Introduction
Laryngeal squamous cell carcinoma (LSCC) is the
second most common malignancy in the head and neck region and is
reported to account for ~2.4% of all new malignancies worldwide
each year (1). The incidence of
LSCC has been rising gradually, particularly in Northeast China
(2). Although rapid progress has
recently been made in treatment, the therapeutic outcomes and the
overall 5-year survival rate remain unsatisfactory (3). Together with invasive and metastatic
behaviors, resistance to chemotherapy has been a major obstacle in
improving the 5-year survival rate of LSCC patients (4). Furthermore, total laryngectomy, the
recommended treatment for advanced patients, leads to serious lung
infection and the loss of speech (5). Therefore, more effective diagnostic
techniques for early stage LSCC have long been warranted. The
improved detection of useful biological and molecular markers in
the diagnosis and therapy of LSCC, and a new strategy for the
treatment of LSCC, are urgently required.
It has been suggested that conventional pathological
prognostic parameters are insufficient to accurately evaluate the
clinical prognosis of patients with LSCC (6). However, biomarkers are considered to
be beneficial not only in evaluating the prognosis but also in
guiding personalized therapy for patients with cancer (7), which further indicates the importance
of identifying potential biomarkers for human LSCC. MicroRNAs
(miRNAs) are a family of endogenous small single-strand RNAs with a
length between 21 and 25 nucleotides, which have emerged as an
important class of gene regulators (8). It has been demonstrated that miRNAs
serve critical roles in tumor cell viability, differentiation,
metastasis and apoptosis in vitro and in vivo
(9). In addition, miRNAs have been
functionally classified as tumor suppressors or proto-oncogenes and
are aberrantly expressed indifferent cancers, including lung
(10), liver (11), stomach (12), leukemia (13), lymphoma (14), breast (15), colorectal (16), and head and neck cancer (17). It has been suggested that miRNAs
may act as the molecular targets for human cancer diagnosis and
personalized therapy (18,19). miRNA-195 has been identified to be
downregulated in a variety of human solid tumors, but the potential
biological roles of miRNA-195 in LSCC remain to be elucidated.
The present study initially examined the expression
of miRNA-195 in LSCC and identified downregulation of miRNA-195 in
LSCC cancer tissue. Subsequently, the role of miRNA-195 in cell
viability, migration and apoptosis in AMC-HN-8 cells was
demonstrated. It was identified that miRNA-195 modulated vascular
endothelial growth factor receptor 2 (VEGFR2) expression, and was
thereby associated with the VEGFR2 signaling network. These
findings demonstrated that miRNA-195 serves a key role in the
pathologic progression of LSCC and revealed the potential molecular
mechanisms of miRNA-195 in human LSCC.
Materials and methods
Patient samples
A total of 23 cases of laryngeal squamous cell
carcinoma tissue samples and adjacent healthy tissue samples
between February 2016 and March 2017 were obtained from the
Department of Otolaryngology in the Fourth Affiliated Hospital of
Harbin Medical University (Harbin, China). There were 10 males and
13 females in the disease group, with a median age of 61 years. The
present study was performed in accordance with the ethical codes of
the World Medical Association (20) and was approved by the Ethics
Committee for Use of Human Samples of Harbin Medical University.
All human participants provided informed written consent and all
clinical investigations were conducted according to the principles
expressed in the Declaration of Helsinki. All specimens were coded
and identified. The specimens comprised a panel of 23 LSCC patient
cases obtained during surgical procedures, which were immediately
stored in liquid nitrogen or fixed in formalin.
Cell culture
AMC-HN-8 cells used in the present study were
purchased from American Type Culture Collection (ATCC; Manassas,
VA, USA). AMC-HN-8 cells were cultured in RPMI-1640 (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C with 5%
CO2 in a humidified incubator. The cultures were
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 100 µg/ml penicillin/streptomycin.
Oligo transfection, miRNA-195
knockdown, and miRNA-195 overexpression in AMC-HN-8 cells
Cells were seeded in antibiotic-free RPMI-1640
medium for 24 h prior to transfection. For upregulation of
miRNA-195, the cells (1×105) in a six-well plate were
transfected with miRNA-195 mimic (Shanghai GenePharma Co., Ltd.,
Shanghai, China) using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) in serum-free Opti-MEM (Gibco;
Thermo Fisher Scientific, Inc.) medium according to the
manufacturer's instructions. For knockdown ofmiRNA-195, the cells
(1×105) in a six-well plate were transfected with
miRNA-195 inhibitor (Shanghai GenePharma Co., Ltd.) using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.).
Transfection complexes were added to medium at a final
oligonucleotide concentration of 50 nM. miRNA-195 mimic negative
control (NC) and miRNA-195 inhibitor NC were also purchased from
Shanghai GenePharma Co., Ltd. and were transfected at a final
concentration of 50 nM using Lipofectamine® 2000
according to the manufacturer's instructions. Opti-MEM medium was
replaced at 4 h post-transfection with regular culture medium and
incubated at 37°C for a further 48 h. The sequences of miRNA-195
mimic and mimic-NC were: 5′-UAGCAGCACAGAAAUAUUGGC-3′ and
5′-UUCUCCGAACGUGUCACGUTT-3′, respectively. The sequences of
miRNA-195 inhibitor and inhibitor-NC were:
5′-GCCAAUAUUUCUGUGCUGCUA-3′ and 5′-CAAUAUUUCUGUGCUGCUAUU-3′,
respectively. Following transfection, the subsequent experiments
were performed within 8 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from human LSCCs was isolated using TRIzol
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Total RNA (0.5 µg) was then reverse
transcribed using the High-Capacity cDNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) to obtain cDNA
according to the manufacturer's instructions. The RNA levels of
miRNA-195 were determined using Power SYBR™ Green PCR Master Mix
(Thermo Fisher Scientific, Inc.) incorporation method on an ABI
7500 fast Real Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) at 95°C for 10 min, followed by 40 cycles of 95°C
for 15 sec, 60°C for 30 sec and 72°C for 30 sec, with U6 as an
internal control. The relative quantitative expression of gene was
calculated using method 2ΔΔCq (21). The sequences of primers were as
follows: hsa-miRNA-195 forward, 5′-GGGGTAGCAGCACAGATT-3′ and
reverse, 5′-TCCAGTGCGTGTCGTGGA-3′; and U6 forward,
5′-GCTTCGGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′.
Evaluation of cell viability via MTT
assay
AMC-HN-8 cells were plated in 96-well plates and
transfected with miRNA-195 mimics, miRNA-195 mimics-NC, miRNA-195
inhibitor and miRNA-195 inhibitor-NC respectively for 4 h.
Following transfection, the serum-free medium was removed and cells
were cultured at 37°C with regular culture medium for a further 48
h. To monitor cell survival, AMC-HN-8 cells in each well were
incubated at 37°C for 4 h with 0.5 mg/ml MTT (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). Subsequently, the MTT solution was
removed and the formazan crystals in the cells dissolved in 150 ml
sterile dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) by
incubating at 37°C for 15 min. Absorbance was recorded at 490 nm
using an Easy Reader 340 AT (SLT-Lab Instruments, Salzburg,
Austria). Relative cell survival was calculated by setting control
absorbance from untreated cells at 100%. Experiments were performed
in triplicate.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
Apoptotic AMC-HN-8 cells in the different groups
were detected using a TUNEL assay as previously described (22). TUNEL staining was detected using
the In Situ Cell Death Detection kit (Roche Diagnostics
GmbH, Mannheim, Germany) according to the manufacturer's
instructions. Sections were also counterstained at 37°C for 5 min
with DAPI (1:5 dilution; Invitrogen; Thermo Fisher Scientific,
Inc.) for nuclei. The number of the TUNEL-positive nuclei ratio in
≥10 representative microscopic fields (magnification, ×200) was
calculated by fluorescence microscopy (Nikon Corporation, Tokyo,
Japan) to compare the apoptosis ratio within the different
groups.
Invasion assays
Invasion assays were conducted using 8 µM
polyethylene terpthalate filters (BioCoat Matrigel Invasion
Chambers; BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA),
as previously described (23).
AMC-HN-8 cells (5×104/well) transfected with miRNA-195
mimics, inhibitor or NC were allowed to invade through
matrigel-coated filters for 16 h in a Transwell plate. The volume
of medium plated in the upper chamber was 200 µl. Cells invaded to
the lower chamber of the Transwell plate and were fixed using 4%
(w/v) paraformaldehyde at room temperature for 30 min and stained
using 0.05% crystal violet at room temperature for 20 min; the cell
numbers were counted (magnification, ×200) by a fluorescence
microscopy (Nikon, Tokyo, Japan) as previously described (23).
Measurement of cell migration with
Transwell migration assay
A Transwell migration assay was used to perform cell
migration. Nuclepore filters with 8-nm pores (Corning, Inc.,
Corning, NY, USA) were coated with type IV collagen (Sigma-Aldrich;
Merck KGaA) overnight at 37°C prior to the assay. AMC-HN-8 cells
(5×104) in 200 µl medium (Gibco; Thermo Fisher
Scientific, Inc.) from different treatment groups were added to the
upper chambers, and the lower chambers were filled with normal
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.). Following 48 h of incubation at 37°C, the cells
on the upper side were removed and discarded, and the cells that
migrated to the lower side of the membrane were fixed with 4%
paraformaldehyde at room temperature for 30 min, stained with 0.1%
crystal violet for 30 min at room temperature and washed three
times with PBS. The migrated cells in the lower side of the
membrane were observed and imaged under an inverted microscope
(magnification, ×200). Images were captured from three randomly
selected fields and the migrated cells counted.
Western blot analysis
The total amount of protein was extracted by RIPA
buffer (Beyotime Biotechnology, Shanghai, China) from AMC-HN-8
cells for immunoblotting analysis. Briefly, the protein
concentrations were determined with a bicinchoninic acid protein
assay kit using bovine serum albumin (Beyotime Institute of
Biotechnology) as the standard. Equal amounts of protein (100 µg)
were separated by 10% SDS-PAGE and blotted onto PVDF membranes (EMD
Millipore, Billerica, MA, USA). The blots were blocked at room
temperature with 5% non-fat milk dissolved in PBS for 2 h, then
probed overnight at 4°C in 5% milk tris-buffered saline/Tween-20
with the following primary antibodies: VEGFR2 (9698S, 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA), Raf (ab33899,
1:1,000; Abcam, Cambridge, UK), extracellular signal-regulated
kinase (ERK, sc-514302, 1:1,000), phosphorylated (p)-ERK (sc-81492,
1:1,000) (both from Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), total mitogen activated protein kinase kinase (MEK; 601121,
1:200), and p-MEK (558375, 1:1,000) (both from BD Biosciences),
p-SRC (12432; 1:1,000), focal adhesion kinase (FAK; 3285; 1:1,000),
p-FAK (8556; 1:1,000) (all from Cell Signaling Technology, Inc.),
protein kinase B (AKT, 610860; 1:1,000) and p-AKT (550747; 1:1,000)
(both from BD Biosciences), B-cell lymphoma 2 (Bcl-2; 15071;
1:1,000; Cell Signaling Technology, Inc.), Bcl-2-like protein 4
(Bax; 5023; 1:1,000), caspase-3 (9662; 1:500) and GAPDH (5174;
1:500) (all from Cell Signaling Technology, Inc.). Membranes were
washed three times, 15 min each, with PBS containing 0.5% Tween-20
and incubated with the following secondary antibodies: Alexa
Fluor® 700 goat anti-mouse immunoglobulin G (IgG) [heavy
and light chains (H+L); A-11029; 1:8,000] or Alexa
Fluor® 800 goat anti-rabbit IgG (H+L; A32730; 1:8,000;
Invitrogen; Thermo Fisher Scientific, Inc.) in PBS at room
temperature for 1 h. Images of western blot bands were captured
using the Odyssey Infrared Imaging System and quantified with
Odyssey software, version 1.2 (both from LI-COR Biosciences,
Lincoln, NE, USA) by measuring the band intensity (area × optical
density) in each group and normalizing to GAPDH as an internal
control. Unless otherwise stated, western blot analyses were
repeated four times.
Statistical analysis
All quantitative data are expressed as the mean ±
standard error of the mean and analyzed using SPSS software version
13.0 (SPSS, Inc., Chicago, IL, USA). Two-tailed unpaired Student's
t-tests were used between two groups and one-way analysis of
variance were used for statistical evaluation of the data between
multiple groups with post hoc contrasts by Student-Newman-Keuls
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
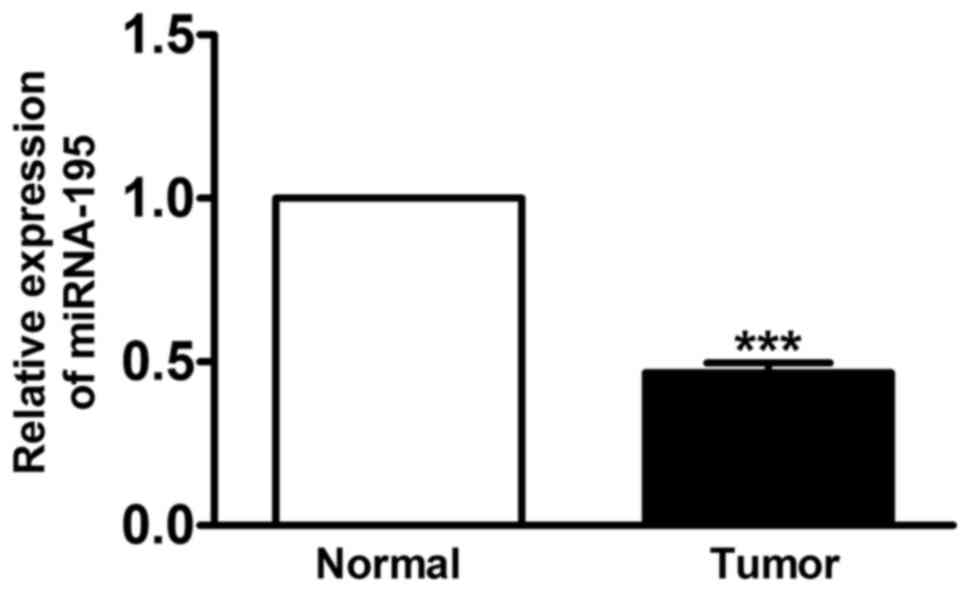
miRNA-195 is downregulated in human
LSCC in vivo
RT-qPCR was used to determine the expression of
miRNA-195 for the LSCC and the matched normal tissue samples
obtained from 23 patients diagnosed with LSCC. For the LSCC
samples, the mean miRNA level for miRNA-195 was significantly
decreased by 55% compared with the corresponding matched samples
(Fig. 1; P<0.01).
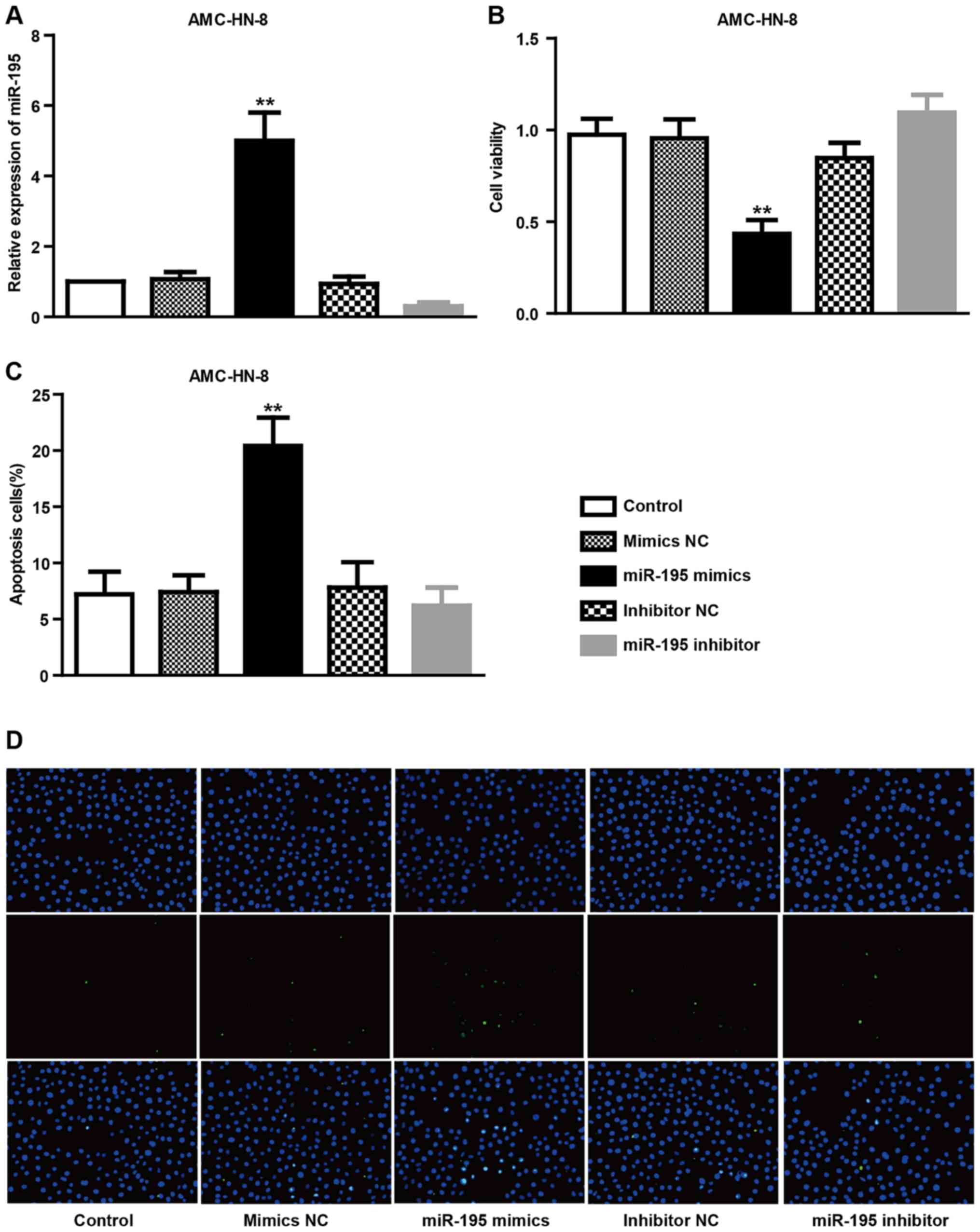
Overexpression of miRNA-195 decreases
cell viability and induces apoptosis in AMC-HN-8 cells
The present study evaluated whether miRNA-195
contributes to the survival rates of AMC-HN-8 cells by
overexpression with specific miRNA-195 mimics (Fig. 2). The results demonstrated that the
expression level of miRNA-195 in AMC-HN-8 cells from the miRNA-195
mimics treatment group was markedly higher compared with those from
the control or miRNA-195 NC treatment groups, whereas the miRNA-195
expression from inhibitor treatment was markedly lower compared
with the control or NC treatment groups (Fig. 2A). In comparison with control
cells, the viability of AMC-HN-8 cells as determined by MTT
analysis was reduced significantly in cells treated with miRNA-195
mimics (P<0.01), but not with the miRNA-195 inhibitor and
scramble, which did not significantly affect the viability of
AMC-HN-8 cells (Fig. 2B). In
addition, TUNEL staining results demonstrated that transfection
with miRNA-195 mimics, but not with the inhibitor, increased the
percentage of TUNEL-positive cells in the AMC-HN-8 cell population,
suggesting that miRNA-195 may provide an anti-apoptotic effect in
AMC-HN-8 cells (P<0.01; Fig. 2C and
D). Together, these data suggest that upregulation of miRNA-195
may serve anti-proliferative and pro-apoptotic roles in AMC-HN-8
cells.
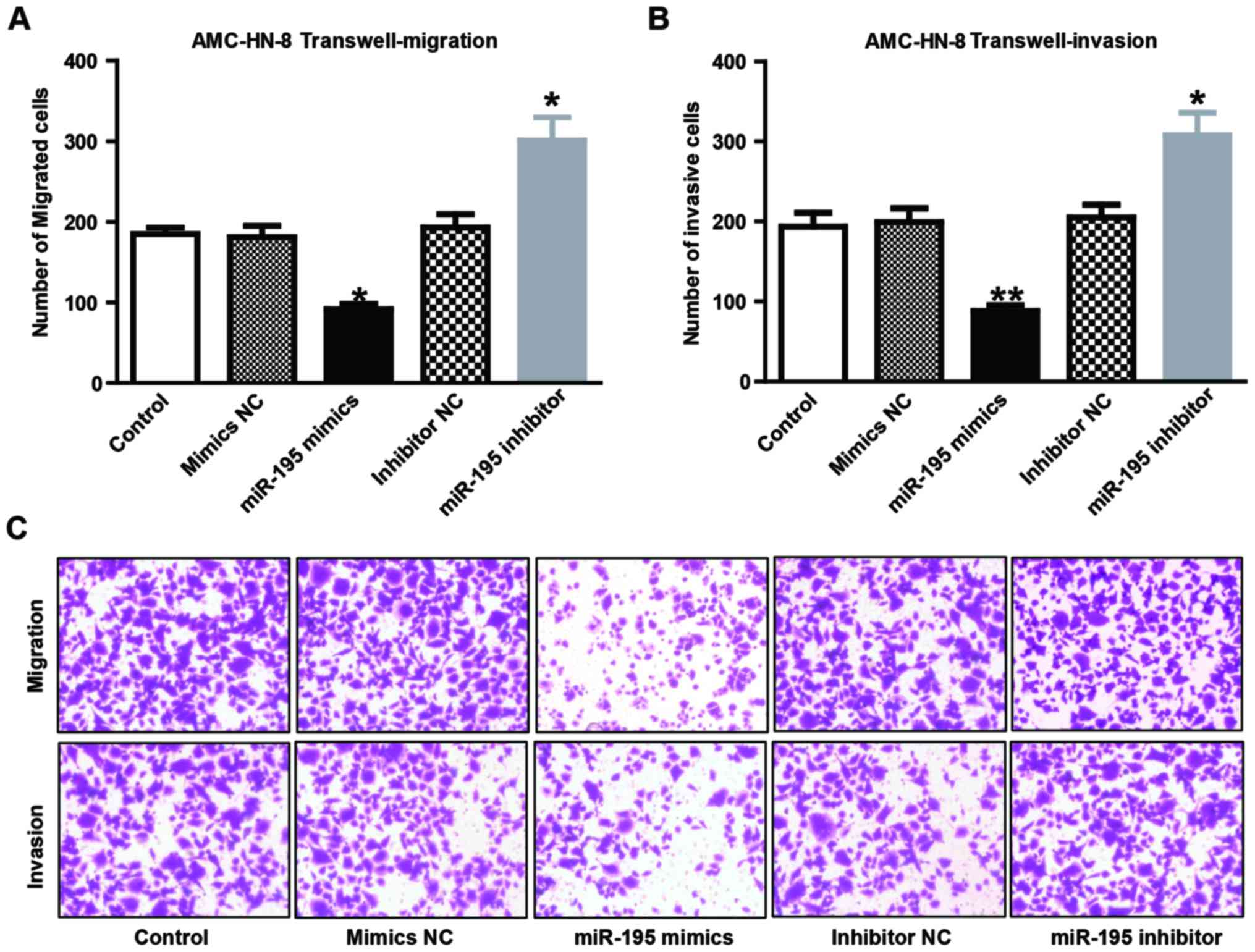
Effects of miRNA-195 on migration and
invasion of AMC-HN-8 cells
The potential effects of miRNA-195 on cell migration
and invasion were assessed using Transwell migration and invasion
assays. AMC-HN-8 cells were selected for overexpression and
knockdown of miRNA-195 using transient transfection, and Transwell
migration and Matrigel invasion assays demonstrated that miRNA-195
overexpression resulted in a significant reduction of AMC-HN-8 cell
migration (Fig. 3A; P<0.05) and
invasion rate (Fig. 3B; P<0.01)
compared with the NC group. By contrast, miRNA-195 knockdown
resulted in an increase of AMC-HN-8 cell migration (Fig. 3A; P<0.05) and invasion rate
(Fig. 3B; P<0.05) compared with
the NC group. Fig. 3C shows the
representative pictures of invasion and migration of AMC-HN-8
cells.
Association of VEGFR2/PI3K/AKT signal
pathways with the miRNA-195-induced pro-apoptotic effect on
AMC-HN-8 cells
The present study investigated whether miRNA-195
produced a pro-apoptotic effect by targeting VEGFR2 and downstream
signaling pathway proteins in AMC-HN-8 cells (Fig. 4). As was hypothesized, negative
regulation was identified between miRNA-195 and VEGFR2 in AMC-HN-8
cells (Fig. 4D). To evaluate the
effect on cell apoptotic protein expression, AMC-HN-8 cells were
transiently transfected with miRNA-195 mimics, miRNA-195 inhibitor
or their NC. Lower levels of Bcl-2 (Fig. 4B) were detected in the miR-195
mimics-transfected cells compared with cells transfected with the
NC or untransfected cells. In addition, higher levels of Bax
(Fig. 4A) and caspase-3 (Fig. 4C) were detected compared with cells
transfected with NC or untransfected cells. Additionally, since the
AKT/p-AKT is the upstream of Bax, Bcl-2 and caspase-3, the
expression levels of AKT/p-AKT were also detected. As shown in
Fig. 4E and F, the expression
level of p-AKT, but not AKT, was significantly decreased in the
miR-195 mimics-transfected cells compared with cells transfected
with the NC or untransfected cells.
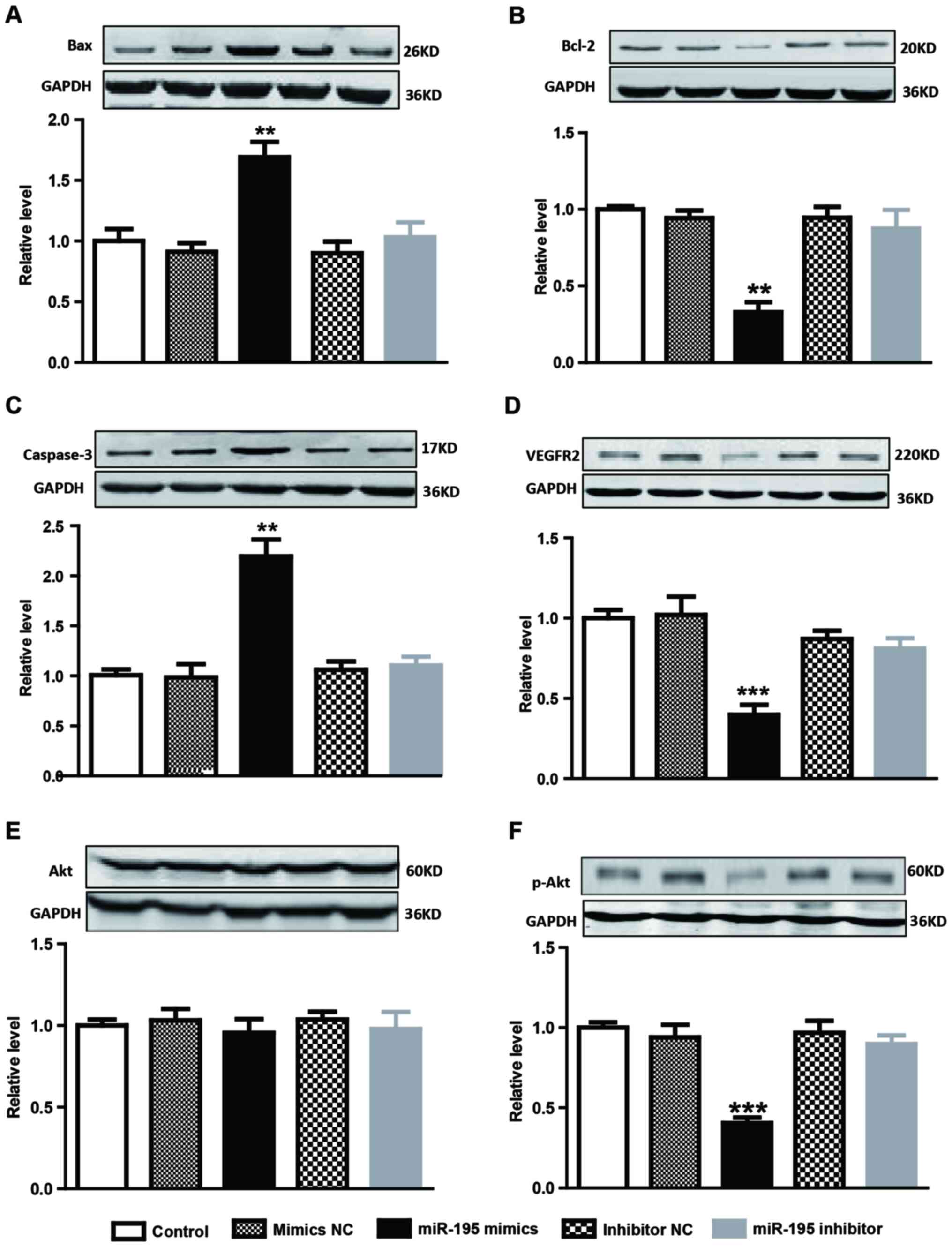 | Figure 4.Protein levels in differently treated
AMC-HN-8 cells were determined by western blotting. Protein levels
of (A) Bax, (B) Bcl-2, (C) caspase-3, (D) VEGFR2, (E) AKT and (F)
p-AKT were detected. Data are expressed as mean ± standard error of
the mean, n=4. **P<0.01 or ***P<0.001 vs. control group.
VEGFR2, vascular endothelial growth factor receptor 2; AKT, protein
kinase B; p, phosphorylated; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-like protein 4. |
Overexpression of miRNA-195 inhibits
viability, migration and invasion via regulating Raf/MEK/ERK and
SRC/FAK signal pathways respectively in AMC-HN-8 cells
To evaluate whether overexpression of miRNA-195
serves a role in the regulation of the viability, migration and
invasion of AMC-HN-8 cells, AMC-HN-8 cells were transiently
transfected with miRNA-195 mimic, miRNA-195 inhibitor or their NC.
Among various signaling molecules downstream of VEGFR2, the
Raf/MEK/ERK pathway mainly promotes cell growth and
differentiation. The western blot analysis data from the present
study demonstrated that the RAF, p-MEK and p-ERK genes were
significantly inhibited by upregulation of miRNA-195 at the protein
level in AMC-HN-8 cells (Fig. 5A;
P<0.05) compared with the control group. In addition, it was
also identified that miRNA-195 overexpression resulted in lower
expression of p-SRC and p-FAK proteins in AMC-HN-8 cells (Fig. 5B; P<0.05) compared with the
control group. Together, these data reveal that overexpression of
miRNA-195 is able to inhibit VEGFR2 and downstream signaling
pathways, including Raf/MEK/ERK and SRC/FAK, in AMC-HN-8 cells,
which inhibit LSCC cell viability, migration and invasion,
respectively.
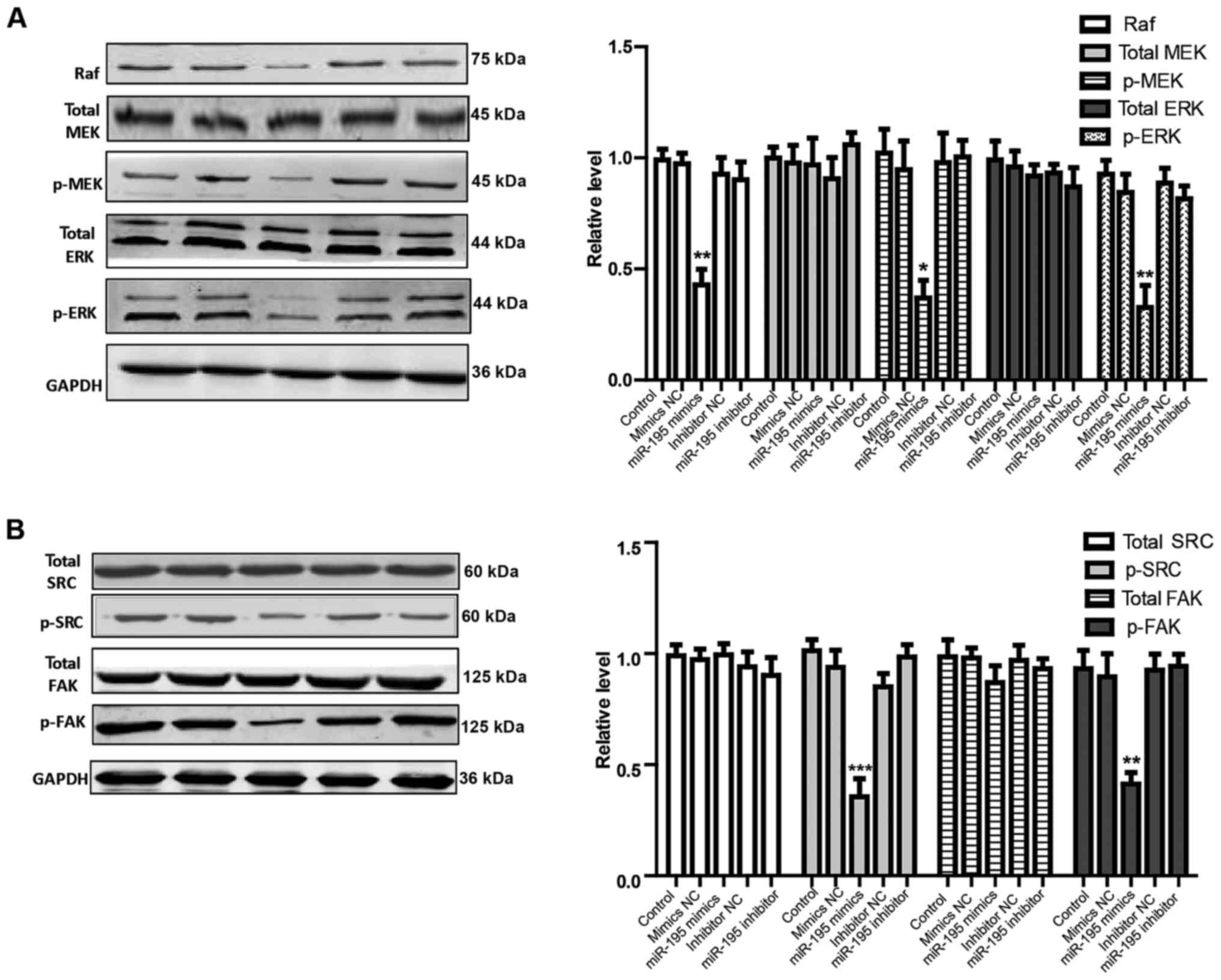 | Figure 5.Effects of miRNA-195 overexpression on
Raf/MEK/ERK and SRC/FAK signaling pathways in cultured AMC-HN-8
cells. Proteins expression of (A) Raf, total-MEK, p-MEK, total-ERK
and p-ERK, and (B) total SRC, p-SRC, total FAK, and p-FAK were
determined by western blotting. Data are expressed as mean ±
standard error of the mean, n=4. *P<0.05, **P<0.01 or
***P<0.001 vs. control group. miRNA/miR, microRNA; NC, negative
control; MEK, mitogen activated protein kinase kinase; p,
phosphorylated; ERK, extracellular signal-regulated kinase; FAK,
focal adhesion kinase. |
Discussion
The present study identified downregulation of
miRNA-195 in LSCC tissue samples compared with matched normal
tissue samples and that overexpression of miRNA-195 in AMC-HN-8
cells was able to suppress cell viability, migration and invasion,
and induce cell apoptosis. In addition, the data suggest that
suppression of VEGFR2 and its downstream signal proteins, including
Raf/MEK/ERK1/2, SRC/FAK and apoptosis-related proteins, mediate the
antitumor effect of miRNA-195 (Fig.
6). The results of the present study aid the understanding of
the mechanisms of miRNA-195 in regulating the viability, migration
and apoptosis in LSCC cell lines and support the suggestion that
miRNAs may serve as potential therapeutic and drug targets.
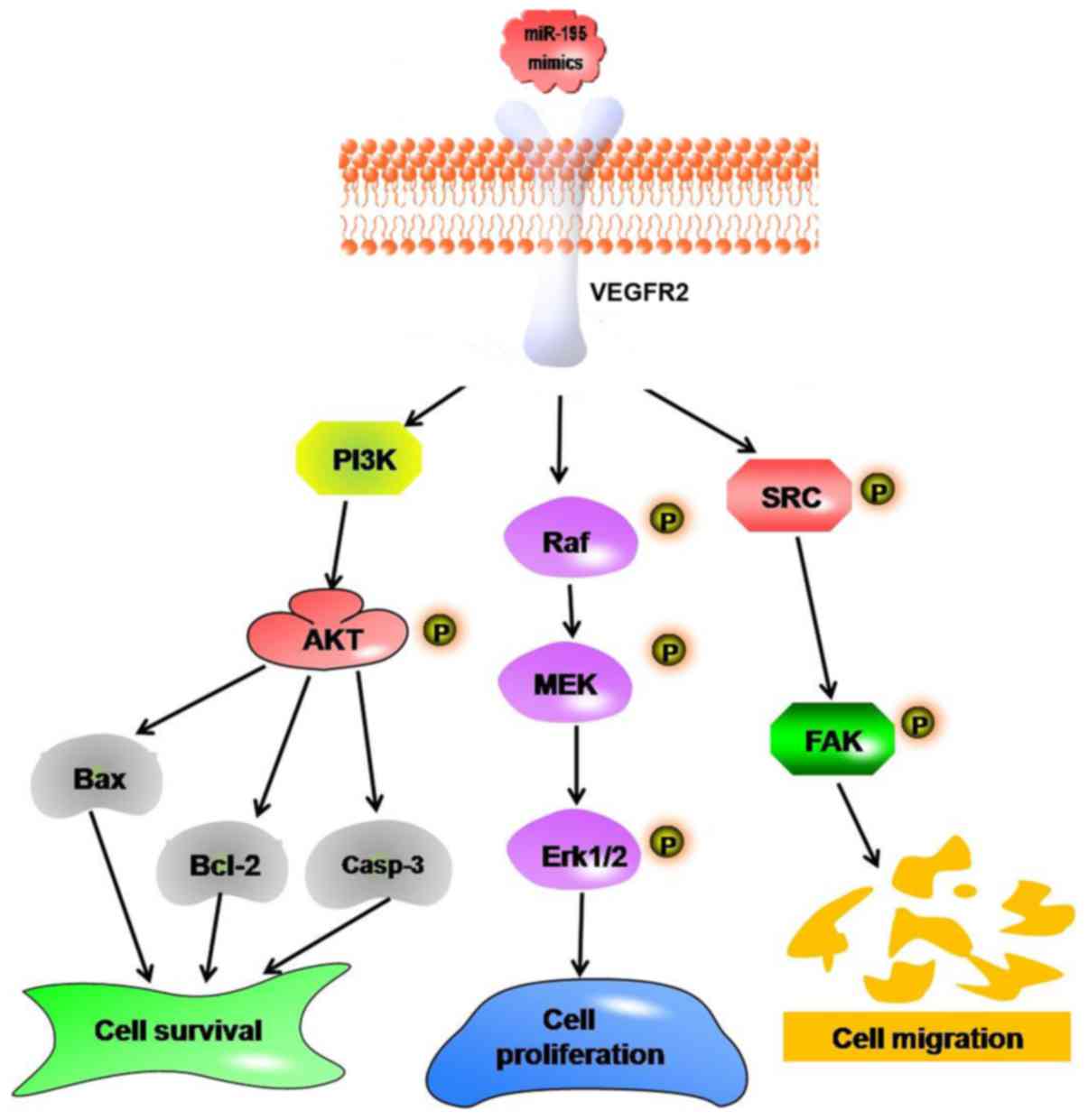 | Figure 6.Schematic illustration of the possible
targeting and signaling mechanisms by which miRNA-195 produces
antitumor effects in cultured AMC-HN-8 cells. Increased miRNA-195
represses cells viability, migration and invasion by targeting
VEGFR2, which leads to decreases in the activation of the
Raf/MEK/ERK and SRC/FAK signaling pathways in cultured AMC-HN-8
cells. In addition, miRNA-195 may induce AMC-HN-8 cell apoptosis by
downregulating the PI3K/AKT signaling pathway. miRNA/miR, microRNA;
VEGFR2, vascular endothelial growth factor receptor 2; PI3K,
phosphoinositide 3-kinase; AKT, protein kinase B; Bcl-2, B-cell
lymphoma 2; Casp-3, caspase-3; MEK, mitogen activated protein
kinase kinase; ERK, extracellular signal-regulated kinase; FAK,
focal adhesion kinase; p, phosphorylated. |
With the development of RT-qPCR and microarrays, a
number of recent studies (24,25)
have identified that dysregulation of miRNAs is closely associated
with the development and progression of various types of cancer,
via cell viability, cell invasion and apoptosis, by acting as
oncogenes or tumor suppressor genes. However, the mechanism of
miRNAs in LSCC remains to be elucidated. miRNA-195 downregulation
has been reported in various types of cancer, including human
hepatocellular carcinoma (26),
breast cancer (18), esophageal
squamous cell carcinoma (27),
adrenocortical cancer (28),
colorectal cancer (29) and human
tongue squamous cell carcinoma (30). The present study examined the
expression level of miRNA-195 in human LSCC tissues using RT-qPCR,
which demonstrated that miRNA-195 was downregulated in primary LSCC
tissues compared with matched adjacent non-cancerous tissues. These
data suggest that downregulation of miRNA-195 in human LSCC tissues
may be one of the molecular events causing its development. Ina
previous study (31), miRNA-195
was observed to regulate a number of target proteins, which are
associated with the cell cycle, apoptosis and viability in multiple
diseases, such as cancer, schizophrenia and heart failure (31–33).
However, to the best of our knowledge, the expression level of
miR-195 in human LSCC, its clinical role and its prognostic value
have not been fully investigated thus far. The present study
demonstrated the role of miRNA-195 in regulating cell biological
functions during the development of LSCC. It was identified that
overexpression of miRNA-195 significantly inhibited cell viability,
migration and invasion, and promoted apoptosis in AMC-HN-8 LSCC
cells. The identification of miRNA-195 target genesis is
significant for the improved understanding of the role of miRNA-195
in tumorigenesis. At present, certain genes such as WEE1 (34), cyclin dependent kinase 6 (35), and Bcl-2 have been confirmed as
targets of the miRNA-195 gene. According to the expression level
ofmiRNA-195 in LSCC, the present study selected the presumed
tumor-related gene VEGFR2 as a potential target of miRNA-195 among
the predicted genes. The current study identified that
overexpression of miRNA-195 represses the expression level of
VEGFR2 in AMC-HN-8 cells. VEGFR2 mediates a variety of important
molecular signaling pathways via its downstream signal proteins,
including Raf/MEK/ERK1/2, SRC/FAK and apoptosis-related proteins,
which serve a key role in regulating tumor development (36). Data from the present study further
support the hypothesis that inhibition of VEGFR2/Raf/MEK/ERK and
VEGFR2/SRC/FAK pathways regulates cancer cell growth and migration,
respectively, mediated by miRNA-195 upregulation in vitro.
It is noteworthy that not only was the expression of VEGFR2 in
AMC-HN-8 cells treated with miRNA-195 mimics significantly lower
than the control group, but also that the levels of p-AKT and Bcl-2
in LSCC cells with miRNA-195 overexpression were significantly
decreased. These results suggest that the miRNA-195/VEGFR2
signaling pathway may serve an important role in the development of
LSCC. However, it should be noted that the present study was
performed in cell lines and the findings may not be capable of
being extrapolated directly to animal models or to humans. There is
a need for caution when applying the results of the present study
to animals or patients.
The present study identified a novel antitumor
miRNA, miRNA-195, in human LSCC tissue. The data suggest that the
therapeutic potential of miRNA-195 in modulating cell growth,
migration and apoptosis during the pathophysiological progression
of LSCC, and the underlying mechanism, is associated with the
inhibition of VEGFR2 and downstream signaling pathways, including
Raf/MEK/ERK, SRC/FAK and PI3K/AKT. The present study indicates that
exogenous application of miRNA-195 may be a promising intervention
in the management of human LSCC and the associated pathological
processes. The findings provide rationale for additional studies to
investigate whether the miR-195 mechanisms associated with
modulating cell growth, migration and apoptosis also operate in the
clinical setting.
Acknowledgements
The authors thank all members of their laboratory
for helpful discussions and comments on the present study.
References
|
1
|
Siegel RL, Fedewa SA, Miller KD,
Goding-Sauer A, Pinheiro PS, Martinez-Tyson D and Jemal A: Cancer
statistics for hispanics/latinos, 2015. CA Cancer J Clin.
65:457–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGuire S: World cancer report 2014.
Geneva, Switzerland: World Health Organization, International
Agency for Research on Cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li F, Liu Y, Kan X, Li Y, Liu M and Lu JG:
Elevated expression of integrin alphav and β5 subunit in laryngeal
squamous-cell carcinoma associated with lymphatic metastasis and
angiogenesis. Pathol Res Pract. 209:105–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Canis M, Ihler F, Martin A, Wolff HA,
Matthias C and Steiner W: Results of 226 patients with T3 laryngeal
carcinoma after treatment with transoral laser microsurgery. Head
Neck. 36:652–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu ST, Zhou Z, Cai Q, Liang F, Han P, Chen
R and Huang XM: Prognostic value of the C-reactive protein/albumin
ratio in patients with laryngeal squamous cell carcinoma. Onco
Targets Ther. 10:879–884. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu JC, Shen WC, Shih TC, Tsai CW, Chang
WS, Cho Y, Tsai CH and Bau DT: The current progress and future
prospects of personalized radiogenomic cancer study. Biomedicine
(Taipei). 5:22015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J,
Lee J, Provost P, Rådmark O, Kim S and Kim VN: The nuclear RNase
III Drosha initiates microRNA processing. Nature. 425:415–419.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tutar L, Tutar E and Tutar Y: MicroRNAs
and cancer; an overview. Curr Pharm Biotechnol. 15:430–437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu F, Yang L, Ling X, Yang R, Yang X,
Zhang L, Fang W, Xie C, Huang D, Zhou Y and Lu J: Sequence
variation in mature microRNA-499 confers unfavorable prognosis of
lung cancer patients treated with platinum-based chemotherapy. Clin
Cancer Res. 21:1602–1613. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv G, Hu Z, Tie Y, Du J, Fu H, Gao X and
Zheng X: MicroRNA-451 regulates activating transcription factor 2
expression and inhibits liver cancer cell migration. Oncol Rep.
32:1021–1028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ribeiro-dos-Santos Â, Khayat AS, Silva A,
Alencar DO, Lobato J, Luz L, Pinheiro DG, Varuzza L, Assumpção M,
Assumpção P, et al: Ultra-deep sequencing reveals the microRNA
expression pattern of the human stomach. PLoS One. 5:e132052010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brockway S and Zeleznik-Le NJ: WEE1 is a
validated target of the microRNA miR-17-92 cluster in leukemia.
Cancer Genet. 208:279–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim J, Jeong D, Nam J, Aung TN, Gim JA,
Park KU and Kim SW: MicroRNA-124 regulates glucocorticoid
sensitivity by targeting phosphodiesterase 4B in diffuse large B
cell lymphoma. Gene. 558:173–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao D, Tu Y, Wan L, Bu L, Huang T, Sun X,
Wang K and Shen B: In vivo monitoring of angiogenesis inhibition
via down-regulation of mir-21 in a VEGFR2-luc murine breast cancer
model using bioluminescent imaging. PLoS One. 8:e714722013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vishnubalaji R, Hamam R, Abdulla MH,
Mohammed MA, Kassem M, Al-Obeed O, Aldahmash A and Alajez NM:
Genome-wide mRNA and miRNA expression profiling reveal multiple
regulatory networks in colorectal cancer. Cell Death Dis.
6:e16142015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang S, Hu F, Liang H, Liu Y, Yang J and
Zhou W: Association between a miRNA-146a polymorphism and
susceptibility to head and neck squamous cell carcinoma in Chinese
patients: A meta-analysis of 8 case-control studies. PLoS One.
12:e01866092017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heneghan HM, Miller N, Kelly R, Newell J
and Kerin MJ: Systemic miRNA-195 differentiates breast cancer from
other malignancies and is a potential biomarker for detecting
noninvasive and early stage disease. Oncologist. 15:673–682. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Itesako T, Seki N, Yoshino H, Chiyomaru T,
Yamasaki T, Hidaka H, Yonezawa T, Nohata N, Kinoshita T, Nakagawa M
and Enokida H: The microRNA expression signature of bladder cancer
by deep sequencing: The functional significance of the miR-195/497
cluster. PLoS One. 9:e843112014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Collier R: World medical association
updates ethical code for physicians. CMAJ. 189:E13722017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tu Y, Wan L, Fan Y, Wang K, Bu L, Huang T,
Cheng Z and Shen B: Ischemic postconditioning-mediated miRNA-21
protects against cardiac ischemia/reperfusion injury via PTEN/Akt
pathway. PLoS One. 8:e758722013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Justus CR, Leffler N, Ruiz-Echevarria M
and Yang LV: In vitro cell migration and invasion assays. J Vis
Exp. Jun 1–2014.doi: 10.3791/51046. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galoian KA, Guettouche T, Issac B, Qureshi
A and Temple HT: Regulation of onco and tumor suppressor MiRNAs by
mTORC1 inhibitor PRP-1 in human chondrosarcoma. Tumour Biol.
35:2335–2341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H: Predicting cancer-related MiRNAs
using expression profiles in tumor tissue. Curr Pharm Biotechnol.
15:438–444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang X, Yin J, Yu J, Xiang Q, Liu Y, Tang
S, Liao D, Zhu B, Zu X, Tang H and Lei X: miRNA-195 sensitizes
human hepatocellular carcinoma cells to 5-FU by targeting BCL-w.
Oncol Rep. 27:250–257. 2012.PubMed/NCBI
|
|
27
|
Fu MG, Li S, Yu TT, Qian LJ, Cao RS, Zhu
H, Xiao B, Jiao CH, Tang NN, Ma JJ, et al: Differential expression
of miR-195 in esophageal squamous cell carcinoma and miR-195
expression inhibits tumor cell proliferation and invasion by
targeting of Cdc42. FEBS Lett. 587:3471–3479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chabre O, Libé R, Assie G, Barreau O,
Bertherat J, Bertagna X, Feige JJ and Cherradi N: Serum miR-483-5p
and miR-195 are predictive of recurrence risk in adrenocortical
cancer patients. Endocr Relat Cancer. 20:579–594. 2013.PubMed/NCBI
|
|
29
|
Wang X, Wang J, Ma H, Zhang J and Zhou X:
Downregulation of miR-195 correlates with lymph node metastasis and
poor prognosis in colorectal cancer. Med Oncol. 29:919–927. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia LF, Wei SB, Gong K, Gan YH and Yu GY:
Prognostic implications of micoRNA miR-195 expression in human
tongue squamous cell carcinoma. PLoS One. 8:e566342013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He JF, Luo YM, Wan XH and Jiang D:
Biogenesis of MiRNA-195 and its role in biogenesis, the cell cycle,
and apoptosis. J Biochem Mol Toxicol. 25:404–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi W, Du J, Qi Y, Liang G, Wang T, Li S,
Xie S, Zeshan B and Xiao Z: Aberrant expression of serum miRNAs in
schizophrenia. J Psychiatr Res. 46:198–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He X, Ji J, Wang T, Wang MB and Chen XL:
Upregulation of circulating miR-195-3p in heart failure.
Cardiology. 138:107–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bhattacharya A, Schmitz U, Wolkenhauer O,
Schonherr M, Raatz Y and Kunz M: Regulation of cell cycle
checkpoint kinase WEE1 by miR-195 in malignant melanoma. Oncogene.
32:3175–3183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deng H, Guo Y, Song H, Xiao B, Sun W, Liu
Z, Yu X, Xia T, Cui L and Guo J: MicroRNA-195 and microRNA-378
mediate tumor growth suppression by epigenetical regulation in
gastric cancer. Gene. 518:351–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun P, Wang L, Lu Y, Liu Y, Li L, Yin L,
Zhang C, Zhao W, Shen B and Xu W: MicroRNA-195 targets VEGFR2 and
has a tumor suppressive role in ACHN cells via PI3K/Akt and
Raf/MEK/ERK signaling pathways. Int J Oncol. 49:1155–1163. 2016.
View Article : Google Scholar : PubMed/NCBI
|