Introduction
Human mesangial cell (HMC) proliferation and
expansion occurs in major glomerular diseases and is the main
feature of IgA nephropathy (IgAN) (1). It is generally considered that the
immune complexes containing IgA are found in the glomerular
mesangium, and that IgA1 is secreted by B lymphocytes, mediated by
the process of glycosylation and over aggregation. The abnormal
IgA1 can be recognized by anti-glycan auto-antibodies of the IgA1
and/or IgG isotype, resulting in formation of circulative immune
complexes (CIC) (2,3). The pathogenic CIC deposits in the
glomerular mesangium can promote resident mesangial cells to
secrete proinflammatory factors, initiating glomerular injuries
(4–6).
Classical immunology considers that differentiated B
cells are the unique source of immunoglobulins (Igs). However, this
theory has been challenged over the past decade by increasing
evidence reporting that Igs could be expressed in cancer cells. Qiu
and Yang initially reported the existence of Ig-like protein in
malignant tumor cells in 1996 (7,8).
Later studies by Kimoto and Zheng et al have shown that Igs
transcripts are expressed in human carcinoma cell lines (9), and in human epithelial carcinoma cell
lines (10). Qiu et al also
has reported IgG secretion by epithelial cancer cells, and
demonstrated that its function is to promote growth and survival of
tumor cells (11). Subsequently,
Igs were found to be widely expressed in many types of cancer
cells, including breast cancer, colon cancer, lung carcinomas,
nasopharyngeal carcinoma, abnormal cervical epithelial cells and
oral epithelial tumor cells (12–16).
Unlike B-cell-derived Igs, which are the key molecules for humoral
immune responses, cancerous Igs are associated with various cell
functions, such as cell survival, proliferation, transformation,
metastasis and carcinogenesis (11,13,17–22).
Besides the cancer cells, there is growing evidence
showing that normal cells could also express Igs. Huang et
al reported that several types of Igs are expressed in normal
cells, including IgG expression in brain neurons with classic
V-(D)-J gene rearrangements (23),
Ig µ gene expression and rearrangement in myeloid cells (24), Ig gene expression and rearrangement
in germ cells (25), mammary gland
(26) and hematopoietic
stem/progenitor cells (27). Kang
et al revealed the LOX-1 dependent overexpression of Ig κ in
cardiomyocytes in response to angiotensin II (AngII) (28). Previous results detected the IgG
expression in the eye (29), and
the IgG, IgA, IgM expression in the liver (30) and in the hippocampus (31). These findings demonstrated that
normal cells could express proteins and mRNA transcripts of the
Ig's heavy chains, light chains, and enzymes required for V(D)J
recombination, suggesting a significant role in maintaining the
organs' microenvironment, and regulating the development and
function of cells.
In the present study, we have confirmed that IgA is
expressed in primary human renal mesangial cells (HRMCs) and in the
HMCs, and investigated its potential role on cell apoptosis and
cell adhesion.
Materials and methods
Cell culture
Primary HRMCs (Sciencell Research Laboratories,
Carlsbad, CA, USA) were cultured in mesangial cell medium (MCM)
solution containing 2% FBS, 1% mesangial cell growth supplement,
and 1% penicillin/streptomycin. The materials to culture HRMCs were
purchased from the Sciencell Research Laboratories and cultured
according to the manufacturer's protocol. Cells were maintained in
serum-free medium for 48 h prior to harvesting. Cells were used at
passage nos. 4 to 6.
The HMC line, C2M12, which retains many of the
morphological and physiological features of the normal HMCs
(32,33), was kindly donated by Professor
Youfei Guan (Department of Physiology and Pathophysiology, Peking
University Health Science Center, Peking, China). These cells were
cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal bovine serum (FBS;
Biological Industries USA, Inc., Cromwell, CT, USA), 1% insulin
transferrin selenium-A supplement (ITS-A; Invitrogen; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin, and 100 mg/ml streptomycin,
at 37°C in an atmosphere of 95% air and 5% CO2. Cells
were sub-cultured when reaching 90% confluency with 0.05% trypsin
containing 1 mM EDTA for 20 sec at 37°C. AngII and staphylococcus
(SAC; Sigma-Aldrich, St. Louis, MO, USA) were used to stimulate the
HMCs.
Cell cycle synchronization
Cell cycle synchronization of the HMCs was performed
following the double thymidine block protocol described by previous
studies (34,35). Briefly, HMCs were seeded on 10 cm
culture dishes at a density of 1×105 cells per dish. In
order to collect cells arrested at G1/S phase, the cell culture was
grown until it reached confluence of 50%, then arrested with 2
mmol/l thymidine in complete culture media for 12 h, washed twice
with phosphate-buffered saline (PBS), and recovered in fresh
complete culture media for 12 h, followed by a second arrest with 2
mmol/l thymidine for another 12 h. After the second arrest, the
supernatant was replaced by fresh complete culture media to recover
the cells. A sample of each cell culture was collected on cover
slips every 2 h after the second cell cycle release.
Cell cycle assay
Cell cycle progression was assessed by flow
cytometry based on the DNA content of cells (36). DNA content of cells at distinct
phases of the cell cycle (G0/G1, S, and G2/M phase) was analyzed
using propidium iodide (PI) staining. HMCs were harvested and
washed twice in cold PBS by centrifugation at 800 × g for 5 min.
Cells were then suspended in 100 µl ice-cold PBS at a density of at
least 2×104 cells per tube. 3 ml of ice-cold 70% ethanol
was gradually added to the cell suspension for fixation. The
suspended cells were incubated at 4°C overnight, then filtered
through a 48 µm filter screen, spun at 1,500 × g for 5 min and
washed twice with ice-cold PBS to remove traces of ethanol. RNase
(0.5 mg/ml) was added to degrade RNA at 37°C for 30 min. After
washing twice with 300 µl ice-cold PBS, the cells were suspended in
300 of 50 µg/ml PI staining solution to stain the nuclei and
incubated at room temperature for 5 min in the dark. The cell cycle
data for individual samples was acquired using the BD
LSRFortessa™ flow cytometer equipped with BD
FACSDiva™ software (BD Biosciences, San Diego, CA, USA)
and analyzed using ModFit LT™ software (Verity Software
House, Topsham, ME, USA).
Immunofluorescence
For indirect immunofluorescence staining (IF), HMCs
and HRMCs were cultured on cover slips and fixed in cold acetone
for 5 min. After washing three times with PBS, the slides were
blocked with 5% BSA (Invitrogen; Thermo Fisher Scientific, Inc.)
(diluted with PBS) for 30 min at room temperature and incubated
with the primary antibody (diluted with PBS) at 4°C overnight.
Mouse anti-human Ig α1, Ig α2 antibodies (Southern-Biotech,
Birmingham, AL, USA), mouse anti-human monoclonal Ig κ, Ig λ
antibodies (Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China) were used as the primary antibody; PBS was used as
a blank control. After removing the unbound antibodies by washing
in PBS for three times, the slides were incubated with goat
anti-mouse IgG antibody (Zhongshan Golden Bridge Biotechnology Co.,
Ltd.) and labeled with fluorescein isothiocyanate (FITC) for 1 h at
room temperature in dark. For direct immunofluorescence staining,
the slides were incubated with the mouse anti-human Ig α-FITC
(Zhongshan Golden Bridge Biotechnology Co., Ltd.) in the dark
overnight at 4°C. After washing another three times, the slides
were incubated with DAPI (Vector Laboratories, Inc., Burlingame,
CA, USA) for 2 min at room temperature. Fluorescent signals were
detected with a Confocal Laser Scanning microscopy FV1000 (Olympus,
Tokyo, Japan).
Semi-quantitative reverse
transcription-polymerase chain reaction (SqRT-PCR)
Total RNA of cultured cells was extracted with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and the
concentration was assessed using a Nanodrop spectrophotometer
(Thermo Fisher Scientific, Inc.). Then 1.5 µg of total RNA was
reverse-transcribed to cDNA using the GoScript™ Reverse
Transcriptase (Promega, Madison, WI, USA). PCR was performed with
the primers targeting constant regions of Ig α, Ig κ, Ig λ (Ig Cα,
Ig Cκ, Ig Cλ) and nested PCR was performed with external primers at
the first round and internal primers at the second round targeting
variable region of Ig κ (Ig Vκ). The sequences of primers and
reaction conditions are listed in Tables I and II. Amplification products were separated
in a 1% agarose gel by electrophoresis, including a 100 bp DNA
ladder (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China). The amplified DNA fragments were identified by
their molecular mass, under ultraviolet light observations. Human
peripheral blood mononuclear cells (PBMCs) were used as the
positive control. The peripheral blood was obtained from healthy
donors. PBMCs were isolated from 5 ml peripheral blood using
two-step discontinuous Ficoll/Hypaque (Second Chemistry Factory,
Shanghai, China) density gradient centrifugation. The white
gradient layer containing PBMCs was recovered and washed with 0.01
M PBS, and the isolated PBMCs used immediately for total RNA
extraction (37).
 | Table I.Sequences of polymerase chain
reaction primers used in this study. |
Table I.
Sequences of polymerase chain
reaction primers used in this study.
| Gene name | Primer | Primer sequence
5′-3′ | Product length
(bp) |
|---|
| Igα constant region
(Ig Cα) | Forward |
ACCATGCAGGAGAAGGTGTC | 340 |
|
| Reverse |
TCACTTGCACTGCTGCCTAC |
|
| Igκ constant region
(Ig Cκ) | Forward |
TGAGCAAAGCAGACTACGAGA | 231 |
|
| Reverse |
GGGGTGAGGTGAAAGATGAG |
|
| Igλ constant region
(Ig Cλ) | Forward |
GGGACCAAGCTCACCGTCCTAG | 316 |
|
| Reverse |
TCTTCTCCACGGTGCTCCCTTC |
|
| Igκ variable region
(Ig Vκ) | External
forward |
GACATCGAGCTCACCCAGTCTCC | 360–380 |
|
| External
reverse |
CGGGAAGATGAAGACAGATGGTGC |
|
|
| Internal
forward |
GAAATTGAGCTCACGCAGTCTCCA | 340–360 |
|
| Internal
reverse |
TGGTGCAGCCACAGTTCGTT |
|
| β-actin | Forward |
AGAGCTATGAGCTGCCTGAC | 121 |
|
| Reverse |
AATTGAATGTAGTTTCATGGATG |
|
 | Table II.Reaction conditions of polymerase
chain reaction used in this study. |
Table II.
Reaction conditions of polymerase
chain reaction used in this study.
| Gene name | Initial
denaturation (°C/min) | Denaturation
(°C/sec) | Annealing
(°C/sec) | Extension
(°C/sec) | Cycle number | Extension
(°C/min) |
|---|
| Ig Cα | 94/4 | 94/30 | 62/30 | 72/30 | 35 | 72/10 |
| Ig Cκ | 95/4 | 95/30 | 50/30 | 72/30 | 35 | 72/10 |
| Ig Cλ | 95/4 | 95/30 | 56/30 | 72/30 | 35 | 72/10 |
| Ig Vκ (External
reaction) | 94/5 | 94/30 | 60/30 | 72/30 | 3 | – |
|
|
| 94/30 | 58/30 | 72/30 | 3 | – |
|
|
| 94/30 | 56/30 | 72/30 | 3 | – |
|
|
| 94/30 | 54/30 | 72/30 | 3 | – |
|
|
| 94/30 | 52/30 | 72/30 | 3 | – |
|
|
| 94/30 | 50/30 | 72/30 | 3 | – |
|
|
| 94/30 | 48/30 | 72/30 | 20 | 72/7 |
| Ig Vκ (Internal
reaction) | 94/5 | 94/30 | 60/30 | 72/30 | 35 | 72/7 |
| β-actin | 94/5 | 94/30 | 56/30 | 72/30 | 25 | 72/7 |
Analysis of gene rearrangement
PCR products of Ig Vκ were cloned into a pGEM-T Easy
Vector (Promega) and transfected into the competent E. coli
cell line TOP10 [Tiangen Biotech (Beijing) Co., Ltd., Beijing,
China]. The transcripts of individual clones were amplified. After
DNA sequencing with an ABI 3730XL Genetic Analyzer (Applied
Biosystems; Thermo Fisher Scientific, Inc.) which was performed by
Invitrogen; Thermo Fisher Scientific, Inc., the variable sequences
were compared with the published sequences of the germline gene
segments using the BLAST tool of the National Center for
Biotechnology Information (NCBI).
Western blot analysis
Cultured cells were harvested and washed twice with
cold PBS, then re-suspended in TSD lysis buffer (TSD lysis buffer,
1% SDS; 50 mmol/l, pH 7.5 Tris-HCL, 50 mmol/l DTT), sonicated for 1
min, and lysed for 30 min at room temperature. The protein
concentration of the cell lysate was calculated with a BCA kit
(Applygen Technologies Inc., Beijing, China). After centrifugation
at 12,000 × g for 10 min at 4°C, 5X loading buffer was added to the
lysate, boiled at 100°C for 5 min, and the samples were immediately
used for western blot analysis. The proteins in the culture
supernatant were precipitated with 50% ammonium sulfate,
centrifuged at 12,000 × g for 15 min and then dissolved in PBS. The
collected fraction was filtrated with the AmiconR Ultra-0.5
Centrifugal Filter Devices (EMD Millipore, Billerica, MA, USA) to
remove the ammonium sulfate. The measurement of protein
concentration in the culture supernatant was performed with the
same method used as for the cell lysate. Free Ig in the human serum
and the cultural medium were used as control.
The protein samples were separated by 10% sodium
dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to nitrocellulose membranes. The membranes were
incubated with rabbit anti-IgA antibody (1:1,000), mouse anti-IgA1
antibody (1:1,000), mouse anti-IgA2 antibody (1:1,000), rabbit
anti-Ig κ antibody (1:10,000), and rabbit anti-Ig λ antibody
(1:50,000). The above antibodies were purchased from Abcam
(Cambridge, UK). All the membranes were washed three times with
TBST for 10 min before incubated with secondary antibodies for 1 h
at room temperature. Goat anti-rabbit IgG-IRDyeTM800CW (1:10,000
and goat anti-mouse IgG-IRDyeTM680CW (1:10,000; both from LI-COR
Biosciences, Lincoln, NE, USA) were used as secondary antibodies.
Immunoreactivity was observed with the Odyssey Infrared imager
(LI-COR Biosciences).
IgA1 purification and mass
spectrometry
After the HMCs were cultured in RPMI-1640 with 2%
FBS for 48 h, the culture supernatant was collected as described
above. IgA1 was purified according to the manufacturer's
instructions of jacalin-sepharose (BioVision, Milpitas, CA, USA).
After precipitation of the proteins, the pellet was dissolved in
PBS and filtrated with the AmiconR Ultra-0.5 Centrifugal Filter
Devices (EMD Millipore) to remove the ammonium sulfate and elution
buffer. The purified proteins were separated by 10% SDS PAGE,
detected by western blot analysis as described above, and further
analyzed by mass spectrometry in the Beijing Protein Innovation
Co., Ltd. (Beijing, China).
Cell stimulation with AngII
HMC were seeded on 10 cm culture dishes. When the
sub-cultured HMC reached 70% confluency, cells were cultured in
RPIM-1640 containing 0.5% FBS overnight, followed by treatment with
10−8 mol/l AngII for 24 h or with SAC (1:1,000) for 48
h. A sample from each cell culture was placed on cover slips for
immunostaining and the remaining cells were collected for western
blot analysis, as described above.
Transfection of cultured HMCs with
small interfering RNA (siRNA)
Lipofectamine 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for the transfection with siRNA. siRNAs
directed against different regions of the constant region of the Ig
α1 heavy chain (siRNA-1, siRNA-2 and siRNA-3), against GAPDH
(positive control, PC) and against the nonspecific, scrambled,
control siRNA [negative control (NC)], were designed by GenePharma
Company (Shanghai, China) and the sequences are listed in Table III. HMCs were seeded onto 12-well
culture plate (2×104 cells/well) in mesangial cell
culture media and grown overnight. HMCs were then transfected with
each of 50 nmol/l siRNA mixed with Lipofectamine reagent in
Opti-mem medium (Invitrogen; Thermo Fisher Scientific, Inc.). PBS
was added to the control group. HMCs were harvested after
transfection for 48 h and used for western blot analysis.
 | Table III.Sequences of siRNA used in this
study. |
Table III.
Sequences of siRNA used in this
study.
| siRNA | Direction | Sequence
(5′-3′) |
|---|
| siRNA-1 | Forward |
GCUCUUAGGUUCAGAAGCGTT |
|
| Reverse |
CGCUUCUGAACCUAAGAGCTT |
| siRNA-2 | Forward |
GGAACCAUGGGAAGACCUUTT |
|
| Reverse |
AAGGUCUUCCCAUGGUUCCTT |
| siRNA-3 | Forward |
GCCUUCACACAGAAGACCATT |
|
| Reverse |
UGGUCUUCUGUGUGAAGGCTT |
| Positive
control | Forward |
UGACCUCAACUACAUGGUUTT |
|
| Reverse |
AACCAUGUAGUUGAGGUCATT |
| Negative
control | Forward |
UUCUCCGAACGUGUCACGUTT |
|
| Reverse |
ACGUGACACGUUCGGAGAATT |
Cell apoptosis assay
After transfection for 48 h, cells were collected
with 0.05% trypsin solution and harvested by centrifugation at 800
× g for 5 min. The harvested cells were washed twice with cold PBS.
According to the manufacturer's protocol, 1×106 cells
were suspended in 100 µl of 1X Annexin V binding buffer and stained
with 5 µl of Annexin V-FITC and 5 µl of 7-AAD (both from BD
Biosciences) in the dark for 15 min at room temperature. After
adding another 400 µl binding buffer and filtrating through a 48 µm
filter, cell apoptosis was measured by flow cytometry (BD
Biosciences).
Cell adhesion assay
Cell adhesion rate was analyzed by the Cell Counting
kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). After siRNA transfection for 48 h, 3×104 cells
were re-suspended in culture media and 100 µl were aliquoted in
each well of a 96-well plate and incubated at 37°C for 1 h. 3 wells
of each group were washed gently three times with PBS, and 100 µl
of fresh culture media with 8 µl of CCK-8 reagents were added. In
order to analyze the total cell concentration, CCK-8 was directly
added to 3 different unwashed wells. After incubation for 3 h at
37°C, concentration was determined by measuring absorbance at 450
nm using a spectrophotometric microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). The cell adhesion rate was
calculated as follows:
Cell adhesion
rate=ODwashed–ODblankODunwashed–ODblank
Statistical analysis
Data was expressed as the means ± standard deviation
and analyzed using SPSS 20.0 for Windows (SPSS, Inc., Chicago, IL,
USA). The differences between experimental groups were analyzed
with one-way analysis of variance, followed by a Least Square
Difference multiple comparison test. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
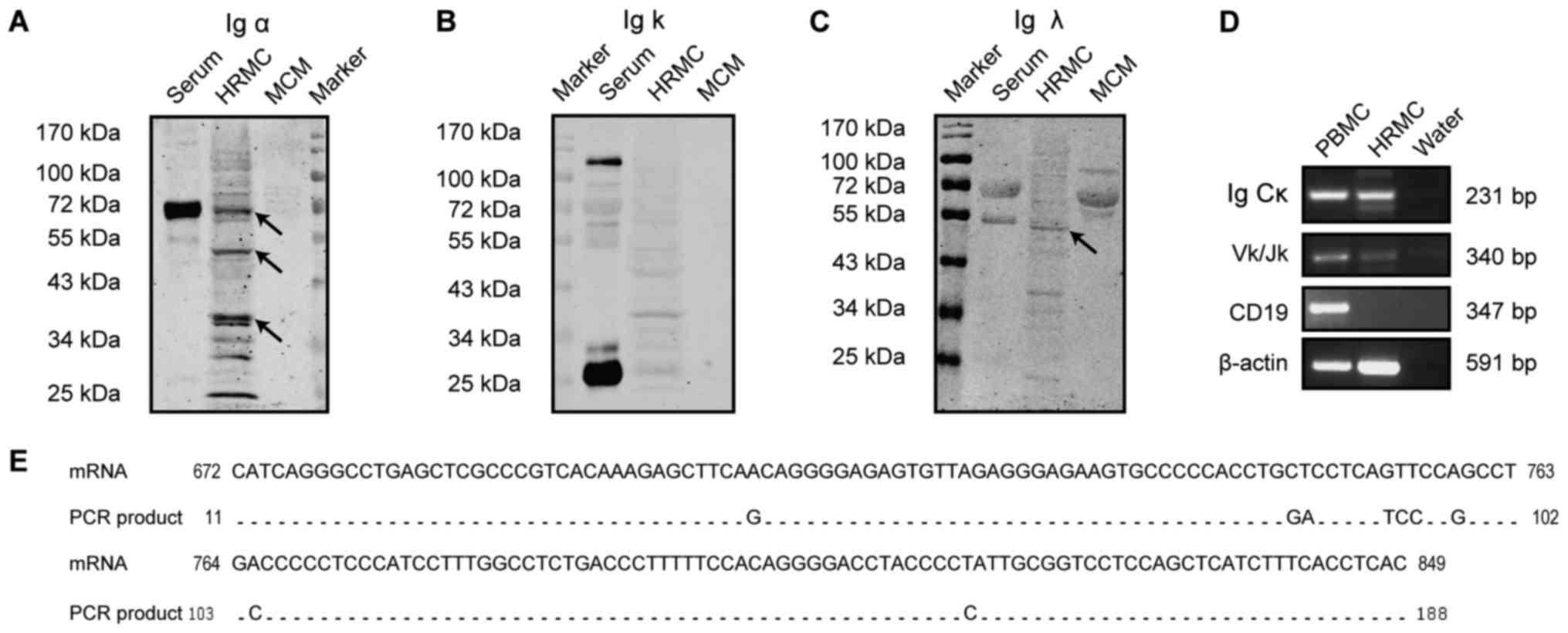
IgA expression in primary HMCs
In this study, the in vivo IgA expression in
HRMCs was investigated. Western blot analysis of the lysed HRMCs
demonstrated that Ig α was present not only at a size of 72 kDa,
which was consistent with the positive control in the serum, but
also at 53 and 38 kDa (Fig. 1A),
indicating that the α chains in the cytoplasm might be truncated or
are at different synthesis stages. There was no obvious band
detected for Ig κ (Fig. 1B). A
positive band for Ig λ was detected at 55 kDa which is similar to
the molecular weight of a dimer and was consisted with the size of
the positive controls in the serum (Fig. 1C). The absence of a band in the MCM
eliminates the possibility of IgA heavy chain and light chain
expression in the culture medium. In addition, RT-PCR revealed Ig
Cκ and Ig Vκ transcripts' expression in HRMCs (Fig. 1D). Further sequencing of the PCR
products showed 95% sequence similarity between the Ig Cκ collected
from the HRMCs and the published sequence obtained from plasma
cells in the NCBI database (Gene Bank, Y14736.1) (Fig. 1E). The predominant Vκ/Jκ
rearrangement pattern was Vκ1-12*01/Jκ4*01, which is different to
the transcripts' pattern observed in the PBMCs (Table IV). The negative expression of
CD19 indicated that there was no B cell contamination in the HRMCs.
The constant region and the Ig Vκ transcripts were strongly
detected, suggesting an IgA expression in the HRMCs.
 | Table IV.Rearrangement patterns of Ig κ
variable region transcripts. |
Table IV.
Rearrangement patterns of Ig κ
variable region transcripts.
| Name | Clone no. | Vκ | Jκ | Identity% |
|---|
| PBMC (n=16) | 1 | Vκ1-27*01 | Jκ1*01 | 90.2 |
|
| 1 | Vκ1-27*01 | Jκ4*01 | 96.1 |
|
| 1 | Vκ1-39*01 | Jκ1*01 | 90.8 |
|
| 6 | Vκ1-39*01 | Jκ4*01 | 86.9–97.6 |
|
| 1 | Vκ1-39*01 | Jκ3*01 | 97.9 |
|
| 1 | Vκ1-39*01 | Jκ5*01 | 93.3 |
|
| 1 | Vκ1-16*02 | Jκ4*01 | 95.5 |
|
| 2 | Vκ1-33*01 | Jκ4*01 | 91.3 |
|
| 1 | Vk4-1*01 | Jk4*01 | 96.6 |
| HMC (n=7) | 7 | Vκ3-20*01 | Jκ1*01 | 94.4 |
| HRMC (n=8) | 2 | Vκ3-20*01 | Jκ1*01 | 92.7–94.4 |
|
| 6 | Vk1-12*01 | Jk4*01 | 94.4–94.7 |
IgA expression in HMC line
IgA expression in mesangial cells was further
confirmed in the HMC cell line with several methods.
Immunofluorescence staining was positive for Ig α, Ig κ, Ig λ in
the mesangial cytoplasm (Fig. 2A).
Similar to a previous study which had reported the absence of IgA
in the FBS (38), the FBS was
negatively stained with rabbit anti-human Ig α, κ and λ antibodies,
indicating that FBS could not interfere with the results. Western
blot analysis of the HMCs lysates displayed similar positive bands
for Ig α at 53 and 38 kDa, and for Ig λ at 55 kDa, but negative
results for Ig κ, which corresponds with the results obtained in
HRMCs (Fig. 2B), further
supporting the IgA expression in mesangial cells.
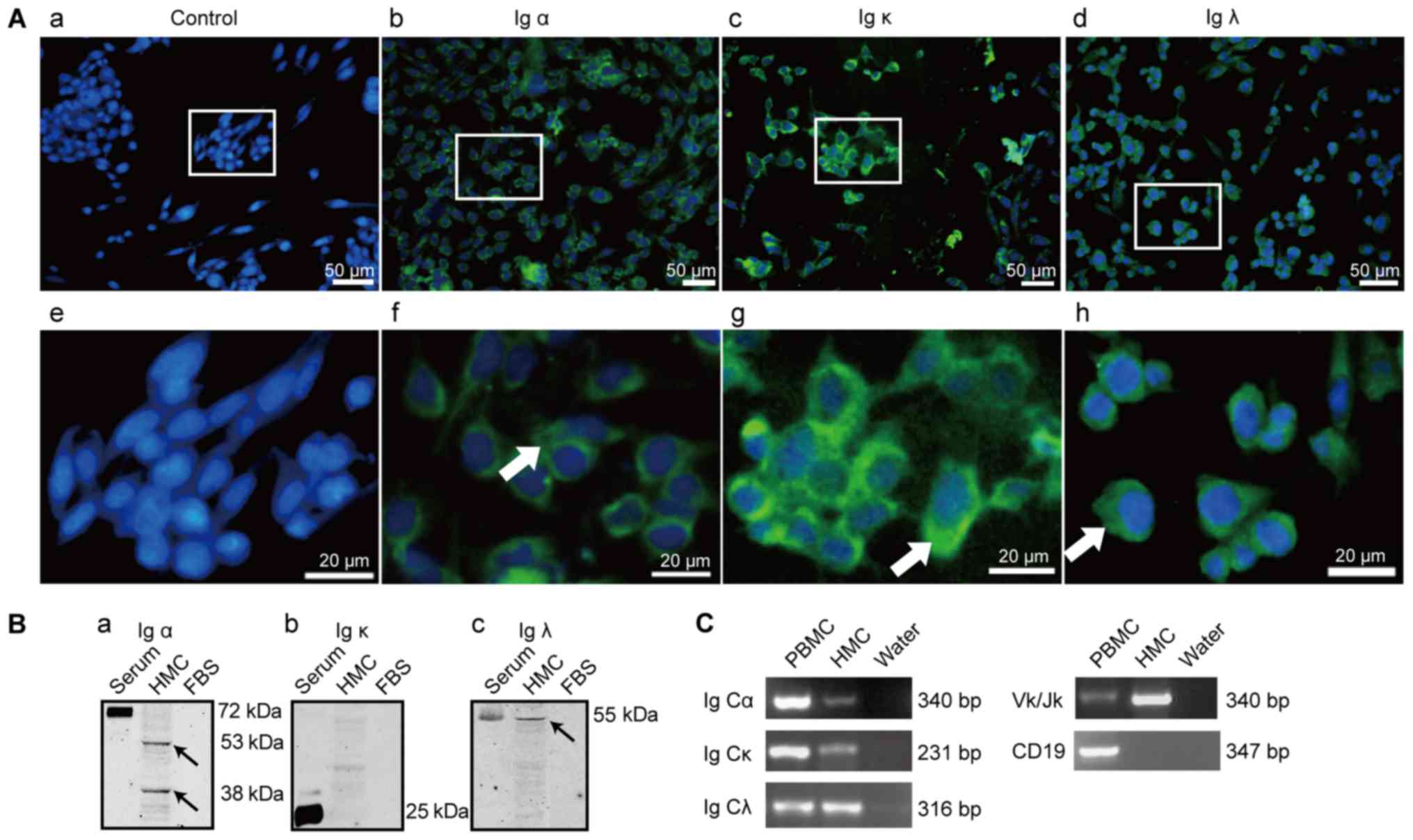 | Figure 2.IgA expression in HMCs. (A) Positive
immunofluorescence staining of Ig α (b and f), Ig κ (c and g) and
Ig λ (d and h) in HMCs, (a and e) were the blank controls with PBS
replacing primary antibodies; Green, antibody staining; blue,
nuclear staining by DAPI; scale bar, 50 µm (upper), 20 µm (lower);
(B) Ig α (a), Ig κ (b), Ig λ (c) detected in human glomerular
mesangial cell line lysates and human serum as a positive control;
arrows indicate immunoreactivity against Ig α and Ig λ; (C) Ig α
heavy chain constant region (Ig Cα), Ig κ light chain constant
region (Ig Cκ), Ig λ light chain constant region (Ig Cλ), and Ig κ
variable region (Vκ/Jκ) transcripts correspond to the 340, 231,
316, and the 340 bp SqRT-PCR products, respectively. HMC, human
mesangial cell; PBS, phosphate-buffered saline; SqRT-PCR,
semi-quantitative reverse transcription-polymerase chain reaction;
PBMC, peripheral blood mononuclear cell as positive control;
negative control: PCR reaction mixture with water; CD19, The B
lymphocyte marker. |
Ig gene rearrangement and transcription is a
prerequisite for Ig expression. To confirm the fact that IgA was
synthesized in HMCs, we further explored the transcripts of Ig α,
Ig κ and Ig λ by examining the mRNA expression of the constant
regions of Ig α, Ig κ, Ig λ and the variable region of Ig κ in the
HMCs (Fig. 2C). The alignment of
the sequences of the RT-PCR products with those of the published Ig
Cα1, Ig Cα2, Ig Cκ and Ig Cλ mRNA sequences in the NCBI database
(Gene Bank, BC016369.1, BC073765.1, Y14736.1, X57823.1)
demonstrated a sequence similarity of 99, 97, 98 and 97%,
respectively (Fig. 3). The DNA
sequencing of the Vκ PCR products showed that the predominant
rearrangement was Vκ3-20*01/Jκ1*01, which was different from the
rearrangements in HRMCs, and less diverse than the transcripts from
PBMCs (Table IV). The HMC-derived
Ig Vκ rearrangement sequence has been submitted to the GenBank
database (GenBank accession no. KX443559).
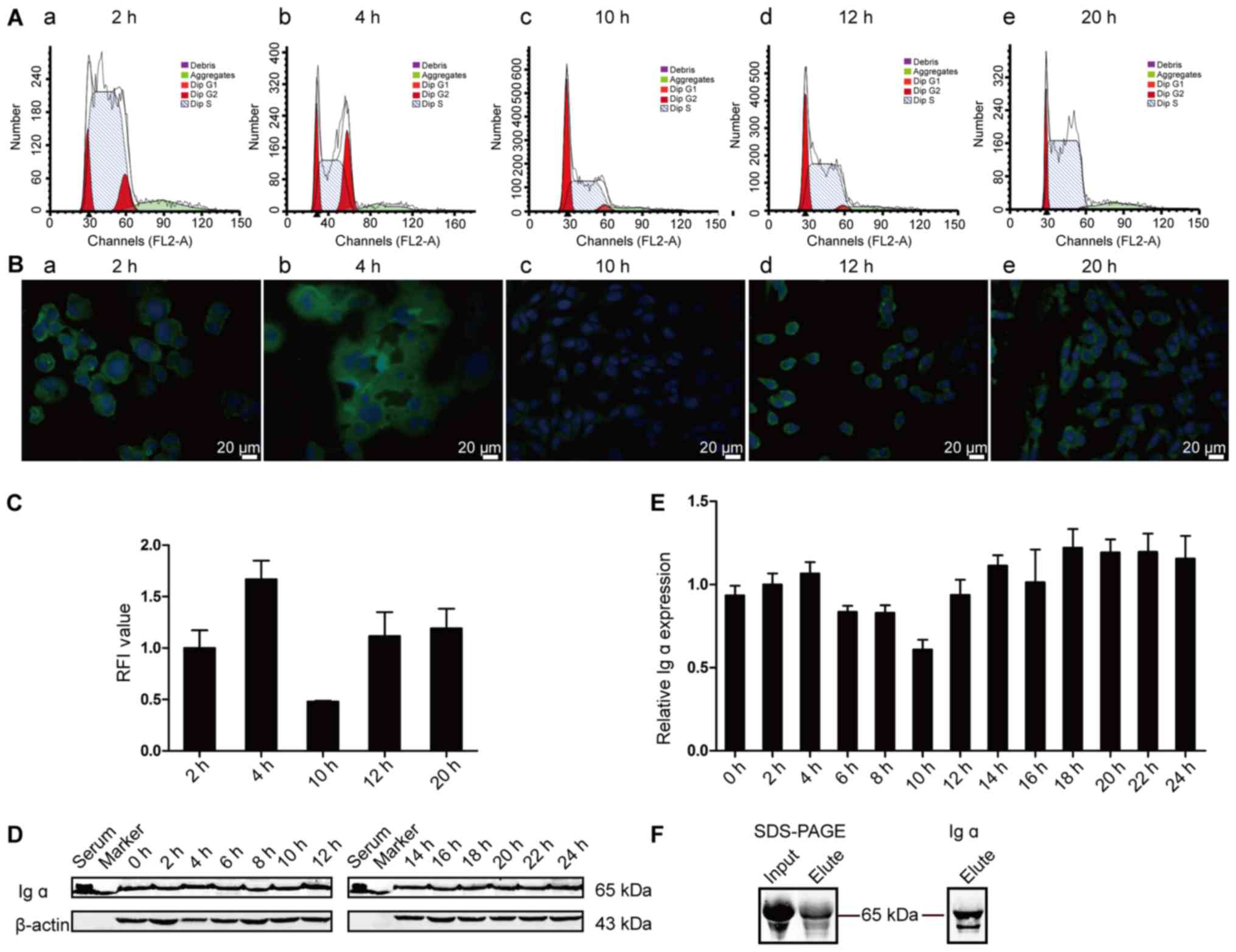
Dynamic expression of IgA in HMCs
during cell cycle and IgA secretion
Double thymidine (TdR) block model was used to
achieve HMCs synchronous growth and the cells were harvested every
2 h to detect the IgA expression at different phases of the cell
cycle. The cell cycle phase was determined by flow cytometry and
demonstrated that HMCs entered S phase at around 2 h, G2/M phase
from 4 to 6 h, G0/G1 phase from 8 h to 10 h, and re-entered into S
phase 12 h later (Fig. 4A).
Consistent with the cell cycle phases, we detected
dynamic expressions of Ig α1 (Fig. 4B
and C) and Ig α2 (data not shown). The immunostaining results
showed that Ig α1 expression was gradually increased from S phase
(2 h), then reached highest levels at the G2/M phase (4–6 h),
decreased after the G0/G1 phase (10 h) and increased in S phase (12
to 24 h) again. Different protein expression levels were also
detected in the cell lysates by Western blot analysis (Fig. 4D and E). The trend of the Ig α
heavy chain expression at 4 and 10 h by Western blot was similar
with that by IF staining. The α chain displayed a differential
localization pattern and expression levels during the 24 h
observation period after synchronization. These changes were in
accordance with the cell G, S and M phases, indicating that the IgA
heavy chain may be associated with cell growth, proliferation and
division.
To find out whether HMCs could secrete IgA, we
purified Ig α1 from the culture supernatant using jacalin-sepharose
which binds to human IgA1 with high specificity. The size of the
eluted protein was 65 kDa, according to the anti-human Ig α and the
Ig α1 antibodies staining, which corresponds to the molecular size
for the Ig α heavy chain (Fig.
4F). Mass spectra results showed that there was high homology
between the amino acid sequences of the band and those of the Ig α1
and Ig α2 constant regions published in the NCBI database (GenBank,
CAC20453.1, AAB30803.1) (Fig.
5).
Up-regulation of IgA in HMCs by
AngII
We utilized AngII, endogenous pro-inflammatory
factor, to examine the effects of pro-inflammatory factors on the
IgA expression in HMCs. 24 h after AngII stimulation, the
immunofluorescence staining was stronger for Ig α, Ig κ, Ig λ in
the cytoplasm (Fig. 6A). Ig α and
Ig λ were more abundant on the fibrous structures in the cytoplasm
and a granular accumulation was observed on the cell membranes.
Similar to the WB results immunostaining revealed that Ig κ was
expressed weakly in HMCs and its accumulation on the membranes was
not obvious.
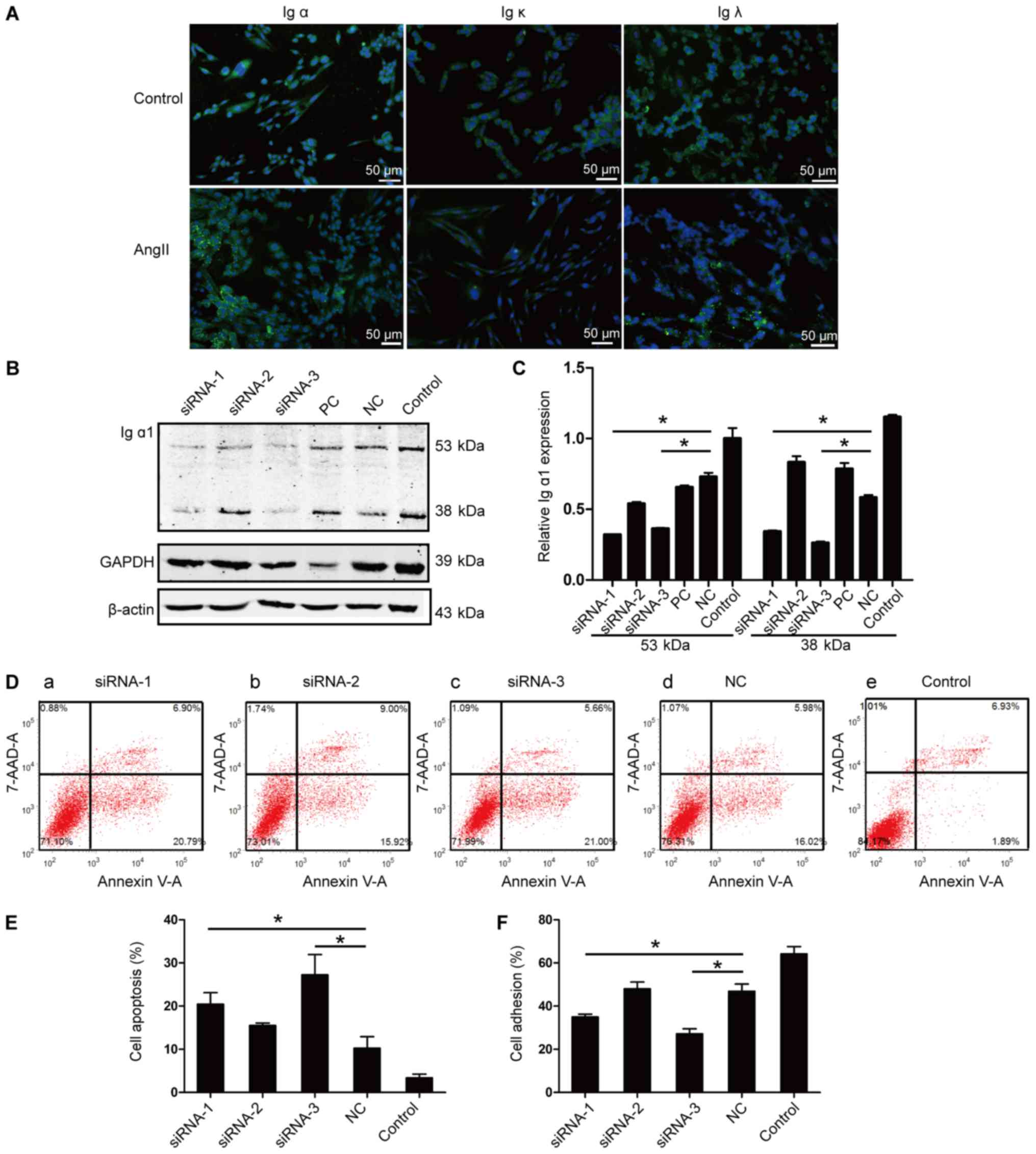 | Figure 6.IgA expression after AngII
stimulation and its effects on cell apoptosis and adhesion. (A) The
positive expression of the Ig α, Ig κ and Ig λ proteins in HMCs,
and after stimulation with AngII for 24 h, detected by
immunofluorescence staining; scale bar, 50 µm; (B) Representative
western blot images of Ig α1 knockdown in HMCs. (C) The expression
rate of the 53 and the 38 kDa band of Ig α1 proteins; after Ig α1
siRNA-1 and siRNA-3 transfection for 48 h; transfection with
siRNA-1 and siRNA-3 was repeated three times. The data were
standardized to the levels of β-actin. (D and E) The rate of cell
apoptosis after Ig α1 siRNA transfection for 48 h; n=4. (F) Cell
adhesion of HMCs after siRNA transfection for 48 h; n=3. *P<0.05
vs. NC. siRNA-1, siRNA-2, siRNA-3: siRNA constructs targeting
different regions of the Ig α1 heavy chain constant region; PC,
positive control: siRNA targeted GAPDH; NC, negative control:
Nonspecific, scrambled siRNA. HMC, human mesangial cell; siRNA,
small interfering RNA. |
The association of IgA with cell
apoptosis and cell adhesion
To investigate the possible effects of HMC-produced
IgA on cell functions, we used the siRNA transfection method to
down regulate the expression of Ig α1 in HMCs. After transfection
with siRNAs for 48 h, the cells were collected to detect the Ig α1
expression in HMCs. The relative expression of the 53 kDa form of
the Ig α1 was significantly downregulated in the siRNA-1 and the
siRNA-3 treated groups compared to the negative control (NC) group
(0.32±0.01 vs. 0.73±0.05, P<0.05; 0.36±0.01 vs.
0.73±0.05, P<0.05, respectively) and this was also similar with
the expression of the 38 kDa form (0.34±0.01 vs. 0.58±0.03,
P<0.05; 0.26±0.02 vs. 0.58±0.03, P<0.05) (Fig. 6B and C). These results indicated
that siRNA-1 and siRNA-3 transfection could effectively
down-regulate IgA in HMCs.
Annexin V assay combined with flow cytometry was
performed to evaluate the apoptosis rates. After siRNA transfection
for 48 h, early apoptosis in HMCs was detected. Apoptosis in the
HMCs siRNA-1 and the siRNA-3 groups showed an early increase
compared with that in the NC group (20.45±5.34 vs.
10.27±5.31%, P<0.05; 27.25±9.81 vs. 10.27±5.31%,
P<0.05, n=4) (Fig. 6D and E),
indicating that IgA expression in HMCs might play an important role
in cell growth and apoptosis. Cell adhesion ability is important
for HMCs to execute functions such as structural support of the
capillary tuft, modulation of glomerular hemodynamics and
phagocytic removal of macromolecules and immune complexes. After
siRNA transfection for 48 h, cell adhesion rates of HMCs in both
the siRNA-1 and the siRNA-3 groups were significantly decreased
compared to the NC group (34.99±2.56 vs. 46.88±6.70%,
P<0.05; 27.16±4.67 vs. 46.88±6.70%, P<0.05) (Fig. 6F). The significant decrease of cell
adhesion rates indicated that the knockdown of IgA in HMCs might
inhibit cell adhesion. These changes in the rate of early apoptosis
and the ability to adhere indicated that IgA expression is
associated with mesangial cell functions.
Discussion
Definitive diagnosis of IgAN requires a kidney
biopsy and IgAN is identified immunohistologically by the presence
of dominant or co-dominant glomerular deposits of IgA (39), which had been generally considered
to be B cell derived. The deposits consist predominantly of
polymeric IgA structures of the IgA1 subclass (40). The pathogenic IgA deposition in the
glomerular mesangium can activate mesangial cells and induce
mesangial hyper-cellularity, apoptosis, oxidative stress,
activation of complement, scarring in the glomerular and
interstitial compartments, and secretion of pro-inflammatory
factors, causing symptoms such as proteinuria, hematuria, and
leading to IgAN (41–43). Igs expression in non-B cells has
been reported in recent years by several studies, which provided
clues for IgA expression in mesangial cells (11,14,37).
Our study demonstrated, for the first time, that mesangial cells
may produce and secret IgA, and that the deposited IgA in the
mesangium of patients with IgAN may be, at least partially,
originated from mesangial cells.
In addition, in this study, we have demonstrated
that the Ig α, Ig κ and Ig λ proteins are present in HRMCs and
HMCs, and that their presence was not due to artificial
contamination by B lymphocytes or by the FBS buffer in the culture
media. These results confirm that the IgA, especially IgA1, is
expressed in the mesangial cells. The different molecular weights
of the Ig α heavy chain suggested that Ig synthesis and assembly
occur at different stages or that it existed in different truncated
or aggregated forms, as it has been previously reported by Hu et
al (44). Furthermore, our
study has shown that the IgA heavy and light chain constant and
variable region gene transcripts and proteins were present in the
HRMCs and HMCs and that their high homology with those mRNA
sequences in the NCBI database strongly supports IgA expression in
mesangial cells,. The unique or dominant Ig Vκ sequences in non-B
cells are consistent with other reports (26,37).
Increase of early apoptosis and decrease of cell
adhesion ability in HMCs after IgA downregulation were observed in
our study, which indicated that expression of Ig α1 might be
associated with mesangial cell functions such as apoptosis,
proliferation, and adhesion. IgA mediated cell proliferation and
apotosis has been reported in human epithelial cancer cells, but
the mechanism was not investigated (45). Previous studies have shown that the
pathogenesis of a variety of renal diseases is highly correlated
with cell apoptosis and changes of apoptotic genes, in which the
Bcl-2 family is one of the most implicated gene families (46,47).
Besides, active effector caspases could proteolytically degrade a
range of intracellular proteins during the apoptosis process
(48,49). IgA expression may participate in
the transcriptional and/or post-translational regulation of
apoptosis related genes or proteins, such as caspases, to inhibit
mesangial cell apoptosis. The specific molecules contributing to
cell adhesion between the mesangial cell and the glomerular
basement membrane are not clear. However, the protein called
Epithelial Protein Lost In Neoplasm (EPLIN) was reported to
strongly express in glomerular mesangial cells (50). EPLIN is implicated in the
organization of the actin cytoskeleton, during the cell-cell or
cell-matrix interactions (51).
The above evidence provide us with clues to explore the underlying
mechanism(s) regulating IgA expression in mesangial cells and
mediating apoptosis and adhesion.
The results of our study have potential application
and significance in clinical practice. First, the facts that IgA
could be expressed in mesangial cell and secreted out of cell can
illustrate that the IgA deposited in the mesangium in patients with
IgAN may be, at least partially, originated from mesangial cells
and may induce mesangial cells proliferation and secretion of
extracellular matrixes. Second, our results have shown that
mesangial cell-derived IgA is required for physiological cell
functions, so exploring the factors which could lead to IgA
deposition in the mesangium would be of great clinical
significance. Third, the upregulation of Ig α, Ig κ, Ig λ
expression by AngII in the cytoplasm of HMCs indicates that an
interaction exists between angiotensin and IgA. This interaction
can be potentially targeted for the clinical treatment of IgAN by
exploiting angiotensin converting enzyme inhibitors or AngII
receptor blockers.
Several points in the study need to be further
clarified. First, IF staining demonstrated the presence of the κ
chain in the cytoplasm and RT-PCR identified the transcript of the
κ chain in the HMCs, but WB was not able to detect the Ig κ band.
Generally speaking, antibodies used in IF recognize the
three-dimensional structure while those in WB bind to the short
line chain of the proteins, therefore the IF staining results are
more convincing. The negative result in the WB might be attributed
to the insufficient recognition by the antibodies. Secondly, a 65
kDa band after jacalin affinity chromatography was positively
detected with antibodies against Ig α and Ig α1 but not Ig α2,
however both α1 and α2 heavy chains were detected in the band by
mass spectrometry. IF demonstrated the staining of both α1 and α2
in the cytoplasm and RT-PCR showed that the transcripts of both α1
and α2 were expressed in HMCs. The dominant expression of Ig α1, as
we found by IF and by WB, may compete with the binding of the
antibody to the Ig α2. It was not easy to explain the affinity of
jacalin to Ig α2, which was considered not to have any
glycosylation sites at the hinge area, and it was unclear if the
glycosylation at the hinge area of Ig α1 and Ig α2 in the mesangial
cells was different from those in the plasm cell.
In conclusion, this study demonstrates that
mesangial cells can express and secret IgA and that this expression
may be associated with cell functions. Our findings provide clues
for the implication of the HMC-produced IgA in the excessive
deposition of IgA in the pathogenesis of IgAN.
Acknowledgements
We thank Professor Youfei Guan (Department of
Physiology and Pathophysiology, Peking University Health Science
Center, Peking, China) for supplying the C2M12 cell line, and the
Department of Immunology, Peking University, for supporting our
work. This study was supported by the National Natural Science
Foundation of China (nos. 91229102 and 81272237).
Glossary
Abbreviations
Abbreviations:
|
HMC
|
human mesangial cell
|
|
AngII
|
angiotensin II
|
|
SAC
|
staphylococcus
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Zhou X, Workeneh B, Hu Z and Li R: Effect
of immunosuppression on the human mesangial cell cycle. Mol Med
Rep. 11:910–916. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomana M, Matousovic K, Julian BA, Radl J,
Konecny K and Mestecky J: Galactose-deficient IgA1 in sera of IgA
nephropathy patients is present in complexes with IgG. Kidney Int.
52:509–516. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomana M, Novak J, Julian BA, Matousovic
K, Konecny K and Mestecky J: Circulating immune complexes in IgA
nephropathy consist of IgA1 with galactose-deficient hinge region
and antiglycan antibodies. J Clin Invest. 104:73–81. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Conley ME, Cooper MD and Michael AF:
Selective deposition of immunoglobulin A1 in immunoglobulin A
nephropathy, anaphylactoid purpura nephritis, and systemic lupus
erythematosus. J Clin Invest. 66:1432–1436. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Novak J, Moldoveanu Z, Renfrow MB,
Yanagihara T, Suzuki H, Raska M, Hall S, Brown R, Huang WQ,
Goepfert A, et al: IgA nephropathy and Henoch-Schoenlein purpura
nephritis: Aberrant glycosylation of IgA1, formation of
IgA1-containing immune complexes, and activation of mesangial
cells. Contrib Nephrol. 157:134–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reily C, Ueda H, Huang ZQ, Mestecky J,
Julian BA, Willey CD and Novak J: Cellular signaling and production
of galactose-deficient IgA1 in IgA nephropathy, an autoimmune
disease. J Immunol Res. 2014:1975482014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiu X and Yang G: Existance of Ig-like
protein in malignant tumor cells. J Norman Bethune Univ Med Sci.
22:572,574,5751996.
|
|
8
|
Qiu X and Yang G: The characteristic and
gene structure of Ig-like protein in maligant tumor. Chin J Immun.
295:1996.(In Chinese).
|
|
9
|
Kimoto Y: Expression of heavy-chain
constant region of immunoglobulin and T-cell receptor gene
transcripts in human non-hematopoietic tumor cell lines. Genes
Chromosomes Cancer. 22:83–86. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng H, Li M, Ren W, et al: Expression
and secretion of immunoglobulin alpha heavy chain with diverse VDJ
recombinations by human epithelial cancer cells. Mol Immunol.
44:2221–2227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiu X, Zhu X, Zhang L, Mao Y, Zhang J, Hao
P, Li G, Lv P, Li Z, Sun X, et al: Human epithelial cancers secrete
immunoglobulin g with unidentified specificity to promote growth
and survival of tumor cells. Cancer Res. 63:6488–6495.
2003.PubMed/NCBI
|
|
12
|
Zhu X, Li C, Sun X, Mao Y, Li G, Liu X,
Zhang Y and Qiu X: Immunoglobulin mRNA and protein expression in
human oral epithelial tumor cells. Appl Immunohistochem Mol
Morphol. 16:232–238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Babbage G, Ottensmeier CH, Blaydes J,
Stevenson FK and Sahota SS: Immunoglobulin heavy chain locus events
and expression of activation-induced cytidine deaminase in
epithelial breast cancer cell lines. Cancer Res. 66:3996–4000.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng H, Li M, Ren W, Zeng L, Liu HD, Hu
D, Deng X, Tang M, Shi Y, Gong J and Cao Y: Expression and
secretion of immunoglobulin alpha heavy chain with diverse VDJ
recombinations by human epithelial cancer cells. Mol Immunol.
44:2221–2227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Z and Gu J: Immunoglobulin G
expression in carcinomas and cancer cell lines. FASEB J.
21:2931–2938. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li M, Feng DY, Ren W, Zheng L, Zheng H,
Tang M and Cao Y: Expression of immunoglobulin kappa light chain
constant region in abnormal human cervical epithelial cells. Int J
Biochem Cell Biol. 36:2250–2257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang C, Xia M, Sun X, He Z, Hu F, Chen L,
Bueso-Ramos CE, Qiu X and Yin CC: IGK with conserved IGΚV/IGΚJ
repertoire is expressed in acute myeloid leukemia and promotes
leukemic cell migration. Oncotarget. 6:39062–39072. 2015.PubMed/NCBI
|
|
18
|
Jiang C, Huang T, Wang Y, Huang G, Wan X
and Gu J: Immunoglobulin G expression in lung cancer and its
effects on metastasis. PLoS One. 9:e973592014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Lin D, Peng H, Huang Y, Huang J
and Gu J: Cancer-derived immunoglobulin G promotes tumor cell
growth and proliferation through inducing production of reactive
oxygen species. Cell Death Dis. 4:e9452013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wen YJ, Mancino A, Pashov A, Whitehead T,
Stanley J and Kieber-Emmons T: Antigen binding of human IgG Fabs
mediate ERK-associated proliferation of human breast cancer cells.
DNA Cell Biol. 24:73–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang PY, Li HY, Zhou ZY, Jin YX, Wang SX,
Peng XH and Ou SJ: Overexpression of immunoglobulin G prompts cell
proliferation and inhibits cell apoptosis in human urothelial
carcinoma. Tumour Biol. 34:1783–1791. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan B, Zheng S, Liu C and Xu Y:
Suppression of IGHG1 gene expression by siRNA leads to growth
inhibition and apoptosis induction in human prostate cancer cell.
Mol Biol Rep. 40:27–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang J, Sun X, Mao Y, Zhu X, Zhang P,
Zhang L, Du J and Qiu X: Expression of immunoglobulin gene with
classical V-(D)-J rearrangement in mouse brain neurons. Int J
Biochem Cell Biol. 40:1604–1615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang J, Sun X, Gong X, He Z, Chen L, Qiu
X and Yin CC: Rearrangement and expression of the immunoglobulin
µ-chain gene in human myeloid cells. Cell Mol Immunol. 11:94–104.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang J, Zhang L, Ma T, Zhang P and Qiu X:
Expression of immunoglobulin gene with classical V-(D)-J
rearrangement in mouse testis and epididymis. J Histochem Cytochem.
57:339–349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, Mao Y, Huang J, Ma T, Zhang L,
Zhu X, Zheng J, Wu L, Yin CC and Qiu X: Immunoglobulin gene locus
events in epithelial cells of lactating mouse mammary glands. Cell
Mol Life Sci. 67:985–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu J, Xia M, Wang P, Wang C, Geng Z,
Cameron Yin C, Zhang C and Qiu X: Immunoglobulin gene expression in
umbilical cord blood-derived CD34+ hematopoietic stem/progenitor
cells. Gene. 575:108–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang BY, Hu C, Prayaga S, Khaidakov M,
Sawamura T, Seung KB and Mehta JL: LOX-1 dependent overexpression
of immunoglobulins in cardiomyocytes in response to angiotensin II.
Biochem Biophys Res Commun. 379:395–399. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niu N, Zhang J, Sun Y, Wang S, Sun Y,
Korteweg C, Gao W and Gu J: Expression and distribution of
immunoglobulin G and its receptors in an immune privileged site:
The eye. Cell Mol Life Sci. 68:2481–2492. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lei Y, Huang T, Su M, Luo J, Korteweg C,
Li J, Chen Z, Qiu Y, Liu X, Yan M, et al: Expression and
distribution of immunoglobulin G in the normal liver,
hepatocarcinoma and postpartial hepatectomy liver. Lab Invest.
94:1283–1295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang S, Huang G, Wang Y, Huang T, Lin S
and Gu J: Up-regulation of immunoglobulin G gene expression in the
hippocampus of rats subjected to acute immobilization stress. J
Neuroimmunol. 258:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ruan XZ, Varghese Z, Fernando R and
Moorhead JF: Cytokine regulation of low-density lipoprotein
receptor gene transcription in human mesangial cells. Nephrol Dial
Transplant. 13:1391–1397. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sraer JD, Delarue F, Hagege J, Feunteun J,
Pinet F, Nguyen G and Rondeau E: Stable cell lines of T-SV40
immortalized human glomerular mesangial cells. Kidney Int.
49:267–270. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prendergast L, van Vuuren C, Kaczmarczyk
A, Doering V, Hellwig D, Quinn N, Hoischen C, Diekmann S and
Sullivan KF: Premitotic assembly of human CENPs-T and -W switches
centromeric chromatin to a mitotic state. PLoS Biol.
9:e10010822011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coldwell MJ, Cowan JL, Vlasak M, Mead A,
Willett M, Perry LS and Morley SJ: Phosphorylation of eIF4GII and
4E-BP1 in response to nocodazole treatment: A reappraisal of
translation initiation during mitosis. Cell Cycle. 12:3615–3628.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jose S, Tan SW, Ooi YY, Ramasamy R and
Vidyadaran S: Mesenchymal stem cells exert anti-proliferative
effect on lipopolysaccharide-stimulated BV2 microglia by reducing
tumour necrosis factor-α levels. J Neuroinflammation. 11:1492014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng J, Huang J, Mao Y, Liu S, Sun X, Zhu
X, Ma T, Zhang L, Ji J, Zhang Y, et al: Immunoglobulin gene
transcripts have distinct VHDJH recombination characteristics in
human epithelial cancer cells. J Biol Chem. 284:13610–13619. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brown TT Jr..Schultz RD, Duncan JR and
Bistner SI: Serological response of the bovine fetus to bovine
viral diarrhea virus. Infect Immun. 25:93–97, 197. PubMed/NCBI
|
|
39
|
Roberts IS: Pathology of IgA nephropathy.
Nat Rev Nephrol. 10:445–454. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Valentijn RM, Radl J, Haaijman JJ, Vermeer
BJ, Weening JJ, Kauffmann RH, Daha MR and van Es LA: Circulating
and mesangial secretory component-binding IgA-1 in primary IgA
nephropathy. Kidney Int. 26:760–766. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Novak J, Julian BA, Mestecky J and Renfrow
MB: Glycosylation of IgA1 and pathogenesis of IgA nephropathy.
Semin Immunopathol. 34:365–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lai KN, Leung JC, Chan LY, Saleem MA,
Mathieson PW, Lai FM and Tang SC: Activation of podocytes by
mesangial-derived TNF-alpha: Glomerulo-podocytic communication in
IgA nephropathy. Am J Physiol Renal Physiol. 294:F945–F955. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lai KN: Pathogenesis of IgA nephropathy.
Nat Rev Nephrol. 8:275–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu D, Duan Z, Li M, Jiang Y, Liu H, Zheng
H, Li L, Bode AM, Dong Z and Cao Y: Heterogeneity of aberrant
immunoglobulin expression in cancer cells. Cell Mol Immunol.
8:479–485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zheng H, Li M, Liu H, Ren W, Hu DS, Shi Y,
Tang M and Cao Y: Immunoglobulin alpha heavy chain derived from
human epithelial cancer cells promotes the access of S phase and
growth of cancer cells. Cell Biol Int. 31:82–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kang BP, Urbonas A, Baddoo A, Baskin S,
Malhotra A and Meggs LG: IGF-1 inhibits the mitochondrial apoptosis
program in mesangial cells exposed to high glucose. Am J Physiol
Renal Physiol. 285:F1013–F1024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qiu LQ, Sinniah R and I-Hong Hsu S:
Downregulation of Bcl-2 by podocytes is associated with progressive
glomerular injury and clinical indices of poor renal prognosis in
human IgA nephropathy. J Am Soc Nephrol. 15:79–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Denault JB and Salvesen GS: Caspases: Keys
in the ignition of cell death. Chem Rev. 102:4489–4500. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Philchenkov AA: Caspases as regulators of
apoptosis and other cell functions. Biochemistry (Mosc).
68:365–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tsurumi H, Harita Y, Kurihara H, Kosako H,
Hayashi K, Matsunaga A, Kajiho Y, Kanda S, Miura K, Sekine T, et
al: Epithelial protein lost in neoplasm modulates platelet-derived
growth factor-mediated adhesion and motility of mesangial cells.
Kidney Int. 86:548–557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Karaköse E, Geiger T, Flynn K,
Lorenz-Baath K, Zent R, Mann M and Fässler R: The focal adhesion
protein PINCH-1 associates with EPLIN at integrin adhesion sites. J
Cell Sci. 128:1023–1033. 2015. View Article : Google Scholar : PubMed/NCBI
|