Introduction
Chronic obstructive pulmonary disease (COPD) is a
chronic inflammatory disease, which is characterized by
progressive, partially reversible airflow limitation. COPD is
considered the third most common life-threatening disease
worldwide, and is associated with high morbidity and mortality
(1,2). In addition, COPD is considered to be
not only a respiratory disease, but also a systemic disorder.
Traditional Chinese medicine (TCM) formulas are comprehensive
medicinal compounds that may provide a systemic approach to COPD
therapy (3).
Bufei Yishen formula (BYF) is a TCM formula, which
is composed of 12 medicinal herbs, that has long been used as a
therapeutic agent for the treatment of COPD. In our previous
clinical study, BYF was reported to exert beneficial effects on
measured outcomes in patients with stable COPD over a 6-month
treatment period and a 12-month follow-up period (4). Subsequently, a systems
pharmacological model was constructed by integrating active
compounds prediction, targets prediction and network pharmacology
to identify 216 bioactive ingredients from BYF and 195 potential
targets. Our previous study demonstrated that BYF was effective for
the treatment of rats with COPD and ventricular hypertrophy, due to
its inhibitory effects on the expression of inflammatory cytokines
and hypertrophic factors, protease-antiprotease imbalance and
collagen deposition in vivo (5). However, the systemic mechanism of BYF
in the treatment of rats with COPD remains unclear. Therefore, the
present study aimed to conduct a systems-level analysis of the
therapeutic mechanism of BYF.
High-throughput molecular biological techniques,
including transcriptomic, proteomic and metabolomic approaches,
have been used to explore complex biological processes and the
function of TCM formulas in systems biology. Transcriptomic
profiling is a promising approach to analyze the entire genome,
which provides details regarding the biological processes
underlying respiratory disease development and medical intervention
(6). Proteomic profiling has been
used to uncover the complexity of the therapeutic effects of TCM
formulas by analyzing expressed proteins and protein function in a
cellular context (7). Furthermore,
metabolomic profiling provides data-rich information regarding the
metabolic alterations that occur as a consequence of the
transcriptome and proteome, which reflects the genetic, epigenetic,
and environmental factors that influence cellular physiology
(8). Therefore, combining
transcriptomics, proteomics and metabolomics has the potential to
provide a system-wide understanding of the complex therapeutic
processes of TCM formulas (9,10).
In our previous studies, the transcriptomic and
metabolomic profiles of rats with COPD and BYF-treated rats were
generated (11,12). The present study aimed to further
analyze the molecular mechanisms of BYF on rats with COPD using
proteomic datasets. Subsequently, systems pharmacology,
transcriptomics, proteomics and metabolomics datasets were
integrated, with the aim of providing a system-wide understanding
of the molecular mechanisms underlying the therapeutic effects of
BYF on rats with COPD.
Materials and methods
Chemicals and animals
Klebsiella pneumoniae (strain ID: 46114) was
obtained from the National Center for Medical Culture Collections
(Beijing, China). Tobacco (Hongqi Canal® Filter tip
cigarette; tobacco type: Tar, 10 mg; nicotine content, 1.0 mg;
carbon monoxide, 12 mg) was purchased from China Tobacco Henan
Industrial Co., Ltd. (Zhengzhou, China). A total of 32
Sprague-Dawley rats (16 male and 16 female; weight, 200±20 g; age,
6–8 weeks) were obtained from the Experimental Animal Center of
Henan Province (Zhengzhou, China). The rats were housed in an
animal room at a constant temperature (25±2°C) under a 12-h
light/dark cycle with free access to food and water. The present
study was approved by the Experimental Animal Care and Ethics
Committee of The First Affiliated Hospital, Henan University of
Traditional Chinese Medicine (Henan, China), and the methods were
conducted in accordance with the approved guidelines of the
Experimental Animal Care and Ethics Committee of The First
Affiliated Hospital, Henan University of Traditional Chinese
Medicine (register no. 2012HLD-0001).
COPD model and drug
administration
The COPD rat model and BYF formula were prepared as
previously described (13).
Briefly, 22 rats (COPD group) were maintained in a closed box and
were exposed to tobacco and repeated K. pneumoniae
infections. The control group rats were untreated. At the end of
week 8, two COPD rats were sacrificed for lung tissue collection,
in order to validate that the rat model was successful. The herbal
drugs contained within BYF were provided by the Department of
Pharmacology, The First Affiliated Hospital, Henan University of
Chinese Medicine, and were prepared in fluid extract. The
components of BYF were as follows: Ginseng Radix et Rhizoma, 9 g;
Astragali Radix, 15 g; Corni Fructus, 12 g; Lycii Fructus, 12 g;
Schisandrae Chinensis Fructus, 9 g; Epimedii Herba, 9 g;
Fritillariae Thunbergii Bulbus, 9 g; Paeoniae Rubra Radix, 9 g;
Pheretima, 12 g; Perillae Fructus, 9 g; Ardisiae Japonicae Herba,
15 g; and Citri Reticulatae Pericarpium, 9 g (5). On week 9, COPD rats were divided to
two groups (10 rats each group) and intragastrically treated with
normal saline (2 ml) or BYF (4.44 g/kg, 0.5 g/ml) every day between
weeks 9 and 20. The control group (10 rats) were also
intragastrically treated with normal saline (2 ml) for the same
time period. On week 20, all rats were sacrificed, and lung tissues
were collected.
Protein expression analysis
Proteins were isolated from the lung tissue from
each of the three experimental groups. Briefly, the lung tissues
were lysed in lysis buffer [4% SDS, 0.1 M DTT, 0.1 M Tris (pH 8.0)]
and homogenized using a mechanical homogenizer (Retsch Technology
GmbH, Haan, Germany). The lysates were cleared by centrifugation at
12,000 × g and 4°C for 5 min, prior to storage at −80°C until
further use. For proteolytic digestion, trypsin (Roche Diagnostics
GmbH, Mannheim, Germany) solution was added to the proteins and
incubated for 24 h at 37°C. Subsequently, each of the samples (30
µl) was individually reconstituted with 70 µl isopropanol, vortexed
for 1 min at room temperature. Tryptic peptides were labeled with
8-plex isobaric tags (AB Sciex Germany GmbH, Darmstadt, Germany)
for relative quantitation according to the manufacturer's
protocol.
Strong cation exchange fractionation was performed
on a Shimadzu Prominence liquid chromatography system (Kyoto,
Japan). Buffers A [10 mM KH2PO4 in 25%
acetonitrile (ACN); pH 3] and B (10 mM KH2PO4
and 2 M KCl in 25% ACN; pH 3) were used as the mobile phase. The
peptide mixtures were diluted 10 times with buffer A and 100 µl was
then loaded onto a PolySULFOETHYL A column (5 µm; 100 Å; 100×4.6 mm
i.d.; PolyLC, Columbia, MD, USA). The following gradients was used:
0–1 min, 0–5% B; 1–21 min, 5–30% B; 21–26 min, 30–50% B; 26–31 min,
50% B; 31–36 min, 50–100% B; 36–46 min, 100% B. The flow rate was 1
ml/min and the column temperature was set at 35°C. A total of 23
fractions (2 min/fraction) were collected and desalted with a C18
SPE column (Phenomenex, Torrance, CA, USA). The dried fractions
were then dissolved in 0.1% formic acid (FA) for liquid
chromatography (LC)-mass spectrometry (MS) analysis.
LC-tandem MS analysis was performed on a Prominence
nano LC system (Shimadzu, Kyoto, Japan) coupled on-line to a
micrOTOF-Q II mass spectrometer (Bruker Daltonik, Bremen, Germany).
Water containing 0.1% FA, and ACN containing 0.1% FA were used as
the mobile phases. The peptide samples (~1 µg of each fraction)
were loaded onto a pulled tip column (15 cmx100 µm i.d.) packed
with C18 Reprosil particles (5 µm; Nikkyo Technos Co., Ltd., Tokyo,
Japan). At the flow rate of 300 nl/min, the gradient was as
follows: 5–34% B, 25 min; 34–60% B, 5 min; 60–80% B, 5 min; 80% B,
4 min. Mass spectrometry analysis was operated in the positive mode
and the ion source settings were as follows: spray voltage, 4500 V;
nebulizer pressure, 5 psi; desolvation gas temperature, 200°C. and
all MS and MS/MS spectra were obtained in data-dependent mode with
one MS full-scan ranging from 300–1,800 m/z followed by 20 MS/MS
scans.
The reporter ion ratio for each identified peptide
was analyzed by Mascot (v2.2; Matrix Science, Inc., Boston, MA,
USA). The proteomics data were analyzed by loess and global median
normalization, and then underwent log2 transformation. All
statistical analyses were performed using Student's t-test with the
SPSS 19.0 software package (IBM Corp., Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant difference.
A fold-change >1.0 was considered upregulation, whereas a
fold-change <1.0 was considered downregulation.
Gene/protein set enrichment, network
and pathway analyses
The molecular function of proteins was explored
using the Cytoscape v3.1.1 plugins ClueGO and BiNGO (14,15).
Pathway enrichment analysis of transcripts and proteins were
analyzed using the Database for Annotation, Visualization and
Integrated Discovery (https://david.ncifcrf.gov/) and Kyoto Encyclopedia of
Genes and Genomes (KEGG; http://www.genome.jp/kegg/) database. Regulated
pathways were considered statistically significant if the P-value
was ≤0.05. For correlation analyses on pathway levels, KEGG
pathways, including the pathways of transcripts, proteins, target
proteins of system pharmacology and metabolomics, were compared.
Pathway enrichment analysis was performed using KEGG and the
aforementioned pathways. In addition, Metscape was used to analyze
the integrated pathway of gene, protein, target proteins of system
pharmacology and metabolomics data (16).
Results and Discussion
Proteomic analysis results of
BYF-treated COPD rats
In our previous study (5), systems pharmacology was used to
identify the bioactive ingredients, and the potential targets of
BYF. In addition, BYF treatment was confirmed to exert beneficial
effects on rats with COPD, due to its inhibitory effects on
inflammatory cytokine expression, protease-antiprotease imbalance
and collagen deposition (5).
Furthermore, we identified molecular alterations at the
transcriptomic and metabolomic level (11,12).
To investigate the system-wide mechanism of BYF in COPD treatment,
the present study examined the effects of BYF on the proteomic
profiles of lung tissues.
Using an LC-MS-based proteomic analysis, 191 and 195
proteins were revealed to be regulated in the COPD model (vs. the
control) and the BYF-treated rats (vs. the COPD model),
respectively. According to a further analysis, the 191 proteins
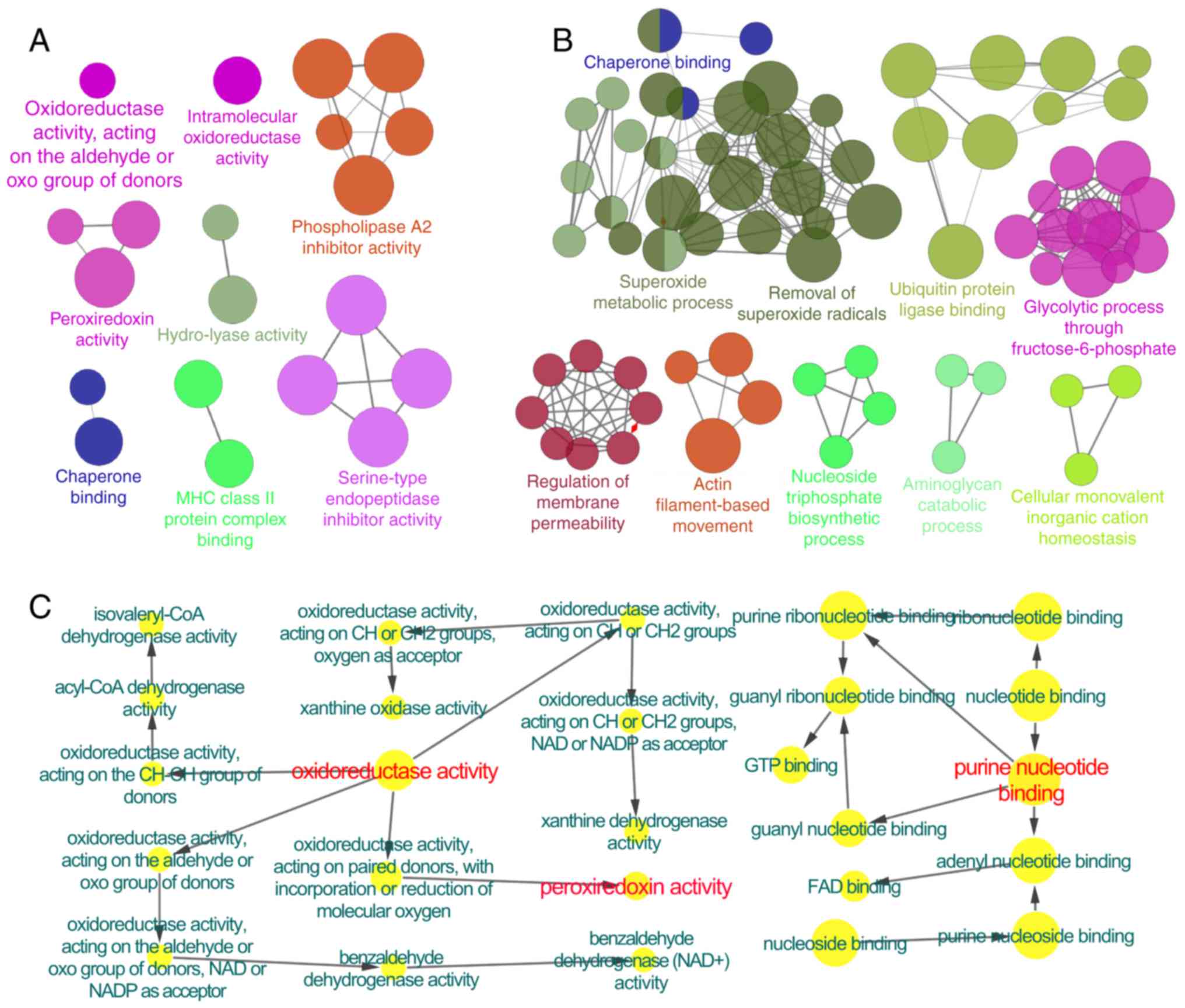
regulated in rats with COPD were predominantly associated with
phospholipase A2 inhibitor activity, peroxiredoxin activity,
oxidoreductase activity and major histocompatibility complex class
II protein complex binding, etc. (Fig.
1A). In BYF-treated rats, the 198 regulated proteins were
attributed to various molecular functions, including superoxide
metabolic process, removal of superoxide radicals and glycolytic
process through fructose-6-phosphate (Fig. 1B). These regulated proteins were
involved in numerous pathways, including focal adhesion, tight
junction, leukocyte transendothelial migration and regulation of
actin cytoskeleton (Tables I and
II).
 | Table I.Pathways associated with the proteins
regulated in lung tissue from rats with chronic obstructive
pulmonary disease. |
Table I.
Pathways associated with the proteins
regulated in lung tissue from rats with chronic obstructive
pulmonary disease.
| Term | Count | % | P-value |
|---|
|
Glycolysis/Gluconeogenesis | 10 | 0.4179 |
6.64×10−6 |
| Hypertrophic
cardiomyopathy | 9 | 0.3761 |
5.29×10−5 |
| Dilated
cardiomyopathy | 9 | 0.3761 |
8.70×10−5 |
| Pyruvate
metabolism | 6 | 0.2507 |
3.62×10−4 |
| Glyoxylate and
dicarboxylate metabolism | 4 | 0.1672 |
7.99×10−4 |
| Tight junction | 9 | 0.3761 | 0.001079 |
| Citrate cycle
(tricarboxylic acid cycle) | 5 | 0.2089 |
0.00125 |
| Leukocyte
transendothelial migration | 8 | 0.3343 | 0.002374 |
| Focal adhesion | 10 | 0.4179 | 0.003903 |
| Tryptophan
metabolism | 5 | 0.2089 | 0.004803 |
| Valine, leucine and
isoleucine degradation | 5 | 0.2089 | 0.006122 |
| Adherens
junction | 6 | 0.2507 |
0.00651 |
| Cardiac muscle
contraction | 6 | 0.2507 | 0.008115 |
 | Table II.Pathways associated with the proteins
regulated in lung tissue from Bufei Yishen formula-treated
rats. |
Table II.
Pathways associated with the proteins
regulated in lung tissue from Bufei Yishen formula-treated
rats.
| Term | Count | % | P-value |
|---|
| Focal adhesion | 13 | 0.5527 |
8.96×10−5 |
| Tight junction | 10 | 0.4252 |
3.02×10−4 |
| Hypertrophic
cardiomyopathy | 8 | 0.3401 |
4.79×10−4 |
| Regulation of actin
cytoskeleton | 12 | 0.5102 |
6.92×10−4 |
|
Glycolysis/Gluconeogenesis | 7 | 0.2976 | 0.002728022 |
| Leukocyte
transendothelial migration | 8 | 0.3401 | 0.003040578 |
| Dilated
cardiomyopathy | 7 | 0.2976 | 0.003855419 |
| Adherens
junction | 6 | 0.2551 | 0.007806313 |
| Extracellular
matrix-receptor interaction | 6 | 0.2551 | 0.011328586 |
| Glyoxylate and
dicarboxylate metabolism | 3 | 0.1276 | 0.016548236 |
| Metabolism of
xenobiotics by cytochrome P450 | 5 | 0.2126 | 0.017848404 |
| Prion diseases | 4 | 0.1701 | 0.020435392 |
| Pyruvate
metabolism | 4 | 0.1701 | 0.027198439 |
| Drug
metabolism | 5 | 0.2126 | 0.032368833 |
| Fatty acid
metabolism | 4 | 0.1701 | 0.032961744 |
| Tryptophan
metabolism | 4 | 0.1701 | 0.035013439 |
| Valine, leucine and
isoleucine degradation | 4 | 0.1701 | 0.041555659 |
| Cardiac muscle
contraction | 5 | 0.2126 | 0.041625416 |
Furthermore, the COPD model group (191 proteins)
shared 98 common proteins with the BYF treated-group (195
proteins). Of these 98 proteins, the alterations in the expression
of 61 proteins in the COPD model were suppressed by BYF treatment
(Table III). These proteins were
attributed to numerous biological functions and two pathways,
including oxidoreductase activity, peroxiredoxin activity, purine
nucleotide binding (Fig. 1C), as
well as focal adhesion and the leukocyte transendothelial migration
pathway (data not shown).
 | Table III.Overlapping proteins between the
chronic obstructive pulmonary disease and Bufei Yishen-treated
groups. |
Table III.
Overlapping proteins between the
chronic obstructive pulmonary disease and Bufei Yishen-treated
groups.
| Accession
number | Molecular weight
(kDa) | log2(A/B) | A/B | log2(B/F) | B/F |
|---|
| IPI00190577 | 404 |
0.1 | 1.071773 | −0.1 | 0.933033 |
| IPI00191728 | 48 |
0.3 | 1.231144 | −0.1 | 0.933033 |
| IPI00192301 | 22 |
0.1 | 1.071773 | −0.1 | 0.933033 |
| IPI00193716 | 46 |
0.3 | 1.231144 | −0.2 | 0.870551 |
| IPI00194097 | 54 |
0.3 | 1.231144 | −0.4 | 0.757858 |
| IPI00195516 | 51 |
0.3 | 1.231144 | −0.2 | 0.870551 |
| IPI00196994 | 23 |
0.3 | 1.231144 | −0.1 | 0.933033 |
| IPI00197770 | 56 | −0.1 | 0.933033 |
0.1 | 1.071773 |
| IPI00198887 | 57 | −0.3 | 0.812252 |
0.2 | 1.148698 |
| IPI00200593 | 47 |
0.1 | 1.071773 | −0.2 | 0.870551 |
| IPI00201300 | 44 |
0.1 | 1.071773 | −0.1 | 0.933033 |
| IPI00201561 | 22 |
0.1 | 1.071773 | −0.1 | 0.933033 |
| IPI00203214 | 95 |
0.2 | 1.148698 | −0.5 | 0.707107 |
| IPI00205135 | 77 |
0.3 | 1.231144 | −0.2 | 0.870551 |
| IPI00205332 | 35 |
0.8 | 1.741101 | −0.5 | 0.707107 |
| IPI00206403 | 38 | −0.3 | 0.812252 |
0.4 | 1.319508 |
| IPI00207014 | 45 |
0.1 | 1.071773 | −0.2 | 0.870551 |
| IPI00207146 | 16 | −0.2 | 0.870551 |
0.3 | 1.231144 |
| IPI00208422 | 88 |
0.3 | 1.231144 | −0.3 | 0.812252 |
| IPI00209113 | 226 | −0.1 | 0.933033 |
0.1 | 1.071773 |
| IPI00211448 | 61 | −0.1 | 0.933033 |
0.2 | 1.148698 |
| IPI00212314 | 68 |
0.4 | 1.319508 | −0.3 | 0.812252 |
| IPI00212523 | 20 | −0.6 | 0.659754 |
0.2 | 1.148698 |
| IPI00214457 | 21 | −0.3 | 0.812252 |
0.3 | 1.231144 |
| IPI00215564 | 24 |
0.1 | 1.071773 | −0.1 | 0.933033 |
| IPI00230787 | 29 | −0.3 | 0.812252 |
0.5 | 1.414214 |
| IPI00231423 | 64 | −0.1 | 0.933033 |
0.5 | 1.414214 |
| IPI00231643 | 16 |
0.1 | 1.071773 | −0.3 | 0.812252 |
| IPI00231694 | 146 |
0.3 | 1.231144 | −0.5 | 0.707107 |
| IPI00231825 | 16 |
0.2 | 1.148698 | −0.2 | 0.870551 |
| IPI00231925 | 41 | −0.5 | 0.707107 |
1.1 | 2.143547 |
| IPI00324986 | 51 |
0.1 | 1.071773 | −0.4 | 0.757858 |
| IPI00325189 | 17 |
0.3 | 1.231144 | −0.1 | 0.933033 |
| IPI00326140 | 167 | −0.5 | 0.707107 |
0.3 | 1.231144 |
| IPI00326179 | 50 |
0.3 | 1.231144 | −0.1 | 0.933033 |
| IPI00326972 | 62 |
0.5 | 1.414214 | −0.2 | 0.870551 |
| IPI00327502 | 26 | −0.2 | 0.870551 |
0.2 | 1.148698 |
| IPI00337168 | 58 |
0.6 | 1.515717 | −0.3 | 0.812252 |
| IPI00358087 | 37 | −0.2 | 0.870551 |
0.3 | 1.231144 |
| IPI00360930 | 28 | −0.4 | 0.757858 |
0.8 | 1.741101 |
| IPI00362072 | 45 | −0.2 | 0.870551 |
0.2 | 1.148698 |
| IPI00362755 | 204 | −0.1 | 0.933033 |
0.4 | 1.319508 |
| IPI00363395 | 21 |
0.2 | 1.148698 | −0.1 | 0.933033 |
| IPI00364890 | 42 |
0.6 | 1.515717 | −0.4 | 0.757858 |
| IPI00365929 | 49 | −0.1 | 0.933033 |
0.6 | 1.515717 |
| IPI00368347 | 118 |
0.8 | 1.741101 | −0.4 | 0.757858 |
| IPI00370654 | 29 |
0.3 | 1.231144 | −0.3 | 0.812252 |
| IPI00371990 | 27 | −0.3 | 0.812252 |
0.2 | 1.148698 |
| IPI00372839 | 110 |
0.3 | 1.231144 | −0.1 | 0.933033 |
| IPI00373505 | 39 | −0.2 | 0.870551 |
0.3 | 1.231144 |
| IPI00388249 | 80 | −0.6 | 0.659754 |
0.6 | 1.515717 |
| IPI00389571 | 54 |
0.1 | 1.071773 | −0.1 | 0.933033 |
| IPI00392216 | 68 |
0.1 | 1.071773 | −0.1 | 0.933033 |
| IPI00411230 | 26 | −0.1 | 0.933033 |
0.2 | 1.148698 |
| IPI00421428 | 29 |
0.4 | 1.319508 | −0.4 | 0.757858 |
| IPI00421517 | 53 | −0.1 | 0.933033 |
0.2 | 1.148698 |
| IPI00470288 | 43 |
0.3 | 1.231144 | −0.1 | 0.933033 |
| IPI00471584 | 83 | −0.1 | 0.933033 |
0.1 | 1.071773 |
| IPI00480679 | 48 | −0.7 | 0.615572 |
0.4 | 1.319508 |
| IPI00948384 | 146 |
0.4 | 1.319508 | −0.2 | 0.870551 |
| IPI01016479 | 375 |
0.7 | 1.624505 | −0.1 | 0.933033 |
Association between genes, proteins
and metabolites
In our previous study, 18 samples were randomly
chosen from three experimental groups for gene expression
experiments. According to a cut-off value of P<0.05, 2,463 and
2,292 differentially expressed genes were detected between the
control and COPD groups, and the COPD and BYF-treated groups,
respectively (11). In addition,
49 and 31 regulated metabolites were detected in lung tissues from
COPD rats and BYF treated-rats, respectively (12). The present study aimed to provide a
system-wide view of the therapeutic mechanism of BYF in COPD
treatment by integrating transcriptomics, proteomics and
metabolomics data.
Metscape software was used to investigate the latent
relationships between the gene, protein and metabolite
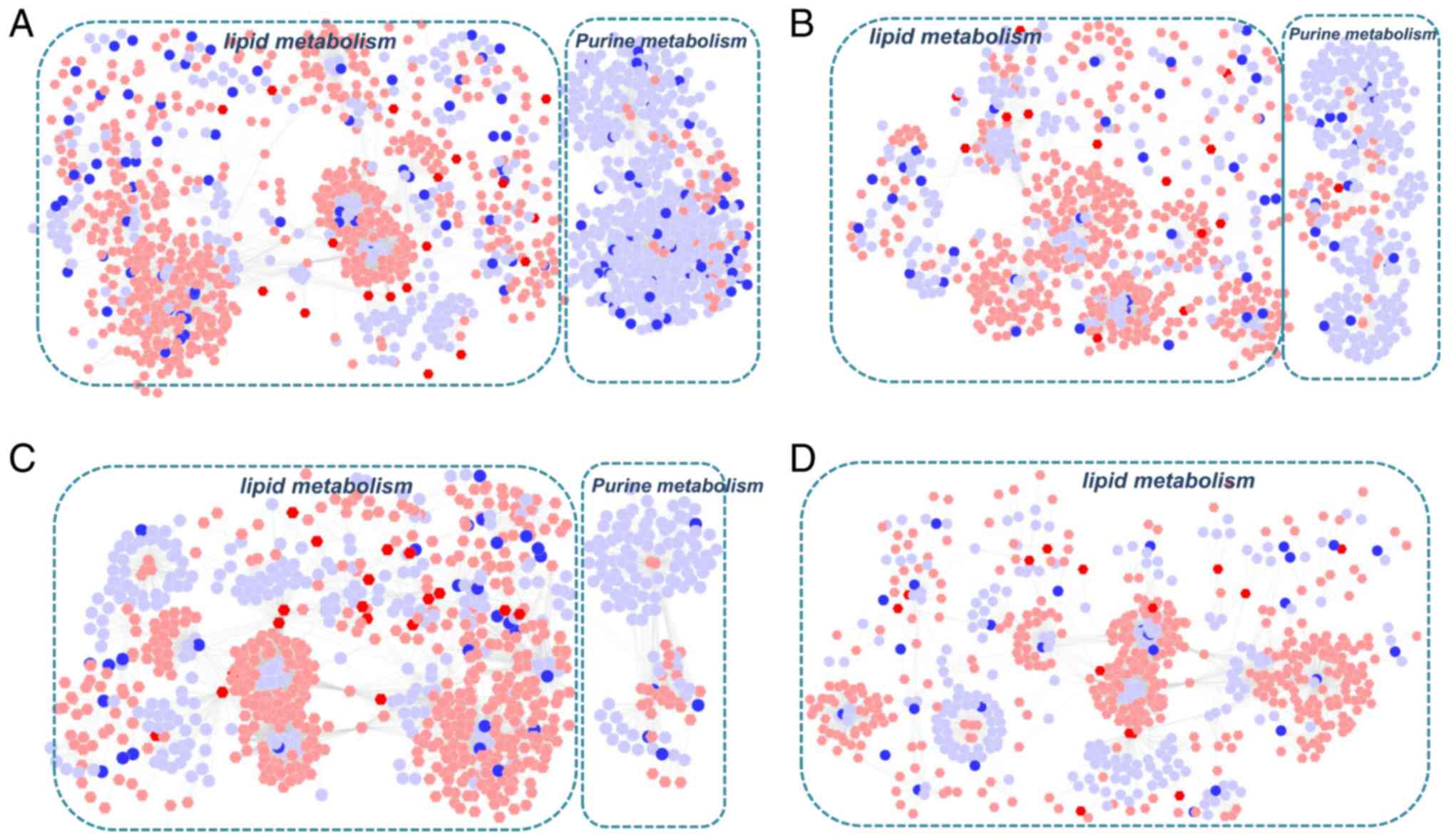
measurements. Initially, two gene-metabolite networks were
generated based on the transcriptomics and metabolomics data of
COPD and BYF-treated rats. As shown in Fig. 2A and B, the metabolite-gene
networks were mainly associated with lipid and purine metabolism.
Subsequently, protein-metabolite networks were generated using the
metabolomics and proteomics data from COPD and BYF-treated rats
(Fig. 2C and D). The results
demonstrated that these proteins and metabolites were also
predominantly associated with lipid and purine metabolism.
Furthermore, the majority of metabolites were involved in lipid
metabolism in the gene/protein-metabolite networks. These findings
suggested that lipid metabolism may be the critical biological
process associated with COPD development and medical
intervention.
Comprehensive analysis of systems
pharmacology, transcriptomics, proteomics and metabolomics
data
Systems pharmacology was previously applied to
identify the active compounds and potential targets of BYF
(5). To provide a more in-depth
understanding of the systemic mechanism of BYF in treating COPD
rats, the systems pharmacology, transcriptomics, proteomics and
metabolomics data were integrated.
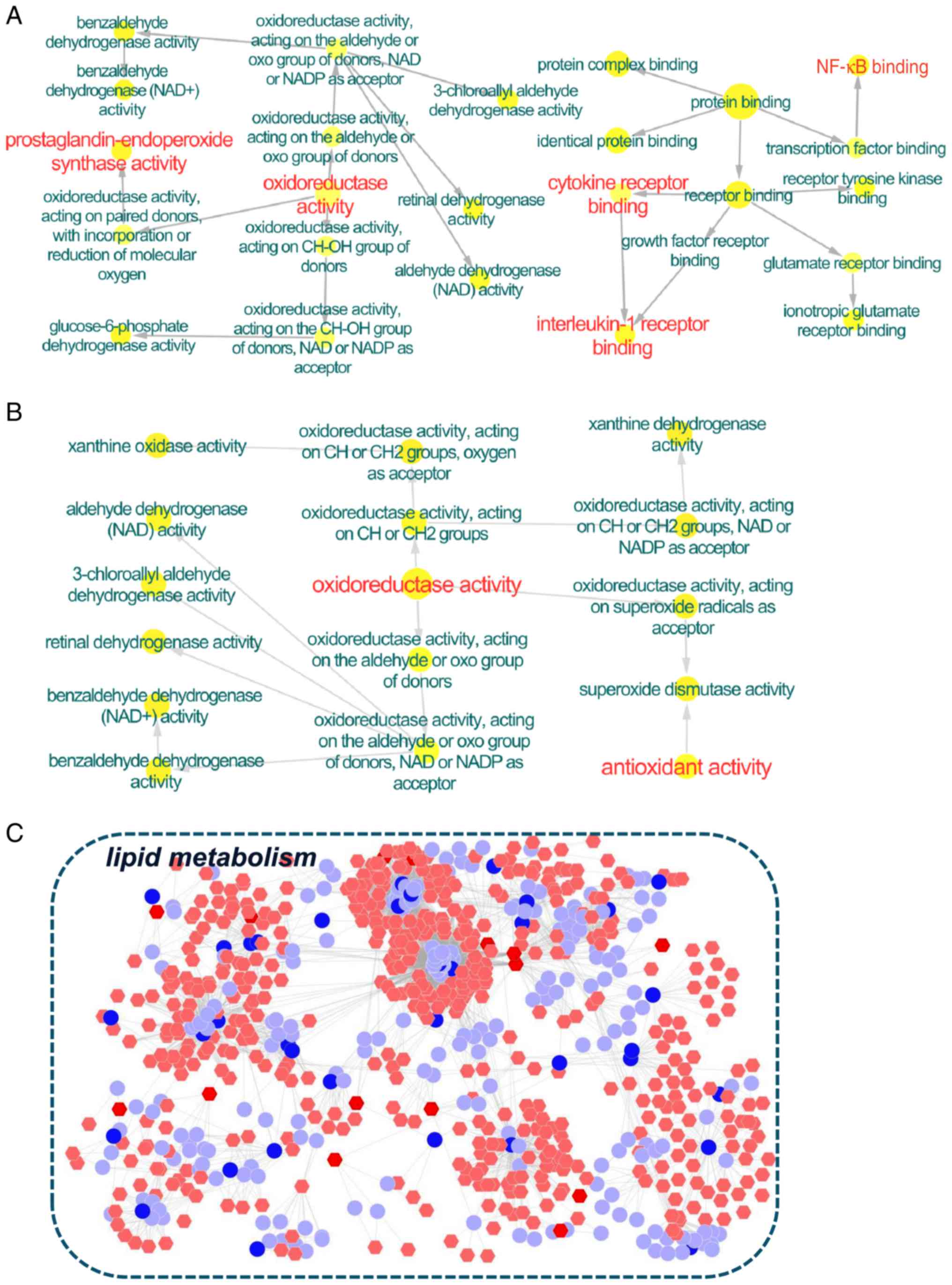
Initially, the direct correlation between the
potential targets and transcripts were analyzed. A total of 10
overlapping proteins [aldehyde dehydrogenase, mitochondrial
(ALDH2); cell division protein kinase 4; checkpoint kinase 1;
dopamine receptor D2; glucose-6-phosphate 1-dehydrogenase (G6PD);
glutathione S-transferase Mu (GSTM)2; interleukin (IL)-1β; vascular
endothelial growth factor receptor 2; glucocorticoid receptor;
prostaglandin G/H synthase (PTGS)1] between the targets of BYF and
the transcripts regulated in the BYF-treated group were detected,
which could be attributed to various molecular functions, including
oxidoreductase activity, nuclear factor (NF)-κB binding and IL-1
receptor binding (Fig. 3A).
Subsequently, 8 overlapping proteins [GSTM1; GSTM2; glutathione
S-transferase P; superoxide dismutase (Cu-Zn) (SOD1); xanthine
dehydrogenase/oxidase; 78 kDa glucose-regulated protein; ALDH2; ATP
synthase subunit β, mitochondrial] were identified between the
potential targets and the proteins regulated in BYF-treated rats
(Fig. 3B). These proteins were
primarily involved in oxidoreductase and antioxidant activity.
Subsequently, the latent correlation between the target proteins
and metabolites regulated in the BYF-treated group was determined
using Metscape software (Fig. 3C).
The results demonstrated that the metabolites and target proteins
were predominantly involved in lipid metabolism.
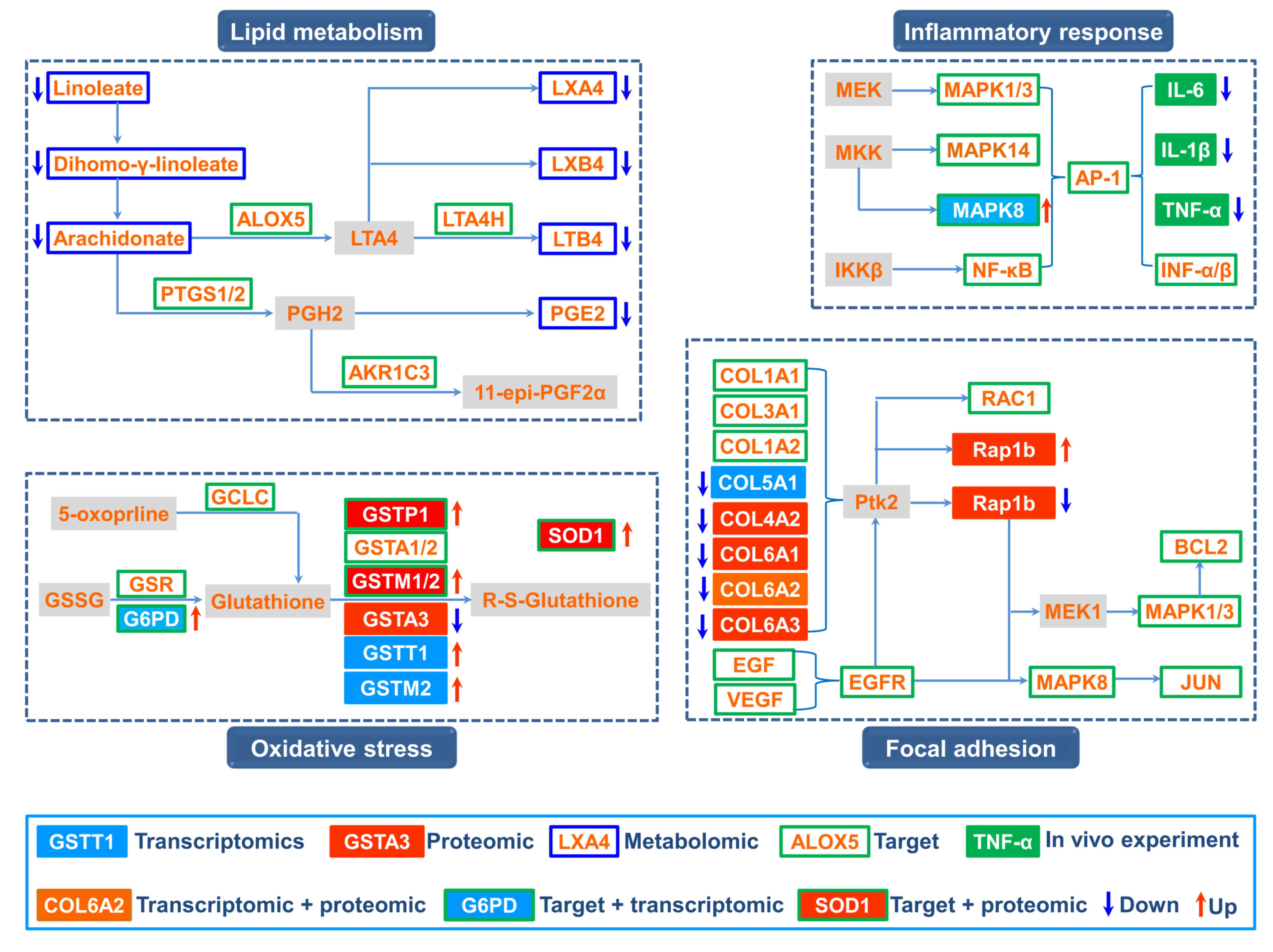
Based on the integrated analysis, a comprehensive
image of the therapeutic mechanism of BYF in COPD treatment was
generated (Fig. 4). The
comprehensive image predominantly consisted of four groups: Lipid
metabolism, inflammatory response, oxidative stress and focal
adhesion.
Our previous study demonstrated that BYF achieved
its ameliorative effect on rats with COPD by suppressing the
expression of inflammatory cytokines, including IL-1β, IL-6 and
tumor necrosis factor-α (5). The
systems pharmacology results indicated that extracellular
signal-regulated kinase (ERK), p38, c-Jun N-terminal kinase (JNK)
and NF-κB were the potential targets of the active compounds
contained in BYF. These data suggested that BYF may decrease the
expression levels of inflammatory cytokines, potentially by
regulating the activation of ERK, p38, JNK and NF-κB.
In our metabolomics study, it was demonstrated that
arachidonic acid metabolism was a significantly dysregulated
pathway; with the metabolites linoleate, dihomo-γ-linoleate,
lipoxin (LX)A4, LXB4, leukotriene (LT)B4, and prostaglandin (PG)E2,
decreased by BYF treatment (12).
Specifically, LXA4, LXB4, LTB4 and PGE2 participated in
inflammatory processes in the airways of patients with COPD
(17,18), and metabolic enzymes, including
arachidonate 5-lipoxygenase, PTGS1/2, LTA4 hydrolase and aldo-keto
reductase family 1 member C3, were potential targets of BYF.
Therefore, these findings indicated that BYF achieved its
anti-inflammatory activity probably through suppressing lipid
metabolism, including arachidonic acid metabolism.
Oxidative stress can trigger sustained inflammatory
responses and is the major contributing factor to obstructive lung
disorders (19,20). In the present study, 8 overlapping
proteins were identified between the targets of BYF and proteomic
measurements of the BYF-treated group, which were predominantly
involved in oxidative stress. For example, the potential targets
(glutamate-cysteine ligase catalytic subunit; glutathione
reductase, mitochondrial; G6PD; glutathione S-transferase P;
glutathione S-transferase A1/2; GSTM1/2), were involved in
glutathione metabolism (21,22).
In addition, the levels of antioxidant proteins, including SOD1,
which were involved in the pathogenesis of COPD, were increased by
BYF treatment (23,24). These results suggested that
regulating oxidative stress status may be one of the main causes of
the anti-inflammatory activity of BYF.
In addition, focal adhesion, which is an overlapping
pathway among proteomic measurements and the potential targets of
BYF (Tables II and IV), was significantly regulated by BYF
treatment. Specifically, the activation of mitogen-activated
protein kinase 1/3 and 8, and JUN, which are potential targets of
BYF, results in upregulation of the transcript levels of
preinflammatory cytokines (25–27).
Taken together, the present study demonstrated that BYF provided
therapeutic benefits against COPD through modulating numerous
biological functions, including lipid metabolism, oxidative stress,
inflammatory response and focal adhesion pathway.
 | Table IV.Associated pathways of the potential
targets of Bufei Yishen formula. |
Table IV.
Associated pathways of the potential
targets of Bufei Yishen formula.
| Term | Count | % | P-value |
|---|
| Neuroactive
ligand-receptor interaction | 30 | 0.7413 | P<0.0001 |
| Amyotrophic lateral
sclerosis | 12 | 0.2965 | P<0.0001 |
| Pathways in
cancer | 27 | 0.6672 | P<0.0001 |
| Drug
metabolism | 12 | 0.2965 | P<0.0001 |
| Calcium signaling
pathway | 19 | 0.4695 | P<0.0001 |
| Bladder cancer | 10 | 0.2471 | P<0.0001 |
| Metabolism of
xenobiotics by cytochrome P450 | 11 | 0.2718 | P<0.0001 |
| Non-small cell lung
cancer | 10 | 0.2471 | P<0.0001 |
| Glutathione
metabolism | 9 | 0.2224 | 0.0001 |
| Colorectal
cancer | 11 | 0.2718 | 0.0001 |
| Small cell lung
cancer | 11 | 0.2718 | 0.0001 |
| Pancreatic
cancer | 10 | 0.2471 | 0.0001 |
| Prostate
cancer | 11 | 0.2718 | 0.0001 |
| Vascular
endothelial growth factor signaling pathway | 10 | 0.2471 | 0.0002 |
| Gap junction | 10 | 0.2471 | 0.0007 |
| Thyroid cancer | 6 | 0.1483 | 0.001 |
| Focal adhesion | 15 | 0.3706 | 0.0011 |
|
Gonadotropin-releasing hormone signaling
pathway | 10 | 0.2471 | 0.0013 |
| Glioma | 8 | 0.1977 | 0.0015 |
| Alzheimer's
disease | 13 | 0.3212 | 0.0015 |
|
Progesterone-mediated oocyte
maturation | 9 | 0.2224 | 0.0022 |
| Prion diseases | 6 | 0.1483 | 0.0024 |
| T cell receptor
signaling pathway | 10 | 0.2471 | 0.0026 |
| Arginine and
proline metabolism | 7 | 0.173 | 0.003 |
| Melanoma | 8 | 0.1977 | 0.003 |
| Fc epsilon RI
signaling pathway | 8 | 0.1977 | 0.0051 |
| Toll-like receptor
signaling pathway | 9 | 0.2224 | 0.0061 |
| Neurotrophin
signaling pathway | 10 | 0.2471 | 0.0065 |
| Nucleotide-binding
oligomerization domain-like receptor signaling pathway | 7 | 0.173 | 0.0065 |
| Apoptosis | 8 | 0.1977 | 0.0093 |
| Adipocytokine
signaling pathway | 7 | 0.173 | 0.0095 |
| Vascular smooth
muscle contraction | 9 | 0.2224 | 0.0111 |
| Insulin signaling
pathway | 10 | 0.2471 | 0.0111 |
| Renal cell
carcinoma | 7 | 0.173 | 0.0117 |
| Endometrial
cancer | 6 | 0.1483 | 0.0132 |
| Mitogen-activated
protein kinase signaling pathway | 15 | 0.3706 | 0.0137 |
| Caffeine
metabolism | 3 | 0.0741 | 0.0142 |
| B cell receptor
signaling pathway | 7 | 0.173 | 0.0161 |
| Arachidonic acid
metabolism | 6 | 0.1483 | 0.0178 |
| Graft-versus-host
disease | 5 | 0.1235 | 0.021 |
| Phenylalanine
metabolism | 4 | 0.0988 | 0.021 |
| Tryptophan
metabolism | 5 | 0.1235 | 0.0228 |
| Oocyte meiosis | 8 | 0.1977 | 0.0302 |
| ErbB signaling
pathway | 7 | 0.173 | 0.031 |
| Tyrosine
metabolism | 5 | 0.1235 | 0.0312 |
| p53 signaling
pathway | 6 | 0.1483 | 0.0374 |
| Epithelial cell
signaling in Helicobacter pylori infection | 6 | 0.1483 | 0.0374 |
| Type II diabetes
mellitus | 5 | 0.1235 | 0.0385 |
| Complement and
coagulation cascades | 6 | 0.1483 | 0.0395 |
Our previous systems pharmacology study identified
the active compounds and potential targets of BYF, and demonstrated
that BYF treatment was able to exert therapeutic effects against
rats with COPD (5). In addition,
the transcriptomic and metabolomic profiles of lung tissues derived
from COPD and BYF-treated rats were generated. In the present
study, a systems biology approach was used to analyze the
therapeutic mechanism of BYF in treating COPD rats. Initially,
proteomic profiles were obtained from the lung tissues of COPD and
BYF-treated rats, and the three levels of omics data were then
integrated. The gene/protein-metabolite model was generated from
the regulated genes, proteins and metabolites, and was used to
identify the affected pathways and to examine them according to the
measured abundances of genes, proteins and metabolites. The results
indicated that these transcripts, proteins and metabolites were
attributed to various functions, including oxidoreductase activity,
antioxidant activity and lipid metabolism. Subsequently, a
comprehensive method was used to integrate the systems pharmacology
and 3-omics data. The system-wide findings demonstrated that BYF
potentially achieved its therapeutic effects over COPD rats by
regulating lipid metabolism, the inflammatory response, oxidative
stress and focal adhesion pathways at the systems level. In
conclusion, the present study suggested that an integrated systems
pharmacology and transcriptomics, proteomics and metabolomics
approach has the potential to considerably advance understanding
regarding the therapeutic mechanism of TCM.
Acknowledgements
The present study was supported by the National
Natural Science Fund of China (grant no. 81130062).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vestbo J, Hurd SS, Agustí AG, Jones PW,
Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ,
Nishimura M, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease GOLD
executive summary. Am J Respir Crit Care Med. 187:347–365. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beran D, Zar HJ, Perrin C, Menezes AM and
Burney P; Forum of International Respiratory Societies working
group collaboration, : Burden of asthma and chronic obstructive
pulmonary disease and access to essential medicines in low-income
and middle-income countries. Lancet Resp Med. 3:159–170. 2015.
View Article : Google Scholar
|
|
3
|
Gan WQ, Man SF, Senthilselvan A and Sin
DD: Association between chronic obstructive pulmonary disease and
systemic inflammation: A systematic review and a meta-analysis.
Thorax. 59:574–580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li SY, Li JS, Wang MH, Xie Y, Yu XQ, Sun
ZK, Ma LJ, Zhang W, Zhang HL, Cao F and Pan YC: Effects of
comprehensive therapy based on traditional Chinese medicine
patterns in stable chronic obstructive pulmonary disease: A
four-center, open-label, randomized, controlled study. BMC
Complement Altern Med. 12:1972012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Zhao P, Li Y, Tian Y and Wang Y:
Systems pharmacology-based dissection of mechanisms of Chinese
medicinal formula Bufei Yishen as an effective treatment for
chronic obstructive pulmonary disease. Sci Rep. 5:152902015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su G, Burant CF, Beecher CW, Athey BD and
Meng F: Integrated metabolome and transcriptome analysis of the
NCI60 dataset. BMC Bioinformatics. 12 Suppl 1:S362011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vogel C and Marcotte EM: Insights into the
regulation of protein abundance from proteomic and transcriptomic
analyses. Nat Rev Genet. 13:227–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan KC, Ipcho SV, Trengove RD, Oliver RP
and Solomon PS: Assessing the impact of transcriptomics, proteomics
and metabolomics on fungal phytopathology. Mol Plant Pathol.
10:703–715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilmes A, Limonciel A, Aschauer L, Moenks
K, Bielow C, Leonard MO, Hamon J, Carpi D, Ruzek S, Handler A, et
al: Application of integrated transcriptomic, proteomic and
metabolomic profiling for the delineation of mechanisms of drug
induced cell stress. J Proteomics. 79:180–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meierhofer D, Weidner C and Sauer S:
Integrative analysis of transcriptomics, proteomics, and
metabolomics data of white adipose and liver tissue of high-fat
diet and rosiglitazone-treated insulin-resistant mice identified
pathway alterations and molecular hubs. J Proteome Res.
13:5592–5602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Yang L, Yao Q, Li Y, Tian Y, Li S,
Jiang S, Wang Y, Li X and Guo Z: Effects and mechanism of bufei
yishen formula in a rat chronic obstructive pulmonary disease
model. Evid Based Complement Alternat Med. 2014:3819762014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang LP, Li JS, Li Y, Tian Y, Li S, Jiang
S, Wang Y and Song X: Identification of metabolites and metabolic
pathways related to treatment with Bufei Yishen formula in a Rat
COPD model using HPLC Q-TOF/MS. Evid-Based Complement Alternat Med.
2015:9567502015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Li SY, Li JS, Deng L, Tian YG, Jiang
SL, Wang Y and Wang YY: A rat model for stable chronic obstructive
pulmonary disease induced by cigarette smoke inhalation and
repetitive bacterial infection. Biol Pharm Bull. 35:1752–1760.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maere S, Heymans K and Kuiper M: BiNGO: A
Cytoscape plugin to assess overrepresentation of gene ontology
categories in biological networks. Bioinformatics. 21:3448–3449.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bindea G, Mlecnik B, Hackl H, Charoentong
P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z and
Galon J: ClueGO: A Cytoscape plug-in to decipher functionally
grouped gene ontology and pathway annotation networks.
Bioinformatics. 25:1091–1093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karnovsky A, Weymouth T, Hull T, Tarcea
VG, Scardoni G, Laudanna C, Sartor MA, Stringer KA, Jagadish HV,
Burant C, et al: Metscape 2 bioinformatics tool for the analysis
and visualization of metabolomics and gene expression data.
Bioinformatics. 28:373–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santus P, Sola A, Carlucci P, Fumagalli F,
Di Gennaro A, Mondoni M, Carnini C, Centanni S and Sala A: Lipid
peroxidation and 5-lipoxygenase activity in chronic obstructive
pulmonary disease. Am J Respir Crit Care Med. 171:838–843. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tulah AS, Parker SG, Moffatt MF, Wardlaw
AJ, Connolly MJ and Sayers I: The role of ALOX5AP, LTA4H and LTB4R
polymorphisms in determining baseline lung function and COPD
susceptibility in UK smokers. BMC Med Genet. 12:1732011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barnes PJ: Cellular and molecular
mechanisms of chronic obstructive pulmonary disease. Clin Chest
Med. 35:71–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sunnetcioglu A, Alp HH, Sertogullarindan
B, Balaharoglu R and Gunbatar H: Evaluation of oxidative damage and
antioxidant mechanisms in COPD, lung cancer, and obstructive sleep
apnea syndrome. Respir Care. 61:205–211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lakhdar R, Denden S, Mouhamed MH, Chalgoum
A, Leban N, Knani J, Lefranc G, Miled A, Ben Chibani J and Khelil
AH: Correlation of EPHX1, GSTP1, GSTM1, and GSTT1 genetic
polymorphisms with antioxidative stress markers in chronic
obstructive pulmonary disease. Exp Lung Res. 37:195–204. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Escribano A, Amor M, Pastor S, Castillo S,
Sanz F, Codoñer-Franch P and Dasí F: Decreased glutathione and low
catalase activity contribute to oxidative stress in children with
α-1 antitrypsin deficiency. Thorax. 70:82–83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rahman I and MacNee W: Antioxidant
pharmacological therapies for COPD. Curr Opin Pharmacol.
12:256–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harju T, Kaarteenaho-Wiik R, Sirvio R,
Sirviö R, Pääkkö P, Crapo JD, Oury TD, Soini Y and Kinnula VL:
Manganese superoxide dismutase is increased in the airways of
smokers' lungs. Eur Respir J. 24:765–771. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong X, Liu Y, Du M, Wang Q, Yu CT and Fan
X: P38 mitogen-activated protein kinase inhibition attenuates
pulmonary inflammatory response in a rat cardiopulmonary bypass
model. Eur J Cardiothorac Surg. 30:77–84. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Y, Meng XM, Jiang GL, Yang YR, Liu
J, Lv XW and Li J: Studies on mitogen-activated protein kinase
signaling pathway in the alveolar macrophages of chronic bronchitis
rats. Mol Cell Biochem. 400:97–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kida Y, Kobayashi M, Suzuki T, Takeshita
A, Okamatsu Y, Hanazawa S, Yasui T and Hasegawa K: Interleukin-1
stimulates cytokines, prostaglandin E2 and matrix
metalloproteinase-1 production via activation of MAPK/AP-1 and
NF-kappaB in human gingival fibroblasts. Cytokine. 29:159–168.
2005. View Article : Google Scholar : PubMed/NCBI
|