Introduction
Atherosclerosis, hypertension, diabetes and various
other vascular remodeling diseases pose serious threats to human
health. Restenosis (RS), following percutaneous coronary
intervention, is a pathogenic vascular remodeling disease. At
present, intra-intima migration and proliferation of vascular
smooth muscle cells (VSMCs) are considered to be among the most
prominent pathogeneses associated with vascular remodeling
(1). Considering the high rates of
morbidity and pathogenesis associated with RS, the studies
regarding the negative effects of long-term therapeutics on RS have
been extensively performed. The underlying mechanisms of VSMC
proliferation, migration and extracellular matrix production
post-injury are highly complex and have not yet been completely
determined (2). Therefore,
advancements with regards to the understanding of the mechanisms
associated with post-injury vascular remodeling are of importance,
particularly with regards to the development of preventative and
therapeutic treatments for cardiovascular diseases.
The Hippo signaling pathway was originally
discovered in Drosophila during functional genetic screening
(3). The Hippo signaling pathway
is a kinase chain, which is composed of a series of protein kinases
and transcriptional co-activators, and is involved in organ size
regulation, cell proliferation and apoptosis (4–6). The
Hippo signaling pathway is highly conserved; however, a similar
signaling pathway exists in mammals. The Hippo, Salvador, Warts,
Mats, Yorki and Scalloped genes in Drosophila are
respectively homologous to macrophage stimulating 1 (MST1),
salvador family WW domain containing protein 1 (WW45), large tumor
suppressor kinases 1 and 2 (LATS1/2), MOB kinase activator 1
(Mob1), YY1 associated protein (YAP)/tafazzin (TAZ) and TEA domain
transcription factors (TEAD) in mammals (7). The aforementioned intermolecular
interactions are responsible for the workings of mammalian cell
signaling pathways, in addition to the mediation of cell
proliferation and apoptosis levels in order to regulate organismal
growth and development (7). The
heart of the Hippo pathway comprises a core kinase cassette that
consists of a pair of related serine/threonine kinases, mammalian
STE20-like protein kinase 1 (MST1; also known as STK4) and MST2
(also known as STK3), which are homologues of D.
melanogaster Hippo 6–9 and LATS1 and LATS2 (1–3),
together with the adaptor proteins Salvador homologue 1 (SAV1)1,
10, Mob1A and Mob1B. These proteins limit tissue growth by
facilitating LATS1- and LATS2-dependent phosphorylation of the
homologous oncoproteins YAP (encoded by YAP1) and
transcriptional co-activator with PDZ-binding motif TAZ (also known
as WWTR1) (8). YAP is transported
to the nucleus following inactivation of the Hippo signaling
pathway, which is associated with the activity of numerous
intracellular kinases and the activation of the proteasome system.
Upstream phosphorylation cascades may phosphorylate YAP, and
phosphorylated YAP (p-YAP) is subsequently retained in the
cytoplasm via interaction with 14-3-3 protein. Following this,
p-YAP is degraded by ubiquitin-dependent proteasomes, thus
inhibiting gene expression associated with the promotion of cell
proliferation and resistance to apoptosis (9). In conclusion, the Hippo signaling
pathway regulates YAP/TAZ function depending on the regulation of
its upstream molecules and kinases.
Previous studies have revealed that neointimal
hyperplasia-associated diseases, including atherosclerosis, share
common underlying molecular mechanisms with tumorigenesis (10). Previous studies have additionally
revealed that the Hippo signaling pathway is associated with
embryogenesis and the development of the cardiovascular system
(11). YAP, the effector of Hippo
pathway, is additionally implicated in the phenotypic modulation of
smooth muscle (12,13).
WWC family member 3 (WWC3) is a member of the WWC
protein family. Limited studies focusing entirely upon
investigation of WW and C2 domain containing 1 (WWC1) have revealed
that WWC1 may regulate the Hippo signaling pathway via interaction
with the LATS1 WW domain in order to induce the phosphorylation of
LATS1 (14,15). Members of the WWC protein family
share a number of highly conserved molecular structures, including
the WW, C2 and ADDV domains (16).
Furthermore, in the present study it has been suggested that WWC3
forms a complex with LATS1, via interaction with the WW domain, in
order to regulate the Hippo signaling pathway. Therefore, it is
possible that WWC3 may form a complex with LATS1, via its WW
domain, in order to regulate the Hippo signaling pathway.
The present study aimed to investigate the
expression pattern of WWC3 in VSMCs following vascular injury, the
effects of WWC3 expression on the Hippo signaling pathway, and the
potential underlying molecular mechanisms associated with this
process.
Materials and methods
Cell culture
Rat A10 aortic VSMCs were purchased from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in Dulbecco's modified Eagle's medium (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc., USA), in a humidified
atmosphere of 5% CO2 at 37°C. In order to perform
platelet-derived growth factor BB (PDGF-BB) experiments, rat VSMCs
were grown to 80–90% confluence, serum-starved for 24 h and treated
with recombinant human PDGF-BB (10 ng/ml; PeproTech, Inc., Rocky
Hill, NJ, USA) for a further 48 h. Cells treated with PBS served as
the control.
Animal model
Twenty male Wistar rats (350–400 g, 10 weeks) were
purchased from the Experimental Animal Center (China Medical
University, Shenyang, China). Housing conditions were: Temperature,
24°C, light/dark cycle (12-h), food (LAD 2001; Trophic Animal Feed
High-Tech Co., Ltd., Nantong, China) and sterilized water provided.
All animal studies were granted ethical approval by the
Institutional Animal Care and Use Committee. Following this, rat
carotid artery balloon injury was performed on 10 rats as
previously described and the 10 remaining rats were the controls
(17). The left common, external
and internal carotid arteries were dissected, and vascular injury
was administered to the left common carotid artery using an A
Baxter 2-Fr balloon catheter (Baxter International, Deerfield, IL,
USA). Following this, the balloon catheter was removed, the
external carotid artery was ligated and the wound was subsequently
closed. The rats were administered penicillin to prevent infection
and were then sacrificed at 14 days post-injury. The right carotid
artery served as an uninjured control.
Plasmid construction and
transfection
Empty p-enhanced green fluorescent protein
(EGFP)-C2, pEGFP-C2-WWC3 and pEGFP-C2-ΔWW vectors were obtained
from the University of Münster (Münster, Germany).
pGL3b_8xGTIIC-luciferase and pRL-TK 1 µg and 50 ng, respectively
plasmids were additionally purchased (Addgene, Inc., Cambridge, MA,
USA). Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) transfection reagent was used for plasmid
transfection. The density of cells transfected with 2 pmol
siRNA-WWC3, (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was
80–90%. A total of 48 h later, cells were harvested for the further
experiments.
Hematoxylin and eosin (H&E)
staining and immunohistochemistry
The common carotid arteries were fixed with 10%
neutral formalin for 12 h at 25°C, embedded in paraffin and cut
into sections (4-µm) and, following this, the sections were
deparaffinized using xylene and rehydrated through a graded alcohol
series (100-95-85–75%). H&E staining was performed following a
standard protocol (18).
Immunostaining was performed using the avidin-biotin-peroxidase
complex method (cat. no. KIT-9710; Ultrasensitive™;
Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China). Normal goat serum
(cat. no. KIT-9710, Ultrasensitive™; Fuzhou Maixin
Biotech Co., Ltd.) was used to reduce nonspecific binding at 25°C
for 30 min. The primary antibodies used were as follows: WWC3 (cat.
no. HPA039814; 1:200; Merck KGaA, Darmstadt, Germany), YAP (cat.
no. 14074; 1:100; Cell Signaling Technology, Inc., Danvers, MA,
USA), connective tissue growth factor (CTGF) and cyclin E (cat.
nos. sc-25440 and sc-481; 1:100; Santa Cruz Biotechnology, Inc.) at
4°C overnight. Staining for primary antibody was performed at room
temperature for 2 h. Biotinylated serum IgG (cat. no. KIT-9710,
ready-to-use; Fuzhou Maixin Biotech Co., Ltd.,) was used as the
secondary antibody. Following rinsing in PBS, the sections were
incubated with horseradish peroxidase-conjugated
streptavidin-biotin, followed by 3,3′-diaminobenzidine
tetrahydrochloride to develop the peroxidase reaction. The sections
were counterstained with hematoxylin at 25°C for 5 min and
dehydrated in ethanol before mounting. All slides were examined per
view at magnification, ×400.
Protein extraction and western
blotting analysis
VSMCs were washed using PBS and collected for
protein extraction. The rat carotid artery was dissected into small
pieces and cells were lysed using a homogenizer in cold
radioimmunoprecipitation assay lysis buffer (Santa Cruz
Biotechnology, Inc.). Following sonication (frequency: 30% of
maximum, at 4°C for 5 sec) and centrifugation (12,000 × g, 4°C, 15
min) of the cell lysate, protein samples were quantified using
ultraviolet spectrophotometry and loaded (40 µg/lane) onto a 10%
SDS-PAGE gel. The following primary antibodies were used in said
analyses: WWC3 (cat. no. HPA039814; 1:500; Merck KGaA), YAP (cat.
no. 14074; 1:500; Cell Signaling Technology, Inc.), p-YAP (cat. no.
13008; 1:500; Cell Signaling Technology, Inc.), phospho-LATS1 (cat.
no. 8654; 1:500; Cell Signaling Technology, Inc.), CTGF and cyclin
E (cat. nos. sc-25440 and sc-481; 1:200; Santa Cruz Biotechnology,
Inc.), GFP-tag (cat. no. 66002-1-Ig; 1:2,000; Wuhan Sanying
Biotechnology, Wuhan, China), β-actin (cat. no. sc-47778; 1:1,000;
Santa Cruz Biotechnology, Inc., USA), α-tubulin (cat. no. sc-5286;
1:1,000; Santa Cruz Biotechnology, Inc.) and lamin B1 (cat. no.
ab16048; 1:1,000; Abcam, Cambridge, MA, USA) incubated at 4°C
overnight. Samples were incubated with peroxidase-coupled secondary
antibodies (cat. nos. sc-516102 and sc-2007; 1:2,000, Santa Cruz
Biotechnology, Inc.) at 25°C for 2 h. 5% skimmed milk (BD
Biosciences, Franklin Lakes, NJ, USA) was used for blocking at 25°C
for 1 h. Proteins were transferred to polyvinylidene difluoride
membranes and visualized using an enhanced chemiluminescence kit
(GE Healthcare, Chicago, IL, USA). Images were obtained using a
BioRad Imaging System (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The relative protein levels were quantified with respect to
the use of β-actin, α-tubulin and lamin B1 proteins as loading
controls.
Immunoprecipitation
Supernatants of the cell lysate were collected
following centrifugation, (12,000 × g, 15 min, 4°C) precleared with
protein G-agarose for 2 h at 4°C and incubated with the
aforementioned antibodies overnight at 4°C. LATS1 (5 µg/ml for IP;
Santa Cruz Biotechnology, Inc.), WWC3 (1:500 for immunoblotting;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Wild-type
immunoglobulin G was used as a negative control. Then detected by
western blotting analysis. Then LATS1 and WWC3 were measured by
western blotting analysis according to the aforementioned protocol.
Cell lysates from A10 cells were subjected to immunoprecipition
with anti-GFP antibody GFP-tag (cat. no. JL-8; 1:3,000; Clontech
Laboratories, Inc., Mountainview, CA, USA) or control IgG (cat. no.
A7028; 2 µg/ml; Beyotime Institute of Biotechnology, Shanghai,
China), and the presence of LATS1 was examined by anti-WWC3
immunoblotting.
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde for 15
min, 25°C, and blocked using 5% goat serum (Invitrogen, Thermo
Fisher Scientific, Inc.) for 2 h at room temperature. Cells were
incubated with the following primary antibodies: WWC3 (cat. no.
HPA039814; 1:50; Sigma-Aldrich; Merck KGaA), LATS1 (cat. no.
sc-398560; anti-mouse; 1:50; Santa Cruz Biotechnology, Inc.), YAP
(cat. no. 14074; 1:50; Cell Signaling Technology, Inc.) overnight
at 4°C. Subsequently, the cells were incubated with secondary
antibodies for 1 h at room temperature (anti-rabbit secondary
antibody and anti-mouse secondary antibody; cat. nos. A11008 and
A32727; 1:200; Invitrogen; Thermo Fisher Scientific, Inc.). Cell
nuclei were counterstained using DAPI (cat. no. D9542;
Sigma-Aldrich; Merck KGaA), and images were captured using a
confocal microscope at magnification, ×400 (Olympus, Corporation,
Tokyo, Japan).
MTT assay
Cells were plated in 96-well plates in medium
containing 10% FBS at ~3,000 cells/well, and the cells were then
harvested for further experimentation at 24, 48, 72, 96, 120-h time
intervals. Cell viability was determined using an MTT assay. A
total of 20 µl 5 mg/ml MTT solution (cat. no. M2128; Sigma-Aldrich;
Merck KGaA) was added to each well, and the plates were incubated
for 4 h at 37°C. The medium from each well was removed and 150 µl
dimethyl sulfoxide (cat. no. D2650; Sigma-Aldrich; Merck KGaA) was
added to dissolve the purple formazan. The results were quantified
using spectrophotometry at a wavelength of 550 nm.
Cell invasion assay
A 24-well Transwell chamber with a pore size of 8-µm
was used in order to determine cell invasion (Costar; Corning
Incorporated, Corning, NY, USA). A total of 5×104 cells
were trypsinized and transferred to the upper Matrigel chamber
containing 100 µl serum-free medium, and incubated for 16 h at
25°C. Medium containing 10% FBS was added to the lower chamber to
act as the chemoattractant. Following this, the non-invaded cells
present on the upper membrane surface were removed using a cotton
swab, and the cells on the bottom surface were fixed using 4%
paraformaldehyde at 25°C for 20 min and stained using hematoxylin
at 25°C for 10 min. The numbers of invaded cells were counted in
five randomly selected, high power fields using a light microscope
at a magnification, ×200 (Olympus Corporation, Tokyo, Japan).
Scratch wound healing assay
VSMCs were seeded into 6-well plates at a confluent
density (1106 cells/well). Mitomycin C (20 µmol/l;
Sigma-Aldrich; Merck KGaA) was added into each well, and the plates
were incubated for 2 h at 25°C. Following this, cell migration was
assayed 24 h post-scratching with a 100 µl pipette micro tip, and
the relative closure distances were determined. Five random fields
were measured by capturing images using a light microscope at
magnification, ×200 (Olympus Corporation).
Dual-luciferase reporter assay
VSMCs were seeded in 24-well plates, and transiently
transfected with either PGL3b-8× plasmid GTIIC (cat. no. 34615),
indicating TEAD transcriptional activity, pRL-TK, WWC3 plasmids or
empty plasmids, using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.). Following a 48-h transfection time
period, cells were lysed and luciferase activity was determined
using the Dual-Luciferase Assay kit (Promega Corporation, Madison,
WI, USA). The activity of thymidine kinase Renilla served as
an internal standard for normalization.
Statistical analysis
The statistical software SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA) was used for all analyses. All data are expressed
as the mean ± standard deviation. All experiments were replicated
triplicate and were analyzed using either the Student's t-test, or
one-way analysis of variance followed by a Dunnett-t test as a
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Suppression of WWC3 expression in
VSMCs is induced by PDGF-BB treatment and balloon injury
Marked neointimal hyperplasia and lumen narrowing
were observed using H&E staining 14 days post-injury in the
balloon-injured rat carotid artery, compared with the control group
(Fig. 1A). Suppression of WWC3
expression and enhancement of YAP, CTGF and cyclin E expression was
determined using immunohistochemistry and western blotting analysis
(Fig. 1B and C). In addition, it
was also revealed that WWC3 expression was suppressed in VSMCs
treated with PDGF-BB (10 ng/ml) for 24 h, whereas YAP, p-YAP, CTGF
and cyclin E levels were markedly enhanced (Fig. 1D). The in vitro results were
consistent with the results of the animal experiments. The
transcriptional activity of TEAD suggested that the activity of the
Hippo signaling pathway was significantly enhanced following
treatment with PDGF-BB (Fig. 1E).
Furthermore, VSMC proliferation was determined, via the MTT assay,
to be enhanced following treatment with PDGF-BB (Fig. 1F). In addition, the scratch wound
healing assay and the Transwell chamber assay revealed that VSMC
migration was markedly enhanced following PDGF-BB-induced injury
(Fig. 1G and H).
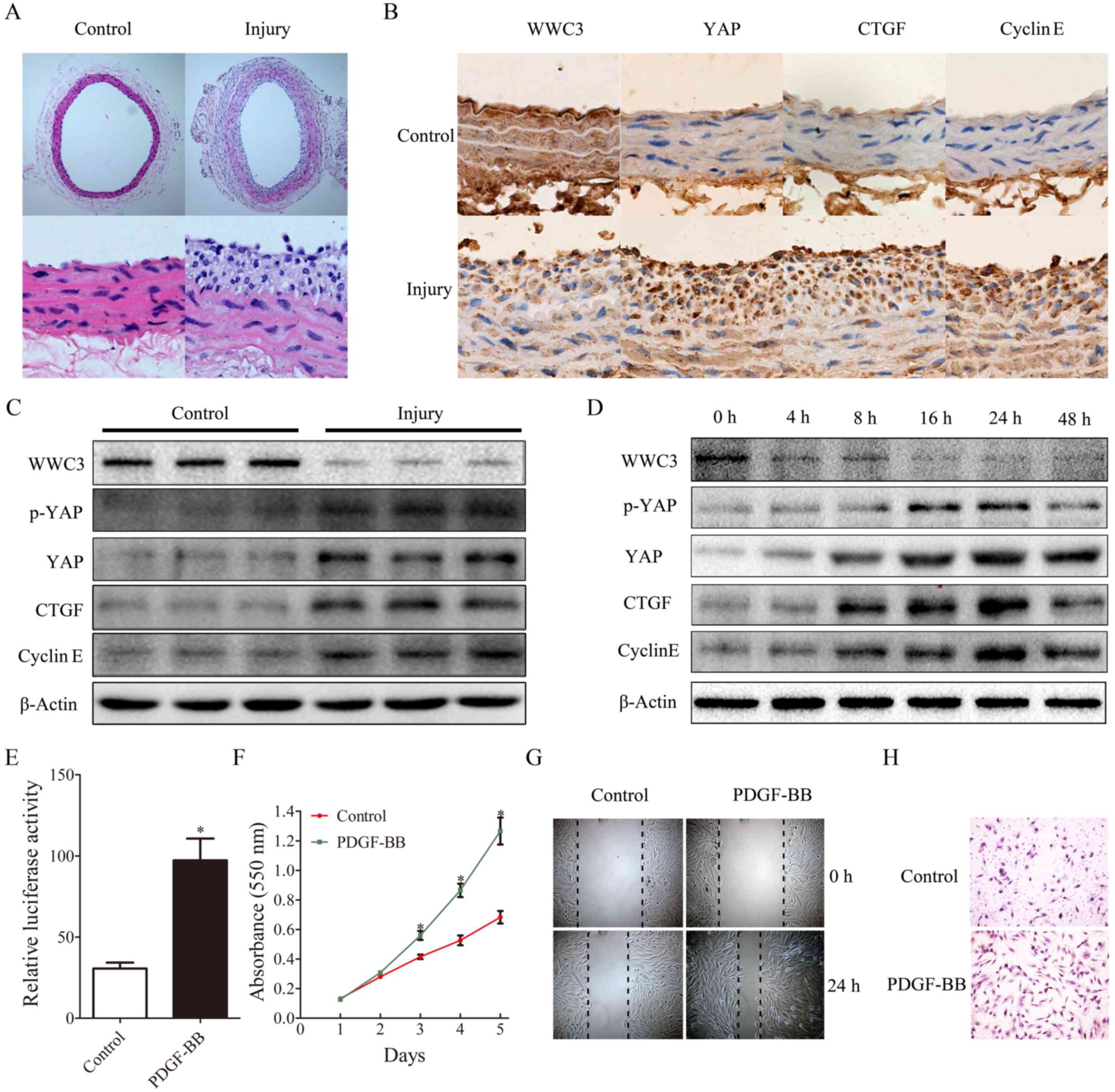 | Figure 1.Suppression of WWC3 expression in
VSMCs is induced by treatment with PDGF-BB and balloon injury. (A)
Hematoxylin and eosin staining of carotid artery tissues obtained
from either control or balloon-injured vessels. I and II
(magnification, ×100), III and IV (magnification, ×400). (B) The
expression levels of WWC3, YAP, CTGF and cyclin E in rat carotid
arteries following balloon injury, revealed by immunohistochemistry
(magnification, ×400). (C) At 14 days post-injury, rat controls or
balloon-injured carotid arteries were harvested for western blot
analysis. (D) VSMCs treated with PDGF-BB (10 ng/ml) for different
time periods were harvested for western blot analysis. (E) The
transcriptional activity of the TEA domain transcription factor was
measured using a dual-luciferase reporter assay following treatment
with PDGF-BB for 24 h. (F) The MTT assay demonstrated that
treatment with PDGF-BB promotes VSMC proliferation. (G) Scratch
wound healing assays and (H) Transwell chamber assays revealed that
treatment with PDGF-BB promotes VSMC migration. The results are
presented as the mean ± standard deviation of three independent
experiments. *P<0.05 vs. control. VSMCs, vascular smooth muscle
cells; WWC3, WW family member 3; PDGF-BB, platelet-derived growth
factor BB; YAP, YY1-associated protein; p-YAP, phosphorylated YAP;
CTGF, connective tissue growth factor. |
WWC3 expression inhibits the
proliferation and migration of VSMCs via upregulation of the Hippo
signaling pathway
In order to investigate the association between WWC3
and the Hippo signaling pathway following treatment with PDGF-BB,
the expression of WWC3 was regulated in a bidirectional manner in
order to investigate the effects upon the predominant components of
the Hippo signaling pathway. Knockdown of WWC3 in VSMCs was
revealed to suppress the levels of p-LATS1 and p-YAP, and to
enhance the expression of YAP, CTGF and cyclin E (Fig. 2A). The transcriptional activity of
TEAD, an indicator of the Hippo-YAP signaling pathway, was
demonstrated to be significantly enhanced following suppression of
WWC3 (Fig. 2B). Furthermore, VSMC
proliferation and migration were enhanced following suppression of
WWC3 (Fig. 2C-G); whereas VSMC
proliferation and migration were suppressed following
overexpression of WWC3 (Fig. 3).
In addition, VSMCs were treated with PDGF-BB following
overexpression of WWC3, and it was demonstrated that PDGF-BB did
not induce a marked effect on the expression of genes associated
with the Hippo signaling pathway, VSMC migration and proliferation
(Fig. 3A-G). Additionally, in
cells transfected with WWC3, treatment with PDGF-BB did not
increase transcriptional activity (Fig. 3B). The results demonstrated that
enhanced levels of VSMC proliferation and migration were associated
with WWC3 activity following treatment with PDGF-BB, and WWC3
inhibited the proliferation and migration of VSMC cells via the
Hippo signaling pathway.
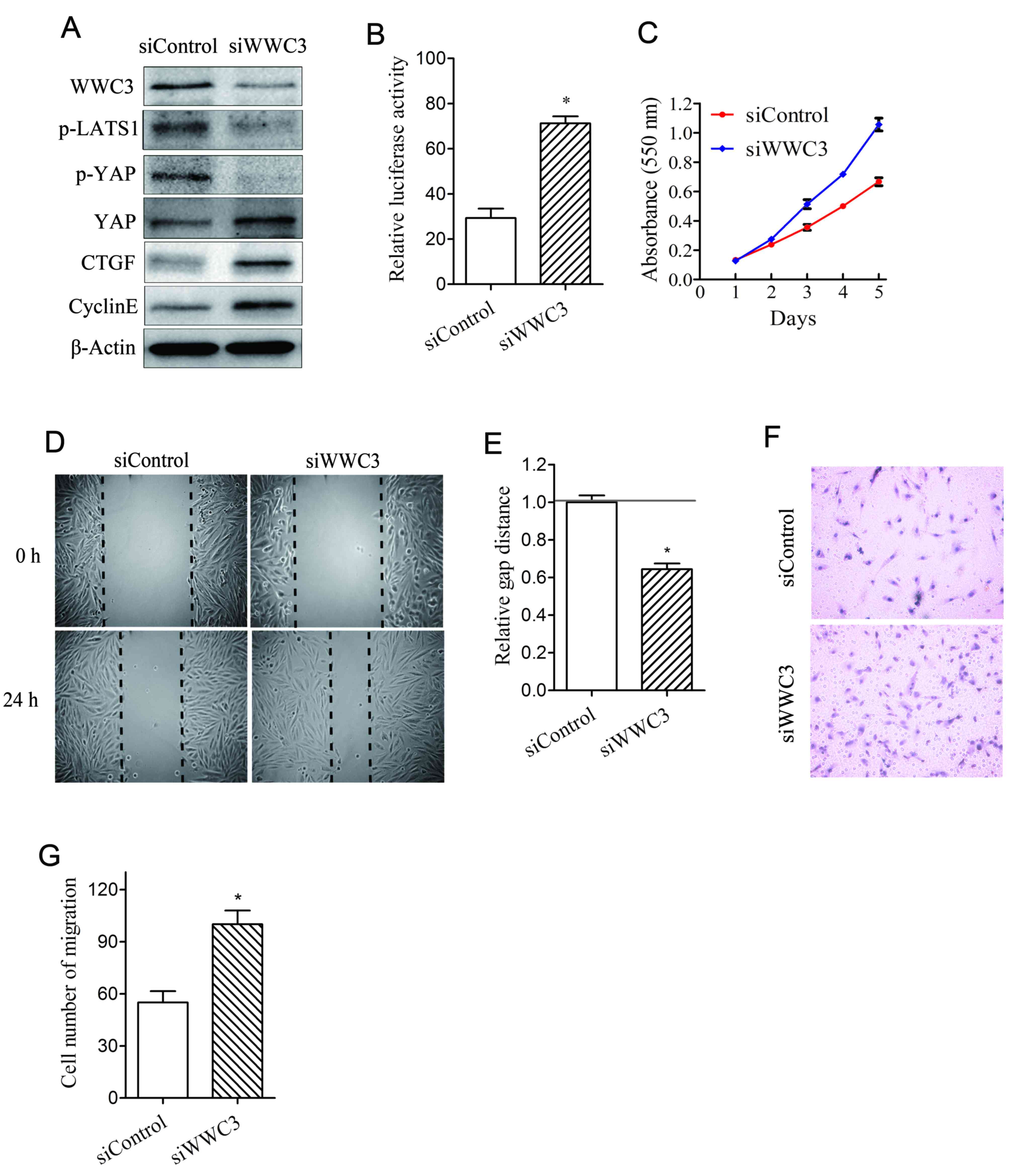 | Figure 2.Knockdown of WWC3 promotes VSMC
proliferation and migration via downregulation of the Hippo
signaling pathway. (A) Western blot analysis for the determination
of the protein expression levels of WWC3, p-LATS1, YAP, p-YAP, CTGF
and cyclin E in WWC3-knockdown VSMCs. (B) Gene knockdown of WWC3
was performed using siWWC3, and the dual-luciferase activity assay
was performed in order to determine the transcriptional activity of
TEA domain transcription factors. (C) An MTT assay was used in
order to determine cell proliferation; (D) a scratch wound healing
assay was performed and (E) analyzed, and a (F) Transwell chamber
assay was performed and (G) analyzed in order to determine cell
migration. The results are presented as the mean ± standard
deviation of three independent experiments. *P<0.05 vs. control.
VSMCs, vascular smooth muscle cells; WWC3, WW domain-containing
protein-3; PDGF-BB, platelet-derived growth factor BB; YAP,
YY1-associated protein; p-YAP, phosphorylated YAP; p-LATS1,
phosphorylated large tumor suppressor kinase 1; CTGF, connective
tissue growth factor; si, small interfering RNA. |
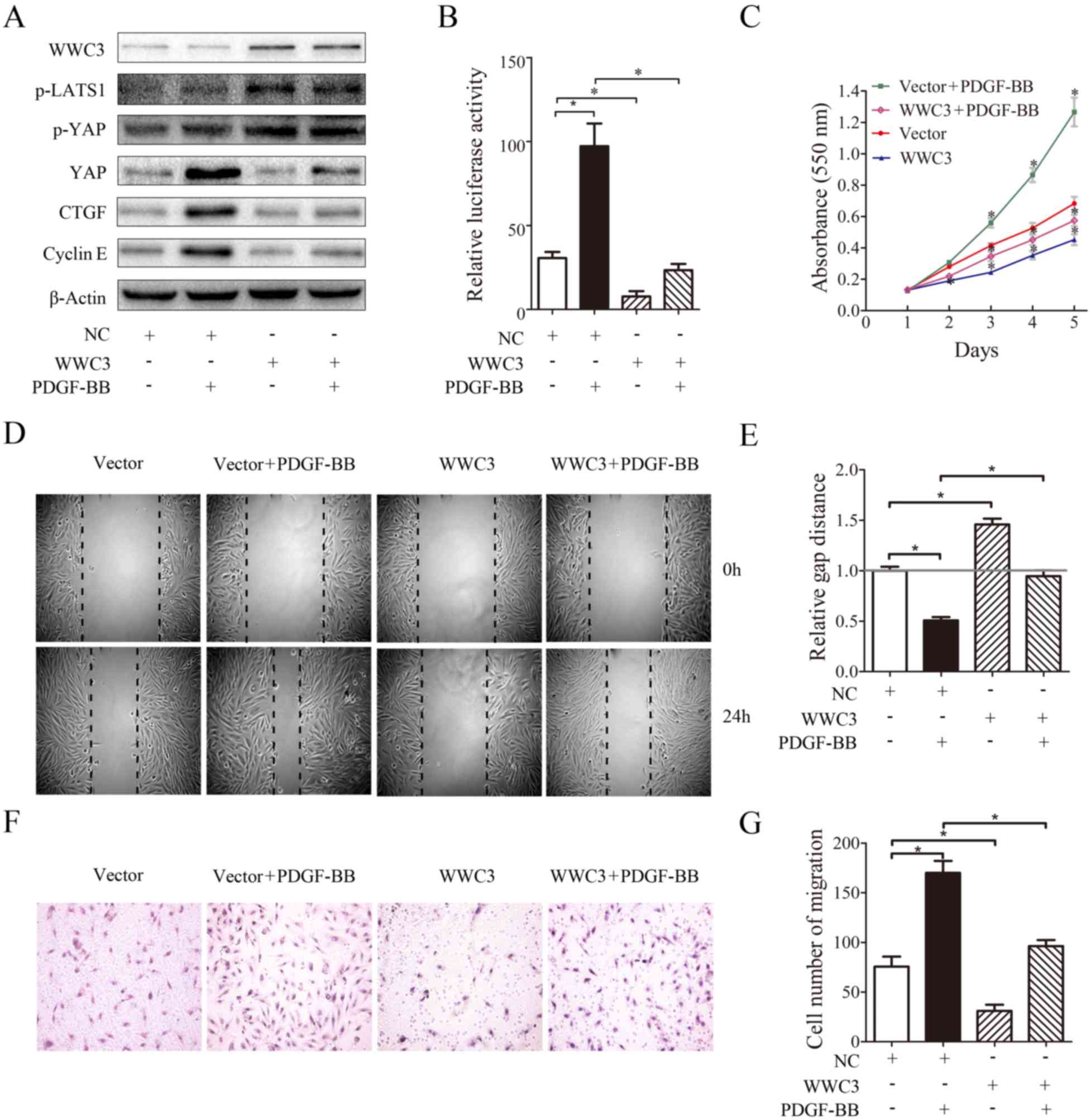 | Figure 3.WWC3 inhibits VSMC proliferation and
migration by upregulating the activity of the Hippo signaling
pathway. (A) Western blotting was used to analyze the expression of
WWC3, phosphorylated LATS1, YAP, p-YAP, CTGF and cyclin E in VSMCs,
following transfection with WWC3 and treatment with PDGF-BB. (B)
VSMCs were transfected with the WWC3 plasmid and subsequently
treated with PDGF-BB. A dual-luciferase reporter assay was used to
determine the transcriptional activity of TEA domain transcription
factors. (C) An MTT assay was used to assess cell proliferation.
(D) A scratch wound healing assay was performed and (E) analyzed,
and a (F) Transwell chamber assay was performed and (G) analyzed in
order to determine cell migration. The results are presented as the
mean ± standard deviation of three independent experiments.
*P<0.05. VSMCs, vascular smooth muscle cells; WWC3, WWC family
member 3; PDGF-BB, platelet-derived growth factor BB; YAP,
YY1-associated protein; p-YAP, phosphorylated YAP; p-LATS1,
phosphorylated large tumor suppressor kinase 1; CTGF, connective
tissue growth factor; NC, negative control. |
WWC3 expression inhibits the
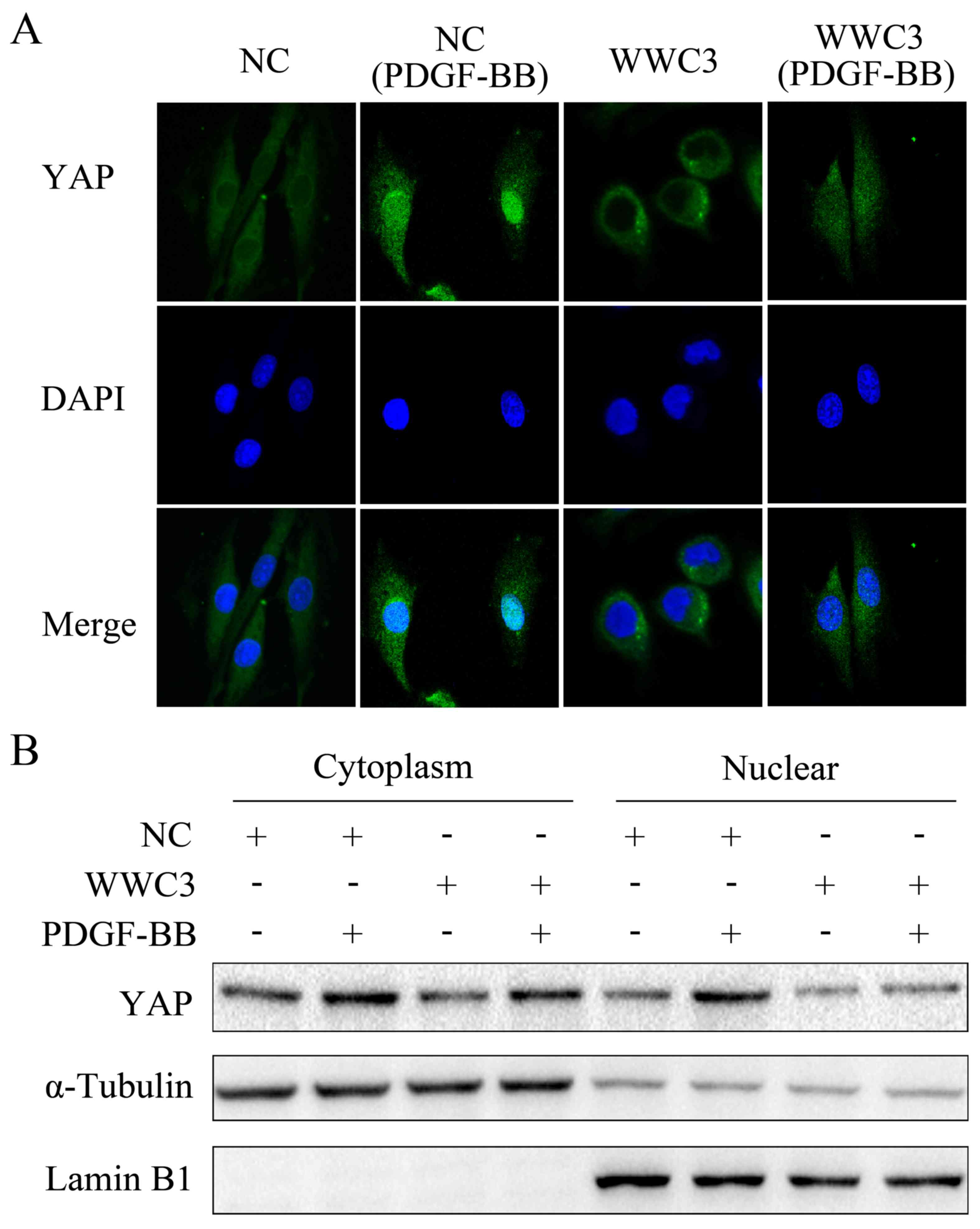
translocation of YAP to the nucleus in VSMCs
Numerous studies have demonstrated that the key
process associated with the activation of the Hippo signaling
pathway is YAP translocation to the nucleus. In the present study,
the expression of YAP was revealed to be enhanced following
treatment with PDGF-BB. Immunofluorescence staining demonstrated
that the location of YAP in the nucleus was markedly increased
following treatment with PDGF-BB, whereas the level of nuclear YAP
was suppressed following overexpression of WWC3. Following PDGF-BB
treatment, YAP expression in VSMC cells that did not overexpress
WWC3 was demonstrated to be more predominantly localized in the
nucleus, compared with VSMC cells overexpressing WWC3 (Fig. 4A). Western blot analyses of the
cytoplasmic and nuclear expression of YAP in VSMCs overexpressing
WWC3, following treatment with PDGF-BB, demonstrated the same
result (Fig. 4B). These results
suggested that WWC3 regulated the localization of YAP in VSMCs
following treatment with PDGF-BB, suppressed YAP translocation into
the nucleus and suppressed the expression of downstream genes in
the Hippo signaling pathway.
WWC3 regulates the Hippo signaling
pathway via interaction with LATS1
Immunoprecipitation analyses were performed, and it
was demonstrated via immunofluorescence staining that WWC3 and
LATS1 were colocalized in the cytoplasm (Fig. 5A). Additionally, it was revealed
that WWC3 may interact with LATS1 (Fig. 5B). In order to further investigate
the interaction between WWC3 and LATS1, a WWC3 mutant plasmid with
deletion of the WW domain was constructed (Fig. 5C). The results of the
immunoprecipitation analyses demonstrated that WWC3 was no longer
able to interact with LATS1 in the absence of the WW domain
(Fig. 5D). Furthermore, western
blot analysis revealed that the expression levels of p-LATS1,
p-YAP, YAP, CTGF and cyclin E in cells transfected with WWC3 with a
deletion of the WW domain (WWC3-ΔWW) did not markedly alter
compared with the control group (Fig.
5E). These results suggested that WWC3 may regulate the Hippo
signaling pathway via interaction with LATS1.
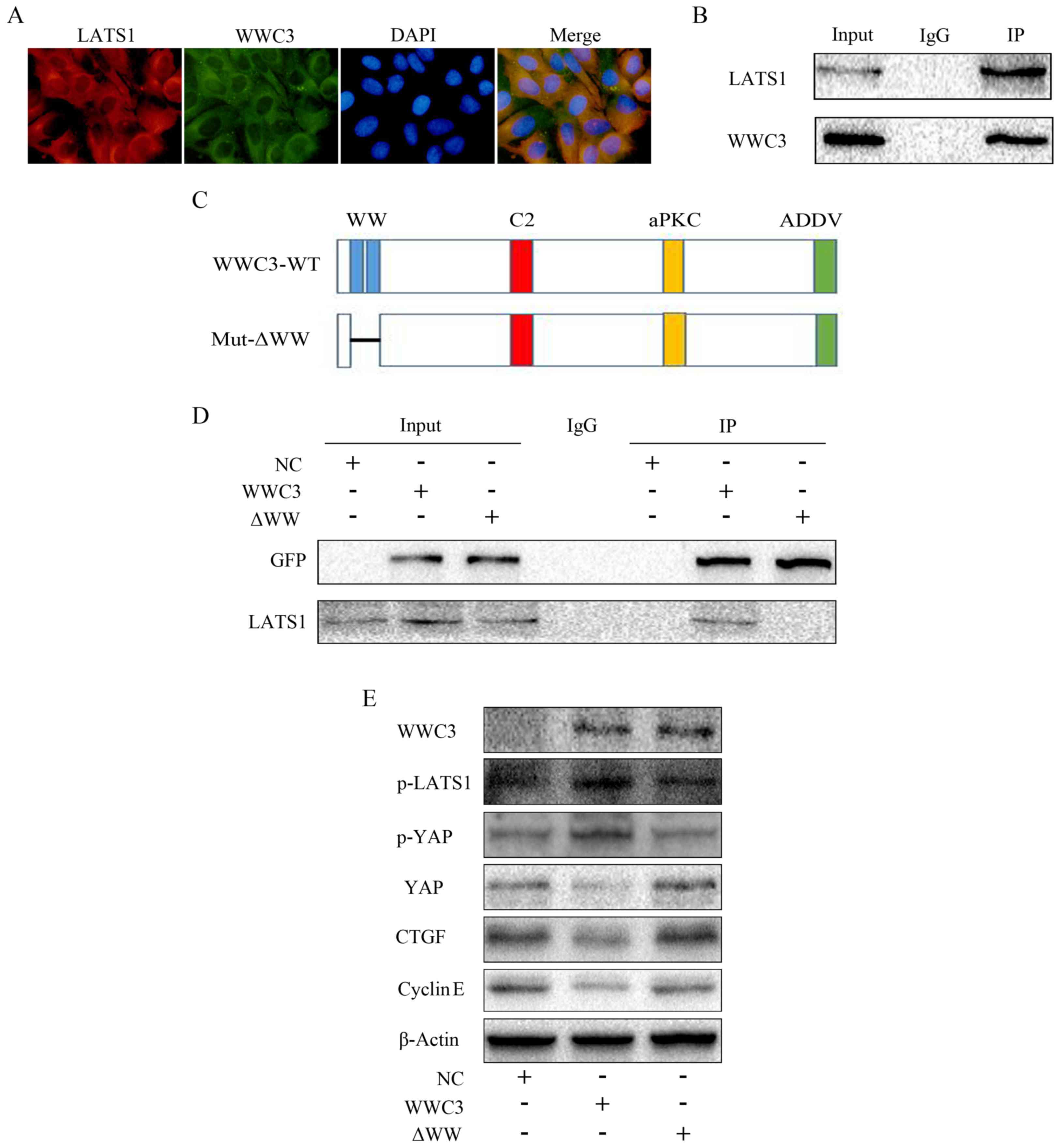 | Figure 5.WWC3 regulates the Hippo signaling
pathway via interaction with LATS1. (A) Colocalization of WWC3 and
LATS1 was observed using immunofluorescence staining
(magnification, ×400). (B) Immunoprecipitation demonstrated that
WWC3 interacted with LATS1 at the endogenous level. WWC3 is the
pulled down protein. (C) Schematic diagram of the structure of
pEGFP-C2-WWC3 WT and pEGFP-C2-ΔWW. (D) Immunoprecipitation results
demonstrated that WWC3 did not interact with LATS1 following
transfection with an empty plasmid and WWC3-ΔWW. (E) The expression
of proteins associated with the Hippo signaling pathway following
transfection with an empty plasmid, WWC3 or WWC3-ΔWW. WWC3, WWC
family member 3; PDGF-BB, platelet-derived growth factor BB; YAP,
YY1-associated protein; p-YAP, phosphorylated YAP; LATS1, large
tumor suppressor kinase 1; p-LATS1, phosphorylated LATS1; CTGF,
connective tissue growth factor; NC, negative control; IP,
immunoprecipitation; IgG, immunoglobulin G; Mut, mutant; ΔWW,
deletion of WW domain; EGFP, enhanced green fluorescent protein;
ADDV, C-terminal PDZ-binding motif; aPKC, atypical protein kinase
C. |
Discussion
The role of the Hippo signaling pathway in cell
development and tumorigenesis has been extensively studied;
(4–6) however, other studies have suggested
that it has an important role in the formation and development of
cardiovascular system (11–13).
A number of studies have also demonstrated that knockdown of LATS2
and MST1/2 results in heart overgrowth and cardiomyocyte
proliferation (11). Further
studies have revealed that the Hippo signaling effector YAP is
involved in the development of the cardiovascular system and is an
important regulator of cardiomyocyte proliferation, cardiac
morphogenesis, myocardial trabeculation and vascular development
(19,20). Furthermore, numerous animal models
of cardiovascular disease have demonstrated that MST, in addition
to a number of LATS targets (including microRNAs), are
over-activated, and the proliferation and migration of VSMCs is
subsequently increased, eventually resulting in vascular remodeling
(21–24). However, the study of the Hippo
pathway in vascular remodeling remains insufficient; associated
factors and mechanisms remain to be determined and thus require
further investigation.
The WWCs protein family, which has a highly
evolutionarily-conserved molecular structure, may form homodimeric
and heterodimeric complexes in order to affect the function and
activity of themselves and other molecules (16). A study using 293T cells
demonstrated that, with regards to structural similarities, all WWC
proteins are able to interact with LATS kinases to negatively
regulate the Hippo signaling pathway via activation of LATS kinases
(16). At present, the majority of
studies have focused on the clinical significance and biological
roles of WWC1 in human cancer; thus, whether WWC3 has an
association with disease pathology remains largely undetermined. To
the best of the authors' knowledge, the present study is the first
to reveal that WWC3 expression is downregulated in VSMCs during
neointimal hyperplasia following injury (treatment with PDGF-BB or
balloon injury), the activity of the Hippo signaling pathway is
suppressed post-injury, YAP translocation to the nucleus is
increased, and the proliferation and migration of VSMCs is enhanced
post-injury. Furthermore, the present study revealed that knockdown
of WWC3 may inactivate LATS1, increase the phosphorylation and
nuclear translocation of YAP, suppress the activity of the Hippo
pathway and enhance the expression of downstream genes, which
subsequently enhances the proliferation and migration of VSMCs.
These results were consistent with the results obtained following
overexpression of WWC3.
YAP, a multifunctional transcriptional coactivator
that predominantly functions as an oncoprotein to promote cell
proliferation and migration, is the primary effector of the Hippo
signaling pathway (25). It was
previously demonstrated that cardiac/SMC-specific YAP knockout mice
exhibited marked cardiac defects and vascular abnormalities
(26). YAP expression is increased
during the VSMC phenotypic switch from a contractile to a synthetic
state, induced by PDGF-BB stimulation during vessel injury. In
addition, YAP overexpression suppresses the expression of VSMC
marker genes, and enhances the proliferation and migration of VSMCs
(12,13). YAP knockout inhibits VSMC
phenotypic modulation otherwise induced by arterial injury, and may
attenuate neointimal formation (12). Overexpression of MST1, a negative
regulator of YAP, may suppress neointimal formation in
balloon-injured rat carotid arteries (23). Such studies have revealed various
functions of YAP and have demonstrated that the most important
regulatory step of the Hippo pathway is the translocation of YAP to
the nucleus. The present study demonstrated that injury enhanced
the total expression of YAP; however, WWC3 overexpression reduced
the expression of YAP and suppressed YAP nuclear import,
subsequently suppressing the expression of downstream genes that
are associated with cell proliferation and migration. These results
suggested that the suppression of YAP nuclear import via WWC may
serve as a novel therapeutic target for the treatment of vascular
diseases.
LATS1 is considered to be a tumor suppressor gene
and may inhibit the proliferation and differentiation of tumor
cells, and induce cellular apoptosis via downregulation of the
primary effector of the Hippo signaling pathway, YAP (27). LATS1/2 directly interact with YAP
and phosphorylate YAP at serine 127 by targeting the HXRXXS motifs
in YAP, and subsequently enhancing YAP cytoplasmic sequestration,
which suppresses the activity of transcription factors (28). In addition, LATS1/2 also
phosphorylate YAP at serine 381, which results in the subsequent
phosphorylation of YAP by casein kinase 1, and the recruitment of
the E3 ubiquitin ligase, resulting in YAP polyubiquitylation and
eventual degradation (29). A
recent study demonstrated that LATS1 is inactivated in vascular
remodeling, and that LATS1 phosphorylation may markedly suppress
vascular remodeling (30). In the
present study, it was revealed that WWC3 affects the
phosphorylation of LATS1, colocalizes with LATS1, and interacts
with LATS1. In the present study, a mutant plasmid of WWC3 with
deletion of the WW domain was constructed, and it was demonstrated
that WWC3 no longer interacted with LATS1 following WW domain
deletion. Furthermore, the levels of p-LATS1, p-YAP, YAP, CTGF and
cyclin E did not significantly alter following transfection with
WWC3-ΔWW. Therefore, it may be hypothesized that the regulatory
effect of WWC3 on the Hippo pathway is dependent on the WWC3-LATS1
interaction via the WW domain.
In conclusion, the present study demonstrated that
WWC3 is able to interact with LATS1 to upregulate the Hippo-YAP
signaling pathway and suppress the proliferation and migration of
VSMCs. This may provide novel therapeutic targets for the
prevention and treatment of vascular remodeling diseases, including
atherosclerotic diseases, vascular restenosis and hypertension.
Further studies are required to investigate the underlying
mechanisms regarding the association between injury and the
suppression of WWC3 expression.
Acknowledgements
The authors would like to thank Dr Enhua Wang for
assistance in the writing of the present manuscript, and Dr Joachim
Kremerskothen for the provision of the WWC3 wild-type and mutant
plasmids.
Glossary
Abbreviations
Abbreviations:
|
VSMC
|
vascular smooth muscle cells
|
|
PDGF-BB
|
platelet-derived growth factor BB
|
|
YAP
|
YY1-associated protein
|
|
CTGF
|
connective tissue growth factor
|
|
LATS
|
large tumor suppressor
|
References
|
1
|
Osherov AB, Gotha L, Cheema AN, Qiang BP
and Strauss BH: Proteins mediating collagen biosynthesis and
accumulation in arterial repair: Novel targets for anti-restenosis
therapy. Cardiovasc Res. 91:16–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodriguez-Menocal L, St-Pierre M, Wei Y,
Khan S, Mateu D, Calfa M, Rahnemai-Azar AA, Striker G, Pham SM and
Vazquez-Padron RI: The origin of post-injury neointimal cells in
the rat balloon injury model. Cardiovasc Res. 81:46–53. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang L, Yang S, Wennmann DO, Chen Y,
Kremerskothen J and Dong J: KIBRA: In the brain and beyond. Cell
Signal. 26:1392–1399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buttitta LA and Edgar BA: How size is
controlled: From hippos to yorkies. Nat Cell Biol. 9:1225–1227.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan D: Hippo signaling in organ size
control. Genes Dev. 21:886–897. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mo JS, Park HW and Guan KL: The Hippo
signaling pathway in stem cell biology and cancer. EMBO Rep.
15:642–656. 2014.PubMed/NCBI
|
|
7
|
Dong J, Feldmann G, Huang J, Wu S, Zhang
N, Comerford SA, Gayyed MF, Anders RA, Maitra A and Pan D:
Elucidation of a universal size control mechanism in Drosophila and
mammals. Cell. 130:1120–1133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han Q, Lin X, Zhang X, Jiang G, Zhang Y,
Miao Y, Rong X, Zheng X, Han Y, Han X, et al: WWC3 regulates the
Wnt and Hippo pathways via Dishevelled proteins and large tumour
suppressor 1, to suppress lung cancer invasion and metastasis. J
Pathol. 242:435–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Doherty TM, Shah PK and Rajavashisth TB:
Cellular origins of atherosclerosis: Towards ontogenetic endgame?
FASEB J. 17:592–597. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heallen T, Zhang M, Wang J,
Bonilla-Claudio M, Klysik E, Johnson RL and Martin JF: Hippo
pathway inhibits Wnt signaling to restrain cardiomyocyte
proliferation and heart size. Science. 332:458–461. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Hu G, Gao X, Wang Y, Zhang W,
Harmon EY, Zhi X, Xu Z, Lennartz MR, Barroso M, et al: The
induction of yes-associated protein expression after arterial
injury is crucial for smooth muscle phenotypic modulation and
neointima formation. Arterioscler Thromb Vasc Biol. 32:2662–2669.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie C, Guo Y, Zhu T, Zhang J, Ma PX and
Chen YE: Yap1 protein regulates vascular smooth muscle cell
phenotypic switch by interaction with myocardin. J Biol Chem.
287:14598–14605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Genevet A, Wehr MC, Brain R, Thompson BJ
and Tapon N: Kibra is a regulator of the Salvador/Warts/Hippo
signaling network. Dev Cell. 18:300–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao L, Chen Y, Ji M, Volle DJ, Lewis RE,
Tsai MY and Dong J: KIBRA protein phosphorylation is regulated by
mitotic kinase aurora and protein phosphatase 1. J Biol Chem.
286:36304–36315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wennmann DO, Schmitz J, Wehr MC, Krahn MP,
Koschmal N, Gromnitza S, Schulze U, Weide T, Chekuri A, Skryabin
BV, et al: Evolutionary and molecular facts link the WWC protein
family to hippo signaling. Mol Biol Evol. 31:1710–1723. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang TR, Yang G and Liu GN: DNA enzyme ED5
depletes egr-1 and inhibits neointimal hyperplasia in rats.
Cardiology. 125:192–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F,
Han X, Feng Y, Zheng C, Wang Z, et al: VGLL4 functions as a new
tumor suppressor in lung cancer by negatively regulating the
YAP-TEAD transcriptional complex. Cell Res. 24:331–343. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morin-Kensicki EM, Boone BN, Howell M,
Stonebraker JR, Teed J, Alb JG, Magnuson TR, O'Neal W and Milgram
SL: Defects in yolk sac vasculogenesis, chorioallantoic fusion, and
embryonic axis elongation in mice with targeted disruption of
yap65. Mol Cell Biol. 26:77–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
von Gise A, Lin Z, Schlegelmilch K, Honor
LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD and Pu
WT: YAP1, the nuclear target of Hippo signaling, stimulates heart
growth through cardiomyocyte proliferation but not hypertrophy.
Proc Natl Acad Sci USA. 109:pp. 2394–2399. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamamoto S, Yang G, Zablocki D, Liu J,
Hong C, Kim SJ, Soler S, Odashima M, Thaisz J, Yehia G, et al:
Activation of mst1 causes dilated cardiomyopathy by stimulating
apoptosis without compensatory ventricular myocyte hypertrophy. J
Clin Invest. 111:1463–1474. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Odashima M, Usui S, Takagi H, Hong C, Liu
J, Yokota M and Sadoshima J: Inhibition of endogenous mst1 prevents
apoptosis and cardiac dysfunction without affecting cardiac
hypertrophy after myocardial infarction. Circ Res. 100:1344–1352.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ono H, Ichiki T, Ohtsubo H, Fukuyama K,
Imayama I, Hashiguchi Y, Sadoshima J and Sunagawa K: Critical role
of Mst1 in vascular remodeling after injury. Arterioscler Thromb
Vasc Biol. 25:1871–1876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Cheng Y, Chen X, Yang J, Xu L and
Zhang C: MicroRNA-31 regulated by the extracellular regulated
kinase is involved in vascular smooth muscle cell growth via large
tumor suppressor homolog 2. J Biol Chem. 286:42371–42380. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sudol M: Yes-associated protein (YAP65) is
a proline-rich phosphoprotein that binds to the SH3 domain of the
Yes proto-oncogene product. Oncogene. 9:2145–2152. 1994.PubMed/NCBI
|
|
26
|
Wang Y, Hu G, Liu F, Wang X, Wu M, Schwarz
J and Zhou J: Deletion of yes-associated protein (YAP) specifically
in cardiac and vascular smooth muscle cells reveals a crucial role
for YAP in mouse cardiovascular development. Circ Res. 114:957–965.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao B, Lei QY and Guan KL: The Hippo-YAP
pathway: New connections between regulation of organ size and
cancer. Curr Opin Cell Biol. 20:638–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim
J, Xie J, Ikenoue T, Yu J, Li L, et al: Inactivation of YAP
oncoprotein by the Hippo pathway is involved in cell contact
inhibition and tissue growth control. Genes Dev. 21:2747–2761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao B, Tumaneng K and Guan KL: The Hippo
pathway in organ size control, tissue regeneration and stem cell
self-renewal. Nat Cell Biol. 13:877–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kudryashova TV, Goncharov DA, Pena A,
Kelly N, Vanderpool R, Baust J, Kobir A, Shufesky W, Mora AL,
Morelli AE, et al: HIPPO-integrin linked kinase cross-talk controls
self-sustaining proliferation and survival in pulmonary
hypertension. Am J Respir Crit Care Med. 194:866–877. 2016.
View Article : Google Scholar : PubMed/NCBI
|