Introduction
Skeletal muscle, the most abundant tissue in the
body, contributes not only to mobility and movement but also to
glucose and lipid metabolism. The loss of skeletal muscle mass
causes decreased locomotion and decreased energy expenditure,
resulting in a higher risk of metabolic diseases such as obesity
and type 2 diabetes (1,2). When skeletal muscle is damaged by
intense resistance training or traumatic injury, regeneration of
muscle cells occurs by highly orchestrated processes (3). The myogenic precursor satellite cells
proliferate and then differentiate into myoblasts. Subsequently,
the mononucleated myoblasts proliferate and differentiate, and then
fuse with each other and pre-existing myofibers, resulting in the
formation of multinucleated myotubes and myofibers. Likewise, the
proliferation and differentiation of muscle cells occurs during
developmental and postnatal myogenesis (4). The activation of myogenic regulatory
factors such as MyoD and myogenin regulates the expression of
myosin heavy chain (MyHC), which is a myotube-specific structural
protein (5). On the other hand, an
increase in the size of individual myotubes and myofibers, called
hypertrophy, causes an increase in skeletal muscle mass (4). Therefore, promotion of myoblast
proliferation and differentiation and an induction of myotube
hypertrophy should be beneficial for muscle regeneration and muscle
mass regulation.
Lactoferrin (Lf) is a multifunctional non-heme
iron-binding glycoprotein, which is present in exocrine fluids such
as saliva, tears, and bile (6).
Neutrophils release Lf into circulation during inflammation
(7), and it exerts antioxidant
(8), anti-infective (9), anti-inflammatory (10), and anti-cancer effects (11,12).
Serum Lf levels are in the range of 2–7 and 1–60 µg/ml in healthy
subjects and patients with rheumatoid arthritis, respectively
(7). Lf levels in synovial fluid
are in the range of 1–100 µg/ml in osteoarthritic patients,
suggesting that local Lf levels are higher during inflammation.
Furthermore, the serum Lf level increases immediately after running
exercise (13). Several receptors
for Lf have been identified on the surface of various cells, and Lf
binds to intelectin (14),
low-density lipoprotein receptor-related protein (LRP)1 (15), nucleolin (16), and Toll-like receptor (TLR)4
(17).
Lf is known to promote the proliferation of murine
C2C12 myoblasts (18), but its
underlying mechanism remains unclear. Furthermore, Lf induces the
conversion of murine C2C12 myoblasts into cells that proceed along
the osteoblastic and chondroblastic lineages (18). Given that chondrogenic, osteogenic,
adipogenic, and myogenic lineages can originate from common
progenitor cells (19), it is of
interest whether Lf has any effect on myoblast differentiation.
Here, we provide evidence that Lf promotes myoblast proliferation
by activating the extracellular signal-regulated kinase (ERK)1/2
signaling pathway, at least partially through LRP1, and induces the
differentiation of myoblasts into myotubes. Moreover, we found that
Lf promotes myotube hypertrophy.
Materials and methods
Animals
All animals were cared for in accordance with the
guidelines of the Animal Care and Use Committee of Osaka Prefecture
University, which also provided ethical approval for the present
study (approval no. 28-185). Male Kwl:ddY mice were obtained from
Kiwa Laboratory Animals (Wakayama, Japan) and had free access to
water and a rodent diet. The mice were housed under controlled
temperature (23±2°C), humidity (60±10%), and light (a 12 h
light-dark cycle starting at 08:00 a.m.) conditions.
Cell culture
Murine myoblast C2C12 cells were obtained from RIKEN
Cell Bank (Tsukuba, Japan) and were maintained as described
previously (20). In brief, cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin, and 100 µg/ml streptomycin (growth medium) at 37°C in
5% CO2 and 95% air at 100% humidity. For proliferation
assays, cells were cultured in DMEM supplemented with 2% FBS and
the antibiotics mentioned above; this medium was termed the
proliferation medium and allowed the myoblasts to proliferate but
not differentiate, in the present study. For the induction of
differentiation, confluent cells were cultured in DMEM supplemented
with 2% horse serum and the antibiotics mentioned above
(differentiation medium).
alamarBlue cell viability assay
Cell viability was determined using the alamarBlue
cell viability reagent (Trek Diagnostic Systems, Cleveland, OH,
USA) (21). For growth curve
experiments, myoblasts were seeded onto 48-well plates at a density
of 0.5×103 cells/cm2 and cultured for six
days. For the determination of cell viability, myoblasts were
seeded onto 48-well plates at a density of 2.0×103
cells/cm2 and cultured for three days. After one day,
this time point was denoted as day 0. Cells were cultured in fresh
proliferation medium supplemented with Lf (iron saturated; approx.
20%) (Morinaga Milk Industry Co., Ltd., Tokyo, Japan) in the
presence or absence of the mitogen-activated protein kinase kinase
(MEK)1/2 inhibitor U0126 (2.5 µM) or the LRP1 inhibitor
receptor-associated protein (RAP, 50 nM) for three days. Before
determination, cells were incubated in phenol red-free
proliferation medium supplemented with Lf for 1 h, followed by
incubation in the above medium containing 5% alamarBlue reagent for
3 h. The fluorescence intensity of the medium was determined by
FluoroSkan Ascent FL (Labsystems, Helsinki, Finland) at an
excitation wavelength of 544 nm and an emission wavelength of 590
nm. Data are expressed as relative values (fluorescence intensity
of the experimental group divided by fluorescence intensity of the
vehicle group at day 0 or at the same time point).
Western blot analysis
For the determination of ERK1/2 signaling, myoblasts
were cultured in serum-free DMEM for one day and incubated with Lf
for the indicated time periods. For the determination of MyHC
expression, myoblasts were cultured in the differentiation medium.
Cells were sonicated in cell lysis buffer (50 mM Tris-HCl, pH 7.5,
containing 150 mM NaCl, 2 mM ethylenediaminetetraacetic acid, 1 mM
dithiothreitol, 1% Triton X-100, 1% sodium deoxycholate, 0.1%
sodium dodecyl sulfate (SDS), 1 µg/ml aprotinin, 10 µg/ml
leupeptin, and 1 mM phenylmethylsulfonyl fluoride) and centrifuged
at 20,000 × g for 15 min. The supernatants were subjected to
SDS-polyacrylamide gel electrophoresis, followed by western blot
analysis with the following primary antibodies: rabbit polyclonal
anti-p-ERK1/2 (Thr202/Thr204; Cell Signaling Technology, Inc.,
Danvers, MA, USA), anti-ERK1/2 (Cell Signaling Technology, Inc.),
rabbit monoclonal anti-LRP1 (Abcam, Cambridge, UK), mouse
monoclonal anti-MyHC (clone MF20; Developmental Studies Hybridoma
Bank, University of Iowa, Iowa city, IA, USA), and anti-β-actin
(Abgent, San Diego, CA, USA). The primary antibodies were detected
using the suitable horseradish peroxidase-conjugated secondary
antibodies (goat anti-mouse or goat anti-rabbit) and the Immobilon
Western Chemiluminescent HRP substrate (EMD Millipore, Billerica,
MA, USA), and exposed to a luminescent image analyzer (LAS-4000 IR
multicolor; Fujifilm Life Sciences, Tokyo, Japan).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from C2C12 myoblasts and
myotubes, murine fresh skeletal muscle (gastrocnemius, soleus, and
extensor digitorum longus), and small intestinal mucosa, and cDNA
was synthesized. The resulting cDNA was amplified by PCR using the
following specific primers: Primers for Itln1 (forward
5′-TGACAATGGCCCAGCATTACC-3′ and reverse
5′-TGACAATGGCCCAGCATTACC-3′); for Lrp1 (forward
5′-ACTATGGATGCCCCTAAAACTTG-3′ and reverse
5′-GCAATCTCTTTCACCGTCACA-3′); for nucleolin (forward
5′-GCACTTGGAGTGGTGAATCAAA-3′ and reverse
5′-AAATGCATACCCTTTAGGTTTGCC-3′); for Tlr4 (forward
5′-GCAGAAAATGCCAGGATGATG-3′ and reverse
5′-AACTACCTCTATGCAGGGATTCAAG-3′); for Myod (forward
5′-TGGGATATGGAGCTTCTATCGC-3′ and reverse
5′-GGTGAGTCGAAACACGGATCAT-3′); for myogenin (forward
5′-CATCCAGTACATTGAGCGCCTA-3′ and reverse
5′-GAGCAAATGATCTCCTGGGTTG-3′); and for Actb (forward
5′-GTGGGCCGCCCTAGGCACCA-3′ and reverse
5′-CTCTTTGATGTCACGCACGATTTC-3′).
siRNA-mediated knockdown
The siRNA duplexes targeting murine LRP1 (siLRP1)
and control siRNA (MISSION siRNA Universal Negative Control) were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The
siLRP1 sequence was as follows: 5′-CCAUGUUUGUGACCCGAAUdTdT-3′. The
siRNA duplexes (20 nM) were transfected into myoblasts using
Lipofectamine RNAiMAX reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 6 h, according to the
manufacturer's transfection protocol.
Immunofluorescence microscopy
Myotubes were fixed in 4% paraformaldehyde in
phosphate-buffered saline (PBS), as described previously (22). Fixed cells were permeabilized with
0.1% Triton X-100 in PBS, blocked with blocking solution (10% FBS
and 5% bovine serum albumin in PBS), and incubated with mouse
monoclonal anti-MyHC antibodies. This was followed by further
incubation with Alexa 488-conjugated anti-mouse IgG. The nuclei
were stained with 4′,6-diamidino-2-phenylindole dihydrochloride
(DAPI) in PBS for 10 min. Fluorescent images were analyzed by a
BIOREVO BZ-9000 fluorescence microscope (Keyence, Osaka, Japan).
The fusion index was calculated as follows: The number of nuclei
within MyHC-positive myotubes containing two or more nuclei was
divided by the total number of nuclei in five random fields, and
the resulting number was termed the fusion ratio. The fusion ratio
in Lf-treated cells was normalized to the fusion ratio in
vehicle-treated cells, resulting in the fusion index. The diameters
of the short axis of myotubes were measured in five random fields.
The mean myotube diameter was determined from more than 20 myotubes
for each sample.
Statistical analysis
Data were analyzed by one-way or two-way ANOVA
followed by Tukey's post-hoc test for multiple comparison analysis.
Statistical analysis was performed using JMP statistical software,
version 8.0.1 (SAS Institute, Cary, NC, USA). Data are expressed as
the mean ± standard deviation (SD), and P<0.05 was considered to
indicate a statistically significant difference.
Results
Involvement of ERK1/2 signaling in
Lf-promoted proliferation of C2C12 cells
C2C12 myoblasts were cultured in the presence of Lf
at various concentrations (0, 1, 10, 50, and 100 µg/ml) for six
days. Lf promoted cell proliferation in a dose- and time-dependent
manner (Fig. 1A), consistent with
previous results (18). When cell
proliferation was determined at the same time points, Lf increased
cell proliferation at day 2 and 4 at concentrations of 50 µg/ml or
higher and at day 6 at concentrations of 10 µg/ml or higher
(Fig. 1B). Lf stimulated cell
proliferation at concentrations of 10 µg/ml or higher when cells
were cultured in the presence of Lf at various concentrations for
three days (Fig. 1C, left panel).
Likewise, Lf enhanced cell proliferation at concentrations of 10
µg/ml or higher when cells were cultured in the presence of Lf for
the first day and in the absence of Lf for the next two days
(Fig. 1C, right panel). These
results indicate that Lf promotes myoblast proliferation and that
Lf stimulation only in the early period (i.e., the first day) is
sufficient to promote cell proliferation. Next, we assessed what
signaling is required for Lf-promoted myoblast proliferation.
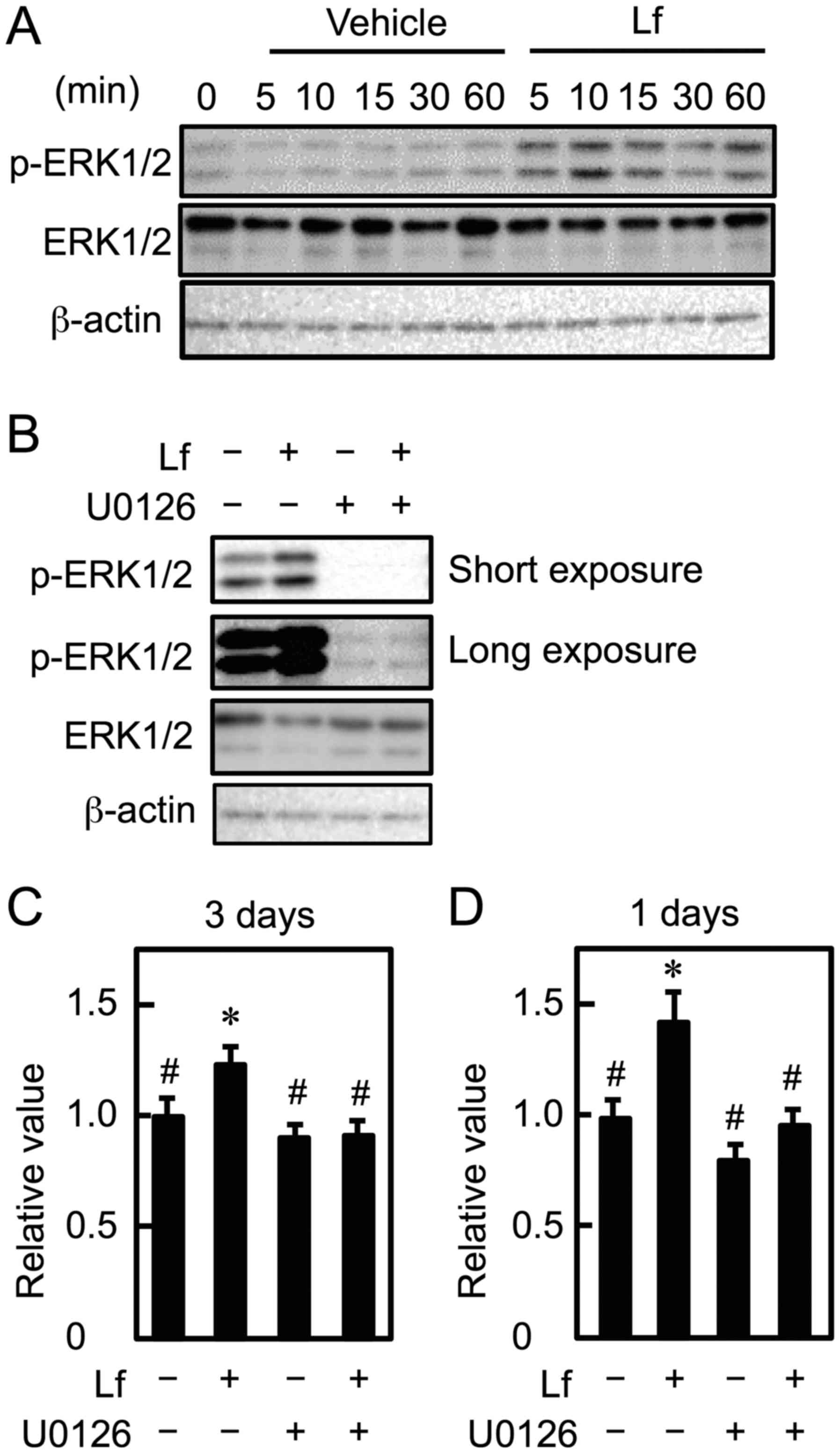
Activation of MEK1/2 causes the phosphorylation of 44 kDa ERK1 and
42 kDa ERK2, which are homologous isoforms. Lf increased ERK1/2
phosphorylation within 5 min in C2C12 myoblasts (Fig. 2A). Furthermore, to determine
whether the ERK1/2 signaling pathway is involved in Lf-stimulated
myoblast proliferation, myoblasts were cultured with Lf in the
presence of U0126 (2.5 µM). Lf-stimulated ERK1/2 activation was
inhibited by U0126, and U0126 decreased basal phosphorylation
levels of ERK1/2 (Fig. 2B). Next,
we determined the effects of U0126 on myoblast proliferation when
cells were cultured in the presence of Lf for three days. The
proliferation-promoting effect of Lf on myoblasts was abolished
following U0126 treatment, regardless of the length of the
incubation time with Lf (Fig. 2C and
D). These results suggest that Lf increases myoblast
proliferation by activating the MEK1/2-ERK1/2 signaling
pathway.
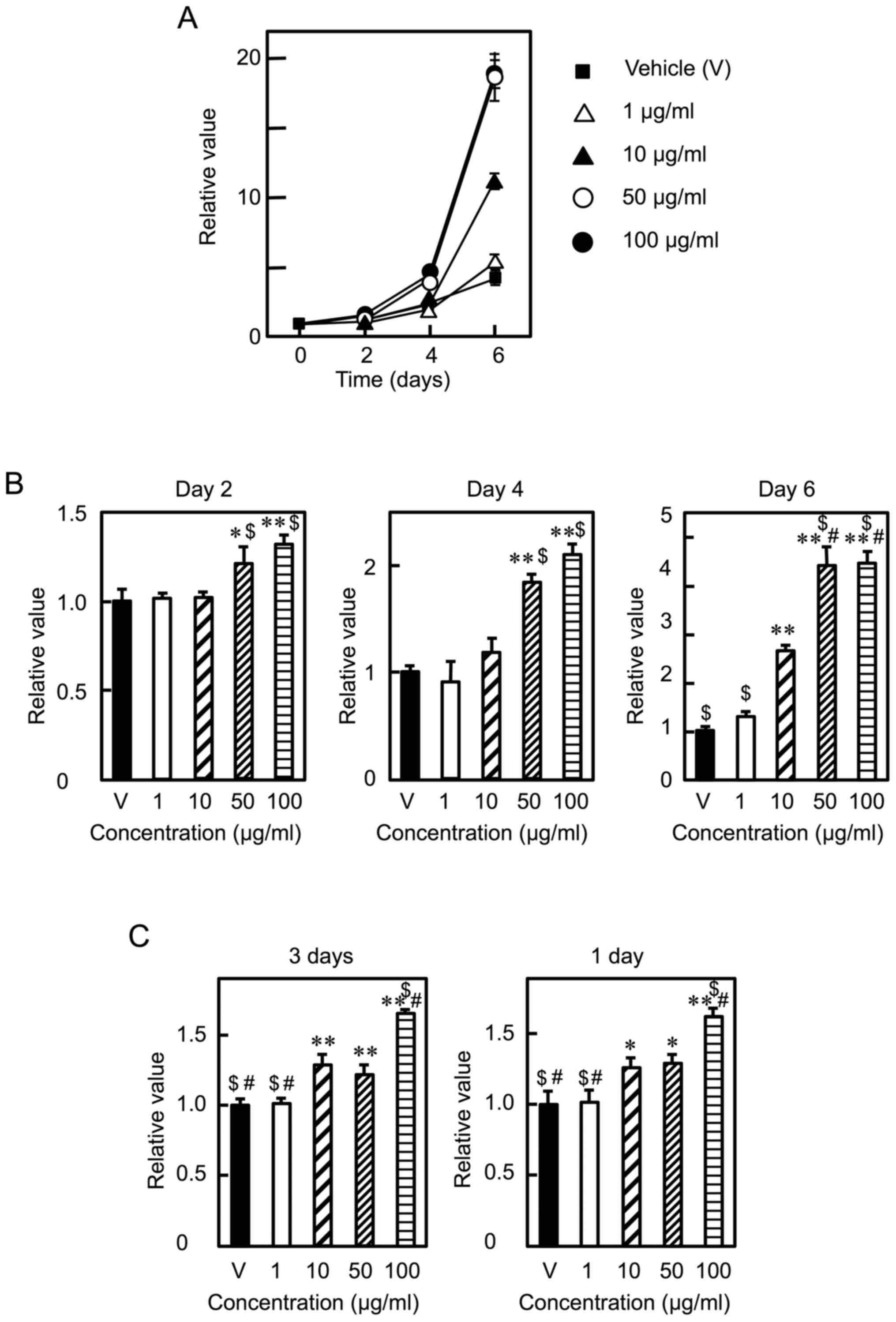 | Figure 1.Effects of Lf on the proliferation of
C2C12 myoblasts. (A) After attachment, myoblasts were cultured in
the presence of the vehicle (V) or Lf (1, 10, 50, and 100 µg/ml)
for the indicated times. Cell viability was determined using the
alamarBlue fluorescent dye. Data are expressed as relative values
(fluorescence intensity (FI) of the experimental group divided by
FI of the vehicle group at day 0). Values are indicated as the mean
± SD (n=3). (B) Data are expressed as relative values (FI of the
experimental group divided by FI of the vehicle group at the same
time point). (C) (left panel) After attachment, myoblasts were
cultured with Lf at various concentrations for three days. (right
panel) After attachment, myoblasts were cultured in the presence of
Lf for one day and in the absence of Lf for the next two days. Data
are expressed as relative values (FI of the experimental group
divided by FI of the vehicle group). Values are indicated as the
mean ± SD (n=4). Statistically significant differences were
determined by one-way ANOVA and Tukey's post-hoc test. *P<0.01,
**P<0.001 vs. the V group. $P<0.001 vs. Lf (10
µg/ml). #P<0.001 vs. Lf (50 µg/ml). Each result is
representative of three or more independent experiments. Lf,
Lactoferrin; FI, fluorescence intensity; ANOVA, analysis of
variance; SD, standard deviation; V, vehicle. |
LRP1 is involved in Lf-stimulated
proliferation of C2C12 myoblasts
We next determined the receptors through which Lf
promotes proliferation and activates ERK1/2 signaling in myoblasts.
To assess whether mRNA for Lf receptors is expressed in C2C12 cells
and murine skeletal muscle, RT-PCR was performed. Myoblasts
expressed Lrp1, Nucleolin, and Tlr4 mRNA before and
after differentiation (Fig. 3A).
These mRNA were also detected in skeletal muscle tissue of the
gastrocnemius, soleus, and extensor digitorum longus. However,
Itln1 mRNA was not detected in C2C12 cells and skeletal
muscle, although it was detected in the small intestine. To
determine whether LRP1 was involved in Lf-stimulated myoblast
proliferation, C2C12 cells were cultured with Lf in the presence of
RAP, an LRP1 antagonist, and cell viability was determined. RAP
attenuated Lf-induced myoblast proliferation (Fig. 3B). Furthermore, C2C12 cells were
transfected with LRP1 siRNA, followed by culture with Lf. Knockdown
of LRP1 inhibited Lf-induced myoblast proliferation and decreased
the basal growth of myoblasts (Fig.
3C). Depletion of LRP1 by approximately 70% did not completely
inhibit, but attenuated Lf-activated phosphorylation of ERK1/2
(Fig. 3D). These results indicate
that LRP1 is involved in Lf-induced myoblast proliferation and
Lf-stimulated ERK1/2 activation.
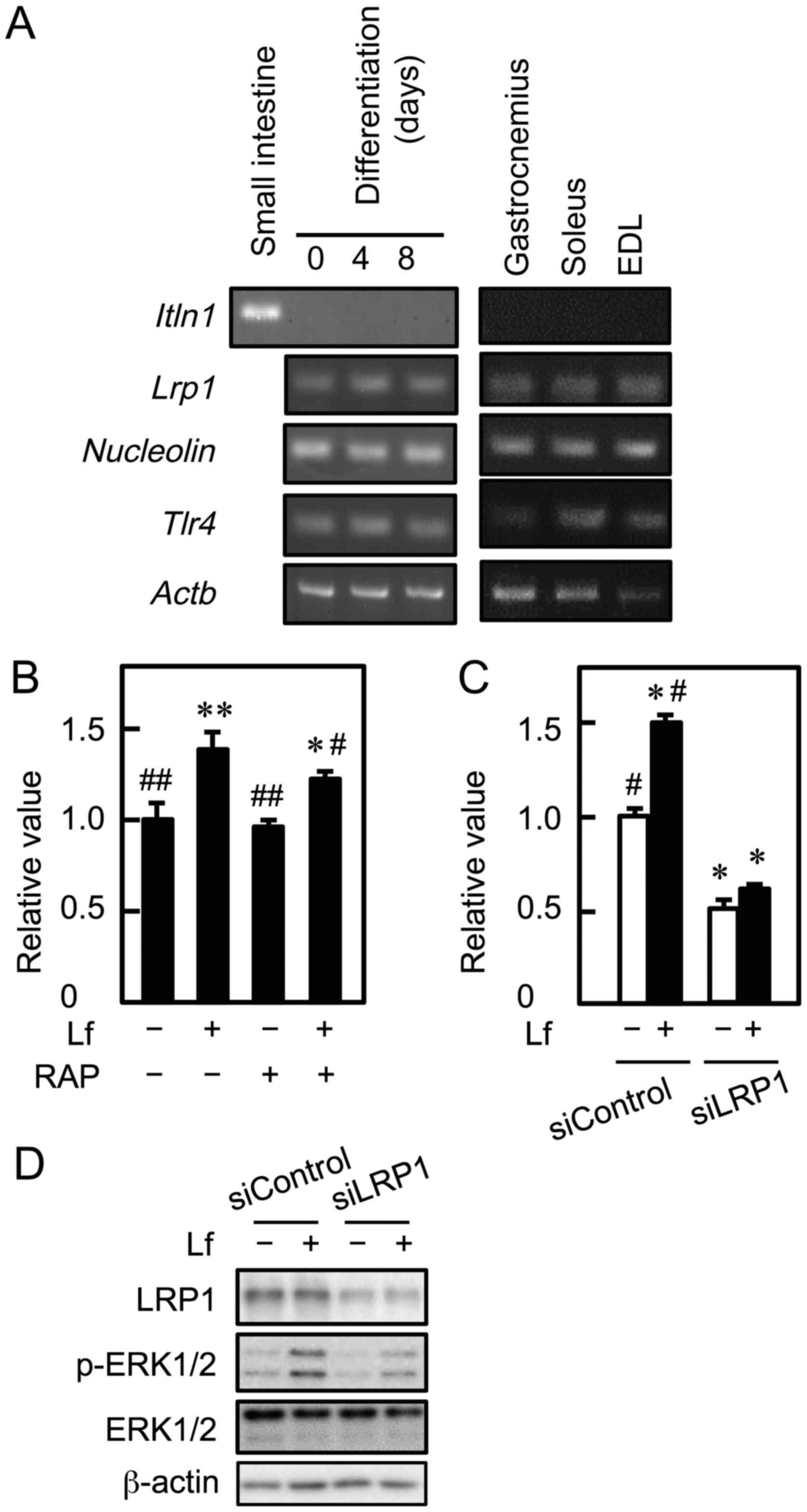 | Figure 3.Involvement of LRP1 in the
Lf-promoted proliferation of myoblasts. (A) cDNA was synthesized
using total RNA from C2C12 cells, skeletal muscle tissue
(gastrocnemius, soleus, and extensor digitorum longus, and small
intestine, and was amplified by PCR. (B) Myoblasts were cultured
with Lf (25 µg/ml) in the presence of RAP for three days. Cell
viability was determined by the alamarBlue assay. Data are
expressed as relative values (FI of the experimental group divided
by FI of the vehicle group (-Lf, -RAP). Values are indicated as the
mean ± SD (n=4). Statistically significant differences were
determined by one-way ANOVA and Tukey's post-hoc test. *P<0.01,
**P<0.001 vs. vehicle group (-Lf, -U0126).
#P<0.05, ##P<0.001 vs. Lf group (+Lf,
-RAP). (C) Myoblasts were transfected with control siRNA
(siControl) or LRP1 siRNA (siLRP1), followed by culture with Lf for
three days. Cell viability was determined by the alamarBlue assay.
Data are expressed as relative values (FI of the experimental group
divided by FI of the vehicle group (-Lf, siControl)). Values are
indicated as the mean ± SD (n=4). Statistically significant
differences were determined by two-way ANOVA and Tukey's post-hoc
test. *P<0.001 vs. siControl group (-Lf, siControl).
#P<0.001 vs. siLRP1 group (-Lf, siLRP1). (D) LRP1
knockdown cells were incubated with Lf for 10 min. The expression
of LRP1, ERK1/2, and phosphorylated ERK1/2 (p-ERK1/2) was analyzed
by western blotting. Each result is representative of three
independent experiments. EDL, extensor digitorum longus; ERK,
extracellular signal-regulated kinase; Itln1, intelectin 1; Lf,
lactoferrin; Lrp1, low-density lipoprotein receptor-related protein
1; RAP, receptor-associated protein; siRNA, small interfering RNA;
Tlr4, Toll-like receptor 4; SD, standard deviation; ANOVA, analysis
of variance. |
Lf induces differentiation of
myoblasts into myotubes
To determine the effect of Lf on myoblast
differentiation, C2C12 cells were cultured in differentiation
medium in the presence of Lf for six days. Expression of MyHC
increased at concentrations ranging from 10–50 µg/ml but not at 100
µg/ml (Fig. 4A). Furthermore, to
determine whether Lf-mediated stimulation only occurs in the early
period (i.e., the first day) and is sufficient to promote cell
differentiation, myoblasts were differentiated into myotubes in the
presence of Lf for the first day and in the absence of Lf for the
next five days. Lf had no influence on myoblast differentiation
(Fig. 4B). The mRNA expression of
MyoD and myogenin was detected earlier when cells
were differentiated in the presence of Lf for six days (Fig. 4C). Furthermore, to determine
whether LRP1 is involved in Lf-induced myoblast differentiation,
C2C12 cells were transfected with LRP1 siRNA and were allowed to
differentiate in the presence of Lf for three days. Knockdown of
LRP1 repressed the Lf-induced increase in MyHC expression (Fig. 4D). Next, to determine the fusion
index, myoblasts were cultured in differentiation medium in the
presence of Lf for six days. The myotubes were reacted with an
anti-MyHC antibody and probed using a fluorescence-tagged secondary
antibody; their nuclei were then counted (Fig. 4E, upper panel). The fusion index
showed that Lf promoted the fusion of myoblasts into myotubes at 10
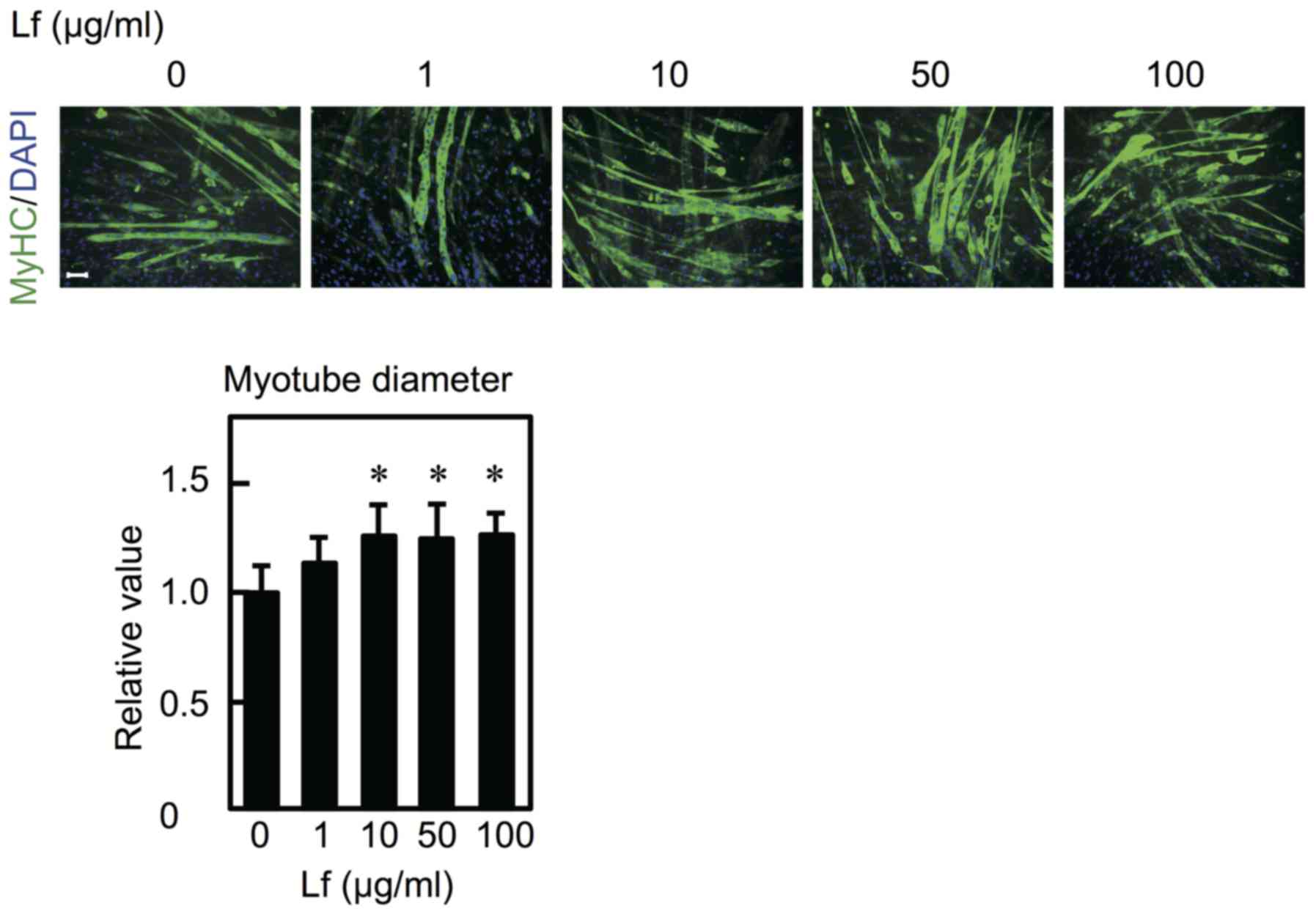
and 50 µg/ml but not at 100 µg/ml (Fig. 4E, lower panel). Furthermore, the
effect of Lf on the diameter of the short axis of myotubes was
determined. After myoblasts were differentiated into myotubes for
six days, myotubes were cultured in the presence of Lf for four
days. The myotubes were labeled with an anti-MyHC antibody and
subjected to immunofluorescent analysis, and the nuclei were
stained (Fig. 5, upper panel). Lf
increased the diameter of the short axis of myotubes at a
concentration of 10 µg/ml or higher (Fig. 5, lower panel). These results
indicate that Lf stimulates myoblast differentiation at
concentrations of 10 and 50 µg/ml, perhaps through LRP1, and
increases myotube size at concentrations of 10 µg/ml or higher.
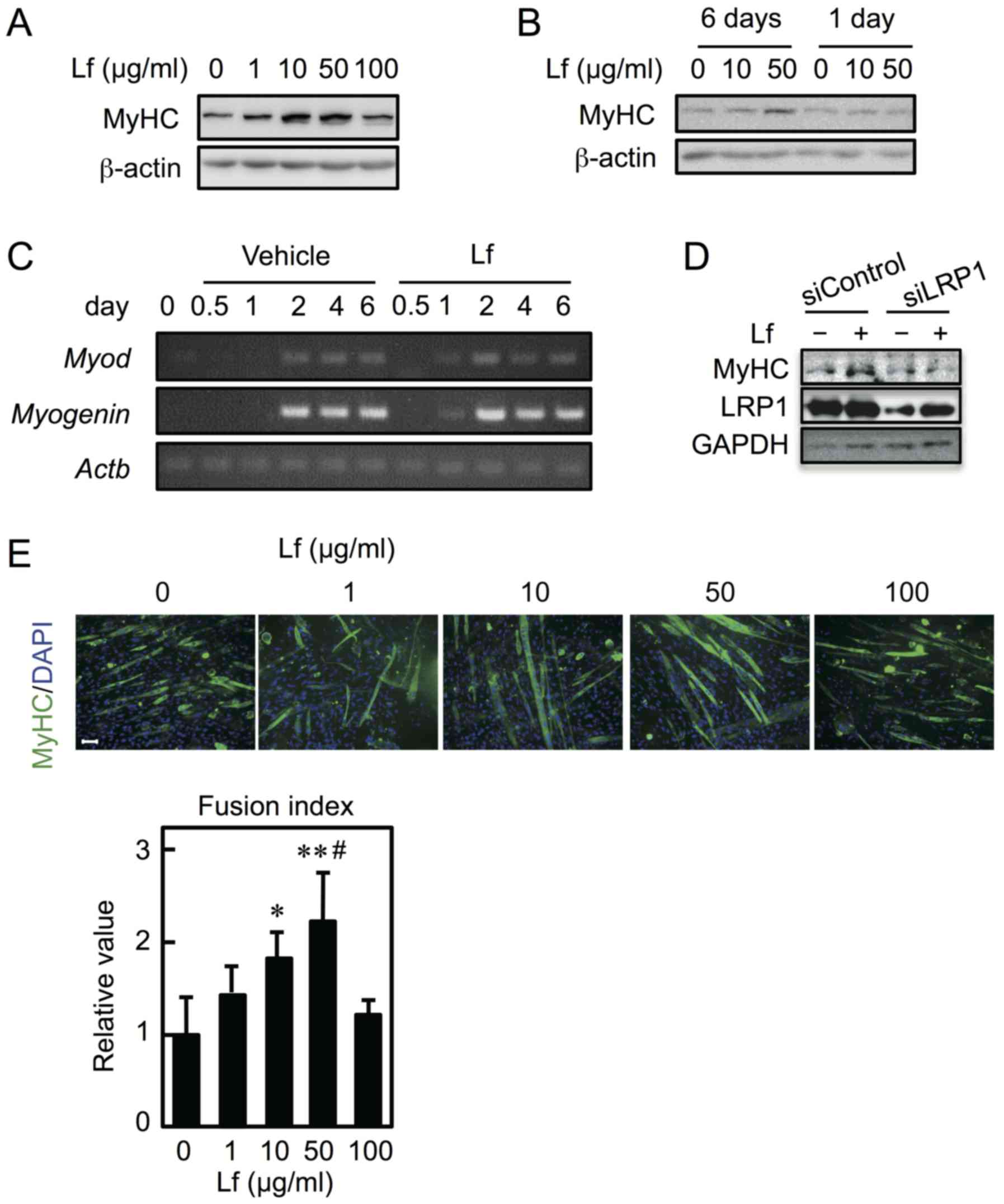 | Figure 4.Effect of Lf on myoblast
differentiation. (A) Myoblasts were cultured in differentiation
medium containing Lf at various concentrations for six days. (B)
Myoblasts were cultured in differentiation medium in the presence
of Lf for six days (6 days) or in differentiation medium in the
presence of Lf for one day and in the absence of Lf for the next 5
days (1 day). (A and B) The expression of MyHC and β-actin was
analyzed by western blots. (C) Myoblasts were cultured in
differentiation medium containing Lf (50 µg/ml). cDNA was
synthesized and genes were amplified by polymerase chain reaction.
(D) Myoblasts were transfected with control siRNA (siControl) or
LRP1 siRNA (siLRP1) and were differentiated in the presence of Lf
(50 µg/ml) for three days. The expression of MyHC, LRP1, and GAPDH
was analyzed by western blotting. (E) (Upper panel) Fixed cells
were reacted with anti-MyHC antibody and a fluorescence-labeled
secondary antibody (green). The nuclei were stained with DAPI
(blue). (Lower panel) The fusion index was calculated.
Statistically significant differences were determined by one-way
ANOVA and Tukey's post-hoc test. *P<0.05, **P<0.001 vs. Lf (0
µg/ml) group. #P<0.05 vs. Lf (1 µg/ml) group. Each
result is representative of three (A, B, C, and E) or two
independent experiments (D). Scale bar, 100 µM for all images. Lf,
lactoferrin; LRP1, low-density lipoprotein receptor-related protein
1; MyHC, myosin heavy chain; siRNA, small interfering RNA. |
Discussion
Lf promotes the proliferation of C2C12 myoblasts and
induces osteoblastic and chondroblastic differentiation of C2C12
myoblasts (18). The present study
demonstrates the mechanism by which Lf promotes myoblast
proliferation and provides information about the roles of Lf in
myoblast differentiation and myotube hypertrophy.
Lf promoted the proliferation of C2C12 myoblasts,
which is consistent with a previous report on the biological effect
of Lf on C2C12 myoblast proliferation (18). In this study, we found that Lf led
to increased cell proliferation when C2C12 myoblasts were exposed
to Lf for only the first day. Thus, stimulation by Lf only in the
early period was sufficient to promote myoblast proliferation. When
skeletal muscle is injured, myoblasts proliferate and then
differentiate to repair and regenerate the injured muscle. Skeletal
muscle regeneration is regulated by the interplay between skeletal
muscle stem cells (satellite cells or myoblasts) and the immune
system (23). With respect to the
latter, injury to skeletal muscle induces an inflammatory response,
resulting in infiltration of inflammatory cells into the local
sites of the injured muscle (24).
Neutrophils are the first inflammatory cells that infiltrate the
injured muscle within 2 h of muscle damage, and their levels peak
1–3 days post-injury before returning to basal levels (25). The first-day stimulation by Lf to
promote cell growth is consistent with the timing of neutrophil
infiltration into the injured area during regeneration. These
results indicate that Lf plays a critical role in myoblast
proliferation and suggest that Lf may function as a critical player
in muscle regeneration.
The proliferation of muscle cells is regulated by
several regulatory signaling pathways, including the ERK1/2
signaling pathway (26). Lf
induced the phosphorylation of ERK1/2 in myoblasts, and U0126
treatment inhibited Lf-stimulated cell proliferation. U0126 is a
selective inhibitor of MEK1 and MEK2, which inhibit ERK1/2
activation. Activation of ERK1/2 is required for myoblast
proliferation (27). Yagi et
al (18) reported that C2C12
cells express LRP1, but the role of LRP1 in C2C12 cells remains
unclear. The present study demonstrated that the administration of
RAP, an LRP1 antagonist, and depletion of LRP1 attenuate
Lf-stimulated cell growth and ERK1/2 phosphorylation. Furthermore,
knockdown of LRP1 repressed Lf-induced MyHC expression. Given that
mesenchymal stem cells are capable of differentiating into multiple
lineages such as chondrogenic, osteogenic, adipogenic, and myogenic
lineages (19), their lineages may
have common Lf receptors and signal transduction pathways. For
example, Lf promotes the proliferation and osteogenic
differentiation of human adipose-derived stem cells (28), although the underlying mechanisms
remain unclear. Grey et al (29) reported that RAP and U0126 inhibited
Lf-induced mitogenesis in osteoblasts and suggested that Lf
promoted osteoblastic cell growth by activating the ERK1/2
signaling pathway through LRP1. In human chondrocytes, Lf promotes
proliferation and activates ERK1/2, although it remains unclear
whether ERK1/2 has any influence on cell proliferation (30). In contrast, Lf promotes the
differentiation of osteoblasts independently of LRP1, although
ERK1/2 is activated through LRP1 (31). Although there are still
contradictory results with regard to the mechanism by which Lf
promotes proliferation of mesenchymal stem cell-derived lineages,
our results indicate that Lf promotes myoblast proliferation by
activating the ERK1/2 signaling pathway, at least partially through
LRP1, and that LRP1 is involved in Lf-promoted myoblast
differentiation.
LRP1 is a member of the low-density lipoprotein
receptor family and exerts two different biological functions: i)
it acts as a scavenger receptor that contributes to the endocytosis
of various ligands (at least 40) and ii) it acts as a signaling
receptor that regulates different cellular processes (32). The conventional LRP1 knockout in
mice is lethal, indicating the indispensability of LRP1 in cellular
physiology (33). Taken together
with the fact that the knockdown of LRP1 decreased the basal growth
of myoblasts (Fig. 3C), LRP1 may
function as a receptor for certain ligands that promote the
proliferation of myoblasts in the absence of Lf.
U0126 completely inhibited Lf-stimulated ERK1/2
activation and cell proliferation. However, knockdown of LRP1 by
approximately 70% did not result in complete inhibition of
Lf-stimulated ERK1/2 activation. Lf promotes proliferation by
activating ERK1/2 signaling through intelectin 1 in intestinal
epithelial cells (34), but no
intelectin 1 was detected in C2C12 myoblasts or skeletal muscle. On
the other hand, knockdown of nucleolin decreased the expression
levels of phosphorylated ERK1/2 in hepatocellular carcinoma
(35) and repressed epidermal
growth factor- or stromal cell-derived factor 1-induced ERK1/2
activation in esophageal squamous cell carcinoma (36). Furthermore, knockdown of TLR4
inhibits the 60-kDa heat shock chaperonin protein-induced ERK1/2
activation in A7r5 vascular smooth muscle cells (37). Future studies will look to
determine if Lf is able to stimulate cell growth via the ERK1/2
signaling cascade through nucleolin and/or TLR4 in myoblasts.
Lf increased the expression of MyHC and the fusion
index. These results indicate that Lf induces myoblast
differentiation and myotube formation. When C2C12 myoblasts are
exposed to differentiation medium (DMEM supplemented with 2% horse
serum), they withdraw from the cell cycle and differentiate into
myotubes. In the present study, C2C12 myoblasts were induced to
differentiate by culturing in the differentiation medium, and Lf
promoted the expression of Myod and myogenin. In
contrast, Yagi et al (18)
suggested that Lf repressed C2C12 myoblast differentiation, because
MyoD expression was suppressed when myoblasts were cultured in the
low-mitogen differentiation medium (DMEM supplemented with 5% FBS)
in the presence of Lf. However, in this study, myoblasts
proliferated when cultured in DMEM supplemented with 2% FBS, but
did not differentiate into myotubes. At this time, we have no
suitable explanation for the discrepancy in the effect of Lf on
C2C12 myoblast differentiation. The effect of Lf on the
differentiation of skeletal muscle stem cells (satellite cells) may
resolve this discrepancy.
Lf promoted the differentiation of myoblasts at
concentrations of 10 and 50 µg/ml but not at a concentration of 100
µg/ml. Thus, the promotion of myoblast differentiation by Lf was
exerted in a limited dose range rather than in a dose-dependent
manner, which is contrary to the observation that Lf promotes
myoblast proliferation in a dose-dependent manner. In contrast, in
other cells derived from mesenchymal stem cells, Lf stimulates
osteogenic differentiation at 100 µg/ml (28) and represses adipogenic
differentiation at concentrations higher than 10 µg/ml (38). Given these seemingly contradictory
results, it is possible that myoblasts express at least two types
of Lf receptors that regulate differentiation. For instance, one
receptor might promote myogenic differentiation and the other might
repress myogenic differentiation; however, further research is
needed to determine if this is indeed the case.
Increased skeletal muscle mass is due to the
expanded cross-sectional area of individual myofibers. In this
study, Lf increased myotube size at concentrations higher than 10
µg/ml. Thus, Lf has the potential to function in both myoblasts and
myotubes, which express common Lf receptors such as LRP1,
nucleolin, and TLR4. We are now attempting to study how Lf acts on
myoblast differentiation and myotube hypertrophy.
Lf has two iron-binding sites and the conformation
of Lf varies depending on whether it is iron-free (apo-Lf) or
iron-saturated (holo-Lf) (39).
Lf. Apo- and holo-Lf exhibit different physiological functions. For
example, apo-Lf represses the proliferation of human intestinal
epithelial Caco-2 cells, whereas holo-Lf enhances it (40). In contrast, apo-Lf promotes
osteoblast proliferation to the same degree as holo-Lf (41). In addition, holo-Lf, but not
apo-Lf, enhances tropoelastin expression through LRP1 in human
dermal fibroblasts (42). The Lf
used in the present study was approximately 20% iron-saturated
bovine Lf. Therefore, it is of interest to determine which Lf
promotes myoblast proliferation and differentiation and myotube
hypertrophy.
In conclusion, this study demonstrates that Lf
stimulation for one day promotes myoblast proliferation and that Lf
seems to stimulate cell proliferation by activating ERK1/2
signaling pathway, at least partially through LRP1. Furthermore, we
found that Lf induced myoblast differentiation and myotube
hypertrophy. This study reveals that Lf may affect skeletal muscle
repair and regeneration, as well as developmental and postnatal
myogenesis.
Acknowledgements
This study was supported in part by Grant-in-Aids
(26292073) for scientific research (to R.Y.) from the Japan Society
for the Promotion of Science. We would like to thank Editage
(www.editage.jp) for English language editing.
References
|
1
|
Kiens B: Skeletal muscle lipid metabolism
in exercise and insulin resistance. Physiol Rev. 86:205–243. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Srikanthan P, Hevener AL and Karlamangla
AS: Sarcopenia exacerbates obesity-associated insulin resistance
and dysglycemia: Findings from the National Health and Nutrition
Examination Survey III. PLoS One. 5:e108052010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rai M, Nongthomba U and Grounds MD:
Skeletal muscle degeneration and regeneration in mice and flies.
Curr Top Dev Biol. 108:247–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
White RB, Bièrinx AS, Gnocchi VF and
Zammit PS: Dynamics of muscle fibre growth during postnatal mouse
development. BMC Dev Biol. 10:212010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Devlin RB and Emerson CP Jr: Coordinate
regulation of contractile protein synthesis during myoblast
differentiation. Cell. 13:599–611. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lonnerdal B and Iyer S: Lactoferrin:
Molecular structure and biological function. Annu Rev Nutr.
15:93–110. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caccavo D, Sebastiani GD, Di Monaco C,
Guido F, Galeazzi M, Ferri GM, Bonomo L and Afeltra A: Increased
levels of lactoferrin in synovial fluid but not in serum from
patients with rheumatoid arthritis. Int J Clin Lab Res. 29:30–35.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou Z, Imam MU, Ismail M, Azmi NH, Ismail
N, Ideris A and Mahmud R: Lactoferrin and ovotransferrin contribute
toward antioxidative effects of Edible Bird's Nest against hydrogen
peroxide-induced oxidative stress in human SH-SY5Y cells. Biosci
Biotechnol Biochem. 79:1570–1578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Actor JK, Hwang SA and Kruzel ML:
Lactoferrin as a natural immune modulator. Curr Pharm Des.
15:1956–1973. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baveye S, Elass E, Mazurier J, Spik G and
Legrand D: Lactoferrin: A multifunctional glycoprotein involved in
the modulation of the inflammatory process. Clin Chem Lab Med.
37:281–286. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamada Y, Sato R, Kobayashi S, Hankanga C,
Inanami O, Kuwabara M, Momota Y, Tomizawa N and Yasuda J: The
antiproliferative effect of bovine lactoferrin on canine mammary
gland tumor cells. J Vet Med Sci. 70:443–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zemann N, Klein P, Wetzel E, Huettinger F
and Huettinger M: Lactoferrin induces growth arrest and nuclear
accumulation of Smad-2 in HeLa cells. Biochimie. 92:880–884. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inoue H, Sakai M, Kaida Y and Kaibara K:
Blood lactoferrin release induced by running exercise in normal
volunteers: Antibacterial activity. Clin Chim Acta. 341:165–172.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawakami H and Lönnerdal B: Isolation and
function of a receptor for human lactoferrin in human fetal
intestinal brush-border membranes. Am J Physiol. 261:G841–G846.
1991.PubMed/NCBI
|
|
15
|
Croy JE, Shin WD, Knauer MF, Knauer DJ and
Komives EA: All three LDL receptor homology regions of the LDL
receptor-related protein bind multiple ligands. Biochemistry.
42:13049–13057. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Legrand D, Vigiè K, Said EA, Elass E,
Masson M, Slomianny MC, Carpentier M, Briand JP, Mazurier J and
Hovanessian AG: Surface nucleolin participates in both the binding
and endocytosis of lactoferrin in target cells. Eur J Biochem.
271:303–317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Curran CS, Demick KP and Mansfield JM:
Lactoferrin activates macrophages via TLR4-dependent and
-independent signaling pathways. Cell Immunol. 242:23–30. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yagi M, Suzuki N, Takayama T, Arisue M,
Kodama T, Yoda Y, Otsuka K and Ito K: Effects of lactoferrin on the
differentiation of pluripotent mesenchymal cells. Cell Biol Int.
33:283–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee KD: Applications of mesenchymal stem
cells: An updated review. Chang Gung Med J. 31:228–236.
2008.PubMed/NCBI
|
|
20
|
Kitakaze T, Sakamoto T, Kitano T, Inoue N,
Sugihara F, Harada N and Yamaji R: The collagen derived dipeptide
hydroxyprolyl-glycine promotes C2C12 myoblast differentiation and
myotube hypertrophy. Biochem Biophys Res Commun. 478:1292–1297.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Brien J, Wilson I, Orton T and Pognan F:
Investigation of the alamar bleu (resazurin) fluorescent dye for
the assessment of mammalian cell cytotoxicity. Eur J Biochem.
267:5421–5426. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ogawa M, Yamaji R, Higashimura Y, Harada
N, Ashida H, Nakano Y and Inui H: 17β-estradiol represses myogenic
differentiation by increasing ubiquitin-specific peptidase 19
through estrogen receptor α. J Biol Chem. 286:41455–41465. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saini J, McPhee JS, Al-Dabbagh S, Stewart
CE and Al-Shanti N: Regenerative function of immune system:
Modulation of muscle stem cells. Ageing Res Rev. 27:67–76. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tidball JG and Villalta SA: Regulatory
interactions between muscle and the immune system during muscle
regeneration. Am J Physiol Regul Integr Comp Physiol.
298:R1173–R1187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Novak ML, Weinheimer-Haus EM and Koh TJ:
Macrophage activation and skeletal muscle healing following
traumatic injury. J Pathol. 232:344–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mebratu Y and Tesfaigzi Y: How ERK1/2
activation controls cell proliferation and cell death: Is
subcellular localization the answer? Cell Cycle. 8:1168–1175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jones NC, Fedorov YV, Rosenthal RS and
Olwin BB: ERK1/2 is required for myoblast proliferation but is
dispensable for muscle gene expression and cell fusion. J Cell
Physiol. 186:104–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ying X, Cheng S, Wang W, Lin Z, Chen Q,
Zhang W, Kou D, Shen Y, Cheng X, Peng L, et al: Effect of
lactoferrin on osteogenic differentiation of human adipose stem
cells. Int Orthop. 36:647–653. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grey A, Banovic T, Zhu Q, Watson M, Callon
K, Palmano K, Ross J, Naot D, Reid IR and Cornish J: The
low-density lipoprotein receptor-related protein 1 is a mitogenic
receptor for lactoferrin in osteoblastic cells. Mol Endocrinol.
18:2268–2278. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brandl N, Zemann A, Kaupe I, Marlovits S,
Huettinger P, Goldenberg H and Huettinger M: Signal transduction
and metabolism in chondrocytes is modulated by lactoferrin.
Osteoarthritis Cartilage. 18:117–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang W, Guo H, Jing H, Li Y, Wang X,
Zhang H, Jiang L and Ren F: Lactoferrin stimulates osteoblast
differentiation through PKA and p38 pathways independent of
lactoferrin's receptor LRP1. J Bone Miner Res. 29:1232–1243. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boucher P and Herz J: Signaling through
LRP1: Protection from atherosclerosis and beyond. Biochem
Pharmacol. 81:1–5. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Herz J, Clouthier DE and Hammer RE: LDL
receptor-related protein internalizes and degrades uPA-PAI-1
complexes and is essential for embryo implantation. Cell.
71:411–421. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang R and Lönnerdal B: Apo- and
holo-lactoferrin stimulate proliferation of mouse crypt cells but
through different cellular signaling pathways. Int J Biochem Cell
Biol. 44:91–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qiu W, Wang G, Sun X, Ye J, Wei F, Shi X
and Lv G: The involvement of cell surface nucleolin in the
initiation of CCR6 signaling in human hepatocellular carcinoma. Med
Oncol. 32:752015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qi J, Li H, Liu N, Xing Y, Zhou G, Wu Y,
Liu Y, Chen W, Yue J, Han B, et al: The implications and mechanisms
of the extra-nuclear nucleolin in the esophageal squamous cell
carcinomas. Med Oncol. 32:452015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao Y, Zhang C, Wei X, Li P, Cui Y, Qin
Y, Wei X, Jin M, Kohama K and Gao Y: Heat shock protein 60
stimulates the migration of vascular smooth muscle cells via
Toll-like receptor 4 and ERK MAPK activation. Sci Rep. 5:153522015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yagi M, Suzuki N, Takayama T, Arisue M,
Kodama T, Yoda Y, Numasaki H, Otsuka K and Ito K: Lactoferrin
suppress the adipogenic differentiation of MC3T3-G2/PA6 cells. J
Oral Sci. 50:419–425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Majka G, Śpiewak K, Kurpiewska K, Heczko
P, Stochel G, Strus M and Brindell M: A high-throughput method for
the quantification of iron saturation in lactoferrin preparations.
Anal Bioanal Chem. 405:5191–5200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Oguchi S, Wakler WA and Sanderson IR: Iron
saturation alters the effect of lactoferrin on the proliferation
and differentiation of human enterocytes (Caco-2 cells). Biol
Beonate. 67:330–339. 1995.
|
|
41
|
Cornish J, Palmano K, Callon KE, Watson M,
Lin JM, Valenti P, Naot D, Grey AB and Reid IR: Lactoferrin and
bone; structure-activity relationshiops. Biochem Cell Biol.
84:297–302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ryu M, Nogami A, Kitakaze T, Harada N,
Suzuki AY and Yamaji R: Lactoferrin induces tropoelastin expression
by activating the lipoprotein receptor-related protein 1-mediated
phosphatidylinositol 3-kinase/Akt pathway in human dermal
fibroblasts. Cell Biol Int. 41:1325–1334. 2017. View Article : Google Scholar : PubMed/NCBI
|