Introduction
Tissue engineering has the potential to treat
millions of patients living with debilitating diseases (1). The requirement for effective bone
repair therapy arises from conditions including congenital
malformation, trauma, tumor resection and skeletal disease
(2). Typically, bone tissue
engineering consists of harvesting cells from a patient, expanding
them in vitro and culturing them into a biomaterial,
additionally termed a scaffold. This functions as a structural
framework to facilitate cell attachment, proliferation and
differentiation into a controlled phenotype (3). Scaffold design is key to effective
tissue engineering. A scaffold should provide an ideal
microenvironment to promote cell and tissue growth. At a minimum,
the scaffold should have adequate mechanical stability to withstand
cellular contractile forces, high porosity with interconnected
pores to facilitate nutrient delivery and remove metabolic waste,
and must be biocompatible to promote tissue formation and
integration (4–9). Growth factors are soluble proteins
that stimulate cell growth and differentiation, which have emerged
as broadly applicable tools for the induction of bone formation.
Bone morphogenetic proteins (BMPs) are growth factors that are
effective at orchestrating novel bone formation in humans by
recapitulating the different stages of bone development (10). Hydroxyapatite (HAP)/collagen (COL)
composites are typically used as bone substitute materials in
dentistry and orthopedic surgery, for the regeneration of damaged
hard tissue (11). Nanometer (n)
HAP in particular has been widely used for tissue engineering
scaffold construction (12–16).
BMP-2 is the most extensively studied BMP in the context of
osteogenesis and has been demonstrated to enhance bone formation
(17–19). Vilquin and Rosset (20) reported that bone marrow-derived
mesenchymal stem cells (BMSCs) lack immunogenicity, making them an
ideal cell source for tissue engineering. Thus, BMSCs may be seeded
into scaffolds and implanted into the body without producing a
marked antigenic response. BMSCs have additionally been used to
determine the biological toxicity of tissue-engineered scaffolds.
In absence of biological toxicity in vitro, it is reasonable
to hypothesize that the same scaffold will avoid antigen rejection
following implantation.
In the present study, blending and freeze-drying
methods were combined to construct the BMP-2-nHAP-COL scaffold. The
scaffold properties were assessed to determine whether the
requirements for bone tissue engineering had been met.
Materials and methods
BMP-2-nHAP-COL scaffold
preparation
Acetic acid solution was diluted in deionized water
to a concentration of 0.005 mol/l, and COL (10 mg; Shengyou
Biotechnology Co., Ltd., Hangzhou, China) was subsequently added
into 10 ml acetic acid solution and stirred (JB-2A; Bante
Instruments Ltd., Shanghai, China) for 50 min. nHAP (10 mg; Emperor
Nano Material Co., Ltd., Nanjing, China) was added to this solution
and stirred overnight. The quality ratio was determined to be 1:1.5
(nHAP:COL). BMP-2 (PeproTech, Inc., Rocky Hill, NJ, USA) was
dissolved using PeproTech protein solution to 10 ng/µl (21), and added to the scaffold solution
prior to stirring for 50 min at 4°C, resulting in a final BMP-2
concentration of 100 ng/ml. The solution was added into a 24-well
Teflon™ culture plate and frozen at −20°C for 24 h, and
lyophilized at −80°C for 48 h (VFD-2000; Boyikang Laboratory
Instruments, Co., Ltd., Beijing, China) to form BMP-2-nHAP-COL
scaffolds. Scaffold morphology and microstructure was observed by
scanning electron microscopy (SEM).
Detection of BMP-2 release from
BMP-2-nHAP-COL scaffolds
A total of three standard BMP-2-nHAP-COL scaffolds
(BMP-2, 100 ng) were placed into a 24-well culture plate and 1X PBS
buffer (1 ml; pH, 7.4) was added into the wells prior to sealing
with a membrane. The plate was placed in an incubator at 37°C, and
PBS from the wells was collected at specific time points (1, 2 and
20 days). PBS samples were subsequently analyzed for BMP-2 content
with a BMP-2 ELISA kit (cat. no. YD-H010379; Yuduo Biotechnology
Company, Shanghai, China). The mean BMP-2 values were calculated to
determine the cumulative release of BMP-2 and a release curve was
subsequently drawn (22). The
experiment was repeated three times.
Animals and ethics statement
A total of eight Sprague-Dawley rats (four male and
four female, 4 weeks old, ~300 g) were obtained from the Center for
Experimental Animals at China Medical University (Shenyang, China;
National Animal Use License no. SCXK-LN2011-0009). Animals were
housed at a temperature of 20–26°C and a 12 h light/dark cycle with
unlimited access to food and water. Animal use was approved by the
Animal Use and Care Committee at China Medical University (protocol
no. CMU62043006). All experiments were approved by the Animal Care
and Use Committee at China Medical University, and complied with
the National Institutes of Health (Bethesda, MD, USA) Guide for the
Care and Use of Laboratory Animals. All efforts were made to
minimize the number of animals used and their suffering.
Isolation, culture and passage of
BMSCs
Rats were sacrificed with excess anesthesia and skin
was sterilized with 75% ethanol. Under aseptic conditions, the
femur and tibia were removed and placed in PBS solution. Following
ultraviolet disinfection, the PBS liquid was drained and the bone
was washed three times with PBS liquid containing penicillin and
streptomycin. The bone marrow cavity was exposed and 5.2 ml
α-Minimum Essential Medium (αMEM; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) containing 10% fetal bovine serum (FBS;
Hyclone; GE Healthcare Life Sciences), 1% penicillin and 1%
streptomycin was drawn through until the majority of the bone
marrow was flushed out. The cell suspension was washed and
precipitated with PBS three times, and transferred to a sterile
container. Cells were counted and seeded into culture dishes
according to the required density. αMEM containing 10% FBS, 1%
penicillin and 1% streptomycin was added, and cells were cultured
at 37°C in a 5% CO2 incubator. The medium was changed
every 24 h. Following adherence to the culture dish, cells were
digested using TrypLe Express enzyme (2.5–3.0 ml; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) for 10 min at 37°C and
αMEM containing 10% FBS was used to terminate the reaction. The
cell suspension was centrifuged at 800 × g for 5 min, and cells
were resuspended in fresh αMEM containing 10% FBS (both Hyclone; GE
Healthcare Life Sciences) and seeded into new culture medium. Cell
medium was changed daily and, once 80–90% confluence was reached,
cells were passaged at a ratio of 1:3. A light-inverted microscope
(cat. no. CKX41; Olympus Corporation, Tokyo, Japan) was used to
observe cell morphology.
BMSC identification
Third-generation BMSCs were used for identification.
The medium was discarded and cells were washed three times with
PBS. TrypLE Express was subsequently added to digest the cells at
37°C for 10 min and αMEM (Hyclone; GE Healthcare Life Sciences)
containing 10% FBS was used to terminate the reaction. The cell
suspension was collected and centrifuged at 500 × g at 4°C for 5
min. Cells were subsequently counted and adjusted to 106
cells/100 µl, and blocked using PBS containing 10% bovine serum
albumin (concentration, 1% w/v; Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) at 37°C. The cell suspension was
transferred to four round-bottom Falcon tubes and
allophycocyanin-labeled anti-cluster of differentiation (CD) 29
(cat. no. 10,225; 1:500; BioLegend, Inc., San Diego, CA, USA),
fluorescein isothiocyanate-labeled anti-CD44 (cat. no. 103,003;
1:500; BioLegend, Inc.), phycoerythrin (PE) -labeled anti-CD45
(cat. no. 103,111; 1:500; BioLegend, Inc.) and PE-labeled anti-CD34
(cat. no. bsm-10820M; 1:500; BIOSS, Beijing, China) antibodies were
added into the respective cell suspensions and incubated at 4°C for
20 min. A flow cytometer (FACSCanto II; BD Biosciences, Franklin
Lakes, NJ, USA) was used to detect the cell surface markers CD44,
CD29, CD45 and CD34 (23), and
Kaluza analysis software (v. 1.3; Beckman Coulter, Inc., Shanghai,
China) was used for analysis. The experiment was repeated three
times.
BMSC culture
Lyophilized BMP-2-nHAP-COL scaffolds were sterilized
with ethylene oxide, washed three times with PBS and αMEM and
subsequently soaked in 10% FBS overnight at 37°C. Individual
scaffolds were placed into wells of a 24-well cell culture plate
and inoculated with 1 ml BMSC suspension (2×105/ml). A
total of 100 µl of Dulbecco's Modified Eagle's Medium (Shanghai
Beinuo Biotechnology, Co., Ltd., Shanghai, China) was gently added
to the well around the scaffold and the cell-seeded scaffold was
cultured in a 5% CO2 incubator at 37°C (24).
BMSC morphology
Following 72 h of incubation, cell-scaffold samples
were collected, washed with PBS and fixed in 2.5% glutaraldehyde
solution overnight at 4°C. The samples were subsequently removed
and washed with PBS prior to dehydration in an ethanol gradient
(50, 70, 80, 90 and 100% for ~20 min each). Samples were air-dried
and sputter-coated with gold. SEM was used to observe the samples.
The experiment was repeated four times.
Adhesion of BMSCs
BMSCs (1×104/ml) were placed in culture
plates pre-coated with BMP-2-nHAP-COL or nHAP-COL scaffolds (1
ml/well). BMSCs placed in culture plates with no scaffolds were
used as a control. A total of six parallel wells were used for each
group and cells were cultured in an incubator at 37°C with 5%
CO2. Non-adherent cell numbers were quantified at 1, 2,
4, 8 and 24 h, and the adhesion rate was calculated according to
the following formula: Adhesion rate (%)=(number of seeded
cells-non-adhered cells)/(number of seeded cells) ×100 (25). The experiment was repeated three
times.
Cell Counting kit-8 (CCK-8) assay
A CCK-8 kit (Nanjing Jiancheng Bioengineering
Institute) was used to detect BMSC proliferation in each group. A
total of three samples per group were analyzed at 1, 3, 5 and 7
days post-inoculation. In each group, 100 µl CCK-8 solution was
added to each well, and the culture plate was placed into an
incubator at 37°C and 5% CO2 for 4 h. A sample of this
liquid (300 µl) was subsequently drawn from each well and added to
a 96-well culture plate. Absorbance values were measured at a
wavelength of 450 nm using a microplate reader (Bio-Rad 550;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) and the average
value of the three samples was calculated. Optical density (OD) at
450 nm was proportional to the number of cells (26). The experiment was repeated three
times.
Alkaline phosphatase (ALP)
activity
A total of three samples per group were analyzed for
ALP activity at 1, 4, 7 and 10 days following initial culture using
an ALP ELISA kit (cat. no. A059-1; Nanjing Jiancheng Bioengineering
Institute). Samples were washed three times in PBS and immersed in
1 ml 0.1% Triton X-100. Cells were lysed by placing the culture
plate in a refrigerator overnight at 4°C. The cell suspension was
further lysed by repeat pipetting and 30 µl suspension was
subsequently transferred to a 96-well plate. Buffer solution (50
µl) and matrix liquid were placed in a water bath at 37°C for 15
min prior to mixing fully. Chromogenic agent (150 µl) was added to
each well and the plate was oscillated. The OD was measured at 520
nm (27). The experiment was
repeated three times.
Statistical analysis
All quantitative data are expressed as the mean ±
standard deviation. Statistical analyses were performed using SPSS
17 (SPSS, Inc., Chicago, IL, USA). The results were analyzed using
either the Student's t-test or one-way analysis of variance (ANOVA)
followed by Scheffé's post hoc test. P<0.05 was considered to
indicate a statistically significant difference. Prior to analysis
with one-way ANOVA, all quantitative data were confirmed to be
normally distributed.
Results
BMP-2-nHAP-COL scaffold
characterization
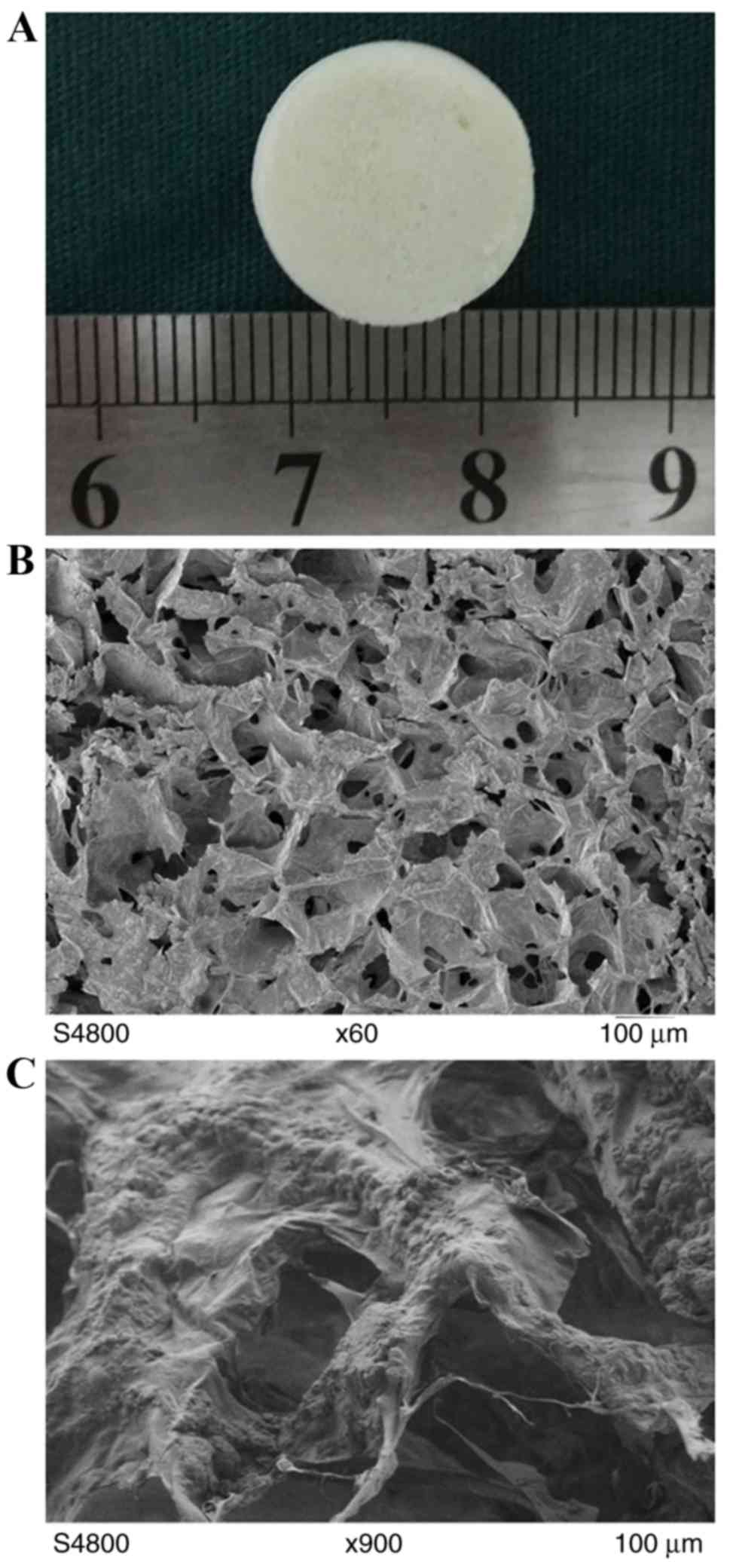
The scaffold was white with a slightly rough surface
and good flexibility. The original state was able to be gradually
restored following compression deformation (Fig. 1A). The scaffold had a
three-dimensional porous structure with a large number of nHAP
particulates adhered on the COL surface. The aperture was 80–200 µm
and the pores were interconnected with no clear fixed direction.
The thickness of the pore wall was 1–2 µm, and nHAP and COL were
well combined (Fig. 1B and C).
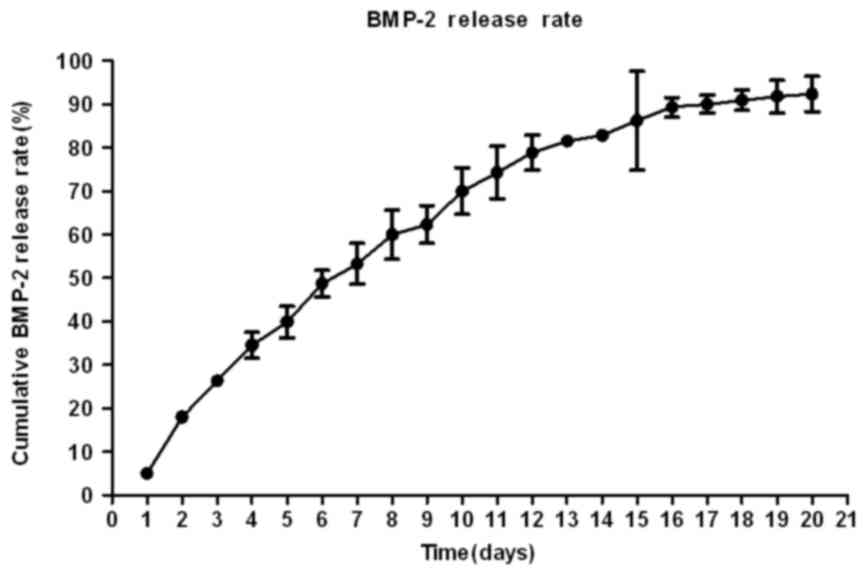
Release of BMP-2 from the
BMP-2-nHAP-COL scaffold
BMP-2 release from the scaffold was detected for 19
days. On day 20, a negligible amount of BMP-2 (<1 ng) was
detected in the supernatant of each sample, and the cumulative
release of BMP-2 from the scaffold material was 90.05±2.08%. The
rate of release was significantly faster in the first few days and
the release curve gradually leveled off prior to slowing (Fig. 2).
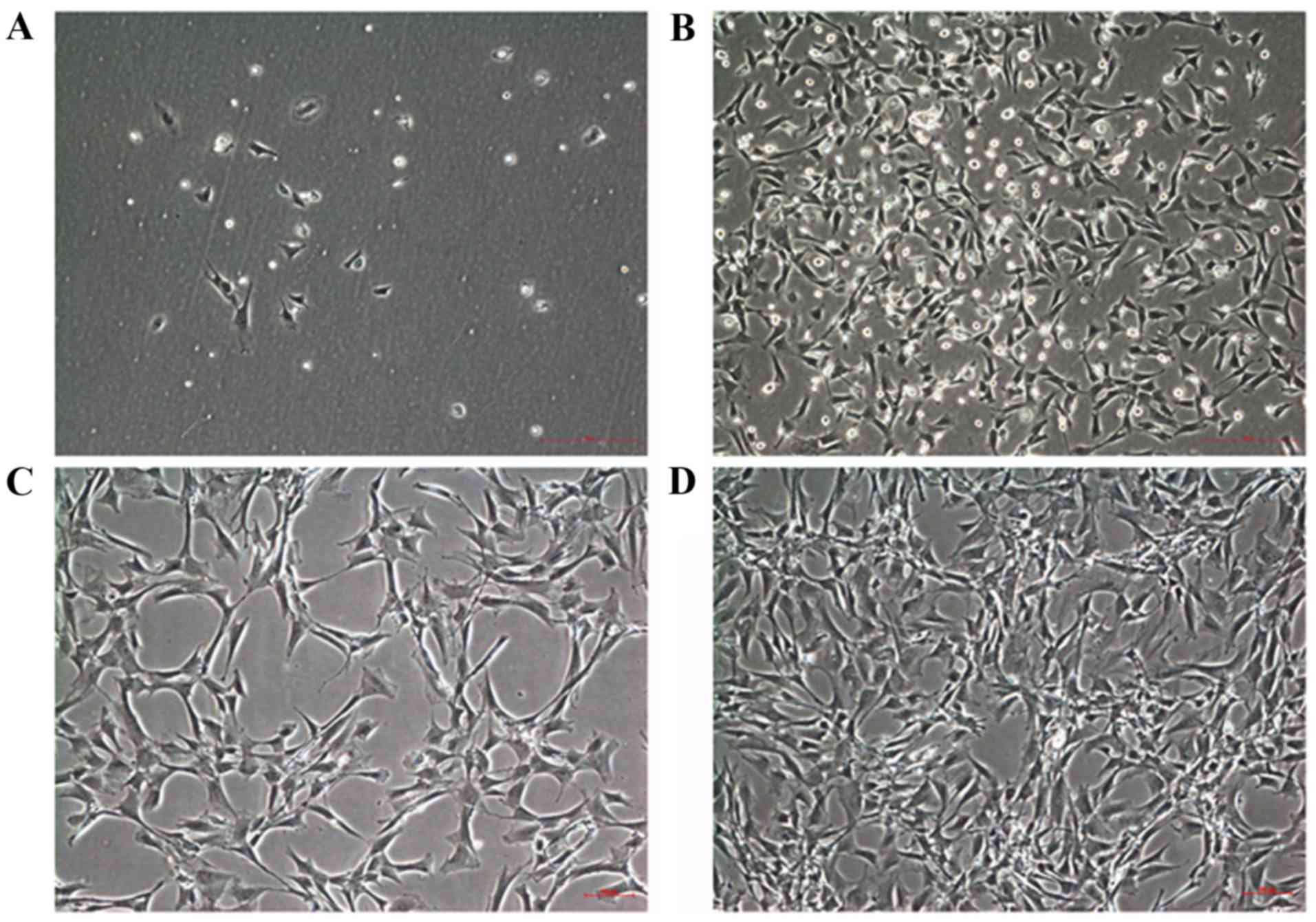
Cell morphology
Primary BMSCs were inoculated for 12 h and gradually
began to adhere to the scaffold. A small number of BMSCs were
deformed after 1 day and were spindle- or polygonal-shaped. A
number of non-adherent red blood cells were additionally observed
(Fig. 3A). Non-adherent and
slowly-adhering cells were removed following the first media
exchange. Approximately 5 days subsequent to the initial culture, a
large number of spindle and polygonal-shaped BMSCs were observed,
combined with a small number of round cells. Additionally,
fibroblast-like and macrophage-like cells were present (Fig. 3B). Cell impurity decreased
significantly following the first passage, and cells adhered to the
culture wall and deformed on day 1 (Fig. 3C). Cell density increased on day 3
following the second passage and BMSCs were approximately 80–90%
confluent on day 5 (Fig. 3D).
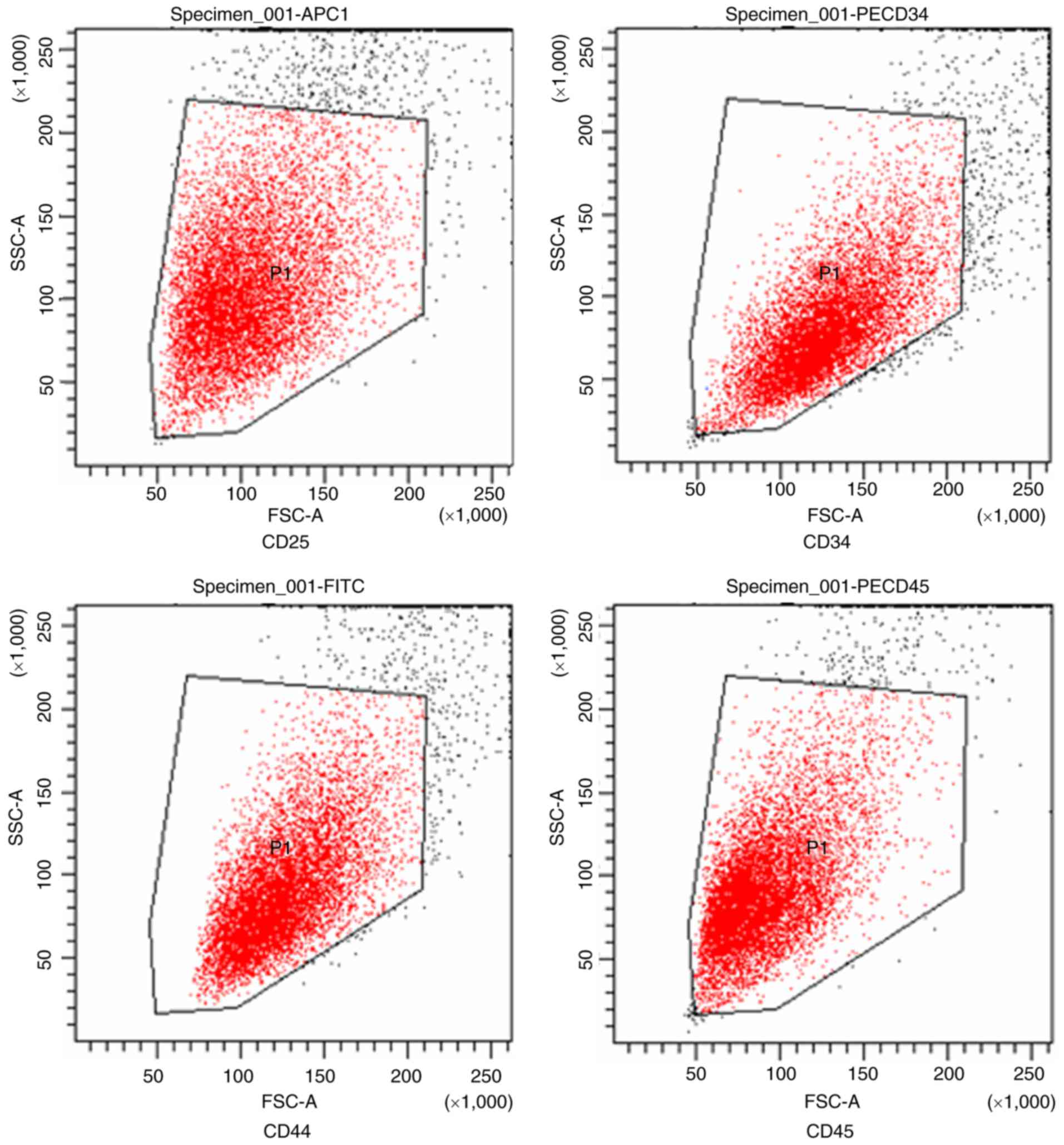
BMSC identification
Flow cytometry for cell surface markers was
performed on third-generation BMSCs. CD45, CD34, CD44 and CD29
expression levels were determined to be 4.4, 6.8, 94 and 100%,
respectively. These results are consistent with flow cytometry BMSC
standards (CD45 and CD34, <10%; CD44 and CD29, >90%; Fig. 4) (23).
BMP-2-nHAP-COL scaffold
biocompatibility
SEM analysis identified a random distribution of
cells on the scaffold surface. At 7 days, an increased number of
cells had adhered to the scaffold surface and the cells were
observed to grow and proliferate well. Typical BMSC morphology was
observed and cells adhered tightly to the scaffold surface via
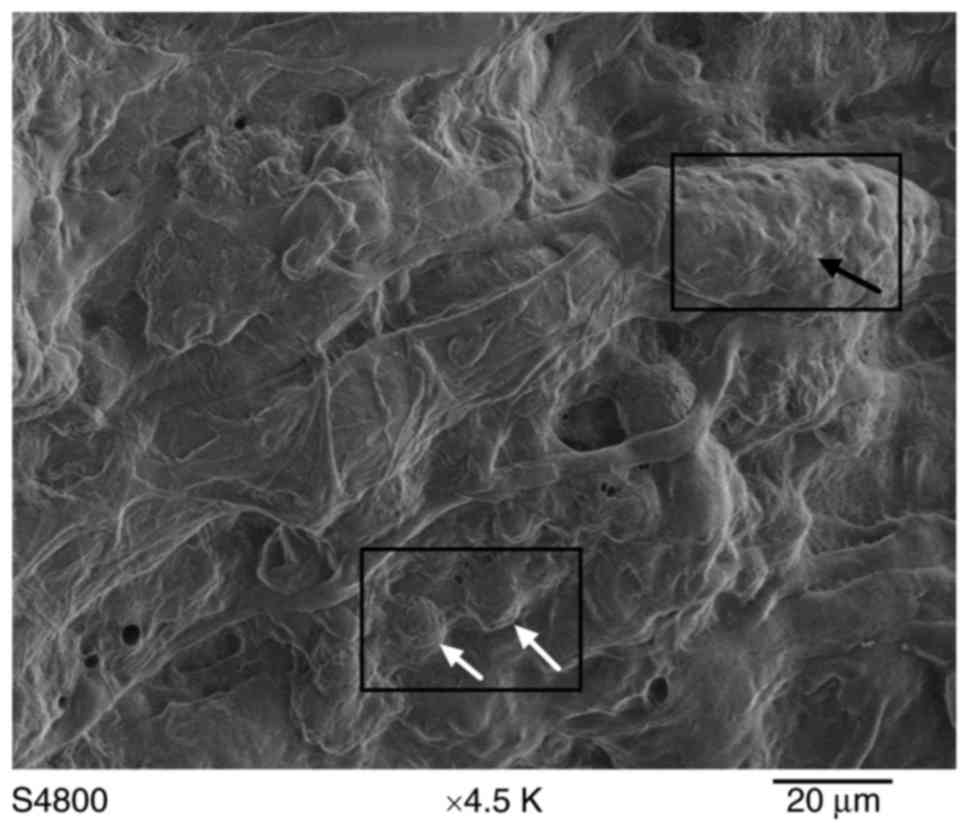
lamellipodia and filopodia, indicative of cell spreading (Fig. 5).
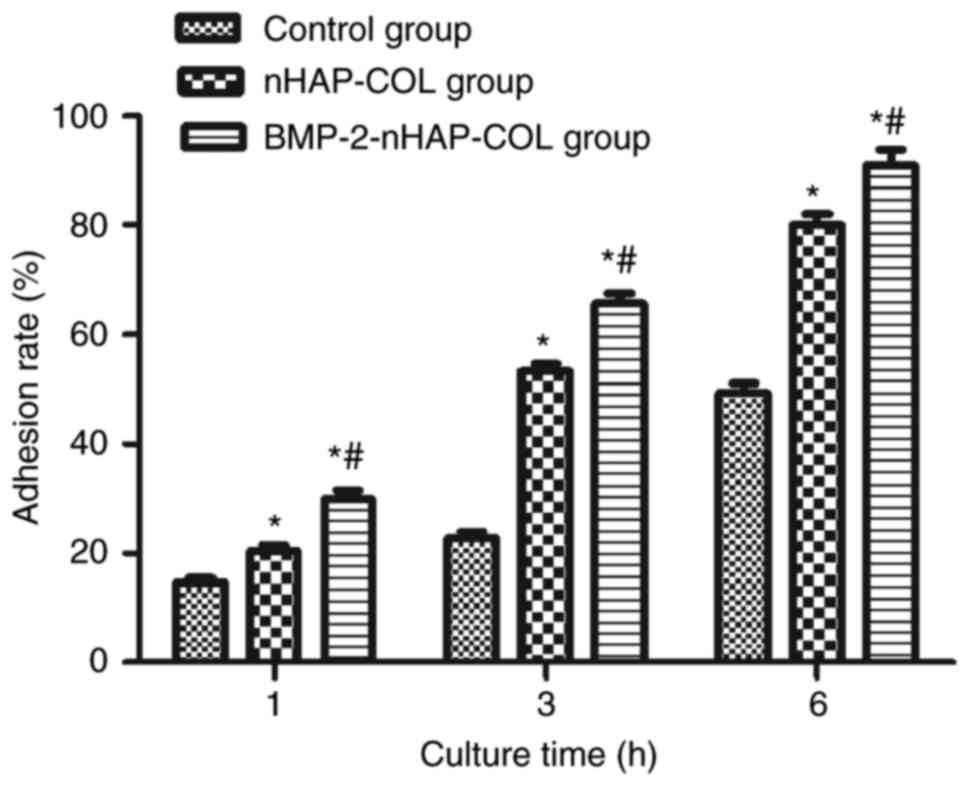
BMP-2 promotes BMSC adhesion
The cell adhesion rate was enhanced with increased
incubation time in all three groups. The adhesion rate was higher
in the scaffold groups compared with the control group (P<0.05)
and was higher in the BMP-2-nHAP-COL group compared with the
nHAP-COL group (P<0.05) following culture for 1, 3 and 6 h. The
adhered cell number was significantly increased following 3 and 6 h
compared with 1 h (P<0.05). These results indicated that BMP-2
increased BMSC adhesion (Fig.
6).
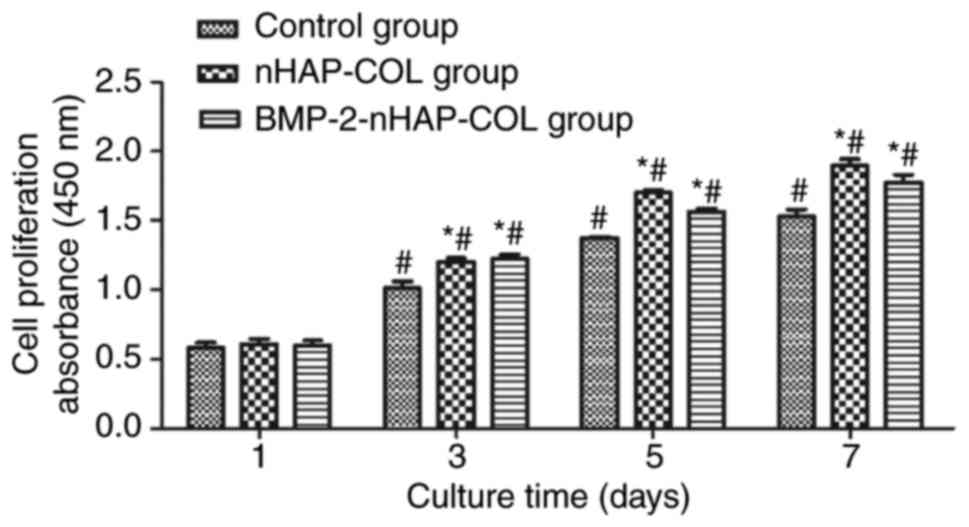
BMSC proliferation is not influenced
by BMP-2
BMSC proliferation at days 1, 3, 5 and 7 in the
BMP-2-nHAP-COL, nHAP-COL and control groups was compared using the
CCK-8 assay. Absorbance values increased with time in the scaffold
groups, indicating significant cell growth within the scaffolds. No
significant difference was detected between the scaffold groups and
the control group at day 1 (P>0.05). However, cell numbers in
the scaffold groups were higher compared with the control group at
day 3 (P<0.05). There was no significant difference detected
between the BMP-2-nHAP-COL and nHAP-COL groups (P>0.05),
indicating that BMP-2 did not increase BMSC proliferation (Fig. 7).
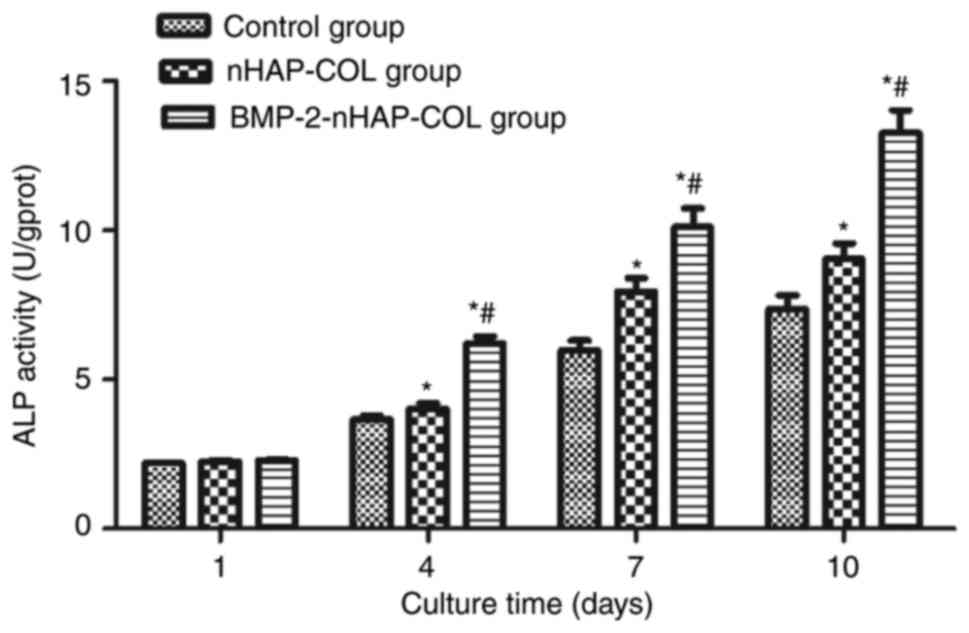
BMP-2 promotes ALP activity in
BMSCs
The ALP activity of BMSCs cultured in the scaffold
and control groups are presented in Fig. 8. No significant differences in the
OD values were identified between the scaffold groups and the
control group (P>0.05) during the first 4 days. However, OD
values were significantly higher in the scaffold groups compared
with the control group between 4 and 10 days (P<0.05).
Furthermore, ALP activity was higher in the BMP-2-nHAP-COL group
compared with the nHAP-COL group, suggesting that the nHAP-COL
scaffold enhanced ALP expression in BMSCs, and that BMP-2 further
enhanced this effect (Fig. 8).
Discussion
COL is a primary component of the extracellular
matrix that has been widely used in constructive remodeling to
facilitate cell growth and differentiation. The widespread use of
COL across numerous clinical applications is due to its desirable
bioinductive, mechanical and degradable properties (28). In the process of constructing
scaffolds and load factors, collagen was repeatedly dried, which
attenuated the decrease of the immunogenicity of COL (29). HAP is a biocompatible material with
osteoconductive properties. It is available in various forms that
determine its bone formation and graft incorporation properties,
accordingly. Unfortunately, bone-graft substitutes consisting
solely of particles are mechanically weak and particles may migrate
from the graft site prior to the ingrowth of new bone tissue that
secures them in place (30).
Nano-scale biomaterials have gained attention from the research
community due to their desirable biological and biomechanical
properties (31). The nHAP-COL
scaffold provides a good, spongy, porous structure that meets the
criteria for an ideal scaffold material, providing a
three-dimensional space for cell nutrient transfer (32). The nHAP particles increase the
surface roughness, thus increasing the surface area of the scaffold
to improve cell adhesion. Li et al (33) used electrospinning to combine BMP-2
with silk fibroin fiber and nHAP, producing a beneficial effect on
the osteogenic differentiation of BMSCs. The present study used
freeze drying to directly combine BMP-2 with the nHAP-COL scaffold.
The rate of BMP-2 release compared with the amount of BMP-2 in the
scaffold following lyophilization revealed that the method resulted
in no significant loss of BMP-2 during the production process. The
sustained release time following lyophilization additionally met
tissue engineering requirements. A number of disinfection methods,
including alcohol immersion, ultraviolet irradiation and ethylene
oxide, were used in the previous experiment. However, each method
resulted in different activity loss due to different disinfection
times (Tong et al, unpublished). Ethylene oxide disinfection
had the lowest rate of BMP-2 activity loss at ~10%, and subsequent
experimental steps confirmed its rationality. No further
experiments were performed to demonstrate factor concentration and
specific disinfection. This may be addressed in future
experiments.
Artificially-extracted BMSCs were mixed with
fibroblast and macrophage-like cells. BMSCs were digested with
TrypLe Express enzyme and subsequently purified. Flow cytometry
analysis revealed that the isolated and purified cells met the
requirements for application in further experiments. SEM images
confirmed successful BMSC growth on the scaffolds. The cell
adhesion rate assay confirmed that the cells adhered well to the
scaffold and that BMP-2 enhanced this adherence rate. There are
numerous methods of detecting cell proliferation, including CCK-8,
MTT, proliferating cell nuclear antigen and Ki67. The CCK-8 assay
was used in the present study. The result proved the effect of the
composite scaffold on promoting proliferation. However, at a
concentration of 10 ng/µl, BMP-2 had no further role in
proliferation promotion. The ALP assay confirmed that the nHAP-COL
scaffold promoted BMSC differentiation and that this effect was
enhanced further by the presence of BMP-2 in the scaffold. Although
a number of reports have used COL or nHAP as a tissue engineering
material (34), to the best of our
knowledge, a combination of these two materials with BMP-2 has not
yet been achieved by lyophilization. The process of freeze-drying
rarely causes a loss of growth factor efficiency (35). Therefore, it may be assumed that
scaffold implantation into the body may facilitate sustained BMP-2
release from the scaffold and may contribute to enhanced bone
formation.
In conclusion, construction of the BMP-2-nHAP-COL
scaffold using a freeze-drying method enables good biocompatibility
in vitro. On this basis, further research may be performed
to develop a more optimal bone tissue engineering scaffold.
Acknowledgements
The present study was supported by the Science and
Technology Plan Project of Liaoning Province (grant no.
2012-B-00002012225082).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou H and Xu HH: The fast release of stem
cells from alginate-fibrin microbeads in injectable scaffolds for
bone tissue engineering. Biomaterials. 32:7503–7513. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mao JJ, Vunjak-Novakovic G, Mikos AG and
Atala A: Regenerative medicine: Translational approaches and tissue
engineering. Artech House; Boston, MA: 2007
|
|
3
|
Boccaccio A, Ballini A, Pappalettere C,
Tullo D, Cantore S and Desiate A: Finite element method (FEM),
mechanobiology and biomimetic scaffolds in bone tissue engineering.
Int J Biol Sci. 7:112–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu X and Ma PX: Polymeric scaffolds for
bone tissue engineering. Ann Biomed Eng. 32:477–486. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma L, Gao C, Mao Z, Zhou J and Shen J:
Biodegradability and cell-mediated contraction of porous collagen
scaffolds: The effect of lysine as a novel crosslinking bridge. J
Biomed Mater Res A. 71:334–342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harley BA, Leung JH, Silva EC and Gibson
LJ: Mechanical characterization of collagen-glycosaminoglycan
scaffolds. Acta Biomater. 3:463–474. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ikada Y: Challenges in tissue engineering.
J R Soc Interface. 3:589–601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Appleford MR, Oh S, Oh N and Ong JL: In
vivo study on hydroxyapatite scaffolds with trabecular architecture
for bone repair. J Biomed Mater Res A. 89:1019–1027. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karageorgiou V and Kaplan D: Porosity of
3D biomaterial scaffolds and osteogenesis. Biomaterials.
26:5474–5491. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
King WJ and Krebsbach PH: Growth factor
delivery: How surface interactions modulate release in vitro and in
vivo. Adv Drug Deliv Rev. 64:1239–1256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hatakeyama W, Taira M, Chosa N, Kihara H,
Ishisaki A and Kondo H: Effects of apatite particle size in two
apatite/collagen composites on the osteogenic differentiation
profile of osteoblastic cells. Int J Mol Med. 32:1255–1261. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng P, Niu M, Gao C, Peng S and Shuai C:
A novel two-step sintering for nano-hydroxyapatite scaffolds for
bone tissue engineering. Sci Rep. 4:55992014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He Y, Dong Y, Cui F, Chen X and Lin R:
Ectopic osteogenesis and scaffold biodegradation of
nano-hydroxyapatite-chitosan in a rat model. PLoS One.
10:e01353662015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu J, Zhou Y, Huang L, Liu J and Lu H:
Effect of nano-hydroxyapatite coating on the osteoinductivity of
porous biphasic calcium phosphate ceramics. BMC Musculoskelet
Disord. 15:1142014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nukavarapu SP, Kumbar SG, Brown JL,
Krogman NR, Weikel AL, Hindenlang MD, Nair LS, Allcock HR and
Laurencin CT: Polyphosphazene/nano-hydroxyapatite composite
microsphere scaffolds for bone tissue engineering.
Biomacromolecules. 9:1818–1825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ganesh N, Ashokan A, Rajeshkannan R,
Chennazhi K, Koyakutty M and Nair SV: Magnetic resonance functional
nano-hydroxyapatite incorporated poly (caprolactone) composite
scaffolds for in situ monitoring of bone tissue regeneration by
MRI. Tissue Eng Part A. 20:2783–2794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vanhatupa S, Ojansivu M, Autio R, Juntunen
M and Miettinen S: Bone morphogenetic protein-2 induces
donor-dependent osteogenic and adipogenic differentiation in human
adipose stem cells. Stem Cells Transl Med. 4:1391–1402. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang YK, Yu X, Cohen DM, Wozniak MA, Yang
MT, Gao L, Eyckmans J and Chen CS: Bone morphogenetic
protein-2-induced signaling and osteogenesis is regulated by cell
shape, RhoA/ROCK and cytoskeletal tension. Stem Cells Dev.
21:1176–1186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Castro-Govea Y, Cervantes-Kardasch VH,
Borrego-Soto G, Martínez-Rodríguez HG, Espinoza-Juarez M,
Romero-Díaz V, Marino-Martínez IA, Robles-Zamora A, Álvarez-Lozano
E, Padilla-Rivas GR, et al: Human bone morphogenetic protein
2-transduced mesenchymal stem cells improve bone regeneration in a
model of mandible distraction surgery. J Craniofac Surg.
23:392–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vilquin JT and Rosset P: Mesenchymal stem
cells in bone and cartilage repair: Current status. Regen Med.
1:589–604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujioka-Kobayashi M, Sawada K, Kobayashi
E, Schaller B, Zhang Y and Miron RJ: Recombinant human bone
morphogenetic protein 9 (rhBMP9) induced osteoblastic behavior on a
collagen membrane compared with rhBMP2. J Periodontol.
87:e101–e107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su J, Xu H, Sun J, Gong X and Zhao H: Dual
delivery of BMP-2 and bFGF from a new nano-composite scaffold,
loaded with vascular stents for large-size mandibular defect
regeneration. Int J Mol Sci. 14:12714–12728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song K, Huang M, Shi Q, Du T and Cao Y:
Cultivation and identification of rat bone marrow-derived
mesenchymal stem cells. Mol Med Rep. 10:755–760. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tong S, Xue L, Xu DP, Liu ZM, Du Y and
Wang XK: In vitro culture of hFOB1.19 osteoblast cells on
TGF-β1-SF-CS three dimensional scaffolds. Mol Med Rep. 13:181–187.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z, Lin M, Xie Q, Sun H, Huang Y,
Zhang D, Yu Z, Bi X, Chen J, Wang J, et al: Electrospun silk
fibroin/poly (lactide-co-ε-caprolactone) nanofibrous scaffolds for
bone regeneration. Int J NanoMedicine. 11:1483–1500.
2016.PubMed/NCBI
|
|
26
|
Zhang H, Ma X, Zhang L, Guan X, Bai T and
Xue C: The ability to form cartilage of NPMSC and BMSC in SD rats.
Int J Clin Exp Med. 8:4989–4996. 2015.PubMed/NCBI
|
|
27
|
Gao P, Zhang H, Liu Y, Fan B, Li X, Xiao
X, Lan P, Li M, Geng L, Liu D, et al: Beta-tricalcium phosphate
granules improve osteogenesis in vitro and establish innovative
osteo-regenerators for bone tissue engineering in vivo. Sci Rep.
6:233672016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chan EC, Kuo SM, Kong AM, Morrison WA,
Dusting GJ, Mitchell GM, Lim SY and Liu GS: Three Dimensional
Collagen Scaffold Promotes Intrinsic Vascularisation for Tissue
Engineering Applications. PLoS One. 11:e01497992016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu C, Lu W, Bian S, Liang J, Fan Y and
Zhang X: Porous collagen scaffold reinforced with surfaced
activated PLLA nanoparticles. Scientific World Journal.
2012:6951372012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee MJ, Sohn SK, Kim KT, Kim CH, Ahn HB,
Rho MS, Jeong MH and Sun SK: Effect of hydroxyapatite on bone
integration in a rabbit tibial defect model. Clin Orthop Surg.
2:90–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xia Y, Zhou P, Cheng X, Xie Y, Liang C, Li
C and Xu S: Selective laser sintering fabrication of
nano-hydroxyapatite/poly-ε-caprolactone scaffolds for bone tissue
engineering applications. Int J Nanomedicine. 8:4197–4213.
2013.PubMed/NCBI
|
|
32
|
Zeng S, Liu L, Shi Y, Qiu J, Fang W, Rong
M, Guo Z and Gao W: Characterization of silk fibroin/chitosan 3d
porous scaffold and in vitro cytology. PLoS One. 10:e01286582015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li C, Vepari C, Jin HJ, Kim HJ and Kaplan
DL: Electrospun silk-BMP-2 scaffolds for bone tissue engineering.
Biomaterials. 27:3115–3124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Polo-Corrales L, Latorre-Esteves M and
Ramirez-Vick JE: Scaffold design for bone regeneration. J Nanosci
Nanotechnol. 14:15–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang W: Lyophilization and development of
solid protein pharmaceuticals. Int J Pharm. 203:1–60. 2000.
View Article : Google Scholar : PubMed/NCBI
|