Introduction
Diabetes-induced kidney disease is a common
complication in patients with diabetes, and may lead to end-stage
renal failure (1–3). Diabetic nephropathy (DN) is the most
severe complication of diabetes and is also a major contributor to
end-stage renal failure (4).
Podocytes are highly specific cells that are located at the outer
surface of the glomerular basement membrane; they aid in
maintaining the structure and function of the glomerular filtration
barrier (5). Podocyte damage may
lead to kidney dysfunctions such as diabetic proteinuria (6,7).
Damage and loss of podocytes is observed in patients with diabetes
and may represent an early phase of DN, and the loss of podocytes
is considered to be a key factor that causes DN (8–10).
The abnormal expression of fibroblast-specific
protein in renal tubular epithelial cells was suggested to indicate
that some myofibroblasts may have been derived from the
transformation of epithelial cells (11). A previous study reported that,
during renal fibrosis, a large portion of tubular epithelial cells
may undergo epithelial-to-mesenchymal transition (EMT) to become
myofibroblasts (12). However, the
specific mechanisms involved in podocyte EMT in DN remain to be
characterized.
During the early phase of DN, podocyte EMT may be
promoted by a number of factors, such as matrix metalloproteinases
(MMPs). Diabetes has been associated with the abnormal expression
of MMP proteins, particularly MMP9 (13). The expression of MMP9 may be
induced by exposure to external stimuli such as reactive oxygen
species (ROS). Generation of excessive ROS levels in podocytes may
activate the extracellular signal-regulated kinase 1/2 signaling
pathway and ultimately induce the expression of MMP9 (14); therefore, increased ROS levels may
cause podocyte injury. S-nitrosylation of certain proteins
in the cell is a marker of oxidative stress, and during the onset
of diabetes, protein S-nitrosylationis enhanced (15). S-nitrosylation was reported
previously to activate the expression of MMP9 and to induce
apoptosis of neuronal cells. One of the main components of the
glomerular basement membrane (GBM) is type IV collagen (16), and over expression of MMP9 may
alter the composition of the GBM, which may result in structural
changes inpodocytes and their eventual loss from the GBM (17). MMP9 may suppress the expression of
podocalyxin inpodocytes, reducing the charge barrier that prevents
microalbuminuria. High-glucose (HG) levels or transforming growth
factor (TGF)-β treatment were reported to induce the expression of
MMP9 proteins in podocytes (18).
MMP9 expression has been associated with the activation of
integrin-linked protein kinase, which promotes the adhesion of
podocytes to the GBM (19).
Notably, it was previously reported that the level of podocytes in
the urine of patients with chronic kidney disease was closely
associated with the plasma expression level of MMP9, and MMP9
polymorphisms may influence the incidence rate of DN (20). These data suggested that MMP9 may
serve a key role in podocyte injury and glomerulopathy.
The methylation and demethylation of genes is a form
of epigenetic modification that has been implicated in a number of
biological processes (21).
Site-specific demethylation is able to dynamically regulate gene
expression, which allows cells to adapt to external stimuli
(22). Previous studies indicated
that alterations in the DNA methylation status in mouse thymic
lymphoma cell lines were able to affect the transcriptional
activity of the MMP9 promoter, and thereby affect the expression of
MMP9 (23,24). Demethylation of certain CpG sites
in the MMP9 promoter was reported to disrupt the synthesis of MMP9
in cartilage tissues during osteoarthritis (25). The aim of the present study was to
assess whether demethylation of the MMP9 promoter region may bea
key regulatory factor in determining podocyte EMT in DN, and to
investigate whether MMP9 promoter demethylation may represent a
prognostic marker of DN.
Materials and methods
Cell culture
Human renal epithelial tissues were obtained from
the unaffected pole of tumor-bearing kidneys of adults; kindly
supplied by Department of Urology, Zhujiang Hospital, Southern
Medical University (Guangzhou, China), and human podocytes were
isolated from renal epithelial tissue and developed by transfection
with the temperature-sensitive SV40-T gene by Guangzhou Scirince
(Scirince, Guangzhou, China) (26). Podocytes were maintained at 37°C in
a humidified atmosphere of 95% air and 5% CO2. Cells
were cultured in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) supplemented with 10% fetal bovine serum, 100
U/ml penicillin and 100 mg/ml streptomycin (Sigma-Aldrich; Merck
KGaA). The cell culture medium was changed once every 2 days.
Cell transfections
Podocytes were cultured for 2 weeks at 37°C to
induce cell differentiation, after which 1×106
podocytes/ml were transferred to a 6-well cell culture plate and
transfected with predesigned MMP9-directed small interfering
(si)RNA (sense 5′-GACCUGGGCAGAUUCCAAAtt-3′, antisense
5′-UUUGGAAUCUGCCCAGGUCtg-3′; Guangzhou Ribo Bio Co., Ltd.,
Guangzhou, China) using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 50 nM
transfection concentration at room temperature. Scramble siRNA was
used as a control. Cells were transfected for 24 h at room
temperature, after which the culture medium (RPMI-1640 medium
supplemented with 10% fetal bovine serum, 100 U/ml penicillin and
100 mg/ml streptomycin) was replaced with RPMI-1640 culture medium
containing 10% FBS, 1% insulin-transferr in-sodium selenite (ITS)
and either 5.0 mmol/ld-glucose [normal glucose (NG) group] or 25
mmol/l d-glucose [high glucose (HG) group], and cells were cultured
for an additional 24 h prior to further evaluation at 37°C.
Experiments were performed in triplicate. RPMI-1640 media, FBS and
ITS were all purchased from Sigma-Aldrich (Merck KGaA).
Immunofluorescence assay
Podocytes were fixed with ice-cold 4%
paraformaldehyde for 15 min and blocked with 5% goat serum
(Sigma-Aldrich; Merck KGaA) with 0.3% Triton X-100 for 15 min at
room temperature, then incubated with MMP9 primary antibody (1:200,
ab38898; Abcam, Cambridge MA, USA) overnight at 4°C. The cells were
subsequently washed 3 times with 0.1 mol/l PBS, followed by
incubation with fluorescein isothiocyanate (FITC)-conjugated
secondary antibody (1:600, ab150117; Abcam) for 1 h at 37°C.
Experiments were performed in triplicate. Images were captured
using an Olympus BX51 fluorescence microscope (Olympus Corporation,
Tokyo, Japan).
Flow-cytometric analysis
Following siRNA transfection and induction cells
were cultured for 24 h at 37°C. The cells were collected for 5 min
at 300 × g at 4°C, washed twice and re suspended with PBS at
1×106 cells/ml. Cells were stained with FITC (5 µg/ml)
and propidium iodide (5 µg/ml) for 15 min at room temperature
(20–25°C) in the dark, and the fraction of living, dead, early
apoptotic and late apoptotic cells was assessed by BD FACS Aria II
flow cytometer (BD Biosciences, Franklin Lakes, NY, USA). The
Annexin V-FITC Apoptosis Detection kit was purchased from Abcam
(ab14085). Experiments were performed in triplicate.
MTT assay
To analyze the effects of HG culture on podocyte
activity, we assessed cell proliferation using the Cell
Proliferation Reagent kit I (Sigma-Aldrich; Merck KGaA). Podocytes
were seeded (5×104 cells/well) in a 96-well plate and
cultured at 37°C for 24 h. The different concentrations of glucose
were added to the media, and the plate was incubated at 37°C for 0,
24, 48, 72 and 96 h, after which cells were transferred to fresh
medium [RPMI-1640 medium (Sigma-Aldrich; Merck KGaA) supplemented
with 10% fetal bovine serum, 100 U/ml penicillin and 100 mg/ml
streptomycin (Sigma-Aldrich; Merck KGaA)] containing 1 mg/ml MTT
(Invitrogen; Thermo Fisher Scientific, Inc.) and cultured for 3 h
at 37°C. The medium was removed, dimethylsulfoxide (100 µl) was
added and the plate was agitated for 20 min at room temperature to
completely dissolve the purple formazancrystals, and absorbance was
measured at 570 nm. Experiments were performed in triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from human podocytes using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse transcribed Prime Script TMRT reagent kit (Takara Bio,
Inc., Otsu, Japan) according to the manufacturer's instructions.
qPCR was performed using SYBR Premix ExTaq (Takara Bio, Inc.) and
an ABI7500 Real-Time PCR instrument (Applied Bio systems; Thermo
Fisher Scientific, Inc.) with the following cycling conditions:
95°C for 10 min, followed by 40 cycles of denaturation at 95°C for
10 sec, annealing at 60°C for 10 sec and extension at 72°C for 20
sec. The primer sequences used for qPCR are provided in Table I. RT-qPCR results were analyzed
using ABI7500 system software (7500 v2.3, Applied Bio systems;
Thermo Fisher Scientific, Inc.). The 2−ΔΔCq method was
used to detect the relative expression of Mrna (27), the GAPDH was used to normalize the
mRNA expression levels and the relative quantification cycle (Cq)
values were reported as expression fold alterations. Experiments
were performed in triplicate, the data averaged.
 | Table I.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′→3′) |
|---|
| MMP9 | F:
GGGACGCAGACATCGTCATC |
|
| R:
TCGTCATCGTCGAAATGGGC |
| α-SMA | F:
GTGTTGCCCCTGAAGAGCAT |
|
| R:
GCTGGGACATTGAAAGTCTCA |
| Podocalyxin | F:
AGCTAAACCTAACACCACAAGC |
|
| R:
TGAGGGGTCGTCAGATGTTCT |
| Fibronectin-1 | F:
CGGTGGCTGTCAGTCAAAG |
|
| R:
AAACCTCGGCTTCCTCCATAA |
| GAPDH | F:
CCTTCATTGACCTCAACTACAT |
|
| R:
CCAAAGTTGTCATGGATGACC |
Western blot analysis
The transfected podocytes were washed twice with
ice-cold PBS solution, and lysed inlysis buffer (Sigma-Aldrich;
Merck KGaA). Total protein was quantified with Bicinchoninic Acid
Protein Assay kit (Beyotime Institute of Biotechnology, Haimen,
China) according to the manufacturer's protocols, and 50 µg/well
protein was used for SDS-PAGE (10%) electrophoresis and transferred
to a polyvinylidene fluoridemembrane and then the membrane was
blocked for 1 h at room temperature. Blocking Reagent was purchased
from Beyotime Institute of Biotechnology. The membranes were
incubated overnight with primary antibodies directed against MMP9
(1:500, sc-21736), α-smooth muscle actin (α-SMA, 1:500, sc-71625),
podocalyxin (1:500, sc-23904), fibronectin-1 (1:500, sc-69681) and
GAPDH (1:500, sc-293335) at 4°C; all purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Subsequently, the membranes
were incubated for 2 h with horseradish peroxidase (HRP)-conjugated
secondary antibody (bovine anti-mouse 1:200, sc-2371, Santa Cruz
Biotechnology, Inc.) at room temperature, and protein bands were
visualized using the Super Signal Chemiluminescent Substrate
(Pierce; Thermo Fisher Scientific, Inc.). Experiments were
performed in triplicate. Protein expression of MMP9, α-SMA,
podocalyxin and fibronectin-1 were normalized to GAPDH. The blots
were analyzed using Quantity One software, version 4.6 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
DNA demethylation analysis
Cells (1×106) in the NG and HG groups
were collected, washed with PBS twice, and genomic DNA was
extracted from cells using the QIAamp DNA Mini kit (Qiagen, Inc.,
Valencia, CA USA), according to the manufacturer's instructions.
Following hydrosulphite treatment of DNA using the EpiTect
Bisulfite kit (Qiagen, Inc.) according to the manufacturer's
protocols, the demethylation status of the MMP9 promoter was
assessed by hydrosulphite sequencing PCR using ABI7500 Real-Time
PCR instrument (Applied Bio systems; Thermo Fisher Scientific,
Inc.) with the following clones made under the cycling conditions:
95°C for 10 min, followed by 40 cycles of denaturation at 95°C for
1 min, annealing at 60°C for 1 min and extension at 60°C for 10
min, and promoter-specific primers: MMP9 forward
5′-GATGGGGGATTTTTTTAGTTTTATT-3′ and reverse
5′-TACCCACCTCTACCAACTACCTATC-3′. Ten clones of each DNA sample were
selected via gel extraction for verification.
Dual-luciferase reporter assays
The effects of CpG-site methylation on MMP9 promoter
activity were examined in vitro using the pGL3-Basic vector
(Promega Corporation, Madison, WI, USA). PCR primers were designed
with NotI and XhoI restriction cut sites in the 5′
ends; MMP9 promoter forward
5′-cgcgcggccgcAGAGGAAGCTGAGTCAAAGAAGGC-3′ and reverse
5′-cccctcgagTGGTGAGGGCAGAGGTGTCT-3′. PCR cycling conditions: 95°C
for 10 min, followed by 40 cycles of denaturation at 95°C for 10
sec, annealing at 60°C for 30 sec and extension at 72°C for 20 sec,
1 cycle at 72°C for 7 min; and a final hold at 4°C. The primers
were used to amplify a 260 bp DNA fragment from the human MMP9
promoter region. The obtained DNA fragments were ligated into the
multiple cloning sites of the pGL3-Basicvector. pGL3-MMP9 plasmids
were recovered and plasmid methylation was performed using
EpiXplore™ Methylated DNA Enrichment kit (Takara Bio, Inc.),
according to the manufacturer's instructions. Following
methylation, the plasmids (0.02 µg/µl) were transformed into 293T
(American Type Culture Collection, Manassas, VA, USA) cells
(105 cells/well) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) in 96-wellplate. Cells
were cultured for 24 h at 37°C, luciferase activity was assessed by
using QUANTI-Luc reagent (Invivogen, San Diego, CA, USA) and a
PerkinElmer EnVision 2104 Multi label Plate Reader (PerkinElmer,
Inc., Waltham, MA, USA), according to the manufacturer's protocols.
All experiments were repeated three times.
Animals and treatments
Male adult Wistar rats (180±16 g) were purchased
from the Guangdong Medical Laboratory Animal Center [Guangzhou,
China; Animal license number SYXK (Guangdong): 20130002]. Rats were
acclimated for 7 days at room temperature under normal lighting
conditions. All animal experiments were approved by the Animal
Research Ethics Board of Sun Yat-sen University (Guangzhou, China)
and were carried out in compliance with institutional guidelines on
the care of experimental animals. All efforts were made to minimize
the suffering of animals; experiments with animals were conducted
in Sun Yat-sen university laboratories due to equipment
availability and access. In the DN model group, diabetes was
induced by intravenous injection of streptozotocin (STZ; 65 mg/kg;
Sigma-Aldrich; Merck KGaA), where as normal Control rats received
citric acid (100 mmol/l) administered by intravenous injection
(28). The blood glucose levels
were measured every day from tail vein blood using a Bayer Contour
glucose meter (Bayer, Pittsburgh, PA, USA), if the blood glucose
level was >16.7 mmol/l for >10 days, the rats were defined as
a successful DN model. At week 2 and week 6, rats were
photographed, the blood glucose levels and body weight were
recorded. At week 6 rats were sacrificed and kidney tissues were
collected.
Kidney flush, glomeruli isolation,
podocyte isolation and subculture
Saline was injected into the distal artery of the
renal artery to wash blood from the tissue. Glomeruli were
separated as described previously (29). Briefly, kidney tissue was cut into
pieces, which were digested with collagenase IV (200 U/ml,
Sigma-Aldrich, Merck KGaA) for 1 h under constant rotation at 37°C,
and passed through a 100-mesh sieve (150 µm). The resulting cell
suspension was sieved using a 200-mesh sieve (75 µm), and the
glomeruli were retained on the sieve surface. The podocyte from
isolation was used for DNA demethylation assay and western blot
analysis. The protocol of demethylation analysis here was conducted
as aforementioned.
Immunohistochemistry
The kidney tissues collected from rats were embedded
in paraffin and sectioned (4 µm). Paraffin-embedded tissues
sections were deparaffinized with xylene and rehydrated and fixed
with 2% paraformaldehyde, 4% sucrose in PBS for 10 min at room
temperature and then permeabilized with 0.3% Triton X-100
(Sigma-Aldrich, Merck KGaA) in PBS for 10 min. Sections were washed
3 times in PBS, and incubated for 30 min at room temperature in 3%
bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.) in PBS
to prevent nonspecific binding. The sections were subsequently
incubated with primary antibodies against MMP9, α-SMA, podocalyxin,
fibronectin-1overnight at 4°C in a moist chamber, then HRP-labeled
secondary antibody as aforementioned for 2 h at room temperature.
3,3′-diaminobenzidine (Sigma-Aldrich; Merck KGaA) was added for 2 h
at room temperature, and the sections were counterstained using
hematoxylin for 5 min at room temperature, then air dried, and
images were captured using a Leica DM5000B microscope equipped with
a Leica DFC500 camera and Image Pro Plus software (vs 5.02; Media
Cybernetics, Inc., Rockville, MD, USA). Five randomly chosen
high-power fields (100 cells/visual field) were visualized. The
percentage of positively staining cells were counted manually and
analyzed using Image J software v1.48 (National Institutes of
Health, Bethesda, MD, USA). The staining intensity of MMP9, α-SMA,
podocalyxin and fibronectin-1 were normalized to MMP9 control.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
(IBM Corp., Armonk, NY, USA) and Graph Pad Prism 5 (Graph Pad
Software, Inc., La Jolla, CA, USA) software. Data are presented as
the mean ± standard error of the mean, determined using single
factor analysis of the variance (ANOVA). Statistical significance
was evaluated by one-way ANOVA followed by least significant
difference test or Dunnett's T3 post hoc analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
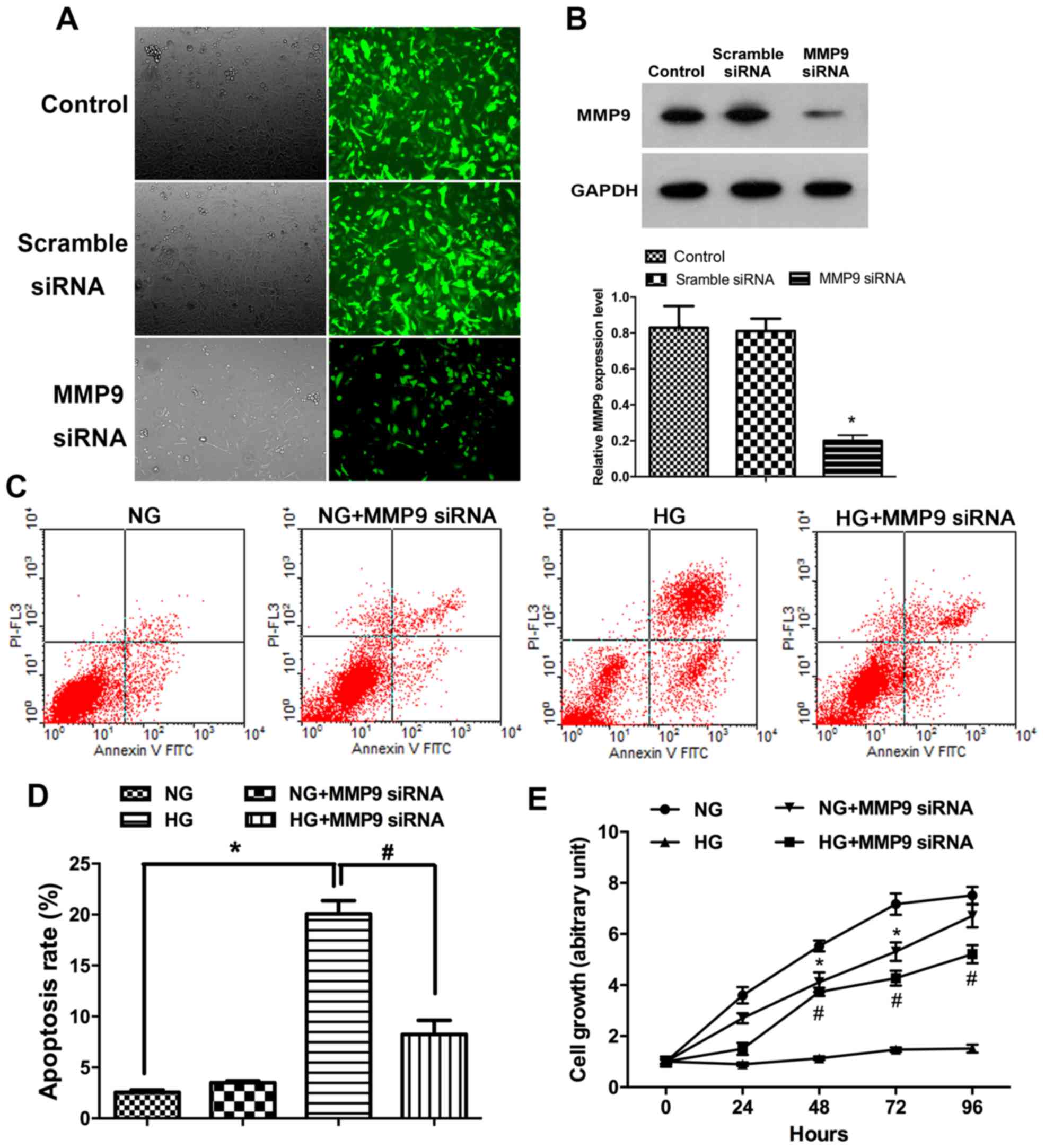
HG-treatment inducespodocyte apoptosis
and suppresses proliferation in vitro
To determine the relationship between MMP9
expression pattern and glucose treatment in podocyte EMT in DN,
MMP9 gene silencing was performed with siRNA in podocyte cultures.
Immunofluorescence assay results demonstrated that the expression
of MMP9 in cells transfected with MMP9-siRNA was lower than the
untransfected control cells (Fig.
1A). Results of western blot analysis confirmed that the
expression of MMP9 protein in podocytes transfected with MMP9-siRNA
was significantly lower compared with the Control group (Fig. 1B; P<0.05). Following incubations
with glucose, the rate of podocytes apoptosis was assessed by flow
cytometry. The data revealed that apoptotic rates were
significantly higher following incubation with high concentrations
of glucose compared with incubation with normal physiological
concentrations of glucose. Following high glucose treatment, the
rate of (early) apoptosis was also significantly higher in
podocytes without siRNA than with MMP9 siRNA treatment (Fig. 1C and D; P<0.05). Results from
the MTT assay indicated that incubation in a high concentration of
glucose significantly reduced human podocyte proliferation compared
with the NG group (Fig. 1E;
P<0.05), but that MMP9 siRNA-transfected podocytes were
resistant to this effect.
HG treatment affects the expression of
MMP9, α-SMA, podocalyxin and fibronectin-1 in podocytes
The expression levels of MMP9, α-SMA, podocalyxin
and fibronectin-1 mRNA and protein in cultured human podocytes were
assessed by RT-qPCR and western blotting, respectively. HG
treatment significantly increased the expression levels of MMP9,
α-SMA and fibronectin-1, and reduced the expression levels
podocalyxin compared with NG-treated cells (Fig. 2A-C). These data further supported
the hypothesis that HG levels may promote podocyte EMT by altering
the expression of MMP9, α-SMA, podocalyxin and fibronectin-1 in
vitro.
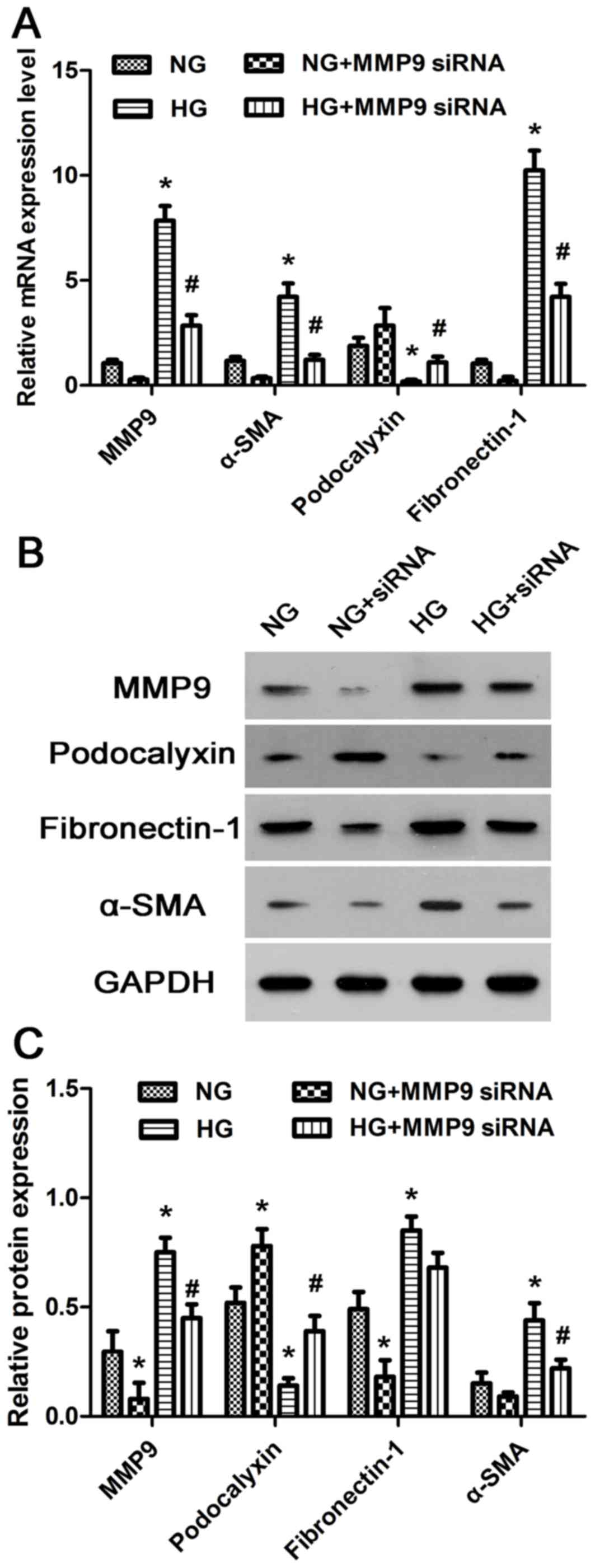 | Figure 2.mRNA and protein expression levels of
MMP9, α-SMA, podocalyxin and fibronectin-1 in cultured human
podocytes treated with HG. (A) Relative mRNA expression levels of
MMP9, α-SMA, podocalyxin and fibronectin-1 wereanalyzed in human
podocytes by reverse transcription-quantitative polymerase chain
reaction. (B) The relative protein expression levels of MMP9,
α-SMA, podocalyxin and fibronectin-1 were detected in human
podocytes by western blot analysis. (C) Densito metric analysis of
protein expression levels from Part B. *P<0.05 vs. NG;
#P<0.05 vs. HG. α-SMA, α-smooth muscle actin; HG,
high glucose; MMP9, matrix metalloproteinase 9; NG, normal glucose;
siRNA, small interfering RNA. |
To further characterize the relationship between
MMP9 and the expression of α-SMA, podocalyxin and fibronectin-1,
MMP9 was down regulated with MMP9-siRNA transfection in cultured
human podocytes. MMP9 knockdown significantly reduced the
expression levels of α-SMA and fibronectin-1, but increased the
expression levels of podocalyxin in both NG and HG-treated human
podocytes compared to the respective untransfected groups (Fig. 2A-C). These results suggested that
MMP9 may alter the physiological characteristics of podocytes by
regulating the expression of α-SMA, podocalyxin and fibronectin-1
in vitro.
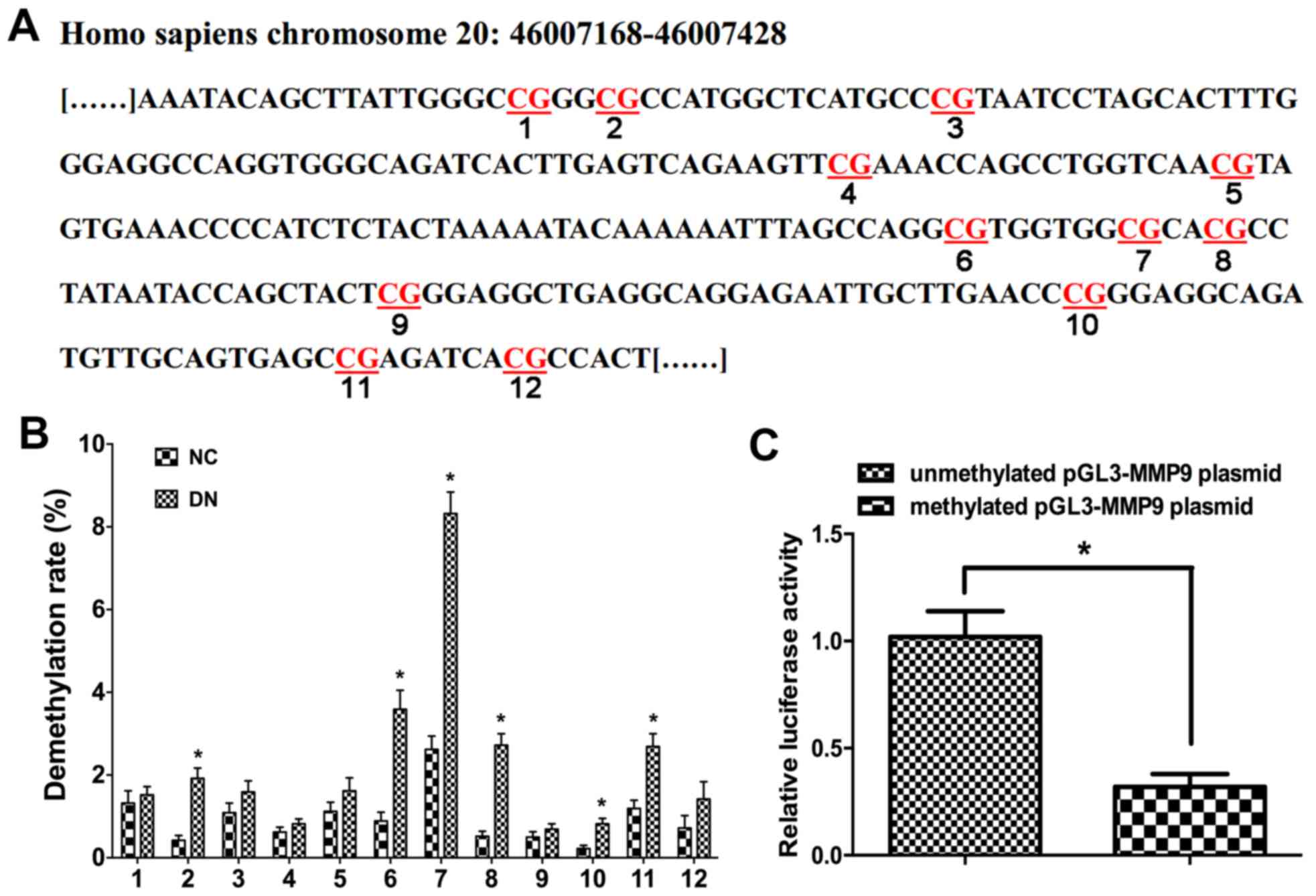
HG treatment induces MMP9 promoter
demethylation
To investigate the relationship between MMP9
promoter demethylation and podocyte EMT following HG treatment, the
methylation status of12 CpG sites within the MMP9 promoter region
were analyzed (Fig. 3A). In the
HG-treated group, CpG sites 3, 6, 7, 8 and 11 exhibited apparent
demethylation compared with NG-treated cells (Fig. 3B), which suggested that HG
treatment may be able to induce demethylation of CpG sites in the
MMP9 promoter region.
Promoter demethylation upregulates
MMP9 expression in vitro
Previously, methylation of promoter region CpG sites
was reported to hinder the binding of transcription factors,
ultimately inhibiting gene expression (30). To confirm that demethylation of the
MMP9 promoter region resulted in an up regulation of MMP9
expression, a reporter construct in which the MMP9 promoter
specific region was ligated to a dual luciferase reporter vector
was used. The results demonstrated that MMP9 promoter demethylation
significantly increased luciferase activity of this reporter, which
suggested that the expression of MMP9 was regulated by
themethylation status of the MMP9 promoter region (Fig. 3C). Therefore, the present study
speculated that methylation of the MMP9 promoter region at the CpG
sites may hinder the binding of transcription factors, thus
inhibiting expression of MMP9.
DN induces podocyte EMT by regulating
expression of MMP9, α-SMA, podocalyxin and fibronectin-1 in
vivo
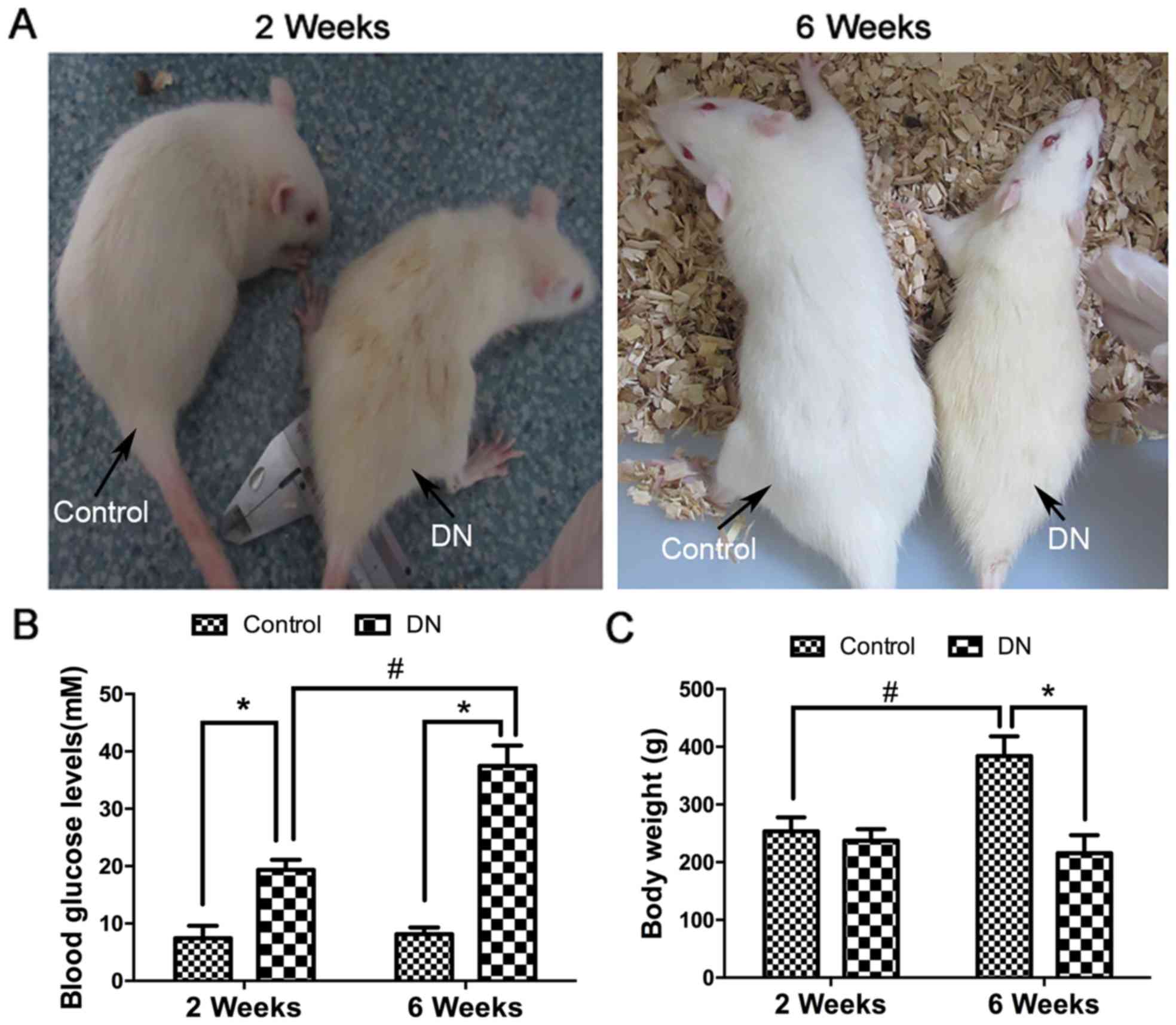
To simulate DN in vivo, Wistar rats were in
traperitoneally injected with STZ (Fig. 4A). The blood glucose levels
significantly increased within the DN group at 2 weeks and 6 weeks
compared with in the control. Furthermore, the blood glucose levels
significantly increased within the DN group at 6 weeks compared
with at 2 weeks (P<0.05; Fig.
4B). There were no marked variations between the control and DN
group body weights at 2 weeks, but were significantly decreased in
the DN group at 6 weeks compared with in the control group.
Furthermore, the body weights significantly increased within the
control group at 6 weeks compared with at 2 weeks (P<0.05;
Fig. 4C).
To investigate the relationship between MMP9
expression and podocyte EMT in vivo, the protein expression
levels of MMP9, α-SMA, podocalyxin and fibronectin-1 in the
podocytes of DN and control rats were examined. It was revealed
that the expression of MMP9, α-SMA and fibronectin-1 was
significantly higher and the expression of podocalyxin was
significantly lower in DN model rats compared with expression
levels in rats in the Control group, as detected by
immunohistochemistry and western blotting (Fig. 5A and B, respectively). In addition,
the level of demethylation of the MMP9 promoter CpGs was
significantly higher in DN rats (Fig.
5C). These in vivo data were consistent with the in
vitro results, and further support the hypothesis that
demethylation of the MMP9 promoter may serve an important role in
podocyte EMT in DN.
 | Figure 5.Effects of STZ treatment on
expression of MMP9, α-SMA, podocalyxin and fibronectin-1 in DN
rats. (A) Expression levels of MMP9, α-SMA, podocalyxin and
fibronectin-1 in the podocytes of DN rats were examined
dimmunohistochemically. Staining intensity (%), the average
positive stained cells percentage to evaluate the slice
immunohistochemistry result, measured by Image J software. All the
experiments were performed in triplicate. (B) The relative protein
expression levels of MMP9, α-SMA, podocalyxin and fibronectin-1
protein in the podocytes of DN rats were examined by western
blotting. (C) Demethylation level analysis of MMP9 promoter in
vitro. *P<0.05 vs. Control. α-SMA, α-smooth muscle actin;
DN, diabetic nephropathy; MMP9, matrix metalloproteinase 9; STZ,
streptozotocin. |
Discussion
DNA methylation involves the addition of a methyl
group to DNA, and is an important epigenetic modification. DNA
methylation is widespread in the human genome and mainly occurs on
the cytosines of CpGdinucleo tides (31). Methylation of gene promoter regions
may lead to long-term silencing of gene expression by preventing
the binding of transcription factors (32). Aberrant promoter demethylation has
been associated with the over expression of genes that are involved
in the pathogenesis of many diseases, and thus may potentially be
used as disease biomarkers (33,34).
The pre-test of the present study indicated that the
expression of MMP9 in podocytes was significantly increased in DN,
which led to investigations of the factors affecting MMP9
expression in podocytes incubated with high concentrations of
glucose. Previous study also indicated that site-specific
demethylation of the MMP9 promoter significantly influenced MMP9
expression in keratinocytes stimulated by tumor necrosis factor α
(TNFα) (35,36). In the present study, human
podocytes were incubated in media containing high concentrations of
glucose. This condition was demonstrated to promote demethylation
of the MMP9 promoter and to significantly up regulate MMP9
expression. Similarly, MMP9 expression was also increased in DN
model rats by demethylation of the MMP9 promoter region. The
results of the present study revealed that HG levels induced
site-specific demethylation of the MMP9 promoter region, enhancing
MMP9 expression. These results suggested that the demethylation
status of the MMP9 promoter may be used as a prognostic marker of
DN in the clinics.
During EMT, polarity and cell-cell adhesions are
lost and the epithelial cells become mesenchymal stem cells,
gaining migratory and invasive properties. In addition to normal
physiological processes such as neural tube formation, EMT occurs
in several pathological events, including cancer, organ fibrosis
and chronic inflammation (37,38).
EMT was first identified as an important differentiation and
morphogenetic process during embryonic development and has been
implicated in renal interstitial fibrosis caused by diabetes
(39). A number of molecular
mechanisms contribute to EMT, including the activation of growth
factors and their receptors by proteases, and the cleavage of
cellular adhesion molecules (40).
MMPs, in particular MMP9, were also previously implicated in EMT
(41,42). For example, serum levels of TNF-α
were demonstrated to be significantly elevated in patients with
renal cell carcinoma (RCC), which induced EMT and promoted
tumorigenicity of RCC by repressing E-cadher in, up regulating
viment in, activating MMP9 and increasing invasion activities
(43).
The present study investigated the association
between MMP9 and EMT in podocytes by assessing the degree of MMP9
promoter demethylation that was induced by HG treatment in
vitro and in vivo, and demonstrated that HG treatment
may up regulate the expression of MMP9 by inducing demethylation of
the MMP9 promoter region. In addition, expression levels of the
mesenchymal cell markers α-SMA and fibronectin-1 were increased in
HG-treated podocytes, whereas the expression levels of podocalyxin,
a marker of podocytes, were significantly reduced. These data
suggested that MMP9 promoter demethylation may induce podocyte EMT
indirectly. First, expression of MMP9 was associated with the
degree of MMP9 promoter demethylation. Second, HG treated podocytes
in vitro had exhibited an increase in MMP9 promoter
demethylation and in the expression levels of MMP9, α-SMA and
fibronectin-1, and reduced expression of podocalyxin. Third, dual
luciferase reporter assays demonstrated that demethylation of MMP9
promoter significantly influenced its promoter activity. Fourth, a
rat model of DN exhibited an increase in the expression levels of
MMP9, α-SMA and fibronectin-1, and a decrease in the expression of
podocalyxin in podocytes. HG levels induced demethylation of the
MMP9 promoter region, enhancing MMP9 expression in podocytes,
ultimately promoting podocyte EMT. However, the mechanism that MMP9
promoter demethylation promotes podocyte EMT remains unknown, and
will need to be further studied in the future.
In summary, the present results provided preliminary
insights into the regulatory roles of MMP9 promoter demethylation
and podocyte EMT. These data indicated that MMP9 promoter
demethylation may serve an important role in podocyte EMT. Further
investigations will be required to determine the precise regulatory
mechanisms involved in this process in vitro and in
vivo. The present data may contribute to the future development
of novel therapeutic strategies to treat DN.
Acknowledgements
This study was supported by The Youth Science Fund
Project of National Natural Science Fund of China (grant no.
81400818), The Provincial Natural Science Foundation of Guangdong
(grant no. 2017A030313783) and The Scientific Research Project of
Shenzhen Municipal Health and Family Planning System (grant no.
201402128).
Glossary
Abbreviations
Abbreviations:
|
α-SMA
|
α-smooth muscle actin
|
|
Cq
|
quantification cycle
|
|
DN
|
diabetic nephropathy
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
GBM
|
glomerular basement membrane
|
|
HG
|
high glucose
|
|
ITS
|
insulin-transferr in-sodium
|
|
MMP9
|
matrix metalloproteinase 9
|
|
NG
|
normal glucose
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
RCC
|
renal cell carcinoma
|
|
ROS
|
reactive oxygen species
|
|
STZ
|
streptozotocin
|
|
TGF-β
|
transforming growth factor-β
|
|
TNFα
|
tumor necrosis factor α
|
References
|
1
|
Gamella-Pozuelo L, Fuentes-Calvo I,
Gomez-Marcos MA, Recio-Rodriguez JI, Agudo-Conde C,
Fernandez-Martin JL, Cannata-Andia JB, Lopez-Novoa JM, Garcia-Ortiz
L and Martinez-Salgado C: Plasma cardiotrophin-1 as a marker of
hypertension and diabetes-induced target organ damage and
cardiovascular risk. Medicine (Baltimore). 94:e12182015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Menini S, Iacobini C, Ricci C, Blasetti
Fantauzzi C and Pugliese G: Protection from diabetes-induced
atherosclerosis and renal disease by D-carnosine-octylester:
Effects of early vs late inhibition of advanced glycation
end-products in Apoe-null mice. Diabetologia. 58:845–853. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khan S, Jena G and Tikoo K: Sodium
valproate ameliorates diabetes-induced fibrosis and renal damage by
the inhibition of histone deacetylases in diabetic rat. Exp Mol
Pathol. 98:230–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leehey DJ, Zhang JH, Emanuele NV,
Whaley-Connell A, Palevsky PM, Reilly RF, Guarino P and Fried LF;
VA NEPHRON-D Study Group, : BP and renal outcomes in diabetic
kidney disease: The veterans affairs nephropathy in diabetes trial.
Clin J Am Soc Nephrol. 10:2159–2169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao L, Wang X, Sun L, Nie H, Liu X, Chen
Z and Guan G: Critical role of serum response factor in podocyte
epithelial-mesenchymal transition of diabetic nephropathy. Diab
Vasc Dis Res. 13:81–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen XW, Du XY, Wang YX, Wang JC, Liu WT,
Chen WJ, Li HY, Peng FF, Xu ZZ, Niu HX and Long HB: Irbesartan
ameliorates diabetic nephropathy by suppressing the
RANKL-RANK-NF-κB pathway in type 2 diabetic db/db mice. Mediators
Inflamm. 2016:14059242016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Ma KL, Liu J, Wu Y, Hu ZB, Liu L,
Lu J, Zhang XL and Liu BC: Inflammatory stress exacerbates lipid
accumulation and podocyte injuries in diabetic nephropathy. Acta
Diabetol. 52:1045–1056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sweetwyne MT, Gruenwald A, Niranjan T,
Nishinakamura R, Strobl LJ and Susztak K: Notch1 and Notch2 in
Podocytes Play Differential Roles During Diabetic Nephropathy
Development. Diabetes. 64:4099–4111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yasuda-Yamahara M, Kume S, Tagawa A,
Maegawa H and Uzu T: Emerging role of podocyte autophagy in the
progression of diabetic nephropathy. Autophagy. 11:2385–2386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andeen NK, Nguyen TQ, Steegh F, Hudkins
KL, Najafian B and Alpers CE: The phenotypes of podocytes and
parietal epithelial cells may overlap in diabetic nephropathy.
Kidney Int. 88:1099–1107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou HL, Wang YT, Gao T, Wang WG and Wang
YS: Distribution and expression of fibroblast-specific protein
chemokine CCL21 and chemokine receptor CCR7 in renal allografts.
Transplant Proc. 45:pp. 538–545. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Omori K, Hattori N, Senoo T, Takayama Y,
Masuda T, Nakashima T, Iwamoto H, Fujitaka K, Hamada H and Kohno N:
Inhibition of plasminogen activator inhibitor-1 attenuates
transforming growth factor-β-dependent epithelial mesenchymal
transition and differentiation of fibroblasts to myofibroblasts.
PLoS One. 11:e01489692016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brandner JM, Zacheja S, Houdek P, Moll I
and Lobmann R: Expression of matrix metalloproteinases, cytokines,
and connexins in diabetic and nondiabetic human keratinocytes
before and after transplantation into an ex vivo wound-healing
model. Diabetes Care. 31:114–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo J, Xu Y, Ji W, Song L, Dai C and Zhan
L: Effects of exposure to benzo[a]pyrene on metastasis of breast
cancer are mediated through ROS-ERK-MMP9 axis signaling. Toxicol
Lett. 234:201–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong Y, Zhang X, Cai X, Wang K, Chen Y
and Deng Y: Puerarin attenuated early diabetic kidney injury
through down-regulation of matrix metalloproteinase 9 in
streptozotocin-induced diabetic rats. PLoS One. 9:e856902014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang XM, Shi K, Li JJ, Chen TT, Guo YH,
Liu YL, Yang YF and Yang S: Effects of angiotensin II intervention
on MMP-2, MMP-9, TIMP-1, and collagen expression in rats with
pulmonary hypertension. Genet Mol Res. 14:1707–1717. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lelongt B, Bengatta S, Delauche M, Lund
LR, Werb Z and Ronco PM: Matrix metalloproteinase 9 protects mice
from anti-glomerular basement membrane nephritis through its
fibrinolytic activity. J Exp Med. 193:793–802. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo W, Hu L, Li W, Xu G, Xu L, Zhang C and
Wang F: Epo inhibits the fibrosis and migration of Müller glial
cells induced by TGF-β and high glucose. Graefes Arch Clin Exp
Ophthalmol. 254:881–890. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang YS, Li Y, Dai C, Kiss LP, Wu C and
Liu Y: Inhibition of integrin-linked kinase blocks podocyte
epithelial-mesenchymal transition and ameliorates proteinuria.
Kidney Int. 78:363–373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakamura T, Ushiyama C, Suzuki S, Hara M,
Shimada N, Ebihara I and Koide H: Urinary excretion of podocytes in
patients with diabetic nephropathy. Nephrol Dial Transplant.
15:1379–1383. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thomas MC: Epigenetic mechanisms in
diabetic kidney disease. Curr Diab Rep. 16:312016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ling L, Ren M, Yang C, Lao G, Chen L, Luo
H, Feng Z and Yan L: Role of site-specific DNA demethylation in
TNFα-induced MMP9 expression in keratinocytes. J Mol Endocrinol.
50:279–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chaturvedi P, Kalani A, Givvimani S, Kamat
PK, Familtseva A and Tyagi SC: Differential regulation of DNA
methylation versus histone acetylation in cardiomyocytes during
HHcy in vitro and in vivo: An epigenetic mechanism. Physiol
Genomics. 46:245–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delgado-Olguin P, Dang LT, He D, Thomas S,
Chi L, Sukonnik T, Khyzha N, Dobenecker MW, Fish JE and Bruneau BG:
Ezh2-mediated repression of a transcriptional pathway upstream of
Mmp9 maintains integrity of the developing vasculature.
Development. 141:4610–4617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jackson MT, Moradi B, Smith MM, Jackson CJ
and Little CB: Activation of matrix metalloproteinases 2, 9, and 13
by activated protein C in human osteoarthritic cartilage
chondrocytes. Arthritis Rheumatol. 66:1525–1536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saleem MA, O'Hare MJ, Reiser J, Coward RJ,
Inward CD, Farren T, Xing CY, Ni L, Mathieson PW and Mundel P: A
conditionally immortalized human podocyte cell line demonstrating
nephrin and podocin expression. J Am Soc Nephrol. 13:630–638.
2002.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baydas G, Nedzvetskii VS, Nerush PA,
Kirichenko SV and Yoldas T: Altered expression of NCAM in
hippocampus and cortex may underlie memory and learning deficits in
rats with streptozotocin-induced diabetes mellitus. Life Sci.
73:1907–1916. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heiland DH, Ferrarese R, Claus R, Dai F,
Masilamani AP, Kling E, Weyerbrock A, Kling T, Nelander S and Carro
MS: c-Jun-N-terminal phosphorylation regulates DNMT1 expression and
genome wide methylation in gliomas. Oncotarget. 8:6940–6954. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lewko B, Golos M, Latawiec E, Angielski S
and Stepinski J: Regulation of cGMP synthesis in cultured podocytes
by vasoactive hormones. J Physiol Pharmacol. 57:599–610.
2006.PubMed/NCBI
|
|
31
|
Pfeifer GP: Mutagenesis at methylated CpG
sequences. Curr Top Microbiol Immunol. 301:259–281. 2006.PubMed/NCBI
|
|
32
|
Carr SM, Poppy Roworth A, Chan C and La
Thangue NB: Post-translational control of transcription factors:
Methylation ranks highly. FEBS J. 282:4450–4465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Z, Zhu LL, Jiang HS, Chen H, Chen Y
and Dai YT: Demethylation treatment restores erectile function in a
rat model of hyperhomocysteinemia. Asian J Androl. 18:763–768.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu W, Li J, Ren M, Zeng Y, Zhu P, Lin L,
Lin D, Hao S, Gao Q, Liang J, et al: Role of the mevalonate pathway
in specific CpG site demethylation on AGEs-induced MMP9 expression
and activation in keratinocytes. Mol Cell Endocrinol. 411:121–129.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zaina S, Goncalves I, Carmona FJ, Gomez A,
Heyn H, Mollet IG, Moran S, Varol N and Esteller M: DNA methylation
dynamics in human carotid plaques after cerebrovascular events.
Arterioscler Thromb Vasc Biol. 35:1835–1842. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ling L, Ren M, Yang C, Lao G, Chen L, Luo
H, Feng Z and Yan L: Role of site-specific DNA demethylation in
TNFα-induced MMP9 expression in keratinocytes. J Mol Endocrinol.
50:279–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
López-Novoa JM and Nieto MA: Inflammation
and EMT: An alliance towards organ fibrosis and cancer progression.
EMBO Mol Med. 1:303–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cufí S, Vazquez-Martin A,
Oliveras-Ferraros C, Martin-Castillo B, Joven J and Menendez JA:
Metformin against TGFβ-induced epithelial-to-mesenchymal transition
(EMT): from cancer stem cells to aging-associated fibrosis. Cell
Cycle. 9:4461–4468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang W, Miao J, Ma C, Han D and Zhang Y:
β-Casomorphin-7 attenuates the development of nephropathy in type I
diabetes via inhibition of epithelial-mesenchymal transition of
renal tubular epithelial cells. Peptides. 36:186–191. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song Y, Gong K, Yan H, Hong W, Wang L, Wu
Y, Li W, Li W and Cao Z: Sj7170, a unique dual-function peptide
with a specific α-chymotrypsin inhibitory activity and a potent
tumor-activating effect from scorpion venom. J Biol Chem.
289:11667–11680. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee WT, Lee TH, Cheng CH, Chen KC, Chen YC
and Lin CW: Antroquinonol from Antrodia Camphorata suppresses
breast tumor migration/invasion through inhibiting ERK-AP-1- and
AKT-NF-κB-dependent MMP-9 and epithelial-mesenchymal transition
expressions. Food Chem Toxicol. 78:33–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee DG, Lee SH, Kim JS, Park J, Cho YL,
Kim KS, Jo DY, Song IC, Kim N, Yun HJ, et al: Loss of NDRG2
promotes epithelial-mesenchymal transition of gallbladder carcinoma
cells through MMP-19-mediated Slug expression. J Hepatol.
63:1429–1439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ho MY, Tang SJ, Chuang MJ, Cha TL, Li JY,
Sun GH and Sun KH: TNF-α induces epithelial-mesenchymal transition
of renal cell carcinoma cells via a GSK3β-dependent mechanism. Mol
Cancer Res. 10:1109–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|