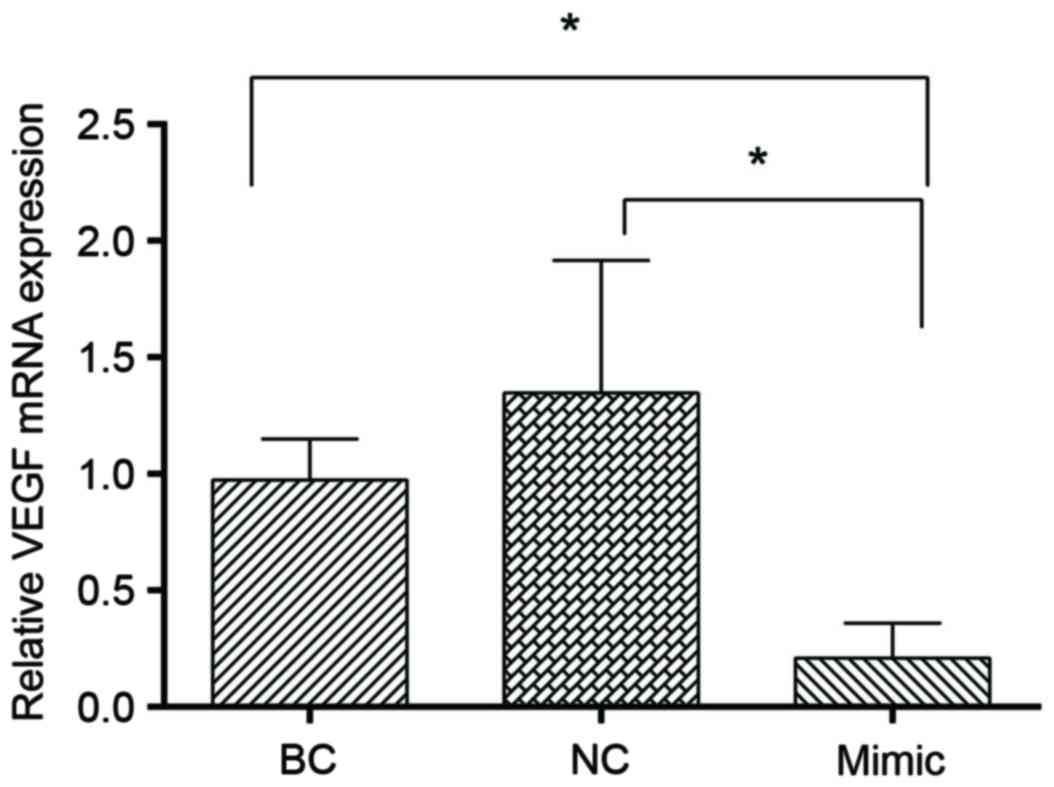
miR-27b inhibits the expression of
VEGFC
Previous studies have demonstrated that VEGFC is a
target of miR-27b and it can be inhibited by miR-27b (15,16).
In order to confirm that miR-27b represses VEGFC expression in
SGC7901 cells, RT-qPCR analysis was performed in the present study,
and it was observed that transfection with miR-27b mimics led to a
significant decrease in VEGFC level (P<0.05; Fig. 7).
Discussion
GC is a lethal malignancy worldwide (17). Due to the nonspecific symptoms of
the early stages of GC, diagnosis is frequently delayed. The 5-year
survival rate of GC is <20% (12). Therefore, it is necessary to
understand the underlying molecular mechanisms and progression
stages of the disease, and to investigate novel therapeutic
targets.
Numerous studies have demonstrated that the abnormal
expression of miRNAs serve a pivotal role in carcinogenesis and
cancer progression (10–12,18).
Circulating miRNAs are able to withstand harsh conditions (6). Therefore, miRNAs may serve as
predictive biomarkers and therapeutic targets for GC.
miR-27b located on chromosome 19p13 which is belongs
to the miR-23b/miR-27b cluster (14). miR-27b has been reported to act as
a tumor suppressor in certain types of human malignancy, including
neuroblastoma (17), clear cell
renal cell carcinoma (18),
pancreatic cancer (19) and lung
cancer (20).
In the present study, it was demonstrated that
miR-27b expression was significantly downregulated in the plasma of
patients with GC, and it was demonstrated that decreased expression
of miR-27b was associated with poor differentiation. Overexpression
of miR-27b inhibited cancer cell proliferation and induced
apoptosis in SGC7901 cells. VEGFC belongs to the platelet-derived
growth factor family, and is a potent inducer of lymphangiogenesis
by activating the vascular endothelial growth factor receptor 3
signaling pathway (21). The
present study confirmed that VEGFC may be inhibited by miR-27b. The
results of the present study demonstrated that miR-27b may be a
candidate tumor suppressor by inhibiting VEGFC in GC. Previous
studies have demonstrated that VEGFC is a target of miR-27b
(15,16). miR-27b was observed to be
downregulated in colorectal cancer, and overexpression of miR-27b
repressed colorectal cancer cell proliferation, colony formation
and tumor growth by targeting VEGFC (17). Downregulation of VEGFC in lung and
colon cancer cells led to a significant inhibition of tumor growth
and metastasis (18). VEGFC is
therefore an attractive target for the development of novel
anticancer strategies.
Overexpression of VEGFA and VEGFC in GC has been
demonstrated to correlate with prognosis, and their silencing may
effectively inhibit cancer growth (22). VEGFC was highly expressed in the
gastric tumor tissues obtained in the present study. Additionally,
the expression of VEGFC was correlated with the pathological
staging of GC. The growth of gastric tumor cells was significantly
inhibited by transfecting with an antisense VEGFC gene (23). Various studies have demonstrated
VEGFC can induce lymphangiogenesis (24–26).
Overexpression of VEGFC has been observed to increase leukemic cell
proliferation in a dose-dependent manner by inducing the expression
of apoptosis regulator Bcl-2 and increasing the Bcl-2/apoptosis
regulator Bax ratio (27).
In the present study, it was observed that miR-27b
significantly decreased VEGFC mRNA expression, inhibited cancer
cell proliferation and induced apoptosis in SGC7901 cells.
Therefore, it is possible that VEGFC is associated with
lymphangiogenesis in addition to cell proliferation and
apoptosis.
In conclusion, the results of the present study
demonstrated that plasma miR-27b was downregulated in GC. The
plasma level of miR-27b is a potential biomarker for the
differentiation between patients with GC and healthy individuals.
Upregulation of miR-27b suppressed cell proliferation and induced
apoptosis in GC cell lines. Blocking the VEGFC signaling pathway
may prove to be an effective treatment for GC by targeting GC cells
in addition to the neovasculature. The results of the present study
suggested that miR-27b may be a potential biomarker and molecular
therapeutic target for GC.
Acknowledgements
The present study was supported by the Guangzhou Key
Project of Medical and Health Science and Technology (grant no.
201102A212011).
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miyahara R, Niwa Y, Matsuura T, Maeda O,
Ando T, Ohmiya N, Itoh A, Hirooka Y and Goto H: Prevalence and
prognosis of gastric cancer detected by screening in a large
Japanese population: Data from a single institute over 30 years. J
Gastroenterol Hepatol. 22:1435–1442. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Correa P: Gastric cancer: Overview.
Gastroenterol Clin North Am. 42:211–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi T, Saikawa Y and Kitagawa Y:
Gastric cancer: Current status of diagnosis and treatment. Cancers
(Basel). 5:48–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hamada S, Satoh K, Fujibuchi W, Hirota M,
Kanno A, Unno J, Masamune A, Kikuta K, Kume K and Shimosegawa T:
miR-126 acts as a tumor suppressor in pancreatic cancer cells via
the regulation of ADAM9. Mol Cancer Res. 10:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hashiguchi Y, Nishida N, Mimori K, Sudo T,
Tanaka F, Shibata K, Ishii H, Mochizuki H, Hase K, Doki Y and Mori
M: Down-regulation of miR-125a-3p in human gastric cancer and its
clinicopathological significance. Int J Oncol. 40:1477–1482.
2012.PubMed/NCBI
|
|
10
|
Chen L, Li H, Han L, Zhang K, Wang G, Wang
Y, Liu Y, Zheng Y, Jiang T, Pu P, et al: Expression and function of
miR-27b in human glioma. Oncol Rep. 26:1617–1621. 2011.PubMed/NCBI
|
|
11
|
Lee JJ, Drakaki A, Iliopoulos D and Struhl
K: miR-27b targets PPARγ to inhibit growth, tumor progression and
the inflammatory response in neuroblastoma cells. Oncogene.
31:3818–3825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishihara T, Seki N, Inoguchi S, Yoshino H,
Tatarano S, Yamada Y, Itesako T, Goto Y, Nishikawa R, Nakagawa M
and Enokida H: Expression of the tumor suppressive miRNA-23b/27b
cluster is a good prognostic marker in clear cell renal cell
carcinoma. J Urol. 192:1822–1830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song J, Bai Z, Han W, Zhang J, Meng H, Bi
J, Ma X, Han S and Zhang Z: Identification of suitable reference
genes for qPCR analysis of serum microRNA in gastric cancer
patients. Dig Dis Sci. 57:897–904. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye J, Wu X, Wu D, Wu P, Ni C, Zhang Z,
Chen Z, Qiu F, Xu J and Huang J: miRNA-27b targets vascular
endothelial growth factor C to inhibit tumor progression and
angiogenesis in colorectal cancer. PLoS One. 8:e606872013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khromova N, Kopnin P, Rybko V and Kopnin
BP: Downregulation of VEGF-C expression in lung and colon cancer
cells decelerates tumor growth and inhibits metastasis via multiple
mechanisms. Oncogene. 31:1389–1397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bera A, VenkataSubbaRao K, Manoharan MS,
Hill P and Freeman JW: A miRNA signature of chemoresistant
mesenchymal phenotype identifies novel molecular targets associated
with advanced pancreatic cancer. PLoS One. 9:e1063432014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Olofsson B, Jeltsch M, Eriksson U and
Alitalo K: Current biology of VEGF-B and VEGF-C. Curr Opin
Biotechnol. 10:528–538. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Chen X, Fang J and Yang C:
Overexpression of both VEGF-A and VEGF-C in gastric cancer
correlates with prognosis, and silencing of both is effective to
inhibit cancer growth. Int J Clin Exp Pathol. 6:586–597.
2013.PubMed/NCBI
|
|
23
|
Zhu P, Zhang J, Chen Q, Wang J and Wang Y:
Expression of vascular endothelial growth factor-C in gastric
carcinoma and the effect of its antisense gene transfection on the
proliferation of human gastric cancer cell line SGC-7901. Am J
Surg. 204:78–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Skobe M, Hawighorst T, Jackson DG, Prevo
R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K and Detmar
M: Induction of tumor lymphangiogenesis by VEGF-C promotes breast
cancer metastasis. Nat Med. 7:192–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steven AS, Achen MG, Jussila L, Baldwin ME
and Alitalo K: Lymphangiogenesis and cancer metastasis. Nat Rev
Cancer. 2:573–583. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tammela T and Alitalo K:
Lymphangiogenesis: Molecular mechanisms and future promise. Cell.
140:460–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dias S, Choy M, Alitalo K and Rafii S:
Vascular endothelial growth factor (VEGF)-C signaling through FLT-4
(VEGFR-3) mediates leukemic cell proliferation, survival, and
resistance to chemotherapy. Blood. 99:2179–2184. 2002. View Article : Google Scholar : PubMed/NCBI
|