Introduction
Due to the development of obstetrical techniques and
perinatal medicine, and advancement of neonatal intensive care, the
survival rate of premature babies has been increased over the years
(1,2). Associated complications, however, are
also rising, particularly in brain injuries (3). The incidence of neonatal brain injury
occurs in >10% of all premature babies (4). Neonatal white matter injury (WMI) is
a common type of brain injury in premature babies. Pathological
features of WMI include abundant infiltration of microglia, damaged
oligodendrocytes, formation of astrocytes, myelin damage and axonal
injury. A severe manifestation is the softening of white matter
around ventricular zones (5,6).
Neonatal WMI is accompanied with neurological injury and
neurodevelopment retardation to different extents (7). Severe WMI can cause intelligence and
body development disorder, epilepsy, visual-auditory disorder and
cerebral palsy, contributing to perinatal mortality and becoming a
public health issue worldwide (8,9).
Multiple factors can induce premature WMI, including intra-uterus
infection, hypoxia, cerebral ischemia and fetal age (9). However, due to insufficient knowledge
of WMI pathogenesis, currently no effective treatment exists,
leaving only symptomatic treatment with unfavorable efficacy
(10). Therefore, studying WMI
pathogenesis should draw attention in pediatric and obstetric
fields.
T helper cells (Th) can be divided into 2 subgroups,
namely Th1 and Th2 cells (11). A
recent study demonstrated the cross-regulation and inhibition
between Th1 and Th2 cells via modulating cytokine secretion,
further maintaining Th1-Th2 balance, and exerting important
functions for the normal immune functions of the body (12). Th1 cells mainly secrete interleukin
(IL)-2 and tumor necrosis factor-α (TNF-α), while Th2 cells secrete
IL-4 and IL-10 (13). Th1/Th2 can
maintain a homeostatic condition via self-regulation and
cross-regulation targeting secreted cytokines, and it participates
in the modulation of cellular and humoral immunity. As a member of
transcriptional factor protein family, NF-κB participates in
regulating cytokine secretion, and inflammatory or immune responses
(14,15). A previous study demonstrated the
close association between Th1-biased Th cell transition, plus the
secretary level of body cytokines and abnormal expression of
nuclear factor (NF)-κB and the occurrence of cerebral injury
(16). The current study
investigated the expression profile of Th1 and Th2 cytokines in WMI
patients, in an attempt to illustrate the role and functions of
Th1/Th1 alternation in WMI pathogenesis.
Patients and methods
General information
A total of 60 premature neonatal babies diagnosed
with WMI were recruited from the Inner Mongolia People's Hospital
(Huhehot, Inner Mongolia) from December 2014 to June 2015. There
were 33 males and 27 females (gestation, 28–34 weeks; average
gestation, 30±2 weeks; body weight at birth, 1,000–2,500 g; average
(mean) body weight, 1,820±350 g). WMI was diagnosed by head
ultrasound, which was performed by at least two pediatric
consultants.
The exclusive criteriaincluded: Perinatal infection,
asphyxia, circulating disorder or ion disorder, those with severe
kidney/liver dysfunction, inherited metabolic disorder, major
malformation of organs, placental abruption, intra-uterus fetal
distress and premature rupture of fetal membranes. The control
group included 60 premature neonatal babies without WMI, recruited
during the same period. The average gestation period was 31±2 weeks
(range, 28–35 weeks), including 22 males and 27 females, with an
average body weight at birth of 1,790±420 g (range, 1,000–2,500 g).
No statistically significant difference was observed between the
two groups regarding body weight. This study was approved by the
ethical committee of the Inner Mongolia People's Hospital. Written
informed consents were obtained by legal guardians for all
participants.
Reagents and equipment
TRIzol reagent, an RNA extraction kit, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
primers, an RT-qPCR kit were all purchased from Invitrogen; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). ELISA kits for IL-2
(cat. no. BMS221-2), TNF-α (cat. no. 88-7346-22), IL-4 (cat. no.
BMS225-2), IL-10 (cat. no. BMS215-2) and NF-κB (cat. no. KHO0371)
were purchased from eBioscience (Thermo Fisher Scientific, Inc.). A
lab system version 1.3.1 micro plate reader was purchased from
Bio-Rad Laboratories, Inc. (Hercules, CA, USA). Cell culture
incubator was purchased from Suzhou Purification Engineering
Installation Co., Ltd. (Suzhou, China). A real time PCR cycler was
purchased from Applied Biosystems; Thermo Fisher Scientific, Inc. A
UV spectrophotometer was purchased from Mettler-Toledo (Greifensee,
Switzerland).
Grouping of WMI patients
Grade of disease was classified into mild [minor
reduction of white matter, perturbation of adjacent groves but no
ventricular dilation, small and limited high-low signal on magnetic
resonance imaging (MRI)], moderate (significantly decreased white
matter leaving little white matter between lateral ventricles and
gray matter, accompanied with dilation of lateral ventricles) and
severe (replacement of white matter by cystic tissues,
significantly high signal fusion with lateral ventricle under
weighted images and significantly dilated ventricles), based on
Flodmar standard and MRI results (17). There were 17, 25 and 18 cases of
mild, moderate and severe diseases, respectively.
Sample collection
Blood samples (5 ml) were collected from veins of
both disease and control groups 24 h within admitting to the
hospital. Blood samples were centrifuged at 3,000 × g for 15 min at
room temperature to collect serum, which was frozen at −80°C.
RT-qPCR for the expression profile of
Th1/Th1 cytokines in WMI patients
TRIzol reagent was used to extract total RNA from
peripheral blood. RNA purity and concentration were determined by
UV spectrometry. Reverse transcription was performed using a test
kit. cDNA was synthesized with specific primers designed using
PrimerPremier 6.0 (www.premierbiosoft.com/primerdesign). RT-qPCR was used
to test target gene expression using specific primers (Table I) synthesized by Sangon Biotech
Co., Ltd., under the following conditions: 52°C for 1 min, followed
by 35 cycles each containing 90°C for 30 sec, 58°C for 50 sec and
72°C for 35 sec. Fluorescent PCR was used to collect all data. Cq
values were calculated using the GAPDH gene as the internal
reference to plot a standard curve, on which quantitative analysis
was performed using the 2−∆∆Cq method (18).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward primer
5′-3′ | Reverse primer
5′-3′ |
|---|
| GADPH |
AGTGCCAGCCTCGTCTCATAG |
CGTTGAACTTGCCGTGGGTAG |
| IL-2 |
GATCTACGCAGCGAAGAACTT |
CTCTGGGACATCTCCGTCA |
| TNF-α |
CTACGGAAGATCTCAATAGCG |
GGGACTCTCAATCCTCGTC |
| IL-4 |
AGCGGATCTACGGAACTCAAT | CTGGCTGAGTCACATC |
| IL-10 |
GAAGATCTCAATAGCGTCA |
AATCTCTCAATCCTCGTC |
| NF-κB |
TCGCGGATCTACGGAAC |
CTCTCAAGTTCCTCATC |
Serum levels of Th1/Th2 cytokines and
NF-κB in WMI patients by ELISA
All samples were measured for Th1/Th2 cytokines
including IL-2, TNF-α, IL-4, IL-10 and NF-κB, using ELISA kits
according to manufacturer's protocol. Linear regression function
was plotted based on standard concentration and optical density
(OD) values. Sample concentration was deduced on the linear
function according to OD values.
Statistical analysis
All data were analysed using SPSS 22.0 software for
statistical analysis (IBM Corp., Armonk, NY, USA). At least three
independent experiments were performed for each assay. Data are
presented as the mean ± standard deviation. Comparison of means
among multiple groups was performed by one-way analysis of variance
with Newman-Keuls multiple comparison post hoc analysis. Student's
t-test was used to compare two groups. Pearson analysis was used
for correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
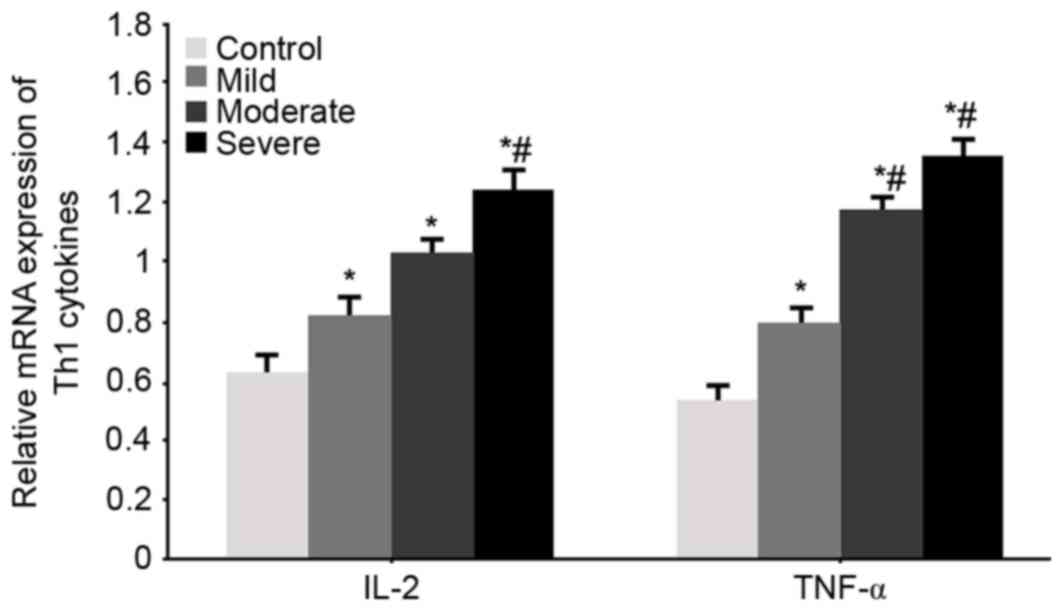
mRNA expression level of the Th1
cytokines IL-2 and TNF-α
RT-qPCR was used to test the mRNA expression levels
of the Th1 cytokines IL-2 and TNF-α in all groups. Significantly
elevated IL-2 and TNF-α mRNA levels were observed in WMI patients
(P<0.05 vs. control group). With aggravated disease condition,
the mRNA expression levels of IL-2 and TNF-α were further
potentiated (P<0.05 vs. mild patients, Fig. 1).
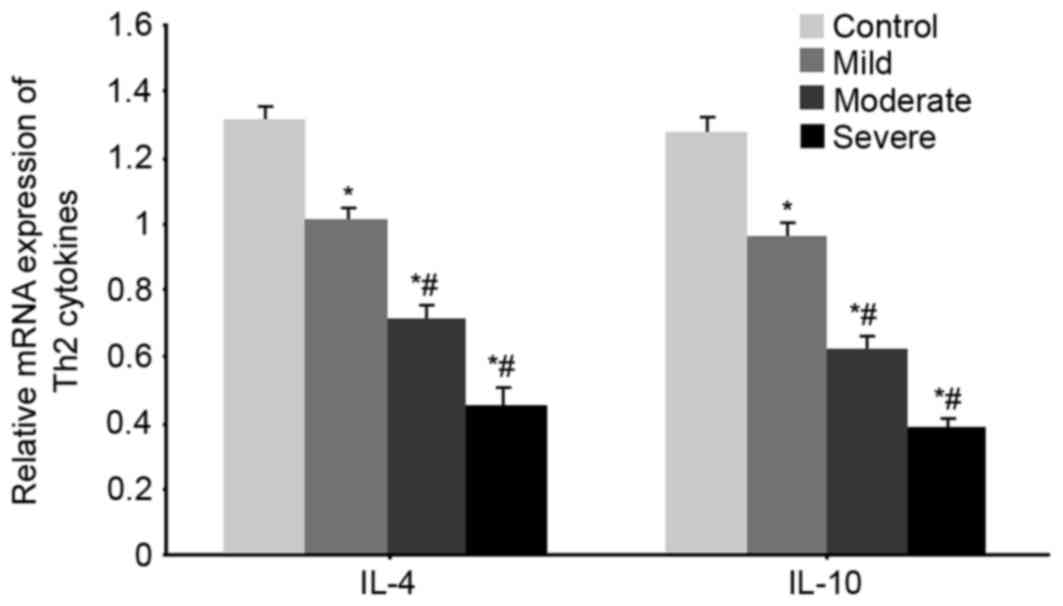
mRNA expression of the Th2 cytokines
IL-4 and IL-10 mRNA expression in WMI patients
RT-qPCR was used to test the mRNA expression levels
of the Th2 cytokines IL-4 and IL-10 in all groups. Significantly
decreased IL-4 and IL-10 mRNA expression levels were observed in
WMI patients (P<0.05 vs. control group). With aggravated disease
condition, the mRNA expression levels of IL-4 and IL-10 were
further decreased (P<0.05 vs. mild patients, Fig. 2).
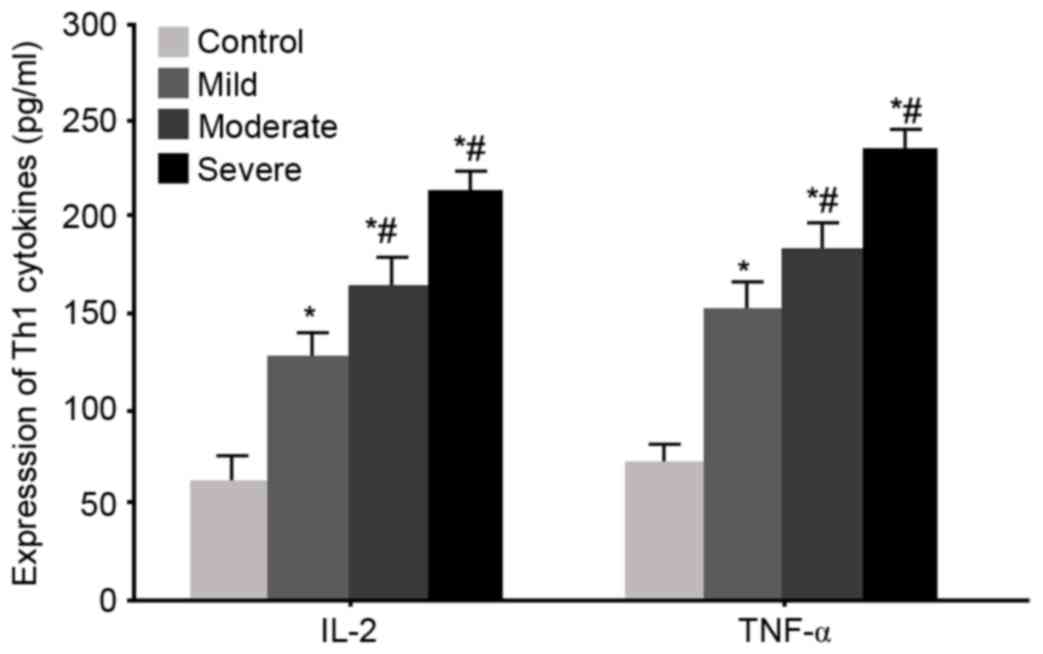
Serum levels of Th1 cytokines IL-2 and
TNF-α in WMI neonates
ELISA was used to measure serum levels of the Th1
cytokines IL-2 and TNF-α in all WMI neonates. The results were
similar results to the mRNA ones, as significantly elevated serum
IL-2 and TNF-α levels were observed in WMI patients (P<0.05 vs.
control group). With aggravated disease condition, serum levels of
IL-2 and TNF-α were further potentiated (P<0.05 vs. mild
patients, Fig. 3).
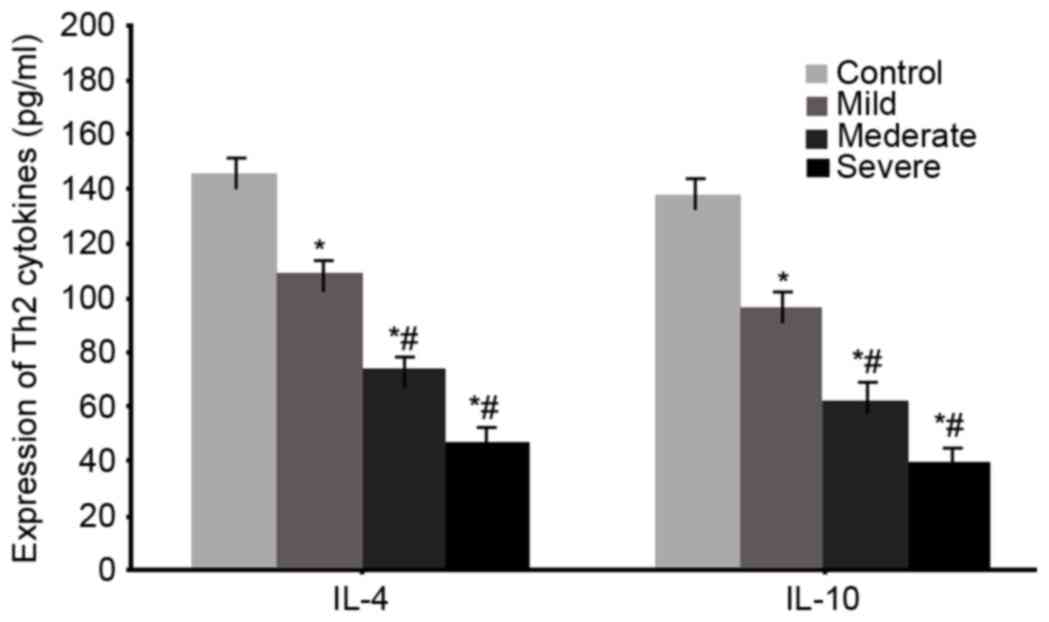
Serum levels of Th2 cytokines IL-4 and
IL-10 in WMI neonates
ELISA was used to measure serum levels of the Th2
cytokines IL-4 and IL-10 in all WMI neonates. The results were
similar results to the mRNA ones, as significantly lowered serum
IL-4 and IL-10 levels were observed in WMI patients (P<0.05 vs.
control group). With aggravated disease condition, serum level of
IL-4 and IL-10 was further suppressed (P<0.05 vs. mild patients,
Fig. 4).
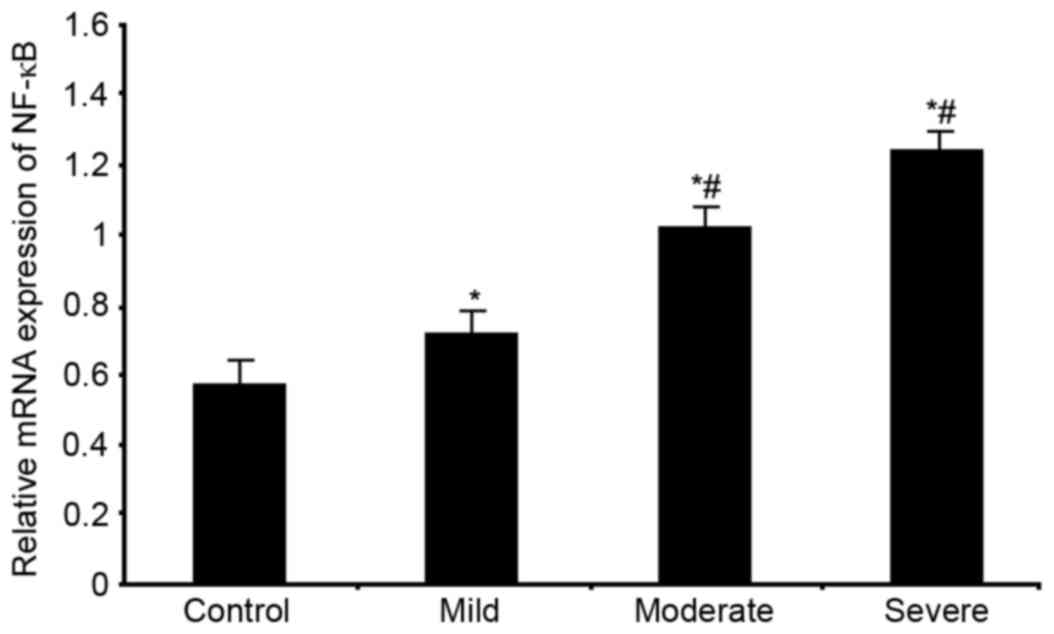
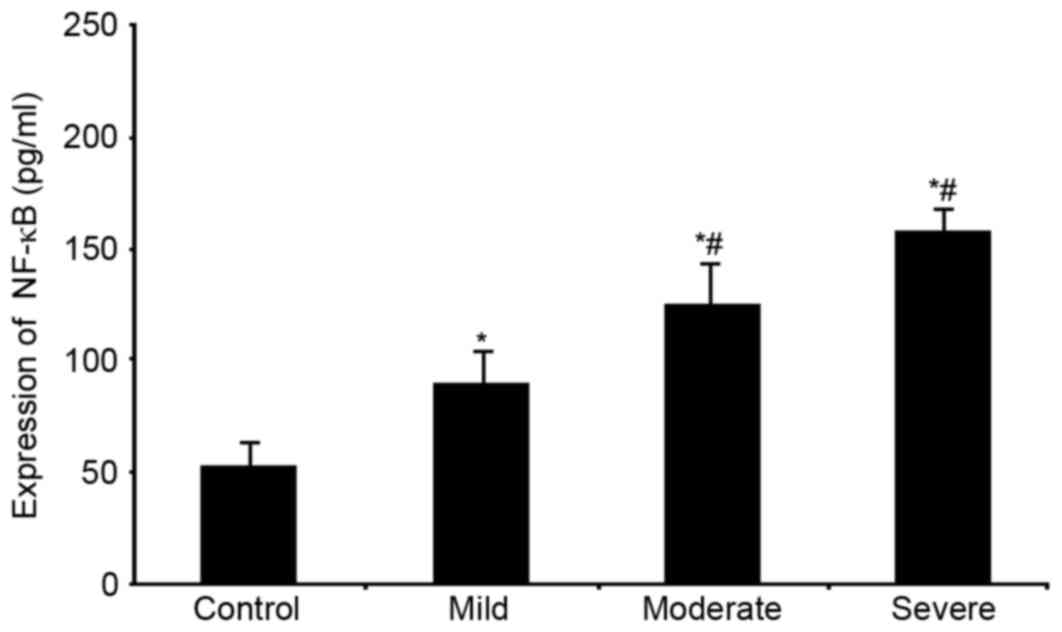
mRNA and protein expression of NF-κB
in serum of WMI premature neonates
RT-qPCR and ELISA were used to analyse the mRNA and
protein expression profiles of NF-κB in premature neonates with
WMI, respectively. Significantly elevated NF-κB mRNA expression
levels were observed in patients (P<0.05 vs. control group,
Fig. 5). With aggravated disease
condition, NF-κB mRNA expression levels were further elevated
(P<0.05 vs. mild group, Fig.
5). Similar to those of mRNA, serum NF-κB levels were
significantly elevated in the disease group, and in the severe
sub-group (P<0.05, Fig. 6).
Correlation between serum Th1 and Th2
cytokines and NF-κB in WMI neonates
The correlation between Th1 and Th2 cytokines in
serum of WMI patients was further analysed. The results
demonstrated that IL-2, TNF-α and NF-κB were negatively correlated
with IL-4 and IL-10 (P<0.05, Table
II).
 | Table II.Correlation between serum Th1 and Th2
cytokines and NF-κB in WMI patients. |
Table II.
Correlation between serum Th1 and Th2
cytokines and NF-κB in WMI patients.
| Cytokines | IL-4 | P-value | IL-10 | P-value |
|---|
| IL-2 | −0.731 | <0.05 | −0.736 | <0.05 |
| TNF-α | −0.657 | <0.05 | −0.622 | <0.05 |
| NF-κB | −0.867 | <0.05 | −0.726 | <0.05 |
Correlation between serum Th1/Th2
cytokines and NF-κB and disease severity
The correlation between Th1/Th2 cytokines in the
serum of WMI patients and their disease severity was further
analysed. The results demonstrated that IL-2, TNF-α and NF-κB
expression were positively correlated with disease severity, whilst
IL-4 and IL-10 expression were negatively correlated with disease
severity (P<0.05, Table
III).
 | Table III.Correlation between serum Th1/Th2
cytokines and disease severity. |
Table III.
Correlation between serum Th1/Th2
cytokines and disease severity.
| Cytokines | Mild | P-value | Moderate | P-value | Severe | P-value |
|---|
| NF-κB | 0.697 | <0.05 | 0.765 | <0.05 | 0.812 | <0.05 |
| IL-2 | 0.815 | <0.05 | 0.436 | <0.05 | 0.702 | <0.05 |
| TNF-α | 0.958 | <0.05 | 0.516 | <0.05 | 0.639 | <0.05 |
| IL-4 | −0.479 | <0.05 | −0.679 | <0.05 | −0.815 | <0.05 |
| IL-10 | −0.652 | <0.05 | −0.581 | <0.05 | −0.562 | <0.05 |
Discussion
A complicated pathogenesis mechanism exists for WMI
in neonates. Intra-uterine factors and ischemia-reperfusion are two
major determinants of WMI (19).
In an intra-uterus infection model by LPS injection, and another
neonatal mouse model of hypoxia-ischemia, ventricular dilation,
significant diffused WMI and softening lesion in cerebral white
matter can be observed (20). In
the intra-uterus infection model, inflammatory cytokines including
TNF-α, IL-6 and interferon-γ are elevated, in addition to an
altered cytokine network in the uterus of pregnant mice (21). This indicates a possible role of
Th1/Th2 in WMI pathogenesis. The change of Th1/Th2 balance and
NF-κB are closely correlated with occurrence of cerebral injury.
The role of Th1/Th2 imbalance and NF-κB, however, remains to be
illustrated. The same stands for the underlying mechanisms.
Among various factors secreted by Th1 cells, TNF-α
and IL-2 are two representative cytokines. Hypoxia and ischemia
cause elevated number of Th1 cells, leading to enhanced secretion
of TNF-α and IL-2, causing chronic inflammation. Therefore,
elevated secretion of Th1-associated cytokines is an important
factor during inflammation. When the body is under inflammatory
change, abundant activation of inflammatory and immune-associated
genes occur, inducing elevated expression of cytokines including
TNF-α and IL-2, thus modulating a complex cytokine network inside
the body (22). NF-κB is a
transcription factor widely distributed in eukaryotic cells,
governing multiple pathological processes including inflammation,
immune response, cell apoptosis and proliferation (23). The present study demonstrated
significantly higher mRNA and protein expression levels of TNF-α
and IL-2 in WMI patients compared with the control group. With
further aggravation of disease severity, TNF-α and IL-2 levels were
further elevated. IL-4 and IL-10 were further suppressed with
advanced disease condition. A correlation analysis between Th1/Th2
cytokines in WMI patients revealed a negative correlation between
TNF-α and IL-2, and Il-4 and IL-10. Disease severity was positively
correlated with TNF-α and IL-2 expression, and was negatively
correlated with IL-4 and IL-10. These results demonstrated that the
facilitation of secretion of the inflammatory factors TNF-α and
IL-2 could activate the inflammatory response, leading to leukocyte
adhesion, maturation and migration of immune cells, which might
lead to further progression of inflammation, eventually leading to
death of oligodendrocytes, impairing myelination in the central
nervous system, and further severity of WMI (24). Cytokines released by Th1 and Th2
cells can antagonize each other and inhibit Th response and release
of associated factors, thus enhancing the humoral immune response
and exerting protective roles (25). NF-κB activation leads to elevated
expression of inflammatory factors, especially the Th1-secreted
cytokines TNF-α and IL-2, suggesting a positive feedback loop
mechanism of TNF-α for secretion of inflammatory factors (25). During WMI pathogenesis, NF-κB
upregulation increases secretion of TNF-α and IL-2, both of which
inhibit secretion of IL-4 and IL-10, leading to Th1/Th2 imbalance,
and making a pathological progression of WMI. Therefore, the
present study provided evidence for studying WMI pathogenesis.
In conclusion, during WMI pathogenesis, NF-κB
upregulation increases the secretion of Th1 cytokines and decreases
the secretion of Th2 cytokines, causing Th1/Th2 imbalance, which
may be a potential risk marker for the early detection or treatment
of WMI.
References
|
1
|
Pagnozzi AM, Dowson N, Doecke J, Fiori S,
Bradley AP, Boyd RN and Rose S: Automated, quantitative measures of
grey and white matter lesion burden correlates with motor and
cognitive function in children with unilateral cerebral palsy.
Neuroimage Clin. 11:751–759. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Z, Bassam B, Thomas AG, Williams M,
Liu J, Nance E, Rojas C, Slusher BS and Kannan S: Maternal
inflammation leads to impaired glutamate homeostasis and
up-regulation of glutamate carboxypeptidase II in activated
microglia in the fetal/newborn rabbit brain. Neurobiol Dis.
94:116–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Yawno T, Sutherland A, Loose J,
Nitsos I, Bischof R, Castillo-Melendez M, McDonald CA, Wong FY,
Jenkin G and Miller SL: Preterm white matter brain injury is
prevented by early administration of umbilical cord blood cells.
Exp Neurol. 283:179–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sandhu MS, Ross HH, Lee KZ, Ormerod BK,
Reier PJ and Fuller DD: Intraspinal transplantation of
subventricular zone-derived neural progenitor cells improves
phrenic motor output after high cervical spinal cord injury. Exp
Neurol. 287:205–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stetler RA, Gao Y, Leak RK, Weng Z, Shi Y,
Zhang L, Pu H, Zhang F, Hu X, Hassan S, et al: APE1/Ref-1
facilitates recovery of gray and white matter and neurological
function after mild stroke injury. Proc Natl Acad Sci USA. 113:pp.
E3558–E3567. 2016; View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ceschin R, Lee VK, Schmithorst V and
Panigrahy A: Regional vulnerability of longitudinal cortical
association connectivity: Associated with structural network
topology alterations in preterm children with cerebral palsy.
Neuroimage Clin. 9:322–337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Serdar M, Herz J, Kempe K, Lumpe K,
Reinboth BS, Sizonenko SV, Hou X, Herrmann R, Hadamitzky M, Heumann
R, et al: Fingolimod protects against neonatal white matter damage
and long-term cognitive deficits caused by hyperoxia. Brain Behav
Immun. 52:106–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng I, Miller SP, Duerden EG, Sun K,
Chau V, Adams E, Poskitt KJ, Branson HM and Basu A: Stochastic
process for white matter injury detection in preterm neonates.
Neuroimage Clin. 7:622–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ranchhod SM, Gunn KC, Fowke TM, Davidson
JO, Lear CA, Bai J, Bennet L, Mallard C, Gunn AJ and Dean JM:
Potential neuroprotective strategies for perinatal infection and
inflammation. Int J Dev Neurosci. 45:44–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bentzley JP, Coker-Bolt P, Moreau NG, Hope
K, Ramakrishnan V, Brown T, Mulvihill D and Jenkins D: Kinematic
measurement of 12-week head control correlates with 12-month
neurodevelopment in preterm infants. Early Hum Dev. 91:159–164.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tian T, Yu S, Liu L, Xue F, Yuan C, Wang
M, Ji C and Ma D: The profile of T helper subsets in bone marrow
microenvironment is distinct for different stages of acute myeloid
leukemia patients and chemotherapy partly ameliorates these
variations. PLoS One. 10:e01317612015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vargas-Rojas MI, Solleiro-Villavicencio H
and Soto-Vega E: Th1, Th2, Th17 and Treg levels in umbilical cord
blood in preeclampsia. J Matern Fetal Neonatal Med. 29:1642–1645.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song KH, Kim MH, Kang SM, Jung SY, Ahn J,
Woo HJ, Nam SY, Hwang SG, Ryu SY and Song JY: Analysis of immune
cell populations and cytokine profiles in murine splenocytes
exposed to whole-body low-dose irradiation. Int J Radiat Biol.
91:795–803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kornete M, Mason ES, Girouard J, Lafferty
EI, Qureshi S and Piccirillo CA: Th1-Like ICOS+ Foxp3+ treg cells
preferentially express CXCR3 and home to β-Islets during
pre-diabetes in BDC2.5 NOD mice. PLoS One. 10:e01263112015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Cao H, Wang H, Yin G, Du J, Xia F,
Lu J and Xiang M: Multiple mechanisms involved in diabetes
protection by lipopolysaccharide in non-obese diabetic mice.
Toxicol Appl Pharmacol. 285:149–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kong F, Zhang W, Feng B, Zhang H, Rao H,
Wang J, Cong X and Wei L: Abnormal CD4 + T helper (Th)1 cells and
activated memory B cells are associated with type III asymptomatic
mixed cryoglobulinemia in HCV infection. Virol J. 12:1002015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Whitaker AH, Feldman JF, Lorenz JM,
McNicholas F, Fisher PW, Shen S, Pinto-Martin J, Shaffer D and
Paneth N: Neonatal head ultrasound abnormalities in preterm infants
and adolescent psychiatric disorders. Arch Gen Psychiatry.
68:742–752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Constable RT, Ment LR, Vohr BR, Kesler SR,
Fulbright RK, Lacadie C, Delancy S, Katz KH, Schneider KC, Schafer
RJ, et al: Prematurely born children demonstrate white matter
microstructural differences at 12 years of age, relative to term
control subjects: An investigation of group and gender effects.
Pediatrics. 121:306–316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Woodward LJ, Edgin JO, Thompson D and
Inder TE: Object working memory deficits predicted by early brain
injury and development in the preterm infant. Brain. 128:2578–2587.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anand G, Vasanthakumar R, Mohan V, Babu S
and Aravindhan V: Increased IL-12 and decreased IL-33 serum levels
are associated with increased Th1 and suppressed Th2 cytokine
profile in patients with diabetic nephropathy (CURES-134). Int J
Clin Exp Pathol. 7:8008–8015. 2014.PubMed/NCBI
|
|
22
|
Zabetian-Targhi F, Mirzaei K, Keshavarz SA
and Hossein-Nezhad A: Modulatory role of omentin-1 in inflammation:
Cytokines and dietary intake. J Am Coll Nutr. 35:670–678. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee CH, Park JH, Ahn JH and Won MH:
Effects of melatonin on cognitive impairment and hippocampal
neuronal damage in a rat model of chronic cerebral hypoperfusion.
Exp Ther Med. 11:2240–2246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coomes SM, Pelly VS, Kannan Y, Okoye IS,
Czieso S, Entwistle LJ, Perez-Lloret J, Nikolov N, Potocnik AJ,
Biró J, et al: IFNγ and IL-12 restrict Th2 responses during
helminth/plasmodium co-infection and promote IFNγ from Th2 cells.
PLoS Pathog. 11:e10049942015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang TY, Lee SY, Chen SL, Chang YH, Wang
LJ, Chen PS, Chen SH, Chu CH, Huang SY, Tzeng NS, et al: Comparing
clinical responses and the biomarkers of BDNF and cytokines between
subthreshold bipolar disorder and bipolar II disorder. Sci Rep.
6:274312016. View Article : Google Scholar : PubMed/NCBI
|