Introduction
Epithelial ovarian cancer (EOC) is the most common
type of ovarian cancer, accounting for over 90% of ovarian cancers,
and is one of the three most common cancers in females (1,2). EOC
is associated with high morbidity and mortality rates owing to the
typical late stage of the disease at diagnosis; up to 75% of
females with EOC are diagnosed at advanced stages because there are
few symptoms in the early stage (3). Ovarian serous carcinoma is the most
common histological type of ovarian cancer, accounting for 70–80%
of all newly diagnosed patients, and the most common and most
aggressive subtype of EOC (4).
Over the past 30 years, advances in surgery and chemotherapy have
had little impact on overall patient survival, and current
treatment leads to relapse in the majority of patients. This
situation calls for investigation of the pathogenesis of ovarian
serous carcinoma and identification of molecular markers for early
diagnosis and treatment.
Erythropoietin producing hepatocellular carcinoma
(Eph) receptors constitute the largest subfamily of receptor
tyrosine kinases that bind membrane-bound ligands called ephrins
(5). The Eph/ephrin interactions
emanate their signals in a bidirectional manner into adjacent
cells, followed by internalization and degradation of the complexes
(6). Eph/ephrin signaling is
proposed to participate in a wide spectrum of developmental
processes through its capacity to regulate cellular adhesion,
migration, or repulsion and tissue/cell boundary formation
(7–11). Beyond their initial role in
developmental processes, Ephs and ephrins are also involved in a
broad range of processes directly related to tumor progression and
metastasis (6,12–15).
Eph receptor-A1 (EphA1), the first member of the Eph
receptor tyrosine kinase family to be discovered, was isolated as a
gene that was amplified in a carcinoma cell line and shown to be
located on chromosome 7q34 (16).
EphrinA1 is the highest affinity binding ligand for EphA1, although
EphA1 also binds ephrinA3 and A4 with lower affinity. Whole-mount
in situ hybridization showed overlapping expression of
EphA1, ephrinA1, and ephrinA3 in the streak and the posterior
paraxial mesoderm during early mouse development (17). Activation of EphA1 can inhibit cell
spreading and migration in a Rho-ROCK-dependent manner (18). These results suggested that
interaction of EphA1 and ephrinA1/A3 plays a role in tumor
development. EphA1 expression has been detected in several types of
human cancer. EphA1 mRNA and protein were detected in human
epidermis at a high level, but EphA1 protein expression was reduced
in non-melanoma skin cancers derived from the epidermis (19). In a previous study, we explored
EphA1 expression in colorectal cancer, gastric cancer, and renal
carcinoma and analyzed the correlation between EphA1 expression and
clinicopathological parameters (20–22).
Our data suggest that EphA1 is expressed in human cancers at highly
varying levels. Expression of EphA1 protein has not yet been
determined in ovarian serous carcinoma. The purpose of this study
is to investigate the expression of EphA1 protein in ovarian serous
adenocarcinoma and its association with clinical parameters.
Materials and methods
Cell lines and tissue samples
Human ovarian cancer cell lines HO8910 and A2780
used in the present study were purchased from the cell resource
center of the Shanghai Institute of life Sciences, Chinese Academy
of Sciences. HO8910 and A2780 were maintained in RPMI 1640 medium
(Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (Gibco; Invitrogen), 100 U/ml penicillin, and 100 mg/ml
streptomycin in a 5% CO2 and 95% atmosphere at 37°C with
cell culture plates (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Clinical specimens were collected from the
Department of Pathology of Affiliated Hospital of Nantong
University from January 2001 to January 2013. Samples consisted of
10 normal fallopian tubes (age, 24–50 years; average, 43.1), 12
ovarian benign serous cystadenoma tumor tissues (age, 23–62 years;
average, 38.5), 15 borderline serous tumors (age, 22–51 years;
average, 34.5) and 76 ovarian serous carcinoma tissues (age, 27–69
years; average, 48.7). Generally, matched normal (or non-tumor) and
tumor tissue from same patient are subjected to detection when we
investigate a gene expression profile in certain cancer. However,
serous ovarian carcinoma is very special. First of all, according
to recent research results, serous ovarian carcinoma is not derived
from ovarian epithelial cells, but from fallopian tube (23). Secondly, there usually almost no
normal ovarian surface epithelial was available in ovarian tumor
tissues. Formalin-fixed and paraffin-embedded tumor tissues were
sectioned at 4-µm thickness. Each tumor was classified according to
WHO Classification Tumors of Female Reproduction Organs (23). Data were acquired with approval
from the Ethics Committee of the Affiliated Hospital of Nantong
University.
EphA1 plasmid transfection
The plasmid EphA1-pCMV6-GFP eukaryotic expression
vector kit was purchased from OriGene Technologies, Inc (Rockville,
MD, USA). HO8910 and A2780 cells were each divided into three
groups: EphA1 transfected group (EphA1-TG), mock group (MG), and
untransfected group (UTG). The MG group and the EphA1-TG group were
transiently transfected with plasmid pCMV6-GFP and plasmid
EphA1-pCMV6-GFP respectively using Lipofectamine 2000 according to
the manufacturer's instructions. The UTG group did not receive any
treatment. The transfection rate for EphA1-pCMV6-GFP and mock was
checked by observation of GFP with a fluorescence microscope and by
RT-PCR amplification of EphA1 mRNA. The protocol for amplification
of EphA1 mRNA was the same as in our previous report (20). For EphA1, the sense primer is
5′-ATCTTTGGGCTGCTGCTTGG-3′ and the antisense primer is
5′-GCTTGTCCTCTCGATCCACATC-3′. For housekeeping gene GAPDH, the
sense primer is 5′-CCAGGTGGTCTCCTCTGACTT-3′ and the antisense
primer is 5′-GTTGCTGTAGCCAAATTCGTTGT-3′.
Determination of cell viability (MTT
assay)
HO8910 and A2780 cells were seeded in 96-well
flat-bottomed plates with 5,000 cells per well in 100 µl of
complete RPMI 1640 medium, followed by incubation at 37°C (5%
CO2 and 95% air) for 24 h to allow the cells to reach
70% confluence. The cells were transiently transfected and cultured
for 48 h. The supernatant was carefully removed, and 100 µl medium
and 20 µl of a 5 mg/ml MTT solution (Thermo Fisher Scientific,
Inc.) were added to each well and incubated for 4 h at 37°C. The
excess MTT was then aspirated. Viable cells internalize the MTT
into their mitochondria. The formazan crystals formed in cells were
dissolved by addition of 150 µl of dimethyl sulfoxide (DMSO). After
shaking for 1 h, the absorbance was measured at 540 nm in a
multiwall scanning spectrophotometer.
Apoptosis
Apoptosis of cells after transfection for 72 h with
EphA1-pCMV6-GFP and mock plasmid was detected using an Annexin
V-FITC apoptosis detection kit (Qiagen GmbH, Hilden, Germany). In
brief, cells were collected after digestion with 0.25% trypsin and
rinsed. The cells were resuspended in binding buffer with 5 µl
Annexin V-FITC and 5 µl propidium iodide (PI). After incubation at
room temperature for 10 min in the dark, Annexin V-FITC/PI binding
was measured by flow cytometry (excitation, 488 nm; emission, 530
nm) using the phycoerythrin emission signal detector (FL1 for
detection of FITC, and FL2 for detection of PI).
Immunocytochemical (ICC) and
immunohistochemical (IHC) staining
ICC and IHC staining was performed by the Envision
method. For ICC staining, cells were grown on glass coverslips to
70% confluence, washed with PBS, and fixed with cold 75% ethanol
for 10 min on ice. The cells were incubated in 3%
H2O2 for 10 min and then at 4°C overnight
with an anti-EphA1 polyclonal antibody (AO1047a, ABGENT) at a 1:100
dilution in Antibody Diluent (Zymed; Invitrogen). After a wash with
PBS, the cells were incubated with secondary antibody (Dako, Ely,
UK) for 20 min at room temperature. Color development was performed
with 3,3-diaminobenzidine (DAB). Nuclei were lightly counterstained
with hematoxylin.
For IHC staining, 4-µm thick sections were
deparaffinized in xylene. After rehydration through a graded
ethanol series, the sections were autoclaved in 10 mM citrate
buffer (pH 6.0) at 120°C for 2 min for antigen retrieval and then
cooled to 30°C and washed with PBS (pH 7.3). After non-specific
sites had been blocked with 3% H2O2 for 10
min, the sections were incubated at 4°C overnight with an
anti-EphA1 polyclonal antibody and washed with PBS. The subsequent
steps were the same as for ICC. Two pathologists independently
assessed the immunostained slides, and any differences in the
staining scores were resolved by consensus.
IHC scoring and quantification
Cytoplasmic staining was considered positive
staining. The scoring for percentage of immunoreactive tumor cells
was as follows: 0, 0%; 1, <20%; 2, 20–50%; and 3, >50%. The
staining intensity was scored and stratified as follows: 0,
negative; 1, weak; 2, moderate; and 3, strong. A final
immunoreactivity score (IRS) was obtained for each of the cases by
multiplying the percentage score and the intensity score. Protein
expression levels were further analyzed by classifying IRS values
as negative (IRS value <4) or positive (IRS value ≥4) (24).
Statistical analysis
Cell experiments were repeated three times and data
were expressed as mean ± standard deviation (mean ± standard
deviation). Results were analyzed by one-way ANOVA. The
χ2 test (Fisher's exact test) was used to assess the
associations of EphA1 protein expression with clinicopathological
variables. Two-sided P-values <0.05 were considered
statistically significant. All analyses were performed by SPSS
software (version 16.0; SPSS, Inc., Chicago, IL, USA).
Results
Expression of EphA1 in ovarian cancer
cell lines
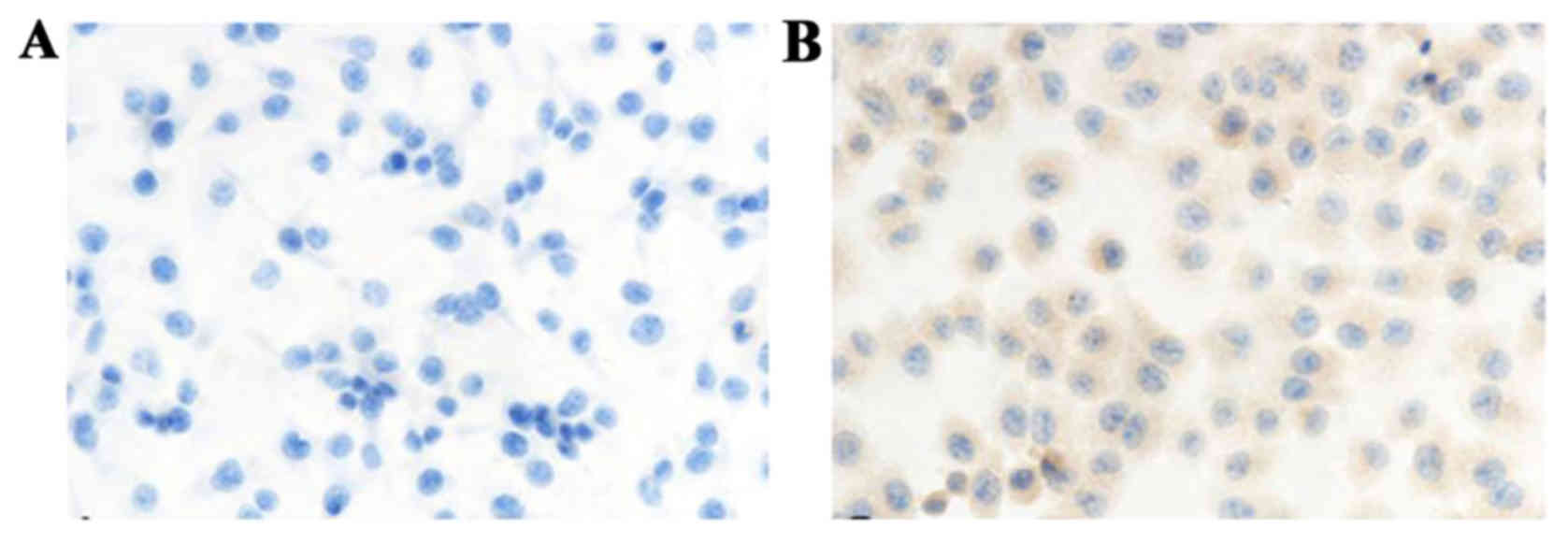
EphA1 expression in human ovarian cancer cell lines
HO8901 and A2780 was examined by immunocytochemistry. EphA1
staining was located in the cytoplasm. The expression of EphA1
protein was negative in HO8910 cells and weakly positive in A2780
cells (Fig. 1).
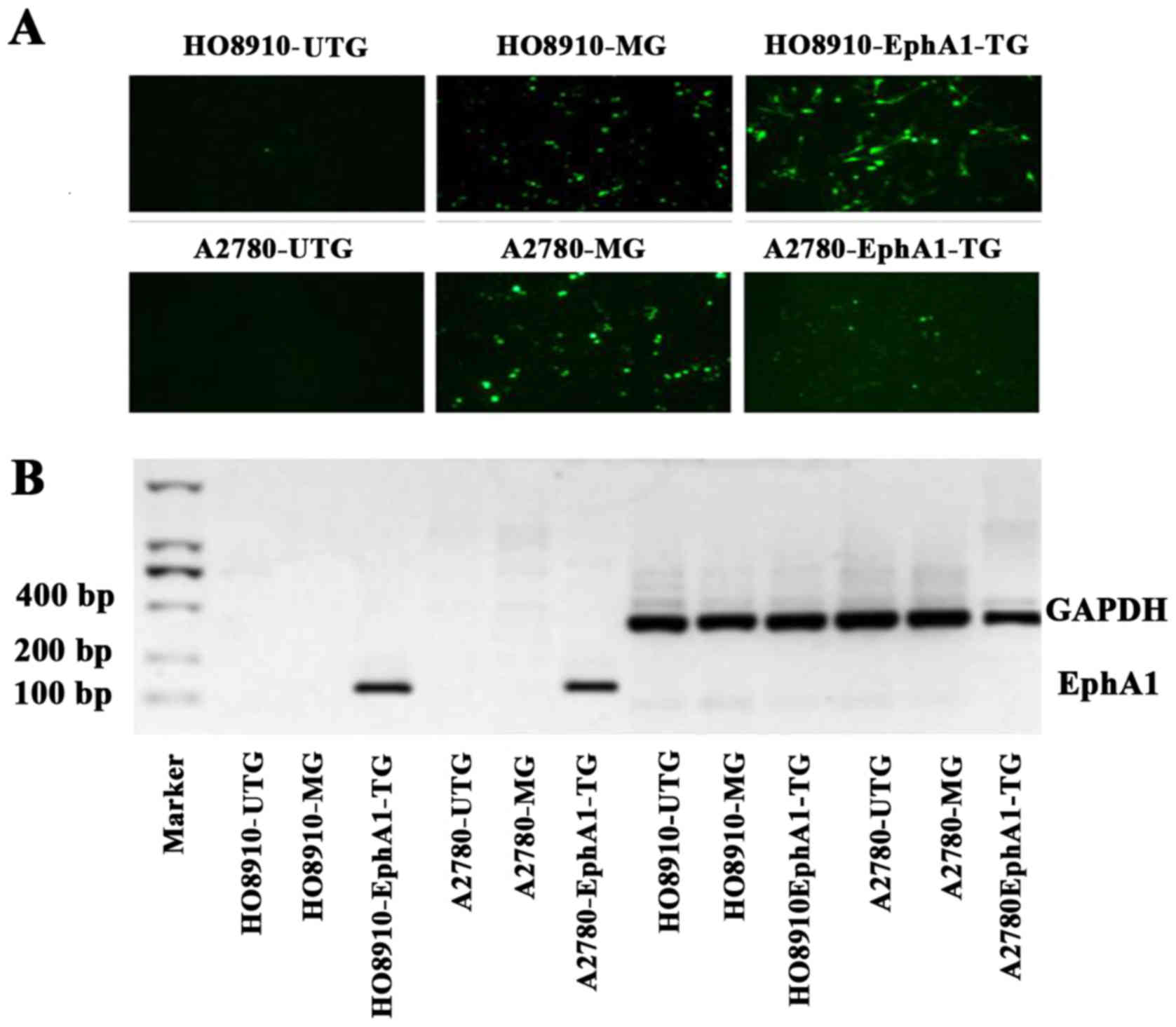
Transfection of EphA1 gene
The ovarian cancer cells were observed using a
fluorescence microscope after transient transfection with pCMV6-GFP
or EphA1-pCMV6-GFP plasmids. Green fluorescence was observed in
ovarian cancer cells transfected with EphA1-TG and MG, but not in
UTG (Fig. 2A). EphA1 mRNA
expression in cells of the EphA1-TG group was detected by RT-PCR
(127 bp), with GAPDH mRNA as an internal control (416 bp) (Fig. 2B).
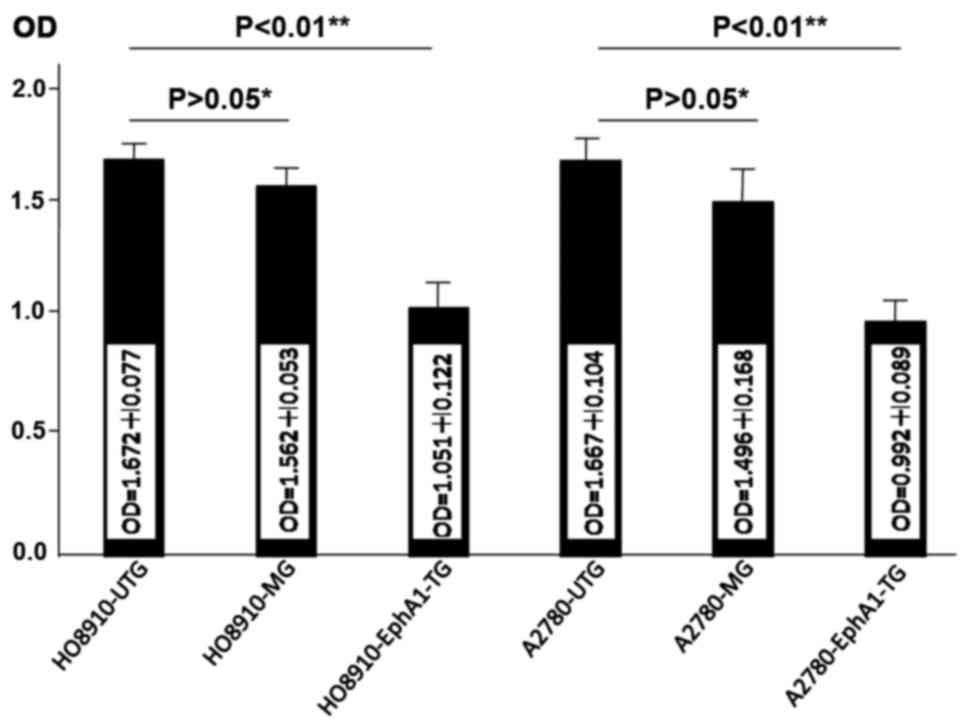
Proliferation of HO8910 and A2780 cell
lines after EphA1 transfection
The MTT assay was performed to determine the
proliferative effect of HO8910 and A2780 ovarian cancer cells
transfected with EphA1 and the data were analyzed using two-sample
independent t-test. The proliferation rate of both HO8910-EphA1-TG
and A2780-EphA1-TG cells was significantly reduced compared with
that in mock and untransfected groups (Fig. 3).
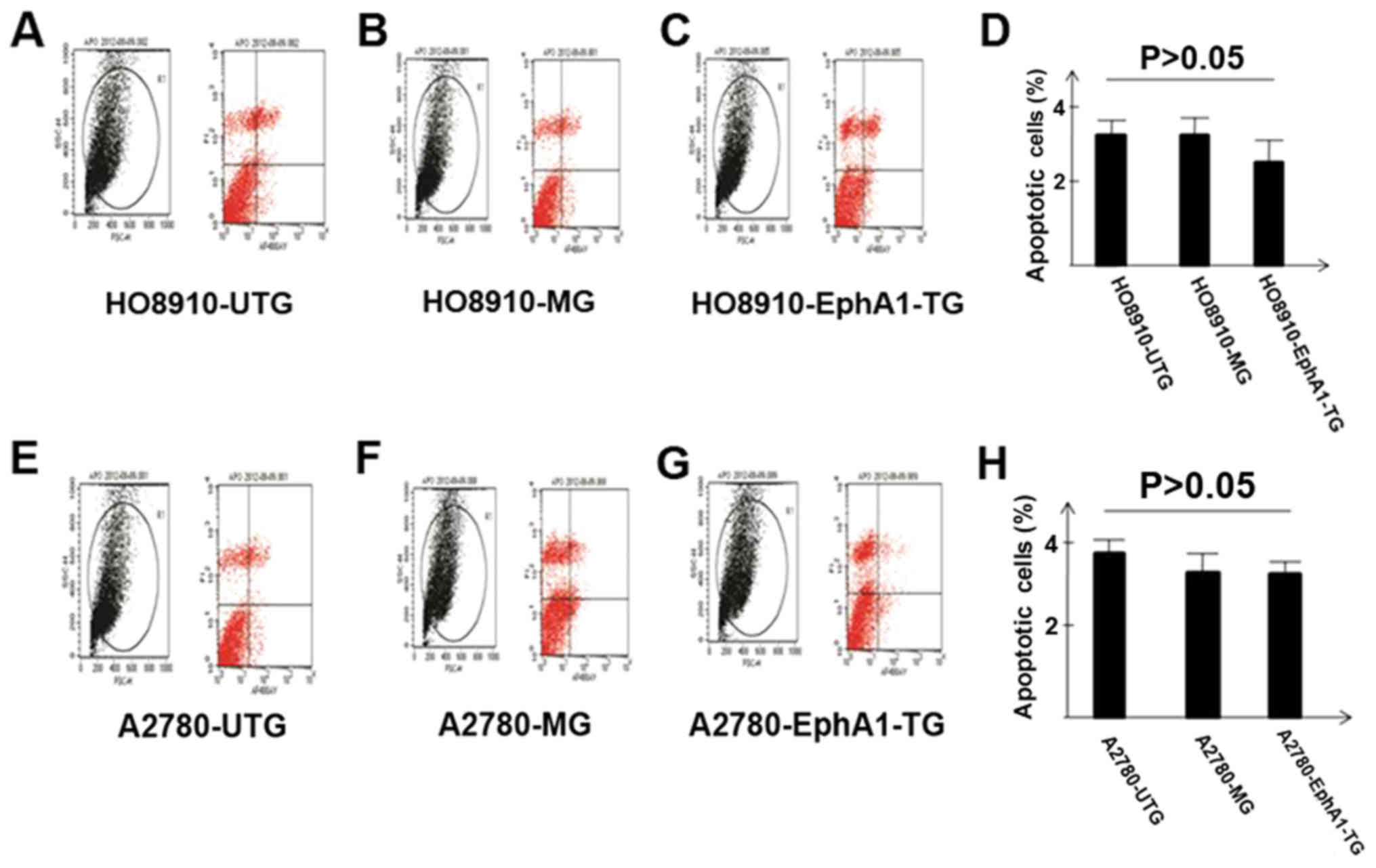
Apoptosis in HO8910 and A2780 cell
lines after EphA1 transfection
Apoptosis was measured in HO8910 and A2780 ovarian
cancer cells using flow cytometry. There was no significant
difference in apoptosis among the EphA1 transfected group, mock,
and untransfected groups for both HO8910 and A2780 cells (Fig. 4).
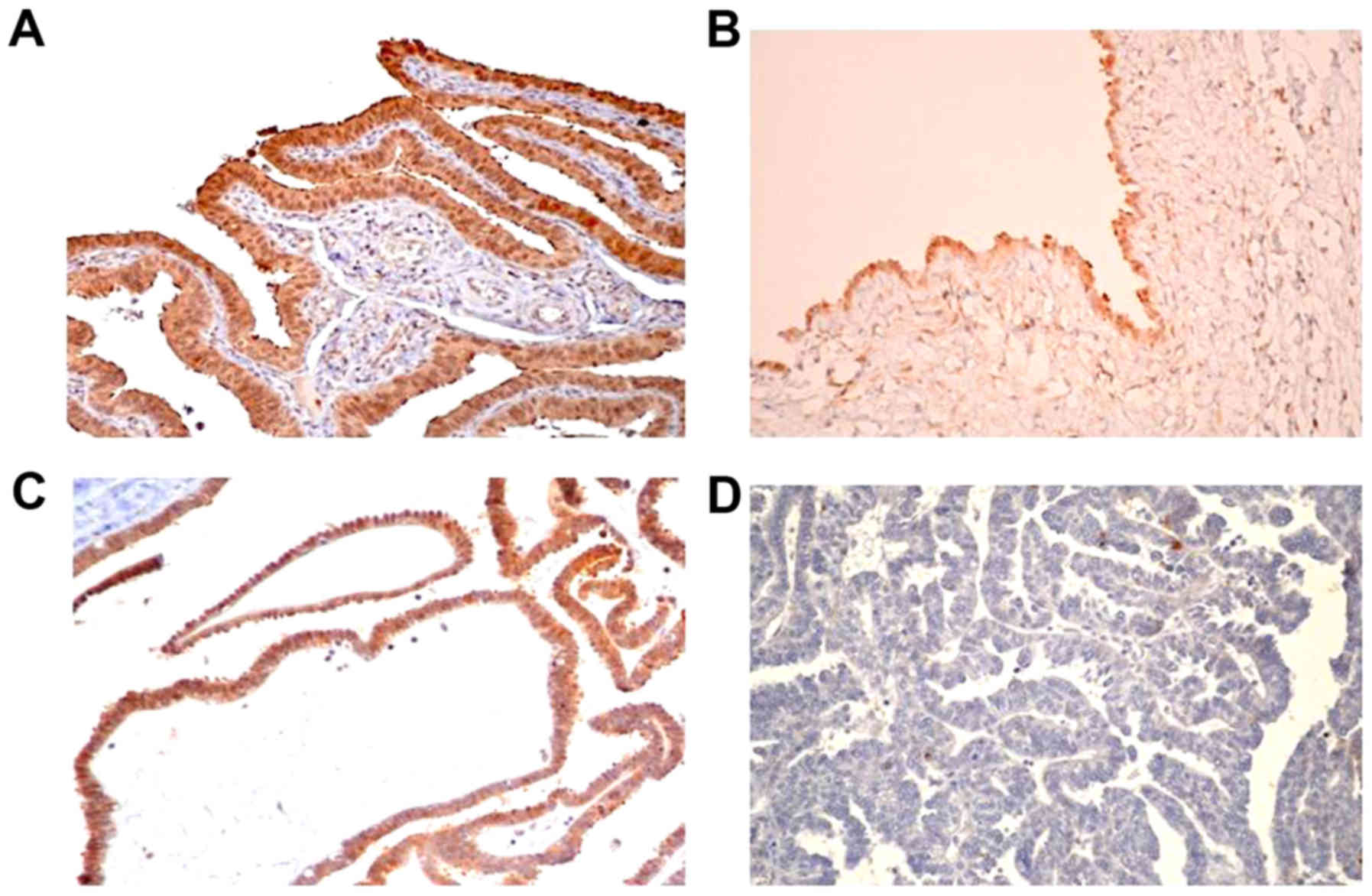
EphA1 expression in normal fallopian
tube and ovarian serous tumors
EphA1 staining in normal fallopian tube, ovarian
benign serous cystadenoma, borderline serous tumors and serous
carcinoma was located predominantly in the cytoplasm with diffuse
positive expression (Fig. 5).
Positive EphA1 staining was detected in all normal fallopian tubes
(10/10) and ovarian benign serous cystadenomas (12/12). EphA1
protein was positively detected in some samples of borderline
serous tumors (9/15) and ovarian serous carcinoma (33/76) (Table I).
 | Table I.EphA1 expression in normal fallopian
tubes, ovarian benign serous cystadenomas, and serous
carcinomas. |
Table I.
EphA1 expression in normal fallopian
tubes, ovarian benign serous cystadenomas, and serous
carcinomas.
|
|
| EphA1 |
|
|---|
|
|
|
|
|
|---|
| Group | No. | Negative | Positive | Positive (%) |
|---|
| Normal fallopian
tube | 10 | 0 | 10 | 100.00 |
| Serous
cystadenoma | 12 | 0 | 12 | 100.00 |
| Borderline serous
tumors | 15 | 6 | 9 |
60.00 |
| Serous carcinoma | 76 | 43 | 33 |
43.42 |
EphA1 expression correlated with
clinicopathological features
The relationship between EphA1 expression and
clinicopathological parameters was shown in Table II. Statistical analysis of the
association between EphA1 expression and clinicopathological
features revealed a significant relationship between EphA1
expression and tumor grade (P=0.016) and Ki67 (P=0.007). No
significant association of EphA1 expression and other features was
found in this study.
 | Table II.Relationship between EphA1 expression
and clinicopathologic parameters in ovarian serous carcinomas. |
Table II.
Relationship between EphA1 expression
and clinicopathologic parameters in ovarian serous carcinomas.
|
|
| EphA1 |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Parameters | No. | − | + | Positive % | χ2 | P-value |
|---|
| Clinical
stages |
|
|
|
|
|
|
|
I+II | 15 | 6 | 9 | 60.00 |
|
|
| III+
IV | 61 | 37 | 24 | 39.34 | 2.091 | 0.148 |
| Grade |
|
|
|
|
|
|
|
Low | 12 | 3 | 9 | 75.00 |
|
|
|
High | 64 | 40 | 24 | 37.50 | 5.784 | 0.016 |
| Metastasis |
|
|
|
|
|
|
|
Yes | 30 | 19 | 11 | 36.67 |
|
|
| No | 46 | 24 | 22 | 47.83 | 0.920 | 0.337 |
| Position of
tumors |
|
|
|
|
|
|
|
Single | 32 | 16 | 16 | 50.00 |
|
|
|
Double | 44 | 27 | 17 | 38.64 | 0.974 | 0.324 |
| Maximum diameter
(cm) |
|
|
|
|
|
|
| ≤5 | 29 | 13 | 16 | 55.17 |
|
|
|
5–10 | 21 | 15 | 6 | 28.57 |
|
|
|
≥10 | 17 | 11 | 6 | 35.29 | 3.938 | 0.140 |
| No
data | 9 | 4 | 5 |
|
|
|
| Age (years) |
|
|
|
|
|
|
|
≤50 | 30 | 17 | 13 | 43.33 |
|
|
|
50–55 | 16 | 12 | 4 | 25.00 |
|
|
|
≥55 | 30 | 14 | 16 | 53.33 | 3.410 | 0.182 |
| Ki67 |
|
|
|
|
|
|
|
>20% | 52 | 35 | 17 | 68.42 |
|
|
|
≤20% | 24 | 8 | 16 | 31.58 | 7.715 | 0.007 |
Discussion
Roles of the receptor tyrosine kinases in both
normal physiology and oncogenesis have been well established. The
genes that encode Eph receptors, the largest subfamily of receptor
tyrosine kinases, are primarily considered to be classic oncogenes.
Overexpression of EphA1 has been reported in several human cancers
(25–27); however, reduced expression of EphA1
also has been detected in prostate cancer cell lines (28), basal cell carcinomas and squamous
cell carcinomas of the skin (19),
and colorectal cancer (20).
Therefore, whether EphA1 is an oncogene has been questioned. We
previously reported that EphA1 expression was associated with
metastasis in esophageal squamous cell carcinoma (29), Gleason score in prostate cancer
(30), invasion and metastasis in
colorectal cancer (20), and
metastasis in gastric cancer (21). Comprehensive studies show obvious
differences in EphA1 expression among different tissues and
different tumor types.
In this study, EphAl expression was negative in
HO8910 and weakly positive in A2780 ovarian cancer cells. In
addition, loss of EphA1 expression was found in most ovarian serous
carcinoma tissues compared with normal fallopian tube and benign
tumor. Our data suggest that EphAl is downregulated in ovarian
serous carcinoma. In contrast, Wong et al reported that
EphA1 mRNA was upregulated in EOC with positive immunostaining of
ephrin receptor A1 (31) and
Herath et al reported that overexpression of EphA1 mRNA
strongly correlated with the high-affinity ligand ephrin A1 in
advanced ovarian cancer (32). We
interpret the difference in results between these papers and ours
as follows: First, the experimental samples used by Herath et
al (32) and Wong et al
(31) included ovarian serous,
mucinous, endometrioid, and clear cell cancer, whereas we focused
on ovarian serous cancer. Second, mechanisms regulating EphA1
protein level may be important in ovarian cancer and EphA1 mRNA
expression may show an inconsistent trend. The possible mechanisms
need to be further explored. We previously proved that
hypermethylation of a CpG island in the EphA1 promoter region leads
to downregulation of EphA1 in colorectal cancer (20). We therefore deduced that
methylation of DNA might be one of the mechanisms for reduced
expression of EphA1 in ovarian serous cancers. Other possible
regulatory mechanisms include EphA1 mutation, microRNA,
deacetylation, and gene deletion. We plan to intensively
investigate these molecular mechanisms in future studies.
Ki67 is a marker of proliferation expressed
exclusively during active phases of the cell cycle. It is commonly
assessed by IHC in clinical settings to judge cell proliferative
activity. It has been correlated with clinical outcome and is
considered to be an indicator of prognosis. Interestingly, our data
show that loss of EphA1 was more often observed in high Ki67 index
tumors (P=0.007). On the other hand, the MTT proliferation assay
showed that overexpression of EhpA1 gene inhibited the
proliferation of HO8910 and A2780 tumor cells, this is consistent
with what observed in tumor tissues. Overexpression of EphA1 in
ovarian cancer cell lines did not affect cell apoptosis. Our
results suggest that EphA1 may play a role in ovarian cancer as a
tumor suppressor but is not a key suppressor gene in ovarian
tumorigenesis.
Histologic grade has been shown to be an important
prognostic factor in cases of ovarian serous carcinoma. Although
the ovarian grading system has evolved over the years, there is no
universally accepted classification. The Federation of Gynecology
and Obstetrics (FIGO) grading system typically analyzes
architectural pattern, nuclear/cytologic atypia, mitotic index, or
a combination of these features. Molecular pathological research
has contributed to improved knowledge of the different subtypes of
ovarian cancer. The World Health Organization Classification System
of Ovarian Cancer, published in 2014 by Kurman et al
(23), eliminated the older
practice of grading serous tumors on a continuum (grade 1, 2, or 3)
and instead differentiates low-grade serous and high-grade serous
ovarian cancers as two distinct diseases. It is now widely accepted
that low-grade and high-grade serous tumors are essentially
distinct diseases exhibiting distinct genetic alterations,
molecular patterns, and clinical behaviors. Low-grade serous
carcinoma develops from well-recognized precursors and behaves in
an indolent fashion. It is characterized by specific mutations
including KRAS, BRAF, and ERBB2 and is relatively genetically
stable (1). In contrast,
high-grade serous ovarian carcinoma is characterized by advanced
stage at diagnosis, frequent TP53 mutation, rapid progression, and
high responsiveness to platinum-based chemotherapy (33). Although high-grade and low-grade
serous carcinomas are usually easily distinguished, it may be
difficult to discriminate between them in some carcinomas and can
especially challenging in small tissue samples (34). This is the first study
demonstrating that EphA1 protein is significantly correlated with
tumor grade in ovarian serous carcinoma, with negative expression
of EphA1 more often found in ovarian high-grade serous cancers
(P=0.016). Our data suggest that EphA1 may be a new molecular
marker for grading ovarian serous carcinoma.
In conclusion, EphA1 expression is decreased in
ovarian serous carcinoma compared with normal fallopian tube and
benign ovarian serous cystadenoma. Decreased EphA1 expression was
more often detected in high-grade tumors. Our data suggest that
EphA1 may be a new marker for grading and prognosis in ovarian
serous adenocarcinoma.
Acknowledgements
The authors wish to thank the patients who
participated in this study. This study was supported by grants from
the National Natural Science Foundation of China (grant no.
81371611) and the National Basic Research Priorities Program 973
Project (grant no. 2014CB744504) from the Ministry of Science and
Technology of China.
References
|
1
|
Ciucci A, Zannoni GF, Buttarelli M,
Martinelli E, Mascilini F, Petrillo M, Ferrandina G, Scambia G and
Gallo D: Ovarian low and high grade serous carcinomas: Hidden
divergent features in the tumor microenvironment. Oncotarget.
7:68033–68043. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seebacher V, Reinthaller A, Koelbl H,
Concin N, Nehoda R and Polterauer S: The impact of the duration of
adjuvant chemotherapy on survival in patients with epithelial
ovarian cancer-a retrospective study. PLoS One. 12:e01692722017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takekuma M, Wong KK and Coleman RL: A
long-term surviving patient with recurrent low-grade serous ovarian
carcinoma treated with the MEK1/2 inhibitor, selumetinib. Gynecol
Oncol Res Pract. 3:52016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Unified nomenclature for Eph family
receptors and their ligands, the ephrins. Eph Nomenclature
Committee. Cell. 90:403–404. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pasquale EB: Eph receptors and ephrins in
cancer: Bidirectional signalling and beyond. Nat Rev Cancer.
10:165–180. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drescher U: The Eph family in the
patterning of neural development. Curr Biol. 7:R799–R807. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mellitzer G, Xu Q and Wilkinson DG: Eph
receptors and ephrins restrict cell intermingling and
communication. Nature. 400:77–81. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu Q, Mellitzer G, Robinson V and
Wilkinson DG: In vivo cell sorting in complementary segmental
domains mediated by Eph receptors and ephrins. Nature. 399:267–271.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holmberg J, Clarke DL and Frisén J:
Regulation of repulsion versus adhesion by different splice forms
of an Eph receptor. Nature. 408:203–206. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cooke J, Moens C, Roth L, Durbin L, Shiomi
K, Brennan C, Kimmel C, Wilson S and Holder N: Eph signalling
functions downstream of Val to regulate cell sorting and boundary
formation in the caudal hindbrain. Development. 128:571–580.
2001.PubMed/NCBI
|
|
12
|
Herath NI and Boyd AW: The role of Eph
receptors and ephrin ligands in colorectal cancer. Int J Cancer.
126:2003–2011. 2010.PubMed/NCBI
|
|
13
|
Takano H, Nakamura T, Tsuchikawa T,
Kushibiki T, Hontani K, Inoko K, Takahashi M, Sato S, Abe H,
Takeuchi S, et al: Inhibition of Eph receptor A4 by
2,5-dimethylpyrrolyl benzoic acid suppresses human pancreatic
cancer growing orthotopically in nude mice. Oncotarget.
6:41063–41076. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Husa AM, Magić Ž, Larsson M, Fornander T
and Pérez-Tenorio G: EPH/ephrin profile and EPHB2 expression
predicts patient survival in breast cancer. Oncotarget.
7:21362–21380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mateo-Lozano S, Bazzocco S, Rodrigues P,
Mazzolini R, Andretta E, Dopeso H, Fernández Y, Del Llano E, Bilic
J, Suárez-López L, et al: Loss of the EPH receptor B6 contributes
to colorectal cancer metastasis. Sci Rep. 7:437022017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hirai H, Maru Y, Hagiwara K, Nishida J and
Takaku F: A novel putative tyrosine kinase receptor encoded by the
eph gene. Science. 238:1717–1720. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duffy SL, Steiner KA, Tam PP and Boyd AW:
Expression analysis of the Epha1 receptor tyrosine kinase and its
high-affinity ligands Efna1 and Efna3 during early mouse
development. Gene Expr Patterns. 6:719–723. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamazaki T, Masuda J, Omori T, Usui R,
Akiyama H and Maru Y: EphA1 interacts with integrin-linked kinase
and regulates cell morphology and motility. J Cell Sci.
122:243–255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hafner C, Becker B, Landthaler M and Vogt
T: Expression profile of Eph receptors and ephrin ligands in human
skin and downregulation of EphA1 in nonmelanoma skin cancer. Mod
Pathol. 19:1369–1377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong Y, Wang J, Sheng Z, Li G, Ma H, Wang
X, Zhang R, Lu G, Hu Q, Sugimura H and Zhou X: Downregulation of
EphA1 in colorectal carcinomas correlates with invasion and
metastasis. Mod Pathol. 22:151–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Dong Y, Wang X, Ma H, Sheng Z, Li
G, Lu G, Sugimura H and Zhou X: Expression of EphA1 in gastric
carcinomas is associated with metastasis and survival. Oncol Rep.
24:1577–1584. 2010.PubMed/NCBI
|
|
22
|
Wang X, Liu Y, Cao G, Zhang X, Xu H, Xu H
and Wang J: Expression of the EphA1 protein is associated with
Fuhrman nuclear grade in clear cell renal cell carcinomas. Int J
Clin Exp Pathol. 8:6821–6827. 2015.PubMed/NCBI
|
|
23
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WOH Classification of Tumours of Female Reproductive
Organs. International Agency for Research on Cancer; Lyon: 2014
|
|
24
|
Miyazaki K, Inokuchi M, Takagi Y, Kato K,
Kojima K and Sugihara K: EphA4 is a prognostic factor in gastric
cancer. BMC Clin Pathol. 13:192013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fox BP and Kandpal RP: Invasiveness of
breast carcinoma cells and transcript profile: Eph receptors and
ephrin ligands as molecular markers of potential diagnostic and
prognostic application. Biochem Biophys Res Commun. 318:882–892.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakagawa M, Inokuchi M, Takagi Y, Kato K,
Sugita H, Otsuki S, Kojima K, Uetake H and Sugihara K:
Erythropoietin-producing hepatocellular A1 is an independent
prognostic factor for gastric cancer. Ann Surg Oncol. 22:2329–2335.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giaginis C, Tsoukalas N, Bournakis E,
Alexandrou P, Kavantzas N, Patsouris E and Theocharis S: Ephrin
(Eph) receptor A1, A4, A5 and A7 expression in human non-small cell
lung carcinoma: Associations with clinicopathological parameters,
tumor proliferative capacity and patients' survival. BMC Clin
Pathol. 14:82014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fox BP, Tabone CJ and Kandpal RP:
Potential clinical relevance of Eph receptors and ephrin ligands
expressed in prostate carcinoma cell lines. Biochem Biophys Res
Commun. 342:1263–1272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Ma J, Dong Y, Shen Z, Ma H, Wang
X, Shi S, Wu J, Lu G, Peng L and Zhoud X: High expression of EphA1
in esophageal squamous cell carcinoma is associated with lymph node
metastasis and advanced disease. APMIS. 121:30–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng L, Wang H, Dong Y, Ma J, Wen J, Wu J,
Wang X, Zhou X and Wang J: Increased expression of EphA1 protein in
prostate cancers correlates with high Gleason score. Int J Clin Exp
Pathol. 6:1854–1860. 2013.PubMed/NCBI
|
|
31
|
Wong YL, Dali AZ, Mohamed Rose I, Jamal R
and Mokhtar NM: Potential molecular signatures in epithelial
ovarian cancer by genome wide expression profiling. Asia Pac J Clin
Oncol. 12:e259–e268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Herath NI, Spanevello MD, Sabesan S,
Newton T, Cummings M, Duffy S, Lincoln D, Boyle G, Parsons PG and
Boyd AW: Over-expression of Eph and ephrin genes in advanced
ovarian cancer: Ephrin gene expression correlates with shortened
survival. BMC cancer. 6:1442006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mignogna C, Staropoli N, Botta C, De Marco
C, Rizzuto A, Morelli M, Di Cello A, Franco R, Camastra C, Presta
I, et al: Aurora kinase a expression predicts platinum-resistance
and adverse outcome in high-grade serous ovarian carcinoma
patients. J Ovarian Res. 9:312016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gu Y, Li F, Qian N, Chen X, Wang H and
Wang J: Expression of EphB6 in ovarian serous carcinoma is
associated with grade, TNM stage and survival. J Clin Pathol.
69:448–453. 2016. View Article : Google Scholar : PubMed/NCBI
|