Introduction
Lung cancer is the most common cancer in the world,
of which 80% were non-small cell lung cancer (NSCLC). Surgical
resection is known as the most effective treatment for NSCLC,
however, due to the fact that most diagnoses were confirmed in an
advanced stage because of its deep location and no specificity of
symptoms in its early stage, only a few patients can be cured by
surgical treatment. Thus cisplatin (DDP) based adjuvant
chemotherapy was studied as a standard treatment for patients with
completely resected NSCLC (1).
However, chemotherapy resistance usually occurs mainly due to DDP
resistance, which contributes to a poor long-term survival rate of
15% (2). As one of the important
mechanisms of drug resistance is resistant to DDP-induced cell
apoptosis in lung cancer (3),
therefore, finding an effective medicine to induce apoptosis of DDP
resistance cells is a reasonable strategy to reverse
resistance.
Accumulating evidence has indicated that
4-aminopyridine (4-AP), one of the most commonly used K+
channel inhibitors, suppresses proliferation and induces apoptosis
in various types of cancer cells, such as malignant astrocytoma
(4), hepatoblastoma (5), acute myeloid leukemia and glioma
(6,7). Therefore, 4-AP is presented as
potential therapeutic agents for various types of cancers.
It has been shown that transmembrane current and
activity of K+ channels is significantly high in NSCLC
(8). However, whether 4-AP could
affect cell growth of A549/CDDP is not clear. In this study, we
examined the effect and possible molecular basis of 4-AP in
A549/CDDP cells and found that 4-AP inhibited cell growth, induced
cell apoptosis and sensitized A549/CDDP cells to DDP via
upregulating phosphatase and tensin homolog (PTEN). Together these
results provide a novel mechanism for 4-AP as a potential
therapeutic agent for patients with DDP resistance.
Materials and methods
Cell culture
DDP resistant lung cancer cell line A549/CDDP was
obtained from Oncology Center of our hospital and maintained in
DMEM medium supplemented with 10% fatal bovine serum (FBS;
PAA Laboratories; GE Healthcare, Chicago, IL, USA), and was
incubated in a humidified chamber with 5% CO2 at
37°C.
Treatment of A549/CDDP cells with
4-AP
4-AP was bought from Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany. Twenty-four hours prior to transfection,
A549/CDDP cells were plated onto a 6-well plate or a 96-well plate
(Nest Biotechnology Co., Ltd., Jiangsu, China) at 30–50%
confluence. Cells were treated with 7 mM 4-AP and collected after
48 h for further experiments.
Cell viability
Cells were treated with different concentrations of
4-AP (0, 1.56, 3.125, 6.25 and 12.5 mM) for 48 h or 7 mM 4-AP for
1, 2 and 3 days. Cell viability was determined by MTT assay, as
previously described (9).
Experiments were performed three times.
Cell cycle analysis
Total of 5×106 cells were collected
following treatment with 7 mM 4-AP for 48 h. Cell cycle analysis
was performed according to the previous description (9). Each experiment was performed in
triplicate.
In vivo tumorigenesis assay in nude
mice
The treated groups were subcutaneously injected to
the left flank of 4–6-week-old 12–13 g male BALB/c nu/nu mice (N=5)
with a suspension of 8×106 A549/CDDP cells with 4-AP (7
mM). The control group were injected to the right flank of nude
mice with a suspension of 8×106 A549/CDDP cells with
0.01 M PBS (7,10). Tumor size was monitored using a
calliper in the process of tumor growth and measured every 3 days.
Mice were sacrificed using cervical dislocation 18 days after
subcutaneous injection and tumor tissues were excised and weighed.
Tumor volumes were calculated as follows: (Dxd2)/2,
where D is the longest diameter and d is the shortest diameter. All
animal studies were conducted in accordance with the principles and
procedures outlined in Guangdong Medical University Guide for the
Care and Use of Animals (Guangdong) 2011–020. All experiments
procedures were approved by the The Animal Care and Use Committee
of the Guangdong Medical University (no. GDY1701068).
Transmission electron microscopy
A549/CDDP cells were cultured in 10 cm-diameter
plates with 7 mM 4-AP treatment for 48 h. 5 ×106 cells
were collected by centrifugation at 2,000 rpm for 10 min and washed
twice with PBS. The pelleted cells were fixed in 2.5% cold
glutaraldehyde supplemented with 0.1 M of sodium cacodylate/1%
sucrose buffer for 24 h. The cells were washed three times with
PBS, then postfixed in 1% osmium tetroxide (60 min), encapsulated
in 1% agar, stained with uranyl acetate and phosphotungstic acid,
and dehydrated in a series of graded ethanolic solutions. Propylene
oxide was added before the cells were finally embedded in Epon
812-Araldite mixture. Ultrathin sections (50 nm) were cut using
ultramicrotome, placed under 200 mesh standard copper grids and
examined under JEM-1400 transmission electron microscope. Each
experiment was performed in triplicate.
Apoptosis assays
A549/CDDP cells were treated with 7 mM 4-AP for 48
h. Apoptosis was demonstrated by Annexin V-APC/7-ADD Apoptosis
detection kit KGA1025 (Kaiji, Nanjing, China). Briefly,
1–5×105 cells were collected, washed twice in cold PBS,
and resuspended in 500 µl binding buffer. The suspension cells were
stained with 5 µl Annexin V-APC and 5 µl 7-ADD, and incubated for
15 min at room temperature in the dark. Apoptotic cells were
assessed using FACS (BD Biosciences, Franklin Lakes, NJ, USA).
Experiments were performed at least three times to qualify
apoptosis by phosphatidylserine (PS) externalization.
Treatment of A549/CDDP cells with 4-AP
and DDP
A549/CDDP cells were treated with different
concentrations of 4-AP (3, 6 and 10 mM) and DDP (0, 12.5, 25, 50,
100 and 200 µM) (Qilu Pharmo Co. Ltd, China) for 48 h at 37°C. Cell
viability was determined using MTT assay, as previously described
(9). Experiments were performed
three times.
Treatment of A549/CDDP cells with 4-AP
and PTEN siRNAs
SiRNA for PTEN was designed and synthesized by
Guangzhou RiboBio Co., Ltd., (Guangzhou, China) (Table I). The sequences of each gene and
their controls are shown in Wang's study (11). A549/CDDP cells were treated with
4-AP (7 mM) and PTEN siRNAs (100 nM) together for 48 h and
collected for further experiments. Each experiments were performed
three times.
 | Table I.Small interfering RNA sequences of
PTEN. |
Table I.
Small interfering RNA sequences of
PTEN.
| Gene | Sequence |
|---|
| PTEN |
|
| 1 | Sense:
5′-GAGCGUGCAGAUAAUGACAdTdT-3′ |
|
| Antisense:
3′-dTdTCUCGCACGUCUAUUACUGU-5′ |
| 2 | Sense:
5′-GUAUAGAGCGUGCAGAUAAdTdT-3′ |
|
| Antisense:
3′-dTdTCAUAUCUCGCACGUCUAUU-5′ |
| 3 | Sense:
5′-GUUAAAGAAUCAUCUGGAUdTdT-3′ |
|
| Antisense:
3′-dTdTCAAGGGCUUAGUAGACCUA-5′ |
Western blot analysis
Cells were lysed in RIPA buffer (Kaiji, Nanjing,
China), and protein concentration was determined using BCA assay
(Beyotime Institute of Biotechnology, Haimen, China). Total protein
(30 µg) was resolved using a 10% SDS-polyacrylamide gel
electrophoresis (PAGE) gel and electro-transferred to
polyvinylidene fluoride membranes (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Membranes were blocked with 5%
non-fat dry milk (for Phosphorylation antibody, adding BSA) in
Tris-buffered saline (pH 7.5) with 0.1% Tween-20, followed by
immunobloting overnight at 4°C with the following primary
antibodies: Anti-pPI3K (Tyr458) (cat no. 4228S, 1:1,000), PI3K (cat
no. 4249S, 1:1,000), pAkt (Ser473) (cat no. 4060S, 1:1,000), Akt
(cat no. 4691S, 1:1,000), CCND1 (cat no. 2978, 1:1,000), CDK4 (cat
no. 12790, 1:1,000) and p21 antibody (cat no. 2947, 1:1,000) were
all purchased from Cell Signaling Technology, Inc., (Danvers, MA,
USA). Anti-PTEN (cat no. ab31392, 1:1,000), Bcl2 (cat no. ab32124,
1:1,000), pro-caspase 9 (cat no. ab135544, 1:1,000) and pro-caspase
3 (cat no. ab32150, 1:1,000), cleaved caspase 9 (cat no. ab2324,
1:1,000) and cleaved caspase 3 (cat no. ab2302, 1:1,000) were
purchased from Abcam (Cambridge, UK). Anti-β-actin (cat no.
14395–1-AP, 1:1,000) was purchased from Proteintech (Rosemont,
Illinois, USA). An HRP-conjugated anti-rabbit (cat no. SA00001-2,
1:1,000) or anti-mouse IgG antibody (cat no. SA00002-1, 1:1,000)
purchased from ProteinTech Group Inc., (Chicago, IL, USA) was used
as the secondary antibody for 1 h at room temperature. Signals were
detected using enhanced chemiluminescence reagents (Pierce; Thermo
Fisher Scientific, Inc.). Bands were analyzed using Image J and
protein expression quantities were determined according to the
following calculation: Integrated optical density (IOD)=density
(mean) × area.
Statistical analysis
All data were analyzed for statistical significance
using SPSS 13.0 software. Two-tailed Student's t test was used for
comparisons of two independent groups. One-way ANOVA was used to
determine differences between groups for all in vitro
analyses followed by S-N-K multiple comparison test. Repeated
measures data of ANOVA was used to determine differences between
groups in in vivo tumorigenesis assay. P<0.05 was
considered to indicate a statistically significant difference.
Results
4-AP inhibits cell growth in vitro and
in vivo
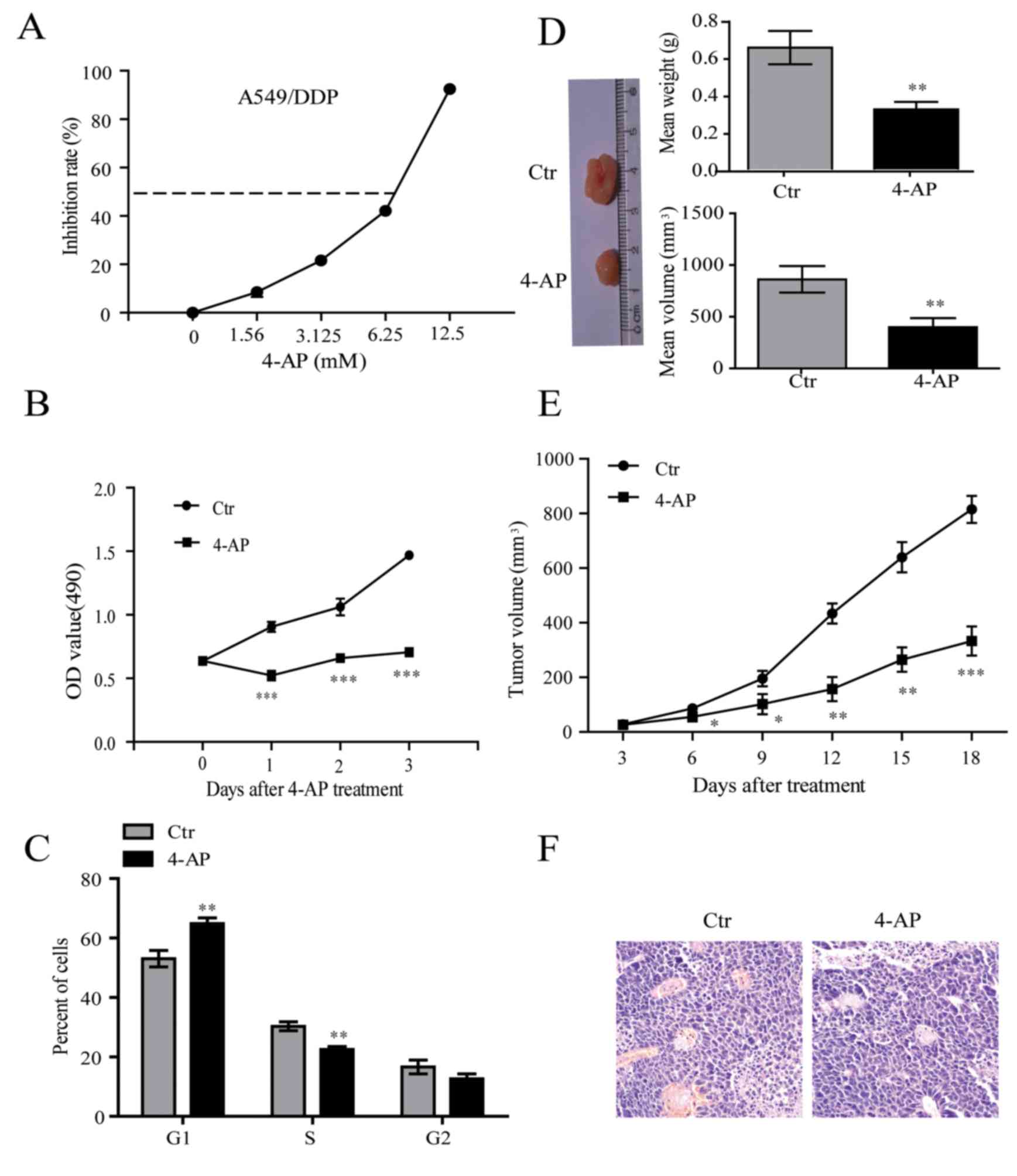
DDP-resistant lung cancer cell line A549/CDDP, was
treated with incremental doses up to 12.5 mM of 4-AP for 48 h. 4-AP
suppressed cell viability of A549/CDDP in a dose-dependent manner.
The IC50 of 4-AP was 7 mM (Fig.
1A), which was chosen for further experiments. The growth
curves showed that 4-AP significantly inhibited cell growth of
A549/CDDP cells (Fig. 1B).
Further, we observed that 4-AP blocked cell cycle transition from
G1 to S and G2 phase in A549/CDDP cells (Fig. 1C). Subsequently, cell proliferation
was measured in vivo by innoculating A549/CDDP cells into
nude mice. The treated groups were subcutaneously injected to the
left flank of nude mice with a suspension of 8×106
A549/CDDP cells with 4-AP (7 mM). The control group were injected
to the right flank of nude mice with A549/CDDP cells suspension
with normal saline (NS). Tumor volume was periodically tested once
every other day until 18 days and growth curve was plotted. We
observed that 4-AP obviously inhibited tumor growth compared with
control (Fig. 1D-F). These above
results suggest that 4-AP exerts a significant inhibitory effect on
A549/CDDP cell growth.
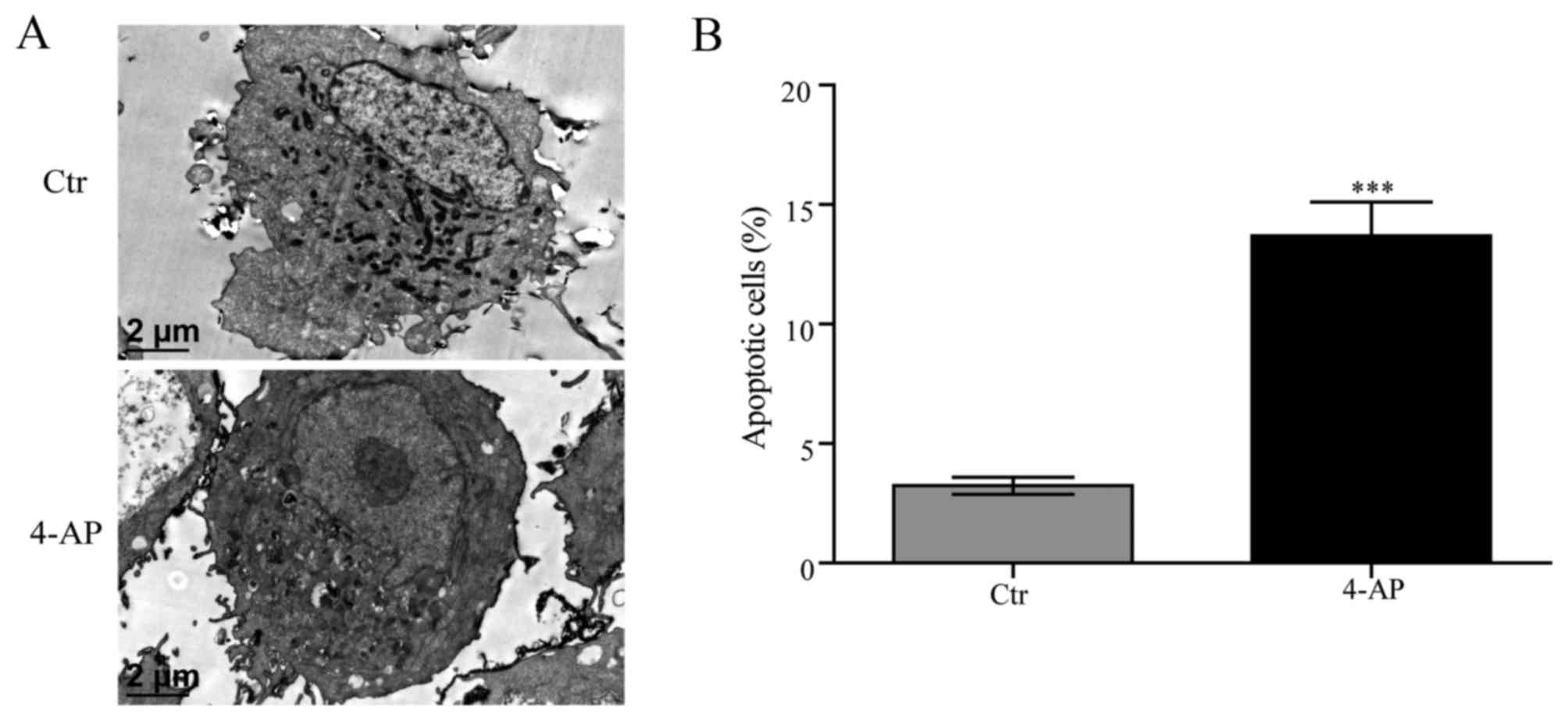
4-AP induces cell apoptosis of
A549/CDDP cells
A549/CDDP cells revealed typical features of
apoptosis including nuclear chromatin condensation and the
appearance of apoptotic body with nuclear membrane observed by
electron microscopy after treated with 7 mM 4-AP for 48 h (Fig. 2A). Annexin V-APC/7-ADD was employed
to explore obvious enhanced cell apoptosis of A549/CDDP induced by
4-AP (Fig. 2B).
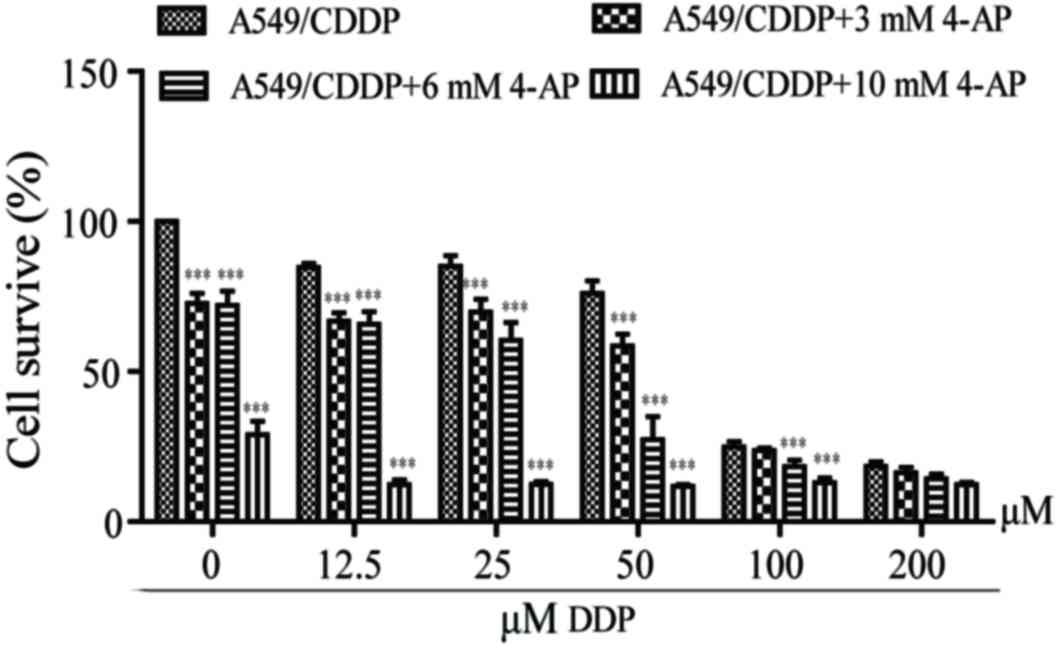
4-AP enhances the sensitivity of
A549/CDDP cells to DDP
The IC50 of DDP for A549/CDDP cells was 75±2.36 µM,
while the IC50 values decreased to 62.5±1.86, 32.14±1.92 and
0.00±0.03 µM respectively in the presence of 3, 6 and 10 mM 4-AP
(Fig. 3). 4-AP significantly
enhanced A549/CDDP cell chemosensitivity to DDP.
4-AP upregulates the expression of
PTEN and modulates PI3K/Akt signal and its downstream cell cycle
and apoptosis-related proteins in A549/CDDP cells
We examined that 4-AP not only significantly
increased the expression of PTEN, but also suppressed the
expression of pPI3K (Tyr458) and pAkt (Ser473) (Fig. 4A). However, no alterations in PI3K
and Akt expression were observed (Fig.
4A).
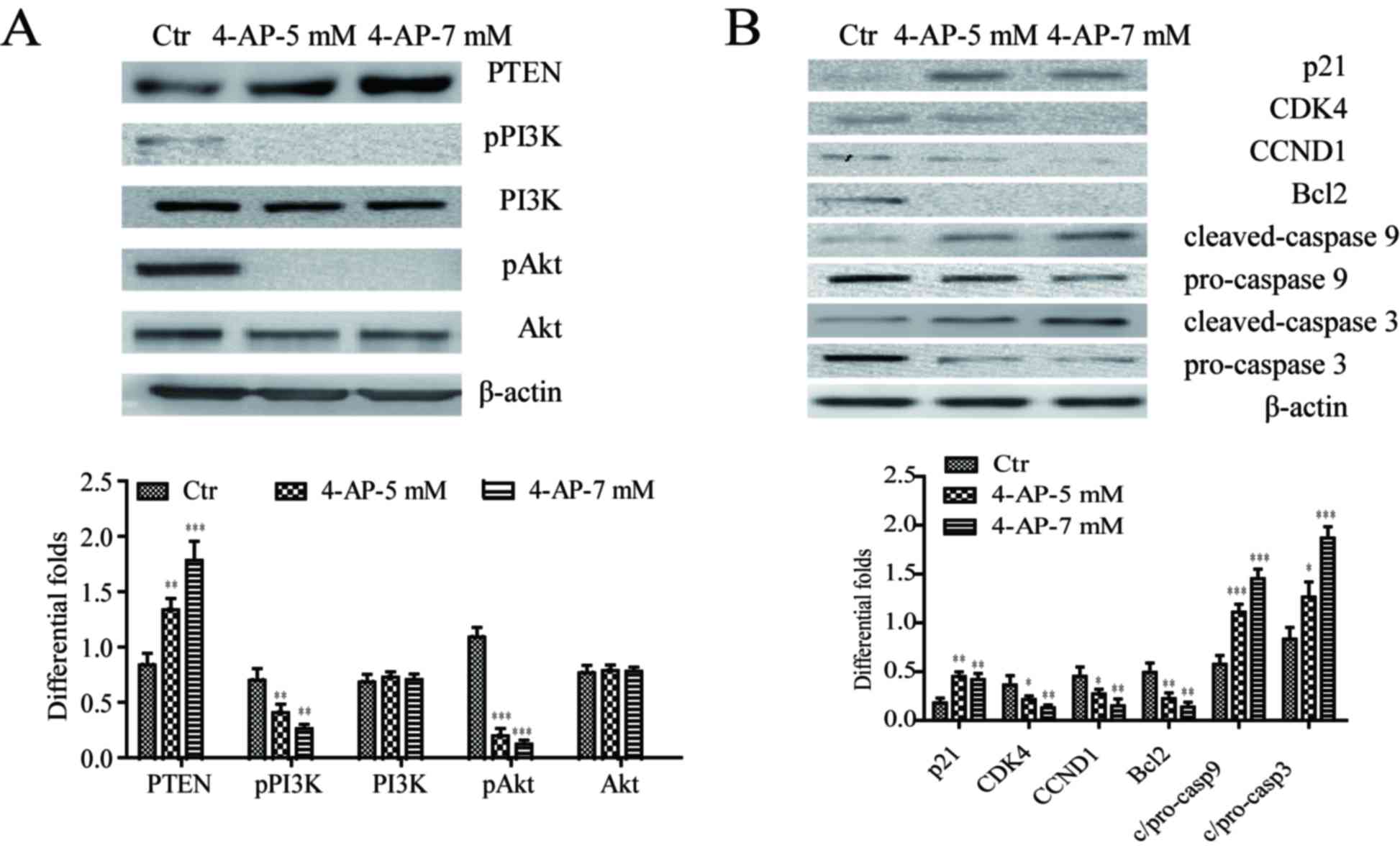 | Figure 4.4-AP upregulates the expression of
PTEN and modulates PI3K/Akt signal and its downstream cell cycle
and apoptosis-related proteins in A549/CDDP cells. (A) 4-AP
suppressed the expression of pPI3K (Tyr458) and pAkt (Ser473), as
well as elevated the expression of PTEN. No alterations in PI3K and
Akt expression were observed. (B) 4-AP suppressed the expression of
CDK4, CCND1, Bcl2, pro-caspase 9 and pro-caspase 3, and elevated
the expression of tumor suppressor p21, cleaved caspase 9 and
cleaved caspase 3. β-actin served as the internal control
*P<0.05, **P<0.01 and ***P<0.001 vs. the control. 4-AP,
4-aminopyridine; PTEN, phosphatase and tensin homolog; PI3K,
phosphoinositide 3-kinase; p, phosphorylated; Bcl2, B-cell lymphoma
2; CDK4, cyclin-dependent kinase 4; CCND1, cyclin-D1; Ctr,
control. |
Cell cycle and apoptosis has been reported as
downstream signal of PI3K/Akt pathway (9). 4-AP treatment suppressed the
expression of CCND1 and CDK4, and elevated the expression of tumor
suppressor p21 (Fig. 4B).
Moreover, the expression of apoptosis-related proteins including
Bcl2, pro-caspase 9 and pro-caspase 3 was inhibited after 4-AP
treatment, while the expression of cleaved caspase 9 and cleaved
caspase 3 was induced (Fig.
4B).
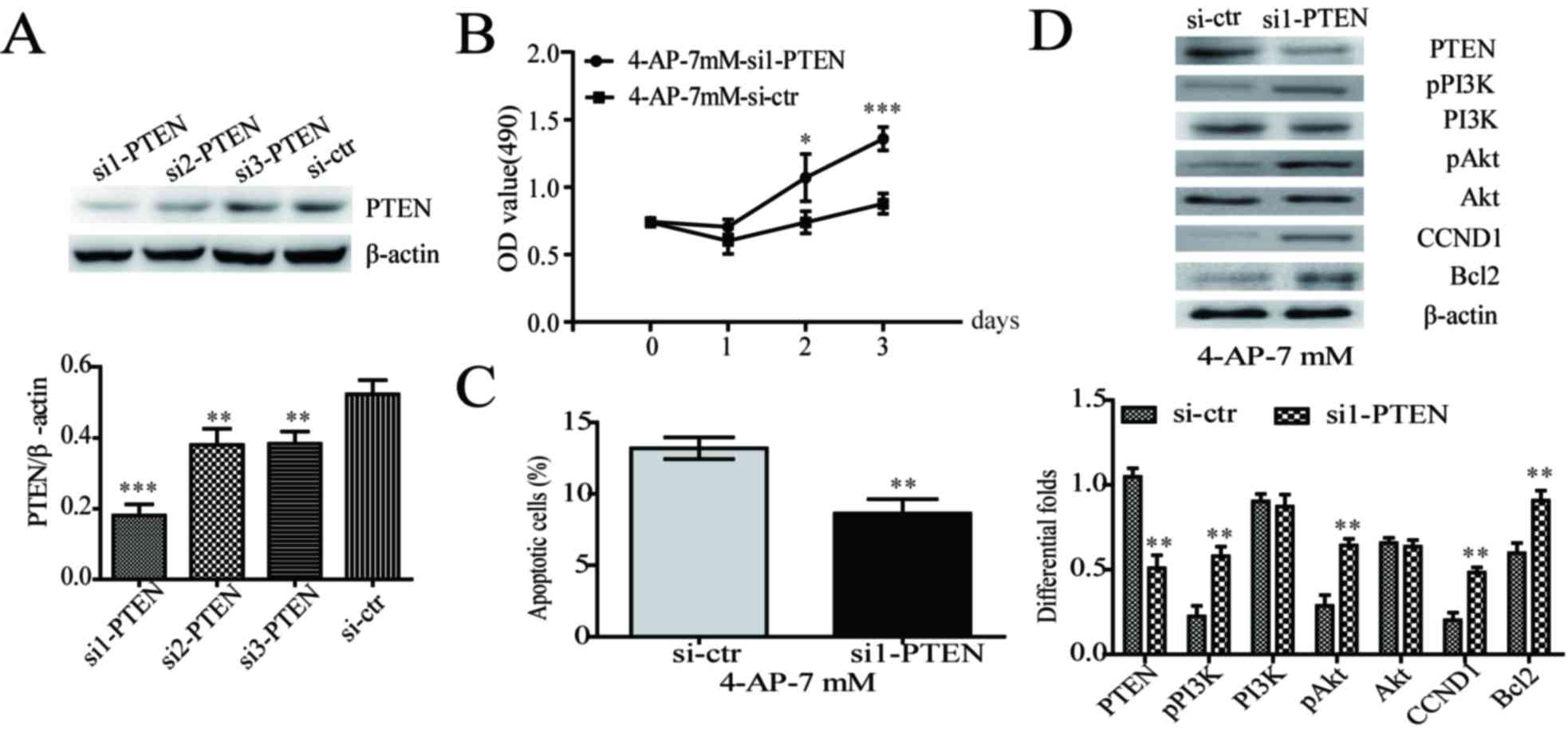
PTEN knockdown partially increased
aggressive phenotypes via upregulating PI3K/Akt signal in
4-AP-treated A549/CDDP cells
To better understand the role of PTEN in
4-AP-treated A549/CDDP cells, siRNA transfection was employed to
knockdown PTEN expression. Knockdown efficiency was evaluated by
western blot (Fig. 5A).
Transiently transfecting PTEN siRNA into 4-AP-treated A549/CDDP
cells not only significantly enhanced cell growth (Fig. 5B), but also inhibited cell
apoptosis in A549/CDDP cells (Fig.
5C). Further, PTEN knockdown upregulated the expression of
pPI3K (Tyr458), pAkt (Ser473), cyclin D1 and Bcl2 (Fig. 5D). These results indicated that
PTEN knockdown could partially increase the aggressive phenotypes
via upregulating PI3K/Akt signal in A549/CDDP cells with 4-AP
treatment.
Taken together, our results demonstrated that 4-AP
inhibited cell growth, induced cell apoptosis and sensitized
A549/CDDP cells to DDP through upregulating PTEN.
Discussion
4-AP, one of the most commonly used K+
channel inhibitors, suppresses proliferation and induces apoptosis
in various types of cancer cells (4–7).
However, the effect of 4-AP in A549/CDDP is largely unknown yet. In
the present investigation, 4-AP played an important role in
proliferation and apoptosis of A549/CDDP cells, which is consistent
with previous results (4–7). In addition, 4-AP enhances the
sensitivity of A549/CDDP cells to DDP. These findings suggest that
4-AP may have a wide range of antitumor effects.
In this study, we detected that 4-AP inhibited cell
growth of A549/CDDP in vitro and in vivo, and
retarded cell cycle progression. It is well known that high
proliferative activity of tumor cells is associated with the
increased cell-cycle transition (12). CCND1, a classic oncogenic protein
of cell cycle signal, promotes cell proliferation and the beginning
of S phase in the cell cycle in many cancers (13,14).
Here, we found that 4-AP-mediated growth suppression attributed to
cell cycle transition obstacle by repressing the expression of cell
cycle G1/S checkpoint proteins CCND1 and CDK4, and inducing the
expression of p21.
Apoptosis, also called type 1 cell death, may be
defined as suicidal cell death with a particular morphology
involving nuclear chromatin condensation (15,16).
As we know the aim of anti-cancer therapy is to induce apoptosis of
tumor cells. In this study, we found that 4-AP induced cell
apoptosis of A549/CDDP. 4-AP-mediated cell apoptosis attributed to
repressing the expression of Bcl2, an oncogenic protein inhibiting
programmed cell death (17), and
suppressing the expression of pro-caspase 9 and pro-caspase 3,
which typically requires processing at caspase cleavage sites to
generate the active enzyme cleaved caspase 9 and 3 (18) Once an initiator caspase is
activated, it processes others that cleave a host of cellular
proteins. A serial cascade reaction of caspase activation sentences
cell to death (17).
PI3K/Akt, a classical signal pathway, inhibits cell
apoptosis and induces cell-cycle progression (9). In this study, we found that 4-AP
treatment significantly suppressed the expression of pPI3K and
pAkt, and its downstream cell cycle and apoptosis signals. Further,
PTEN, a well-known tumor suppressor that inhibits the activation of
PI3K/Akt (19,20), was found to be upregulated in 4-AP
treated cells. Knocking down PTEN expression could increase the
aggressive phenotypes and activate PI3K/Akt signal in A549/CDDP
cells with 4-AP treatment. These findings suggest that
4-AP-mediated promotion of PTEN downregulates PI3K/Akt signaling,
which in turn inhibits cell growth and induces cell apoptosis in
A549/CDDP cells.
DDP is the most commonly used anti-cancer drug
(21). However, chemotherapy
resistance usually occurs mainly due to DDP resistance, the
tolerance of cancer cells to DDP-induced apoptosis in lung cancers
(3). The present study found 4-AP
sensitized A549/CDDP cells to DDP in the presence of 4-AP and DDP
together. These indicate that 4-AP may be used to improve the
efficacy of DDP-based chemotherapy in patients with lung
cancer.
Taken together, our results demonstrated that 4-AP
inhibited cell growth, induced cell apoptosis and enhanced the
sensitivity of A549/CDDP cells to DDP through upregulating PTEN.
4-AP may be used as an adjuvant therapy to improve the efficacy of
patients with DDP-resistant lung cancers.
Acknowledgements
The present study was supported by National Nature
Science Fund of China (grant nos. 81401906, 81502532) (http://www.nsfc.gov.cn), Natural Science Foundation of
Guangdong Province (2014A030310239) and Doctoral Fund of Affiliated
Hospital of Guangdong Medical University (grant no.
BJ20150003).
References
|
1
|
Califano R, Karamouzis MV, Banerjee S, de
Azambuja E, Guarneri V, Hutka M, Jordan K, Kamposioras K,
Martinelli E, Corral J, et al: Use of adjuvant chemotherapy (CT)
and radiotherapy (RT) in incompletely resected (R1) early stage
non-small cell lung cancer (NSCLC): A European survey conducted by
the European society for medical oncology (ESMO) young oncologists
committee. Lung Cancer. 85:74–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stewart DJ: Mechanisms of resistance to
cisplatin and carboplatin. Crit Rev Oncol Hematol. 63:12–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chin LS, Park CC, Zitnay KM, Sinha M,
DiPatri AJ Jr, Perillán P and Simard JM: 4-Aminopyridine causes
apoptosis and blocks an outward rectifier K+ channel in malignant
astrocytoma cell lines. J Neurosci Res. 48:122–127. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JA, Kang YS, Jung MW, Kang GH, Lee SH
and Lee YS: Ca2+ influx mediates apoptosis induced by
4-aminopyridine, a K+ channel blocker, in HepG2 human
hepatoblastoma cells. Pharmacology. 60:74–81. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W, Xiao J, Adachi M, Liu Z and Zhou
J: 4-aminopyridine induces apoptosis of human acute myeloid
leukemia cells via increasing [Ca2+]i through P2X7 receptor
pathway. Cell Physiol Biochem. 28:199–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang L, Li B, Li W, Guo H and Zou F:
ATP-sensitive potassium channels control glioma cells proliferation
by regulating ERK activity. Carcinogenesis. 30:737–744. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dai T, Zhou Q, Zeng X, He J, Yang Y, Li C,
Ren D, Liu L and Liao B: A study on the characteristics of the
membrane potassium channels in human non-small cell lung cancer
cell. Zhongguo Fei Ai Za Zhi. 4:281–286. 2001.(In Chinese).
PubMed/NCBI
|
|
9
|
Zhen Y, Liu Z, Yang H, Yu X, Wu Q, Hua S,
Long X, Jiang Q, Song Y, Cheng C, et al: Tumor suppressor PDCD4
modulates miR-184-mediated direct suppression of C-MYC and BCL2
blocking cell growth and survival in nasopharyngeal carcinoma. Cell
Death Dis. 4:e8722013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ru Q, Tian X, Wu YX, Wu RH, Pi MS and Li
CY: Voltage-gated and ATP-sensitive K+ channels are associated with
cell proliferation and tumorigenesis of human glioma. Oncol Rep.
31:842–848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Wu Q, Liu Z, Luo X, Fan Y, Liu Y,
Zhang Y, Hua S, Fu Q, Zhao M, et al: Downregulation of FAP
suppresses cell proliferation and metastasis through PTEN/PI3K/AKT
and Ras-ERK signaling in oral squamous cell carcinoma. Cell Death
Dis. 5:e11552014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang L, Wang HY, Li JD, Wang JH, Zhou Y,
Luo RZ, Yun JP, Zhang Y, Jia WH and Zheng M: KPNA2 promotes cell
proliferation and tumorigenicity in epithelial ovarian carcinoma
through upregulation of c-Myc and downregulation of FOXO3a. Cell
Death Dis. 4:e7452013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang W, Kahn SM, Zhou P, Zhang YJ, Cacace
AM, Infante AS, Doi S, Santella RM and Weinstein IB: Overexpression
of cyclin D1 in rat fibroblasts causes abnormalities in growth
control, cell cycle progression and gene expression. Oncogene.
8:3447–3457. 1993.PubMed/NCBI
|
|
14
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kroemer G, El-Deiry WS, Golstein P, Peter
ME, Vaux D, Vandenabeele P, Zhivotovsky B, Blagosklonny MV, Malorni
W, Knight RA, et al: Classification of cell death: Recommendations
of the nomenclature committee on cell death. Cell Death Differ. 12
Suppl 2:S1463–S1467. 2005. View Article : Google Scholar
|
|
16
|
Galluzzi L, Maiuri MC, Vitale I, Zischka
H, Castedo M, Zitvogel L and Kroemer G: Cell death modalities:
Classification and pathophysiological implications. Cell Death
Differ. 14:1237–1243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi Y: Mechanisms of caspase activation
and inhibition during apoptosis. Mol Cell. 9:459–470. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong-Dong L, Xi-Ran Z and Xiang-Rong C:
Expression and significance of new tumor suppressor gene PTEN in
primary liver cancer. J Cell Mol Med. 7:67–71. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo H, Yang Y, Duan J, Wu P, Jiang Q and
Xu C: PTEN-regulated AKT/FoxO3a/Bim signaling contributes to
reactive oxygen species-mediated apoptosis in selenite-treated
colorectal cancer cells. Cell Death Dis. 4:e4812013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Florea AM and Büsselberg D: Cisplatin as
an anti-tumor drug: Cellular mechanisms of activity, drug
resistance and induced side effects. Cancers (Basel). 3:1351–1371.
2011. View Article : Google Scholar : PubMed/NCBI
|