Introduction
With respect to prognosis and mortality, esophageal
squamous cell carcinoma (ESCC) is the 8th most common type of
cancer and the 6th most common cancer-associated cause of premature
mortality (1). Globally, ~450,000
people are affected by ESCC and this incidence is growing (2), with ~500,000 new cases diagnosed each
year (3). The 5-year survival for
patients with ESCC remains low (15–20%) (4). The cure rate of early stage ESCC is
as high as 50% following surgical resection (5), although a number of patients with
ESCC are not candidates for surgery due to comorbid conditions,
including advanced age. In these cases, the 30-day mortality is
2–10% (6).
Numerous studies have revealed that smoking and
pre-diagnosis alcohol consumption are risk factors for ESCC, and
the surgical technique, biological behavior, postoperative
treatment and response to chemoradiotherapies contribute to
improving prognosis (7,8). There are additional genetic
alterations that contribute to the prognosis of ESCC, including
somatic mutations, copy number variations and gene expression
alterations (9). MicroRNAs (miRs)
are useful diagnostic and prognostic indicators for human cancer
(10), and miR-377 suppresses the
initiation and progression of ESCC by inhibiting cluster of
differentiation 133 and vascular endothelial growth factor
(11). miR-1290 and miR-613 are
prognostic factors for patients with ESCC (12,13),
and high expression of miR-103/107 is associated with poor survival
in patients with ESCC (14).
Nevertheless, miRs may cooperate to drive the progression and
prognosis of esophageal carcinoma.
miR signatures may aid in the diagnosis and
prognosis of cancer (15). Feber
et al (16) assessed the
association of miR expression with patient survival and lymph node
metastasis by evaluating miR expression in 45 primary tumors. This
previous study identified that miR profiles have prognostic value
for staging patients with ESCC. The present study screened
signature miRs involved in predicting ESCC using miR-sequencing
(seq) and mRNA datasets from The Cancer Genome Atlas (TCGA;
gdc-portal.nci.nih.gov) and the Gene
Expression Omnibus (GEO; www.ncbi.nlm.nih.gov) database. Subsequently, a
prognostic scoring system was created to identify predictive miRs
using sample risk scores. All cancer samples were divided into
high- and low-risk categories and validated using the scoring
system, and the differentially-expressed genes (DEGs) associated
with miRs were functionally annotated.
Materials and methods
Microarray data
miR-seq and mRNA-seq data from early stage ESCC
samples were downloaded from TCGA on March 18, 2017 and 89 samples
with miR and mRNA expression data were obtained by matching
barcodes. These were early stage (stage I and II) cancer samples.
This dataset was used as a test dataset.
A further two miR expression datasets of ESCC
samples, GSE43732 and GSE13937, were downloaded from the GEO
database. The GSE43732 dataset was based on the platform of
GPL16543, and contained 53 early stage cancer samples. The GSE13937
dataset was based on the platform of GPL8835, and contained 31
early stage cancer samples. These two datasets were used as
validation datasets. Clinical feature data for all downloaded
datasets were also collected (Table
I).
 | Table I.Clinical features of cancer samples
downloaded from TCGA and the Gene Expression Omnibus. |
Table I.
Clinical features of cancer samples
downloaded from TCGA and the Gene Expression Omnibus.
| Clinical feature | TCGA (n=89) | GSE43732 (n=53) | GSE13937 (n=31) |
|---|
| Age, mean ± standard
deviation | 63.02±12.44 | 59.21±9.26 | – |
| Gender |
|
|
|
| Male | 62 | 43 | – |
|
Female | 17 | 10 | – |
| Pathologic_M |
|
|
|
| M0 | 79 | – | – |
| M1 | 0 | – | – |
| Pathologic_N
(/N1) |
|
|
|
| N0 | 64 | 43 | – |
| N1 | 24 | 10 | – |
| Pathologic_T |
|
|
|
| T0 | 1 | – | – |
| T1 | 24 | 7 | – |
| T2 | 33 | 15 | – |
| T3 | 31 | 31 | – |
| Alcohol |
|
|
|
| Yes | 66 | 32 | 23 |
| No | 22 | 21 | 7 |
| Smoking |
|
|
|
|
Yes | 17 | 34 | 23 |
| No | 31 | 19 | 7 |
|
Reformed | 35 | – | – |
| New tumor |
|
|
|
|
Yes | 27 | – | – |
| No | 60 | – | – |
| Radiation
therapy |
|
|
|
|
Yes | 18 | – | 14 |
| No | 65 | – | 17 |
| Mortality |
|
|
|
|
Succumbed | 27 | 24 | 14 |
|
Survived | 67 | 29 | 17 |
| Overall survival
time (months) (mean ± standard) | 20.47±20.59 | 44.7±24.05 | 29.78±20.89 |
Prognostic miRs
The overall prognosis of patients with early stage
ESCC is comparatively good. Samples with a <6-month censor time
are not representative samples for analyzing prognostic factors.
Therefore, miR-seq data samples from TCGA with a survival time of
<6 months were removed to avoid introducing more mixed factors,
and the remaining 77 samples assessed with Cox regression analysis
using the survival package in R (17) to identify prognostic miRs
(threshold of P<0.01 for the log rank test).
Prognostic scoring system
Prognostic miRs were matched with miRs in the
GSE43732 and GSE13937 datasets, and common ones were collected.
Selected miRs were ranked according to log rank P-values to
construct a prognosis scoring system. miRs were added singly
subsequent to the first three, until the highest P-value
representing correlation significance between samples and overall
survival time was obtained. When the P-value was greatest, miRs
were considered to be signature miRs, and the scoring system was
created using these miRs.
Risk scores are used to assess risk factors for
large samples (18). Signature
miRs were used to calculate risk scores for samples in the TCGA
dataset using the following formula:
Risk score = β gene 1 × expr gene 1 + β gene 2 ×
expr gene 2 + … + β gene n × expr gene n, where β gene indicates
the regression coefficients of the gene, and the exp gene indicates
its expression levels.
The risk scores of validation samples (GSE43732 and
GSE13937) were computed, and a median risk score was applied to
stratify low- and high-risk samples. Subsequently, survival
correlation coefficients between low- and high-risk samples in the
TCGA and GEO datasets, and correlations among risk scores, were
assessed. In addition, correlations between clinical features and
sample prognosis were analyzed via Cox regression.
Functional annotation of samples with
different prognosis risks
The matched RNA-seq data was downloaded from TCGA
according to the barcodes of the samples used in the prognostic
miRNA analysis. The RNA-seq data was used to screen the DEGs
between high- and low-risk samples using the limma package in R
(bioconductor.org/packages/release/bioc/html/limma.html)
(19). A false discovery rate
(FDR) of <0.05 was set as the threshold. Correlation
coefficients for gene expression and risk scores were computed, and
positively and negatively-correlated genes were annotated with
respect to significant functional terms, and Kyoto Encyclopedia of
Genes and Genomes (KEGG; www.genome.jp/kegg) pathway terms, using DAVID
(david.ncifcrf.gov) (20).
Results
Prognostic miRs
Using Cox regression analysis on samples that
indicated a survival time of >6 months, 34 prognostic miRs from
the miR-seq dataset were screened and 16 common miRs were
identified between the GSE43732 and GSE13937 datasets (Table II).
 | Table II.Common miRs between
prognosis-associated miRs, and miRs in GSE43732 and GSE13937. |
Table II.
Common miRs between
prognosis-associated miRs, and miRs in GSE43732 and GSE13937.
| miR | P-value |
|---|
| hsa-miR-129-2 | 3.29
×10−05 |
| hsa-miR-34b | 1.86
×10−04 |
| hsa-miR-374a | 1.92
×10−04 |
| hsa-miR-412 | 1.99
×10−04 |
| hsa-miR-140 | 4.66
×10−04 |
| hsa-miR-214 | 5.15
×10−04 |
| hsa-miR-144 | 1.57
×10−03 |
| hsa-miR-376b | 1.59
×10−03 |
| hsa-miR-486 | 1.67
×10−03 |
| hsa-miR-33b | 3.99
×10−03 |
| hsa-let-7f-1 | 6.22
×10−03 |
| hsa-miR-494 | 6.24
×10−03 |
| hsa-miR-33a | 6.37
×10−03 |
| hsa-miR-432 | 6.73
×10−03 |
| hsa-miR-219-1 | 7.88
×10−03 |
| hsa-miR-188 | 9.87
×10−03 |
Prognostic scoring system
To create a prognostic scoring system, common miRs
between prognostic miRs and miRs in the GEO datasets were added
singly following the first three, until the highest P-value
representing connection significance between samples and overall
survival time was obtained.
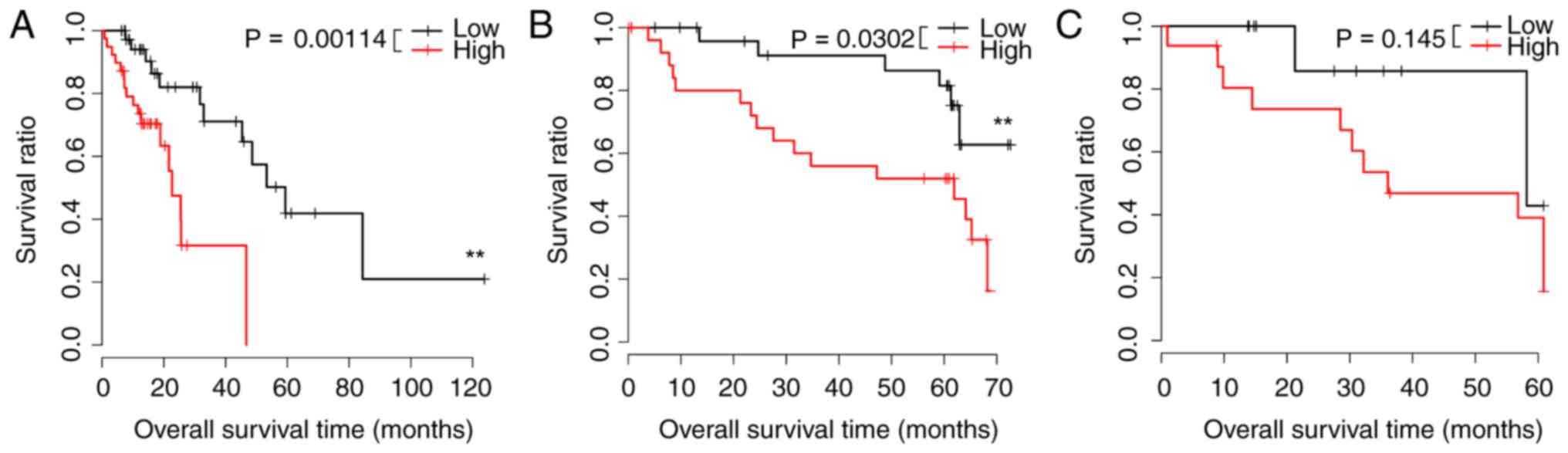
A prognostic scoring system was created using the 10
signature miRs with the greatest P-values, and low-risk samples had
greater survival in the TCGA and GSE43732 datasets. These data
appear in Fig. 1A and B.
Differences in the GSE13937 dataset were not notable (Fig. 1C). Regression analysis revealed
that risk scores were correlated with prognosis (P=0.0141; Table III). Differences in expression
among 10 signature genes in samples stratified by clinical features
were noted, and Table IV shows
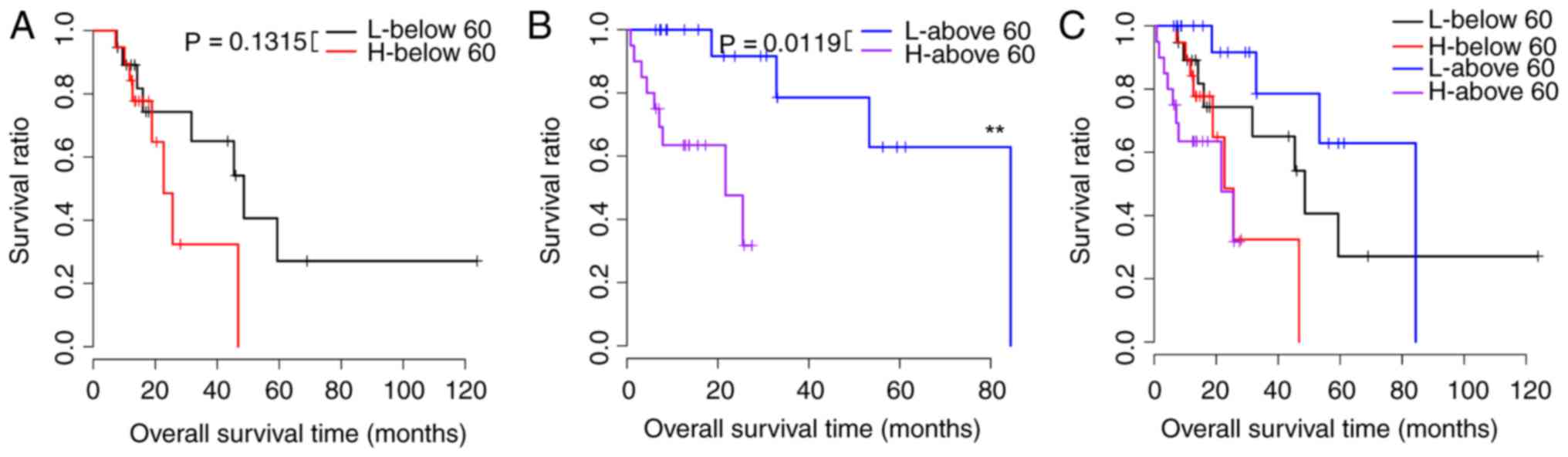
the risk factors that were prognostic for samples with different
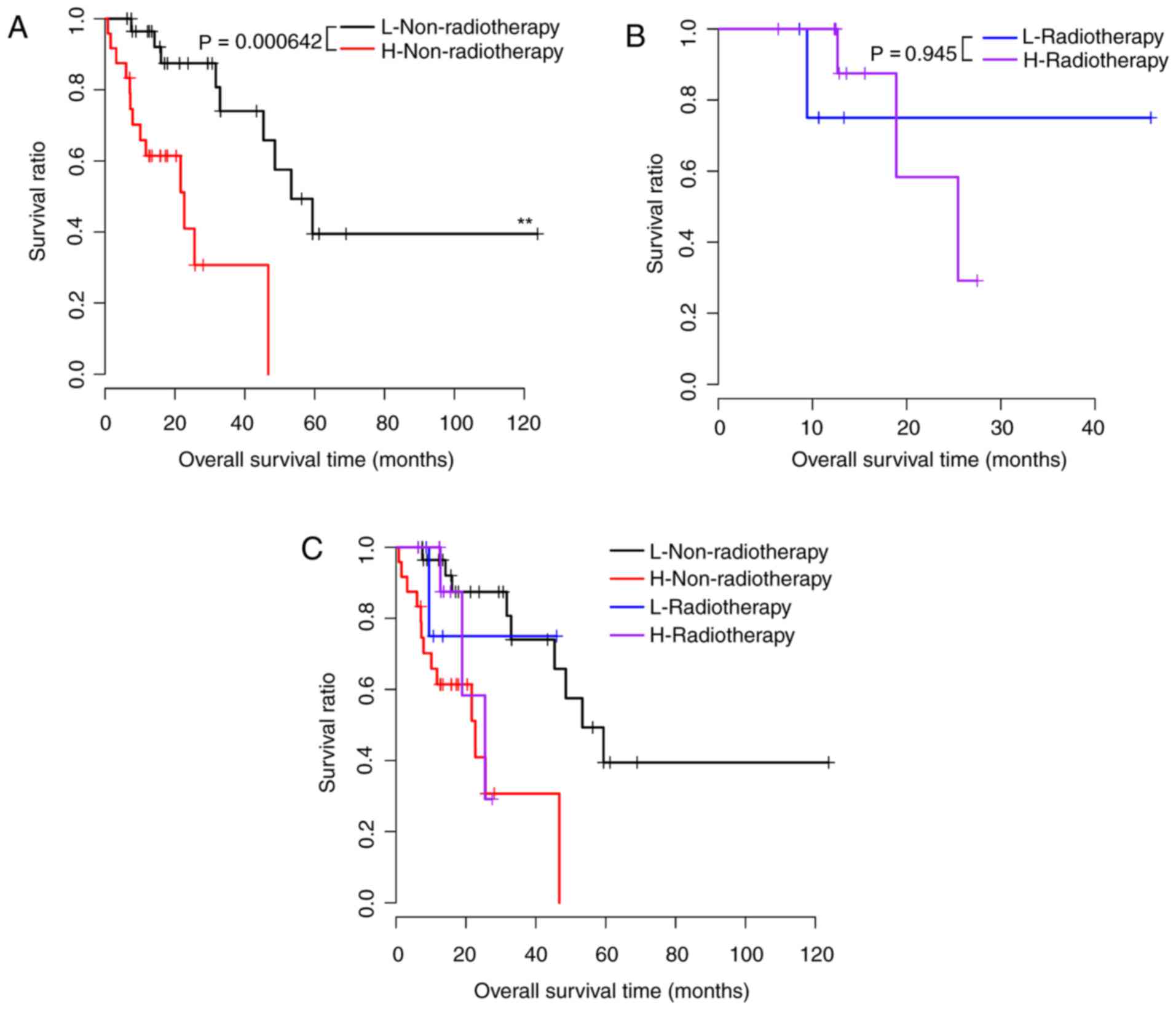
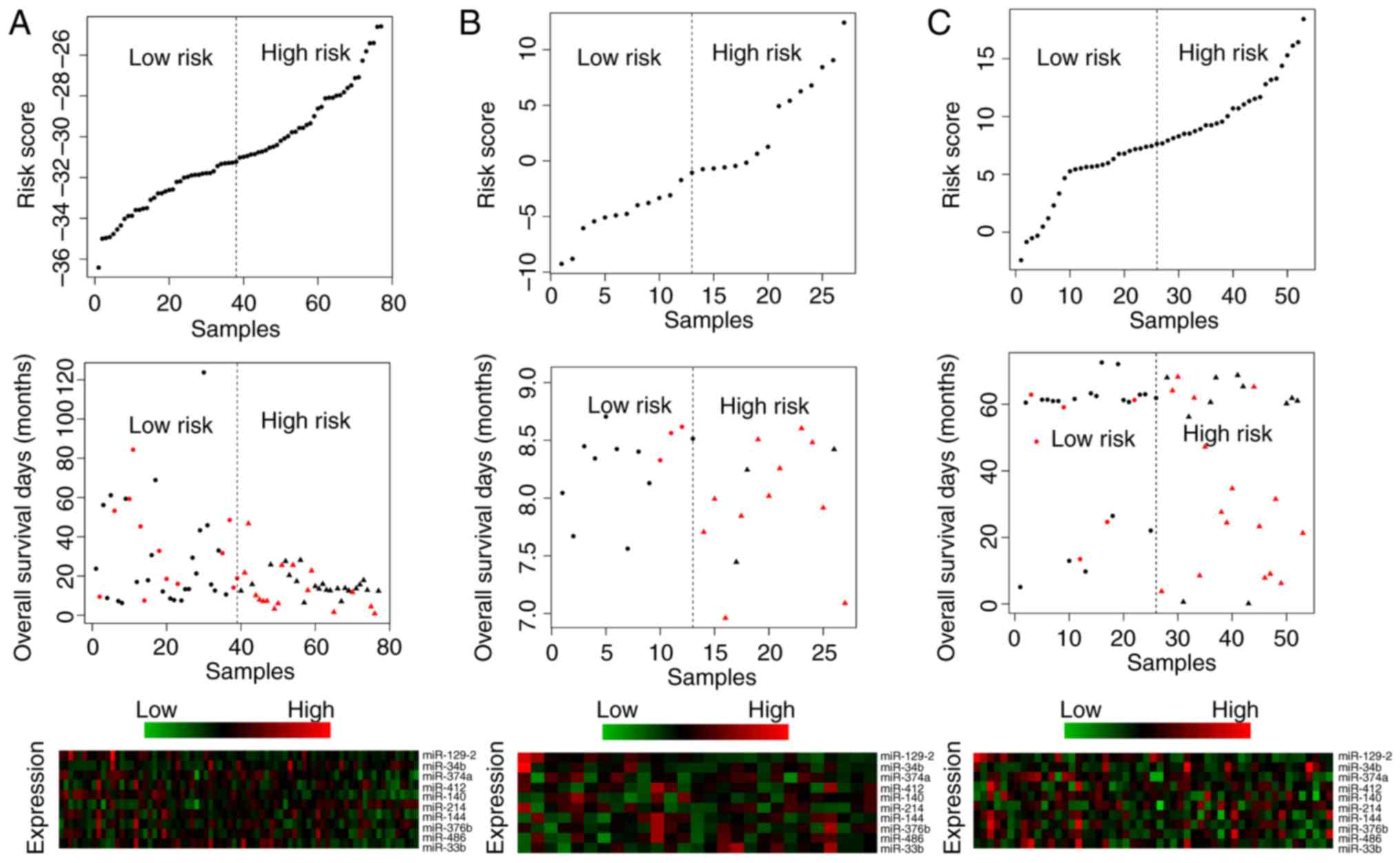
risk scores (P<0.05). Survival curves are presented in Figs. 2–5. Risk scores for samples, survival time
and expression clustering heatmaps of the 10 signature miRs from
the TCGA, GSE13937 and GSE43732 datasets are in Fig. 6.
 | Table III.Cox regression results for the
prognosis-associated clinical features. |
Table III.
Cox regression results for the
prognosis-associated clinical features.
| Clinical
feature | P-value | Hazards regression
(confidence interval) |
|---|
| Age, >60 years
vs. <60 years | 0.97 | 1.016
(0.438–2.356) |
| Sex, male vs.
female | 0.615 | 1.325
(0.442–3.97) |
| Alcohol, yes vs.
no | 0.916 | 0.943
(0.318–2.793) |
| Tobacco, yes vs. no
vs. reformed | 0.56 | 0.872
(0.551–1.382) |
| New tumor, yes vs.
no | 0.726 | 1.168
(0.491–2.778) |
| Radiation therapy,
yes vs. no | 0.9302 | 0.951
(0.3113–2.907) |
| Risk score, high
vs. low | 0.0141 | 1.21
(1.005–1.458) |
 | Table IV.Prognostic factors in high- and
low-risk samples under the same clinical features. |
Table IV.
Prognostic factors in high- and
low-risk samples under the same clinical features.
| Clinical
feature | P-value |
|---|
| Age |
|
| ≥60,
n=39 | 0.0119 |
| ≤60,
n=38 | 0.1315 |
| Gender |
|
| Male,
n=6 | 0.0731 |
| Female,
n=15 | 0.07537 |
| Alcohol |
|
| Yes,
n=59 | 0.002 |
| No,
n=18 | 0.548 |
| Smoker |
|
| Yes,
n=15 | 0.193 |
| No,
n=25 | 0.0253 |
|
Reformed, n=31 | 0.166 |
| New tumor |
|
| Yes,
n=27 | 0.166 |
| No,
n=48 | 0.0175 |
| Radiation
therapy |
|
| Yes,
n=17 | 0.945 |
| No,
n=54 | 0.000642 |
Functional annotation of samples with
different prognosis risks
In total, 168 DEGs were identified, and 58 were
negatively-associated with risk scores, with 110
positively-associated with risk scores. The expression pattern of
the top 20 DEGs positively- and negatively-associated with risk
scores differed significantly between low and high-risk samples
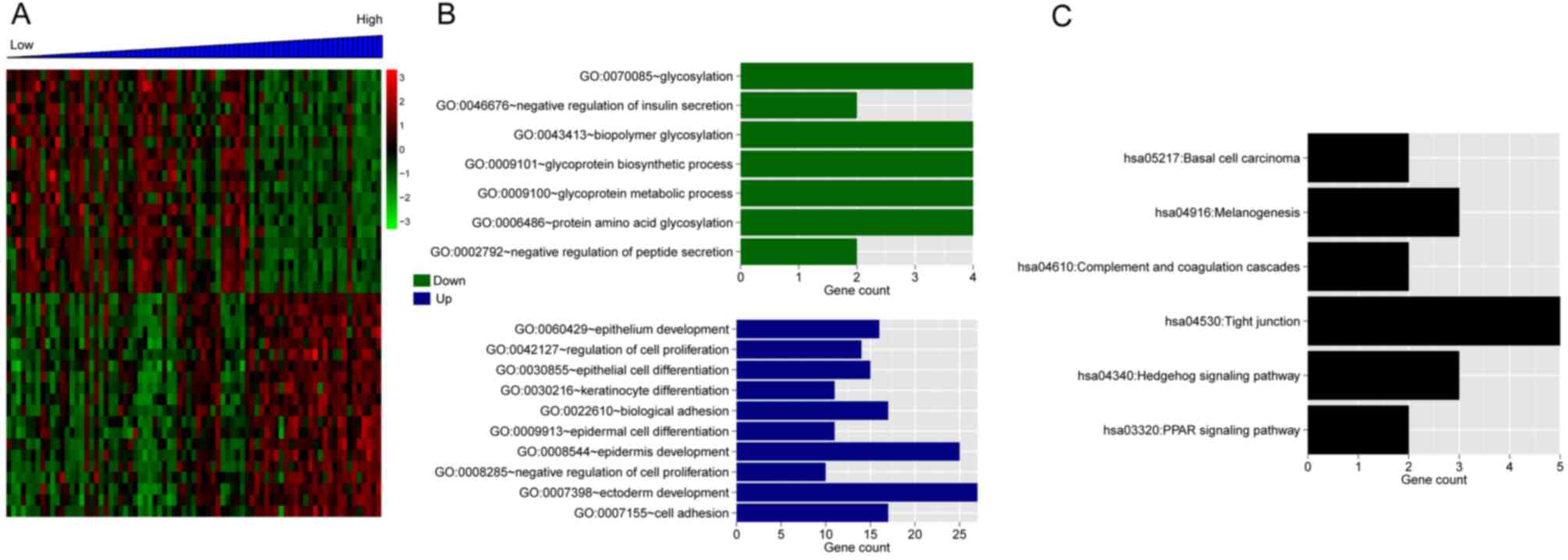
(Fig. 7A). The GO enrichment of
the DEGs is presented in Fig. 7B.
The top 20 positively-associated DEGs were significantly enriched
in six KEGG pathways, including: hsa05217-Basal cell carcinoma,
hsa04916-Melanogenesis, hsa04610-Complement and coagulation
cascades, hsa04530-Tight junction, hsa04340-Hedgehog signaling
pathway and hsa03320-PPAR signaling pathway (Fig. 7C).
Discussion
In order to screen miRs involved in the prognosis of
ESCC, miR-seq and mRNA-seq data for early stage ESCC samples were
downloaded from TCGA, with a further two miR expression datasets,
GSE43732 and GSE13937, downloaded from the GEO database. miR-seq
data samples with a survival time of >6 months were subjected to
Cox regression analysis to assess prognostic value. Common
prognostic miRs, and miRs in the GSE43732 and GSE13937 datasets,
were used for risk score calculations, and a median risk score was
used to stratify low- and high-risk samples. A prognostic scoring
system of 10 signature miRs was made according to survival analysis
between low- and high-risk samples. It was noted that low-risk
samples had greater survival compared with high-risk samples in the
TCGA and GSE43732 datasets. Age, alcohol and tobacco use, and
radiotherapy were prognostic factors for samples with different
risk scores. The present study identified 168 DEGs for all miRs,
110 of which were positively correlated with risk scores. The top
20 positively-correlated and top 20 negatively-correlated DEGs were
significantly enriched in six and 10 functional terms,
respectively. There were six significantly enriched KEGG pathways,
including ‘tight junction’ and ‘melanogenesis’.
Prognostic scoring is used to predict survival and
disease recurrence for a number of types of cancer (21). Wang et al (17) established a 53-gene expression
system to be used to predict overall survival for gastric cancer.
Mao et al (22) created a
12-gene prognostic scoring system to guide adjuvant therapy for
breast cancer. Yang et al (23) created a miR signature to stratify
patients with Barrett's esophagus with different prognostic risks
for targeted chemoprevention.
A number of miRs in the prognostic system used in
the present study have been previously implicated in ESCC or some
other malignant tumors. miR-214, a miR that regulates cancer cell
proliferation, migration and invasion by targeting phosphatase and
tensin homolog in gastric cancer, has been reported to reduce cell
survival via downregulation of Bcl2l2 in cervical cancer cells
(24,25). The predictive value of miR-214 for
prognosis and multidrug resistance has been implicated in ESCC
(26). Overexpression has been
reported to enhance cisplatin sensitivity in ESCC by directly
targeting surviving, and indirectly through CUG triplet repeat RNA
binding protein 1 (27). miR-129-2
suppresses the proliferation and migration of ESCC via
downregulation of SRY-related HMG box 4, and miR-129 is
hypothesized to be a novel therapeutic target and biomarker in
gastrointestinal cancer (28,29).
miR-37a is a prognostic marker for patient survival in early-stage
non-small cell lung cancer (30).
miR-39a has been implicated in cell proliferation, migration and
invasion in gastric cancer by targeting SRC kinase signaling
inhibitor 1 (31). miR-486-5p
expression is frequently decreased in human cancer. Low or
unaltered expression of miR-486-5p compared with neighboring normal
tissues has been demonstrated to be associated with a poor
prognosis, and high expression with a good prognosis, in gastric
cancer (32). miR-486 was observed
to be downregulated in ESCC tissues (33). In patients with ESCC, miR-486-3p
was highly expressed following chemotherapy treatment (34). In conclusion, miR-214, miR-129-2,
miR-37a and miR-486 may predict survival in patients with ESCC,
although these data require validation with larger studies.
Acknowledgements
The present study was funded by the Natural Science
Foundation of China Youth Fund Project of Xinjiang (grant no.
2016D01C322).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
Statistics, 2010: CA Cancer J Clin. 60:1–300. 2010.
|
|
2
|
Herszényi L and Tulassay Z: Epidemiology
of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci.
14:249–258. 2010.PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidenceand mortality worldwide: Sources, methods and major
patternsin GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rice TW, Adelstein DJ, Zuccaro G, Falk GW
and Goldblum JR: Advances in the treatment of esophageal carcinoma.
Gastroenterologist. 5:278–294. 1997.PubMed/NCBI
|
|
6
|
Bennett C, Vakil N, Bergman J, Harrison R,
Odze R, Vieth M, Sanders S, Gay L, Pech O, Longcroft-Wheaton G, et
al: Consensus statements for management of Barrett's dysplasia and
early-stage esophageal adenocarcinoma, based on a Delphi process.
Gastroenterology. 143:336–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim T, Grobmyer SR, Smith R, Ben-David K,
Ang D, Vogel SB and Hochwald SN: Esophageal cancer-the five year
survivors. J Surg Oncol. 103:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Christein JD, Hollinger EF and Millikan
KW: Prognostic factors associated with resectable carcinoma of the
esophagus. Am Surg. 68:258–263. 2002.PubMed/NCBI
|
|
9
|
Wang VE, Grandis JR and Ko AH: New
strategies in esophageal carcinoma: Translational insights from
signaling pathways and immune checkpoints. Clin Cancer Res.
22:4283–4290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang YX and Gao WQ: Roles of microRNAs
during prostatic tumorigenesis and tumor progression. Oncogene.
33:135–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li B, Xu WW, Han L, Chan KT, Tsao SW, Lee
NPY, Law S, Xu LY, Li EM, Chan KW, et al: MicroRNA-377 suppresses
initiation and progression of esophageal cancer by inhibiting CD133
and VEGF. Oncogene. 36:3986–4000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie R, Wu SN, Gao CC, Yang XZ, Wang HG,
Zhang JL, Yan W and Ma TH: Prognostic value of combined and
individual expression of microRNA-1290 and its target gene nuclear
factor I/X in human esophageal squamous cell carcinoma. Cancer
Biomark. 20:325–331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guan S, Wang C, Chen X, Liu B, Tan B, Liu
F, Wang D, Han L, Wang L, Huang X, et al: MiR-613: A novel
diagnostic and prognostic biomarker for patients with esophageal
squamous cell carcinoma. Tumor Biol. 37:4383–4391. 2016. View Article : Google Scholar
|
|
14
|
Guo Y, Chen Z, Zhang L, Zhou F, Shi S,
Feng X, Li B, Meng X, Ma X, Luo M, et al: Distinctive microRNA
profiles relating to patient survival in esophageal squamous cell
carcinoma. Cancer Res. 68:26–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hiroki E, Akahira J, Suzuki F, Nagase S,
Ito K, Suzuki T, Sasano H and Yaegashi N: Changes in microRNA
expression levels correlate with clinicopathological features and
prognoses in endometrial serous adenocarcinomas. Cancer Sci.
101:241–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feber A, Xi L, Pennathur A, Gooding WE,
Bandla S, Wu M, Luketich JD, Godfrey TE and Litle VR: MicroRNA
prognostic signature for nodal metastases and survival in
esophageal adenocarcinoma. Ann Thorac Surg. 91:1523–1530. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang P, Wang Y, Hang B, Zou X and Mao JH:
A novel gene expression-based prognostic scoring system to predict
survival in gastric cancer. Oncotarget. 7:55343–55351.
2016.PubMed/NCBI
|
|
18
|
Zhang CB, Zhu P, Yang P, Cai JQ, Wang ZL,
Li QB, Bao ZS, Zhang W and Jiang T: Identification of high risk
anaplastic gliomas by a diagnostic and prognostic signature derived
from mRNA expression profiling. Oncotarget. 6:36643–36651.
2015.PubMed/NCBI
|
|
19
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang LM, Kevans D, Mulcahy H, O'Sullivan
J, Fennelly D, Hyland J, O'Donoghue D and Sheahan K: Tumor budding
is a strong and reproducible prognostic marker in T3N0 colorectal
cancer. Am J Surg Pathol. 33:134–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mao XY, Lee MJ, Zhu J, Zhu C, Law SM and
Snijders AM: Genome-wide screen identifies a novel prognostic
signature for breast cancer survival. Oncotarget. 8:14003–14016.
2017.PubMed/NCBI
|
|
23
|
Yang H, Gu J, Wang KK, Zhang W, Xing J,
Chen Z, Ajani JA and Wu X: MicroRNA expression signatures in
barrett's esophagus and esophageal adenocarcinoma. Clin Cancer Res.
15:5744–5752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: MiR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cancer Cell Int.
13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang F, Liu M, Li X and Tang H: MiR-214
reduces cell survival and enhances cisplatin-induced cytotoxicity
via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett.
587:488–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y and Hong L: Prediction value of
miR-483 and miR-214 in prognosis and multidrug resistance of
esophageal squamous cell carcinoma. Genet Test Mol Biomarkers.
17:470–474. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Phatak P, Byrnes KA, Mansour D, Liu L, Cao
S, Li R, Rao JN, Turner DJ, Wang JY and Donahue JM: Overexpression
of miR-214-3p in esophageal squamous cancer cells enhances
sensitivity to cisplatin by targeting survivin directly and
indirectly through CUG-BP1. Oncogene. 35:2087–2097. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang M, Li Y, Liu W, Wang R, Tang A, Hao
H, Liu Z and Ou H: miR-129-2 suppresses proliferation and migration
of esophageal carcinoma cells through downregulation of SOX4
expression. Int J Mol Med. 32:51–58. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fesler A, Zhai H and Ju J: miR-129 as a
novel therapeutic target and biomarker in gastrointestinal cancer.
Onco Targets Ther. 7:1481–1485. 2014.PubMed/NCBI
|
|
30
|
Võsa U, Vooder T, Kolde R, Fischer K, Välk
K, Tõnisson N, Roosipuu R, Vilo J, Metspalu A and Annilo T:
Identification of miR-374a as a prognostic marker for survival in
patients with early-stage nonsmall cell lung cancer. Genes
Chromosomes Cancer. 50:812–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu X, Wang W, Su N, Zhu X, Yao J, Gao W,
Hu Z and Sun Y: miR-374a promotes cell proliferation, migration and
invasion by targeting SRCIN1 in gastric cancer. FEBS Lett.
589:407–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen H, Ren C, Han C, Wang D, Chen Y and
Fu D: Expression and prognostic value of miR-486-5p in patients
with gastric adenocarcinoma. PLoS One. 10:e01193842015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu SG, Qin XG, Zhao BS, Qi B, Yao WJ,
Wang TY, Li HC and Wu XN: Differential expression of miRNAs in
esophageal cancer tissue. Oncol Lett. 5:1639–1642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hummel R, Wang T, Watson DI, Michael MZ,
van der Hoek M, Haier J and Hussey DJ: Chemotherapy-induced
modification of microRNA expression in esophageal cancer. Oncol
Rep. 26:1011–1017. 2011.PubMed/NCBI
|