Introduction
Cortistatin (CST) is a endogenous neuropeptide
cloned from human, rat and mouse tissues, which exhibits a
remarkable structural and functional resemblance with somatostatin
(SST) (1,2). Both peptides share the ability to
bind and activate all five cloned SST receptors
(SSTR1–5), with similar efficacy and potency (3). Despite these analogies, the profile
of CST is not simply redundant but shows unique and even opposite
actions from those exerted by SST, especially in immune and central
nervous system (4–9). However, the mechanisms underlying
these biological differences are still unknown.
It is currently known that CST and SST have a wide
distribution in many organs (10),
including the retina (11,12). However, if on one hand there are
numerous experimental evidences on the expression, receptors and
signalling mechanisms of SST that are involved in the
physio-pathology of the retina (13), on the other hand there is a paucity
of published data about CST and retina.
CST is expressed in human retina: more in the
Retinal Pigment Epithelium (RPE) than in the neuroretina. Low
levels of neuropeptide gene expression have been associated with
apoptosis and glial activation, supporting the neuroprotective role
of CST in diabetic retinopathy (12) and other retinal disorders (14). RPE cells, a monolayer of cells
between the neuroretina and the choroid, play an important role in
the maintenance of retina homeostasis. These cells can be exposed
to various extracellular stimuli that promote their death and
consequently to be the trigger of several eye diseases. Exposure to
solar ultraviolet (UV) radiation is an external stressor, which may
induce in these cells the production of reactive oxygen species
(ROS), mitochondrial dysfunction, DNA damage, increase of apoptotic
activity, leading to irreversible cellular necrosis (15–17).
To aid in our understanding on the
pathophysiological role of CST in RPE, we investigated the
modulation of neuropeptide gene expression. In fact, our finds
display that: i) SST and CST mRNA expression are both detected in
basal conditions in a human retinal pigment epithelial cell line
(Arpe-19) after UV-A radiation exposure. Moreover we assessed the
relationship among CST, SST and its receptor subtypes
(SSTR1–5) in the same experimental paradigm.
Materials and methods
Cell lines
Human RPE cells (ARPE-19 cell line, originally
obtained from the American Type Cell Culture-CRL-2302- and kindly
provided by Professor Stefano Cacchione, University of Rome ‘La
Sapienza’) were used. The cell line was verified by the BMR
Genomics S.r.l. Cell Line Authentication Service (Padova, Italy;
ref. Nr 130264) using short tandem repeat analysis and an
amelogenin gender-determining locus. Percent match between the
submitted sample and the database profile was 100% (17). The cells were used between passages
5 and 8 and grown in 50/50 Ham's F12/Dulbecco's modified Eagle's
Medium (Gibco; Thermo Fisher Scientific, Inc., Walthan, MA, Usa),
supplemented with 15% (vol/vol) fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, Usa) and 100 U/ml
penicillin/streptomycin in a humidified incubator at 37°C, 5%
CO2 and 95% O2. The cells were plated into 6
cm of diameters cell culture dishes (1×106 cells) for
the experiments. UV exposure was produced by UV lamp (Vilber
Lourmat VL-62C Power 6W) in a custom designed UV irradiation unit
at 37°C with 5% CO2. The UV-A exposure of cells (at 365
nm) were performed at 10 cm from the source for 30 or 60 min, with
an intensity of approximately 0.06 J/cm2/sec. Following
UV-A treatment the cells were incubated for 24 h and successively
used for different experimental procedures (17).
Cell viability
Cell viability was evaluated by MTS reduction assay,
24 h afterwards UV-treatments. The intracellular soluble ‘formazan’
produced by cellular reduction of the MTS was determined by
recording the absorbance using an automatic microplate photometer
at a wavelength of 490 nm; cell viability was expressed as a
percentage of surviving cell (18).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cells using
PureLink® RNA Mini Kit and the elimination of any
genomic DNA was performed by on-column DNAse treatment (Life
technology; Thermo Fisher Scientific, Inc., Walthan, MA, USA). RNA
concentration was evaluated by spectrophotometric reading at 280
and 260 nm. Total RNA was used for first strand cDNA synthesis with
SCRIPT cDNA Synthesis Kit and Oligo-dT, as random primer (Jena
Bioscience GmbH, Jena, Germany). The PCR was performed with about
150 ng cDNA using PCRBio Classic Taq (PCR Biosystems Ltd; London
Bioscience Innovation Centre, London, NW1 ONH, United Kingdom).
Experimental protocols for semiquantitative PCR reactions were:
Denaturation, 95°C for 3 min; followed by 40 cycles of denaturation
at 95°C for 15 sec. Sequences of primers and temperature of
annealing used for PCR analysis are reported in Table I. PCR products were then analysed
by 1.5% agarose gels electrophoresis in TBE 1X Buffer. Image
acquisition and product analysis was made by Bio-Rad imaging
systems with Quantity One® 1-D analysis software. The
density of the PCR bands were divided by that of the housekeeping
gene (GAPDH) and expressed as a percentage of the control band
density.
 | Table I.Primer sequences used for the PCR
studies. |
Table I.
Primer sequences used for the PCR
studies.
| Gene | Primers | Ta (°C) | Product size
(bp) | Cycles |
|---|
| GAPDH | F
5′-AACGGATTTGGTCGTATTG-3′ | 58 | 208 | 40 |
|
| R
5′-GGAAGATGGTGATGGGATT-3′ |
|
|
|
| SST | F
5′-GGCTGCGCTGTCCATCGTC-3′ | 59 | 285 | 40 |
|
| R
5′-CAGCCAGCTTTGCGTTCTCG-3′ |
|
|
|
| CST | F
5′-CTCCAGTCAGCCCACAAGAT-3′ | 63 | 173 | 40 |
|
| R
5′-CAAGCGAGGAAAGTCAGGAG-3′ |
|
|
|
| SSTR1 | F
5′-CGCTGGCTGGTGGGCTTCGTGTTG-3′ | 62 | 481 | 40 |
|
| R
5′-GCCGCCGGACTCCAGGTTCTCAG-3′ |
|
|
|
| SSTR2 | F
5′-ACAGCTGTGCCAACCCTATC-3′ | 55 | 358 | 40 |
|
| R
5′-AGCTGACTCAAACACCGTTCT-3′ |
|
|
|
| SSTR3 | F
5′-GCGAGCCGGCTTCATCATCTACAC-3′ | 65 | 517 | 40 |
|
| R
5′-GACCCGGCCGTTCATCTCCTTC-3′ |
|
|
|
|
SSTR4 | F
5′-TGGTCGGCAGTCTTCGTGGTCTAC-3′ | 62 | 516 | 40 |
|
| R
5′-CTTGCGGCCGGGTTCTGGT-3′ |
|
|
|
|
SSTR5 | F
5′-GCGGCCTGGGTCCTGTCTCT-3′ | 65 | 627 | 40 |
|
| R
5′-CCCCCGCCTGCACTCTCAC-3′ |
|
|
|
Statistical analysis
Data were analysed by one-way ANOVA, followed by
post-hoc Dunnett's test for comparing all treatment with control or
by post hoc Newman-Keuls for comparisons between group means, when
appropriate, using a PrismTM computer program (GraphPad, San Diego,
CA, USA). All results was presented as the mean ± SEM of at least
three different experiments, unless otherwise specified.
Differences were considered statistically significant if
P<0.05.
Results
ARPE-19 cell viability following
exposure to ultraviolet UV-A irradiation
Preliminary experiments were carried out to
investigate the modulation of CST and SST gene expression in
ARPE-19 cells after 30 min and 1 h of UV-A exposure. The cells
showed no signs of alteration nor cellular degeneration after UV
radiation exposure compared to control group not exposed, as also
demonstrated by experiments of cell vitality [Cell viability (%)
n=6: Control, 100; 30′ radiation, 97.28±2.0; 60′ radiation,
96.52±2.4]. These data confirm what was previously seen from our
group: treatment with UV-A radiation caused a reduction in cell
viability from 2 h onwards as a result of apoptotic events that
were manifested after 30–60 min of exposure (17).
SST and CST gene expression levels in
ARPE-19 cells exposed to ultraviolet UV-A irradiation
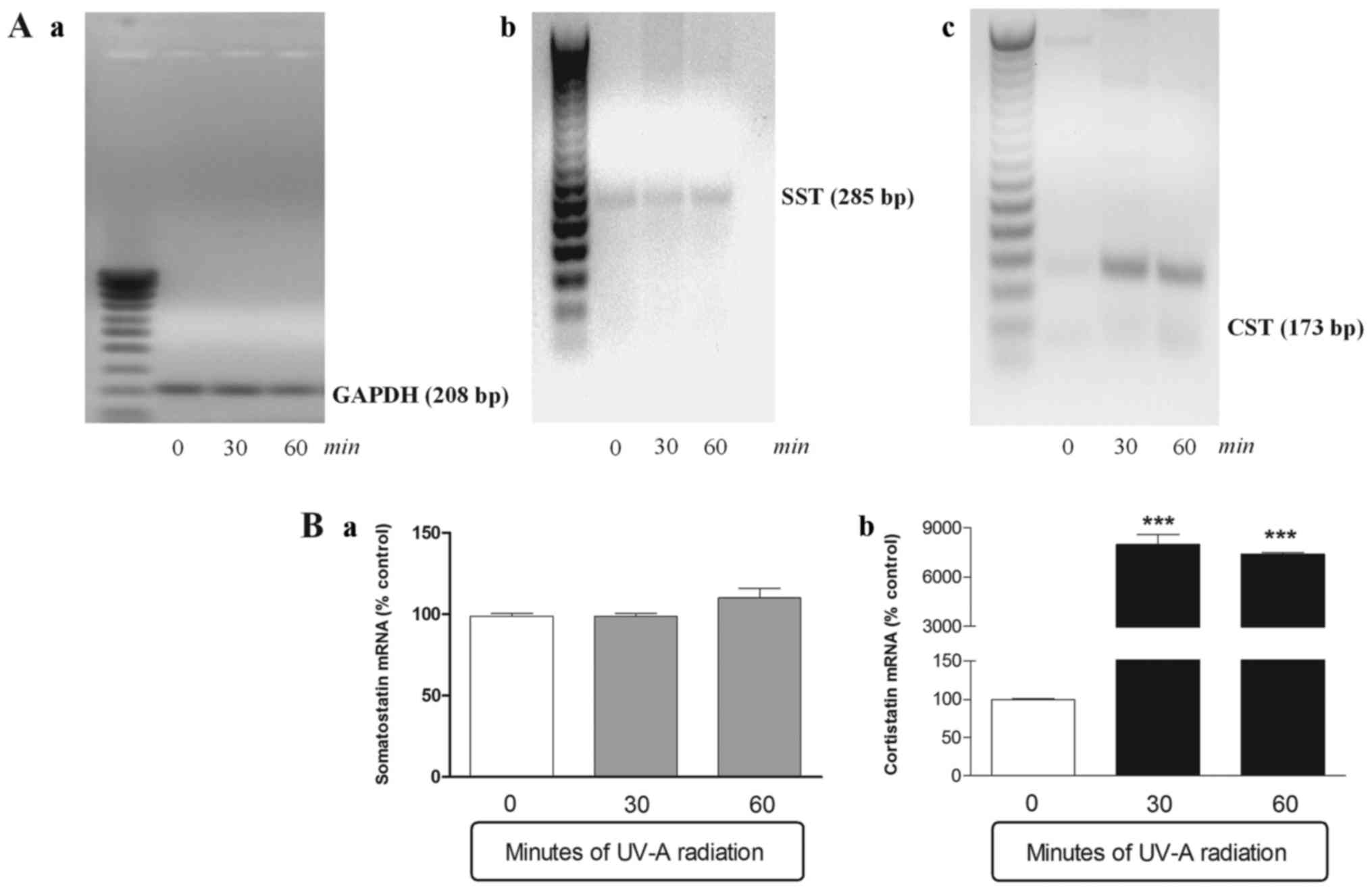
SST and CST mRNA expression were both detected in
basal conditions, although CST levels were significantly lower
compared with those of SST. After 30 and 60 min of UV-A radiation
exposure, the SST mRNA levels remained constant with a slight
tendency to grow (but not in a significant manner) after an hour of
treatment (Fig. 1). On the
contrary the CST, slightly expressed in basal conditions, was
significantly over-expressed already after 30 min of UV-A
treatment. In fact, after half an hour, the CST mRNA levels were 80
times more higher, compared to time 0, and they remained constant
in subsequent 30 min (Fig. 1).
SSTR1, SSTR2,
SSTR3, SSTR4 and SSTR5 gene
expression levels in ARPE-19 cells exposed to ultraviolet UV-A
irradiation
A second series of experiments were performed in
order to ascertain whether CST and SST gene expression variations,
observed after UV-A exposure, were linked to a modulation of SST
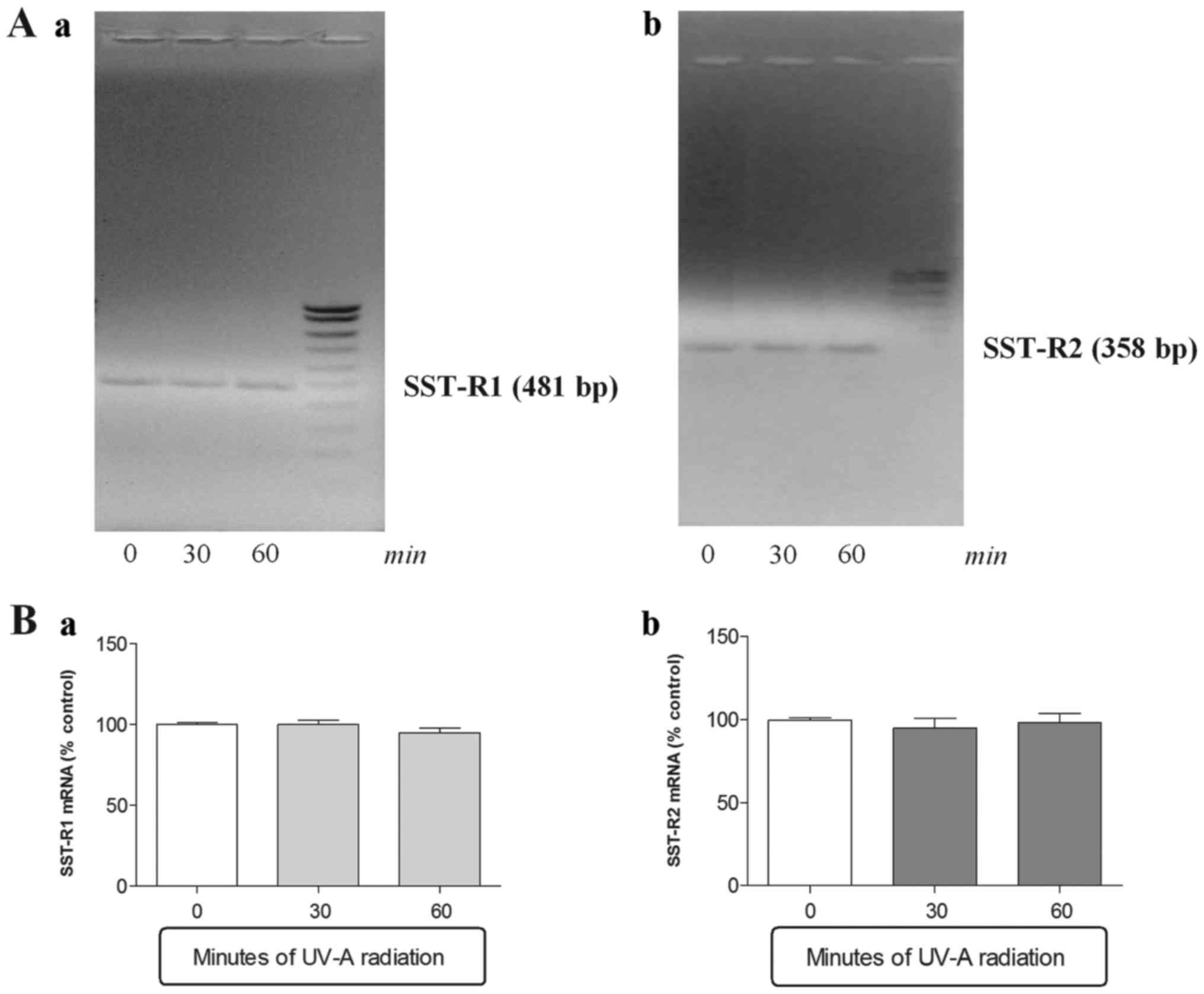
receptors (SSTR1–5). PCR analysis showed the presence of
SSTR1, SSTR2 mRNA and low but detectable
levels of SSTR4 in basal condition. On the contrary
SSTR3 and SSTR5 mRNA were not detected under
same conditions (Figs. 2 and
3).
The SSTR1 and SSTR2 mRNA
levels did not differ after 30 or 60 min UV-A radiation exposure
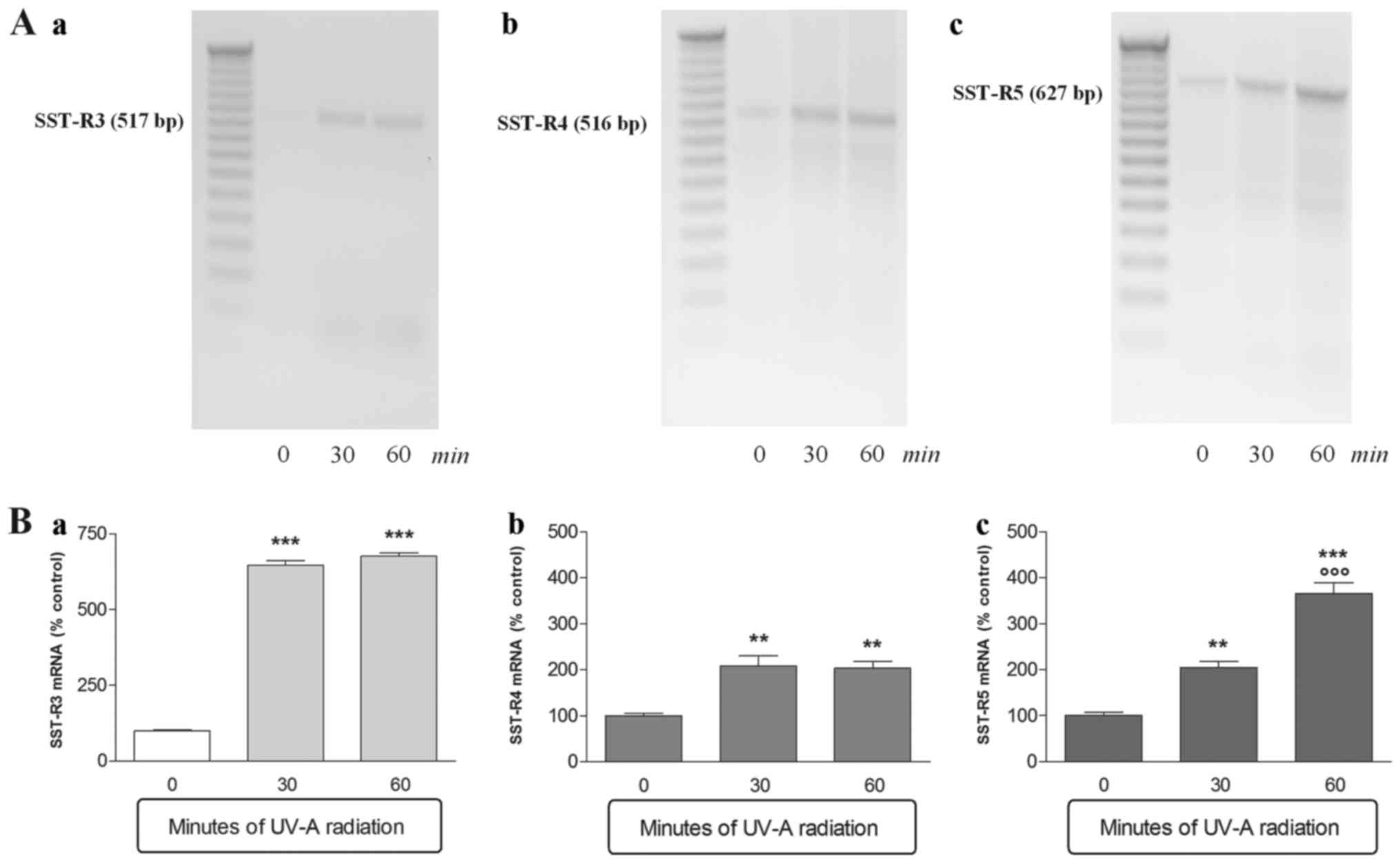
(Fig. 2). In contrast
SSTR3, SSTR4 and SSTR5 were
over-expressed already after 30 min of UV-A treatment (Fig. 3). SSTR3 was most over-expressed
with mRNA levels that were 6 times higher after only 30 min of
treatment compared to control not irradiated (Fig. 3a). SSTR4 expression
doubled after 30 min of UV-A radiations and remained constant until
the end of the experiment (Fig.
3b). Finally, SSTR5 was overexpressed in a
time-dependent manner; indeed the SSTR5 mRNA levels doubled
approximately at each experimental time point (Fig. 3c).
Discussion
The findings of the present study show for the first
time that one-hour of continuous exposition to UV-A radiations
causes in ARPE-19 cells an increase of CST mRNA levels, rather than
SST. The increase of CST mRNA is also associated with an increase
of gene expression of SST receptor subtypes, putative CST
receptors. Recently, we demonstrated that UV-A radiation for five
consecutive hours induced in ARPE-19 cells a rapid increase in ROS
levels, which after one hour led to activation of apoptotic events
(activation of apoptotic genes, Bax and Caspase-3, and decrease of
anti-apoptotic gene, Bcl-2) that in turn contributing to
irreversible cell necrosis subsequent (17). Therefore, there seems to be a link
between increased CST mRNA levels and early apoptotic events
triggered by treatment with UV-A, though the pathophysiological
role of CST remains unclear. Certainly, CST plays a role only in
part that overlaps with that of SST in our experimental paradigm
because both peptides are expressed in basal conditions, even if
CST mRNA levels are significantly lower compared with those of SST,
but only CST is significantly over-expressed after exposure to UV-A
radiation. On the basis of these findings, one may theorize a
protective role for CST in the adaptive response of the RPE exposed
to UV-A radiation. Hypothesis is also supported by several
literature evidence: i) apoptotic events induced by UV-A radiation
coincide temporally with the overexpression of CST observed in our
experimental model (17); ii) CST
retinal levels are inversely associated with apoptosis and degree
of glial activation, two of the characteristics of retinal
neurodegeneration (12); iii) CST
protects the rat retina against the blockade of oxidative
phosphorylation and glycolysis in vitro (14) and iv) CST shows antiangiogenic,
neuroprotective, antioxidant, anti-apoptotic and anti-inflammatory
properties, in different biological systems (9,19,20).
However, further studies will be necessary because we cannot
completely exclude that CST overexpression in our study is rather
associated with the beginning and support of the apoptosis and
necrosis observed in RPE after exposure to UV-A radiation.
CST shares with SST the ability to bind and activate
all five cloned SST receptors (SSTR1–5), with similar
efficacy and potency (3). This
analogy might explain the considerable overlapping between CST and
SST on several biological actions, including neuroprotective
effects in the retina subjected to chemical ischemia or in the
diabetic retinopathy (11,12,14).
SSTR1–5 genes are widely expressed in normal human eye
tissues and in particular in retina. hRPE cells express mainly
SSTR-1 and SSTR-2 in basal condition as shown
by RT-PCR and immunohistochemical techniques(21,22), although all five SST receptors have
been also detected in D407 h-RPE cells by western blot (17,23).
In spite of growing interest about the effects of the SST-receptors
activation by SST in the retina, surprisingly little is known on
the functions correlated to bond between CST and SST-receptors.
Moreover, the role of SST and of its receptors in the RPE is far
more complex than that experimental evidences suggest. In fact, an
examination of the current scientific literature gives only a
sketchy and often conflicting picture of the role of SST and its
receptors (22–24). In the present study, the comparison
between gene expression levels of SST-receptors and CST or SST
shows that: i) SSTR1 and SSTR2 gene
expression are correlated to high levels of SST mRNA rather than to
CST, few expressed, in basal conditions; ii) mRNA levels of
SSTR3, SSTR4 and SSTR5 are little
or no detectable in basal conditions and iii) the treatment with
UV-A radiations causes a significant increase both in mRNA levels
of SSTR3, SSTR4, SSTR5 and CST,
while it doesn't change the SST and SST-R1–2 basal
levels.
At this point in the study, it is important to point
out that profiles of the mRNA do not always correlate with the
level of protein expression. In fact, the transcript analysis gives
us an indication of what genes are active in a specified cell, but
nothing about the protein levels actually expressed. However,
studying the modulation of the expression of a gene means to
investigate in what tissue is expressed, under what conditions it
works and what is the effect of its expression. Therefore this
study, despite its limitations (evidence only at transcriptional
level), allows us to hypothesize that both CST and SSTRs (most
probably SSTR3, SSTR4 and SSTR5)
are involved in the UV-A-induced adaptive response in the hRPE
cells. Certainly, the real physiological role of CST and the
involvement of SSTRs in RPE health and disease, require further
investigation. Anyhow these findings provide novel insights on
pathophysiological role of CST/SST/SSTRs system and encourage new
research avenues for the therapeutic potential of this system in
degenerative processes underlying exposure to UV radiation and that
are implicated in numerous ocular pathologies, particularly macular
degeneration.
Acknowledgements
The present study was financed by UCSC internal
funding (Fondi di Ateneo 2013 to Giuseppe Tringali). Thanks
are due to Stefano Cacchione and Sara Luzzi of the Dipartimento di
Biologia e Biotecnologie, Sapienza-Università di Roma for providing
ARPE-19 cells. We would like also to thank Kathy Lewis for her
contribution to experimental work described.
References
|
1
|
de Lecea L, Criado JR, Prospero-Garcia O,
Gautvik KM, Schweitzer P, Danielson PE, Dunlop CL, Siggins GR,
Henriksen SJ and Sutcliffe JG: A cortical neuropeptide with
neuronal depressant and sleep-modulating properties. Nature.
381:242–245. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spier AD and de Lecea L: Cortistatin: A
member of the somatostatin neuropeptide family with distinct
physiological functions. Brain Res Brain Res Rev. 33:228–241. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siehler S, Seuwen K and Hoyer D:
[125I]Tyr10-cortistatin14 labels all five somatostatin receptors.
Naunyn Schmiedebergs Arch Pharmacol. 357:483–489. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dello Russo C, Lisi L, Navarra P and
Tringali G: Diverging effects of cortistatin and somatostatin on
the production and release of prostanoids from rat cortical
microglia and astrocytes. J Neuroimmunol. 213:78–83. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Córdoba-Chacón J, Gahete MD, Pozo-Salas
AI, Martínez-Fuentes AJ, de Lecea L, Gracia-Navarro F, Kineman RD,
Castaño JP and Luque RM: Cortistatin is not a somatostatin analogue
but stimulates prolactin release and inhibits GH and ACTH in a
gender-dependent fashion: Potential role of ghrelin. Endocrinology.
152:4800–4812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Córdoba-Chacón J, Gahete MD, Durán-Prado
M, Luque RM and Castaño JP: Truncated somatostatin receptors as new
players in somatostatin-cortistatin pathophysiology. Ann N Y Acad
Sci. 1220:6–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Markovics A, Szoke É, Sándor K, Börzsei R,
Bagoly T, Kemény Á, Elekes K, Pintér E, Szolcsányi J and Helyes Z:
Comparison of the anti-inflammatory and anti-nociceptive effects of
Cortistatin-14 and Somatostatin-14 in distinct in vitro and in vivo
model systems. J Mol Neurosci. 46:40–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tringali G, Greco MC, Lisi L, Pozzoli G
and Navarra P: Cortistatin modulates the expression and release of
corticotrophin releasing hormone in rat brain. Comparison with
somatostatin and octreotide. Peptides. 34:353–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Delgado M and Gonzalez-Rey E: Role of
cortistatin in the stressed immune system. Front Horm Res.
48:110–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dalm VA, Van Hagen PM, de Krijger RR, Kros
JM, Van Koetsveld PM, van der Lely AJ, Lamberts SW and Hofland LJ:
Distribution pattern of somatostatin and cortistatin mRNA in human
central and peripheral tissues. Clin Endocrinol (Oxf). 60:625–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carrasco E, Hernández C, Miralles A,
Huguet P, Farrés J and Simó R: Lower somatostatin expression is an
early event in diabetic retinopathy and is associated with retinal
neurodegeneration. Diabetes Care. 30:2902–2908. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carrasco E, Hernández C, de Torres I,
Farrés J and Simó R: Lowered cortistatin expression is an early
event in the human diabetic retina and is associated with apoptosis
and glial activation. Mol Vis. 14:1496–1502. 2008.PubMed/NCBI
|
|
13
|
Vasilaki A and Thermos K: Somatostatin
analogues as therapeutics in retinal disease. Pharmacol Ther.
122:324–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mastrodimou N, Lambrou GN and Thermos K:
Effect of somatostatin analogues on chemically induced ischaemia in
the rat retina. Naunyn Schmiedebergs Arch Pharmacol. 371:44–53.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roduit R and Schorderet DF: MAP kinase
pathways in UV-induced apoptosis of retinal pigment epithelium
ARPE19 cells. Apoptosis. 13:343–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanus J, Zhang H, Wang Z, Liu Q, Zhou Q
and Wang S: Induction of necrotic cell death by oxidative stress in
retinal pigment epithelial cells. Cell Death Dis. 4:e9652013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tringali G, Sampaolese B and Clementi ME:
Expression of early and late cellular damage markers by ARPE-19
cells following prolonged treatment with UV-A radiation. Mol Med
Rep. 14:3485–3489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barltrop JA, Owen TC, Cory AH and Cory JG:
5-(3-Carboxylmethoxyphenyl)-2-(4,5-dimethylthiazolyl)-3-(4-sulfophenyl)
tetrazolium, inner salt (MTS) and related analogs of
3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT)
reducing to purple water-soluble formazans as cell-viability
indicators. Bioorg Med Chem Lett. 1:611–614. 1991. View Article : Google Scholar
|
|
19
|
Delgado-Maroto V, Benitez R, Forte-Lago I,
Morell M, Maganto-Garcia E, Souza-Moreira L, O'Valle F, Duran-Prado
M, Lichtman AH, Gonzalez-Rey E and Delgado M: Cortistatin reduces
atherosclerosis in hyperlipidemic ApoE-deficient mice and the
formation of foam cells. Sci Rep. 7:464442017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gonzalez-Rey E, Pedreño M, Delgado-Maroto
V, Souza-Moreira L and Delgado M: Lulling immunity, pain, and
stress to sleep with cortistatin. Ann N Y Acad Sci. 1351:89–98.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klisovic DD, O'Dorisio MS, Katz SE, Sall
JW, Balster D, O'Dorisio TM, Craig E and Lubow M: Somatostatin
receptor gene expression in human ocular tissues: RT-PCR and
immunohistochemical study. Invest Ophthalmol Vis Sci. 42:2193–2201.
2001.PubMed/NCBI
|
|
22
|
Sall JW, Klisovic DD, O'Dorisio MS and
Katz SE: Somatostatin inhibits IGF-1 mediated induction of VEGF in
human retinal pigment epithelial cells. Exp Eye Res. 79:465–476.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Papadaki T, Tsilimbaris M, Pallikaris I
and Thermos K: Somatostatin receptor activation (sst(1)-sst(5))
differentially influences human retinal pigment epithelium cell
viability. Acta Ophthalmol. 88:e228–e233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vasilaki A, Papadaki T, Notas G, Kolios G,
Mastrodimou N, Hoyer D, Tsilimbaris M, Kouroumalis E, Pallikaris I
and Thermos K: Effect of somatostatin on nitric oxide production in
human retinal pigment epithelium cell cultures. Invest Ophthalmol
Vis Sci. 45:1499–1506. 2004. View Article : Google Scholar : PubMed/NCBI
|