Introduction
Cranial nerve involvement frequently involves neuron
damage, and often leads to psychiatric disorder caused by multiple
inducements (1). Cranial nerve
involvement affects cognitive impairments and mental health, and
can lead to mental disability for patients worldwide (2). Patients with cranial nerve
involvement account for >5% of the population, and is a common
disabling mental disease with patients often suffering from complex
psychiatric with cognitive, language, memory and behavior
impairments (3,4). The key characteristics of cranial
nerve involvement include neuron damage and cognitive impairment
(5). However, the mechanism of
multiple lower cranial nerve involvement associated with neuron
damage and cognitive impairment remains to be fully elucidated.
Therefore, understanding the signaling pathway between cranial
nerve involvement and cognitive impairment is essential for the
treatment of patients with cranial nerve involvement.
The role of histamine in cranial nerve involvement
has received increasing attention in investigations. A previous
study reported that the expression levels of histamine were
upregulated in patients with cranial nerve involvement, formulating
a stable protein complex target for histamine, which is important
for maintaining the normal function of neurons, and critical for
neuronal differentiation and brain development (6,7). The
target for neuroprotective agents for neurocognitive repair and
inhibition of neuron-function damage developed according to
cognitive deficits across multiple domains in substantial
intellectual impairment (8,9). In
addition, although the theoretic mechanism of anti-histamine agents
in cranial nerve involvement are well understood, treatment of this
type of disease remains limited and lacks preclinical investigation
(10–12). Therefore, investing the mechanism
of anti-histamine in the treatment of cranial nerve involvement is
important to better explain therapeutic effects according to
observations.
Pharmaceutical studies have shown that
anti-histamine drugs are efficient for the treatment of cranial
nerve involvement (12,13). Lurasidone, is an azapirone
derivative and a second-generation novel antipsychotic candidate,
which was approved for the treatment of schizophrenia in the USA in
2010, and by the European Medicines Agency in 2014 (14,15).
A previous report indicated that cranial nerve involvement was
cured in some way following treatment with lurasidone in patients
with schizophrenia (12).
Additionally, lurasidone with lithium or valproate was identified
as a therapeutic strategy for the treatment of bipolar I depression
(15). The therapeutic mechanism
underlying the effects of lurasidone involved decreased levels of
serotonin 5-HT7, serotonin 5-HT2A, serotonin
5-HT1A and dopamine D2 by antagonist
activities (16).
In this present study, the therapeutic effects of
lurasidone were investigated in a cranial nerve involvement rat
model. On the basis of the aforementioned evidence, the present
study examined the preclinical outcomes of lurasidone for cranial
nerve involvement therapy. The data obtained suggested that
lurasidone repaired neuron-function loss and improved anxiety,
compared with a placebo. The results also suggested that cognitive
ability was improved following treatment with lurasidone.
Materials and methods
Analysis of serotonin receptors and
neuroprotective protein
Serotonin receptors and neuroprotective proteins in
serum were analyzed using a commercialized ELISA kit in patients
with cranial nerve involvement. A total of 180 patients and 124
healthy volunteers were recruited in Sichuan People's Hospital
between April 2014 and December 2014. All patients were required to
sign informed consent before the examinations. Serum was collected
from 10 ml blood using centrifugation at 6,000 × g for 10 min at
4°C. The ELISA assays were performed according to the
manufacturer's protocols. The results were measured at 450 nm in an
ELISA reader and finally converted to concentrations of serotonin
5-HT7, serotonin 5-HT2A, serotonin 5-HT1A,
serotonin 5-HT6, Forkhead-Box P2 (Foxp2), SxIP and
microtubule end-binding (EB) protein.
Animal experiments
Rats (n=100) of a mutant intravenously mutated
cranial nerve involvement Sprague-Dawley rat model (specific
pathogen-free; 6–8 weeks old) were purchased from Slack Co., Ltd.
(Shanghai, China). All rats were housed under controlled conditions
(temperature, 23±1°C, humidity, 55±5%) in a 12 h light/dark cycle
with free access to food and water. The rats with cranial nerve
involvement were randomly divided into two groups and injected
intravenously with either lurasidone (0.32 mg) or placebo (PBS,
0.32 mg) as a control. The total treatment regime comprised a total
of seven injections, once per day. All experimental procedures were
performed according to the guidelines of Sichuan Academy of Medical
Sciences, Sichuan People's Hospital (Sichuan, China). All
experiments were approved by the Animal Care and Use Committee of
Tianjin Medical (Tianjin, China).
Measurement of blood pressure, heart
rate and blood glucose parameters
Blood levels in the rats with cranial nerve
involvement were measured using a blood glucose gauge
(OneTouch® VerioVue; Johnson & Johnson Medical
(Shanghai) Ltd., Shanghai, China). The pressure was recorded prior
to and following treatment with lurasidone or placebo. Heart rate
and blood pressure parameters were measured every 2 days, as
described in a previous report (17).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from the hippocampal cells
of the rats with cortical cranial nerve involvement following
treatment with lurasidone or placebo using an RNAeasy Mini kit
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNA
(1 µg) was subjected to RT into cDNA using a reverse transcription
kit (Invitrogen; Thermo Fisher Scientific, Inc.). The cDNA (10 ng)
was used for qPCR analysis (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) with a SYBR-Green Master Mix system (Thermo Fisher
Scientific, Inc.) and a total reaction volume of 25 µl (primers, 1
µl, cDNA, 2 µl, buffer, 2 µl, reverse transcriptase, 0.5 µl, SYBR,
0.5 µl and water, 19 µl). All forward and reverse primers were
synthesized by Invitrogen; Thermo Fisher Scientific, Inc. (Foxp2,
forward: 5′-AACAGAGACCACTGCAGGTGCC-3′; reverse:
5′-TCCCTGACGCTGAAGGCTGAG−3′; SxIP, 5′-TATGGTCTCTGCCTGTTGC-3′,
5′-TGCTACTGCCCATTACAATTCC-3′; EB, forward:
5′-GGATTTGAATCACGTTTGTGTC-3′, reverse: 5′-AACTTGCGCTCATCTTAGGC-3′;
5-HT7, forward: 5′-AATAAGGGTAAGCCAATTGTATGGA-3′, reverse:
5′-TGGTGCAAAATCAACATTCC-3′; 5-HT6, forward:
5′-TATTACGAAGGCCAACCTAT-3′, reverse: 5′-TTCTTCTTCAGGCAAATCAT-3′;
5-HT1A, forward: 5′-TCAAAAAGAAAGGAG-3′, reverse:
5′-TCATCTGAGATAAGGGCTG-3′; 5-HT2A forward:
5′-TGTTTTAACGCCATTAGGTCA-3′, reverse: 5′-TCCGAGCAACTGATAAGTCT-3′
and β-actin, forward: 5′-CGGAGTCAACGGATTTGGTC-3′, reverse:
5′-AGCCTTCTCCATGGTCGTGA-3′).
Thermocycling conditions were as follows:
Pre-denaturation at 95°C for 90 sec, denaturation at 94.5°C for 30
sec and annealing at 56°C for 10 sec for 40 cycles. The alterations
in relative mRNA expression levels were calculated using the
2−ΔΔCq method (18),
with the results expressed as the n-fold, compared with the
control.
Western blot analysis
The hippocampal cells from the rats with cranial
nerve involvement treated with lurasidone or placebo were
homogenized in lysate buffer containing protease inhibitor and were
centrifuged at 6,000 × g at 4°C for 10 min. The supernatant was
used to analyze proteins. For detection of proteins, the proteins
were extracted using a protein extraction kit (Qiagen Sciences,
Inc., Gaithersburg, MD, USA) according to the manufacturer's
protocol. Protein concentration was measured using a BCA protein
assay kit (Thermo Fisher Scientific, Inc.). Protein samples (20
µg/lane) were resolved by 15% SDS-PAGE and then transferred onto
polyvinylidene fluoride membranes (Merck KGaA, Darmstadt, Germany)
as previously described (19). For
western blot analysis, primary antibodies: FoxP2 (ab16046), EB
(ab157217), SxIP (ab45142), β-actin (ab8227) (all, 1:200; Abcam,
Shanghai, China) were added for 12 h at 4°C following blocking in
5% skimmed milk for 1 h at 37°C. The sections were washed three
times with PBS to remove primary antibodies and then incubated with
HRP-labeled secondary goat anti-rabbit antibodies (ab150077;
1:2,000 dilution; Abcam) 24 h at 4°C. The results were visualized
using chemiluminescence detection system.
Immunofluorescence analysis
The therapeutic effects of lurasidone on neuronal
repair were evaluated using immunofluorescence staining of anti
neuroprotection-associated proteins in the hippocampus of the
experimental rats. Staining was performed on cerebral neurons of
the hippocampus in randomly-selected animals from the lurasidone or
placebo-treated groups. The immunofluorescence procedures were as
previously reported and captured using a fluorescence microscope
(FV3000; Olympus Corporation, Tokyo, Japan) at ×40 magnification
(20).
Experiments using the elevated plus
maze trial and analysis of swimming duration
The anxiety of the rats was evaluated using an
elevated plus maze trial based on the hypothesis that rat
experience fear of open fields. The details of the elevated plus
maze trial were as described in a previous study and the space was
improved to a size of 80×15×60 cm (21). The rats with cranial nerve
involvement were fixed at the center of the elevated plus maze, and
these rats were positioned facing an open arm for a total of 5 min.
The durations spent in the open and closed arms were recorded and
calculated using the following formula: D2=(B - A)/(B + A). A
represents the time spent in the open arm and the B represents the
time spent in the closed arm. The anxious behavior was measured
using the above formula and the path efficacy was calculated. The
behavioral capacity of the rats with cranial nerve involvement was
analyzed by swimming duration according to the method described in
a previous study (22).
Efficacy and safety assessment
Assessments of efficacy and dose-limiting toxicity
in the presence of lurasidone were performed in the present study.
Safety assessments included the incidence rates (≥10%) of the most
frequent treatment-emergent adverse events in a 30-day treatment
period in the experimental and control groups. The efficacy and
safety data included all rats in the cranial nerve involvement,
therapeutic drug and control groups.
Statistical analysis
All data are presented as the mean ± standard error
of the mean of triplicate experiments. All data were analyzed using
SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA). Unpaired data was
determined using Student's t test and comparisons of data between
multiple groups were analyzed using one-way analysis of variance.
Kaplan-Meier analysis was used to estimate the risk of relapse and
re-treatment during the 368-day treatment period. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of the cranial nerve
involvement rat model
Adult Sprague-Dawley (6–10 weeks old) rats with
cranial nerve involvement were subjected to cerebral artery
occlusion and reperfusion and examined in the designed experiments.
The rats in the cranial nerve involvement model received lurasidone
treatment or placebo treatment as a control. The preclinical
parameters of cranial nerve involvement, including body
temperatures, body weight, blood pressure, heart rate and blood
glucose, were recorded prior to and following treatment. The
characteristics of the rats and patients with cranial nerve
involvement are summarized in Table
I. The data indicated that the states of the rats with cranial
nerve involvement, determined using the Positive and Negative
Syndrome Scale (PANSS) were improved following lurasidone
treatment, compared with the placebo. In addition, physiological
parameters exhibited significant differences between the lurasidone
and placebo groups. Of note, the mean blood pressure and heart rate
during the experiment were significantly different between the
lurasidone and placebo groups. In addition, the MTD was identified
and the most common treatment-associated adverse events were
hypertension, diarrhea, lethargy, rash, proteinuria and vomiting
(Table II; ≥10%). A dose of 0.32
mg of lurasidone met criteria for further preclinical experiments
in terms of the tolerability and therapeutic effects.
 | Table I.Characteristics of patients and rats
with cranial nerve involvement. |
Table I.
Characteristics of patients and rats
with cranial nerve involvement.
| Parameter | n | % |
|---|
| Total healthy
volunteers | 180 | 100 |
| Men | 85 | 47 |
| Women | 95 | 53 |
| Total patients | 124 | 100 |
| Men | 61 | 49 |
| Women | 63 | 51 |
| Total rats with
cranial nerve involvement | 100 | 100 |
| Male | 50 | 50 |
| Female | 50 | 50 |
| Positive and
negative syndrome scale score | 121.4±6.2 | − |
| Body weight
(g) | 132±10.5 | − |
| Blood glucose
(mmol/l) | 8.3±2.5 | − |
| Blood pressure (mm
Hg) | 143±12 | − |
| Heart rate | 370±26 | − |
| Drug therapy | 80 | 100 |
| Placebo | 40 | 50 |
| Lurasidone | 40 | 50 |
 | Table II.Treatment-associated adverse effects
of lurasidone with overall incidence ≥10%. |
Table II.
Treatment-associated adverse effects
of lurasidone with overall incidence ≥10%.
|
| Lurasidone (n=20
per group) |
|---|
|
|
|
|---|
|
| Total | 0.08–0.16 | 0.25–0.32 |
|
|---|
| Adverse effect | (n=60) | mg | mg | 0.40 mg |
|---|
| Hypertension | 12 | 3 | 4 | 5 |
| Diarrhea | 7 | 1 | 3 | 3 |
| Proteinuria | 9 | 2 | 3 | 4 |
| Vomiting | 10 | 2 | 3 | 5 |
| Lethargy | 8 | 2 | 3 | 3 |
| Rash | 9 | 2 | 3 | 4 |
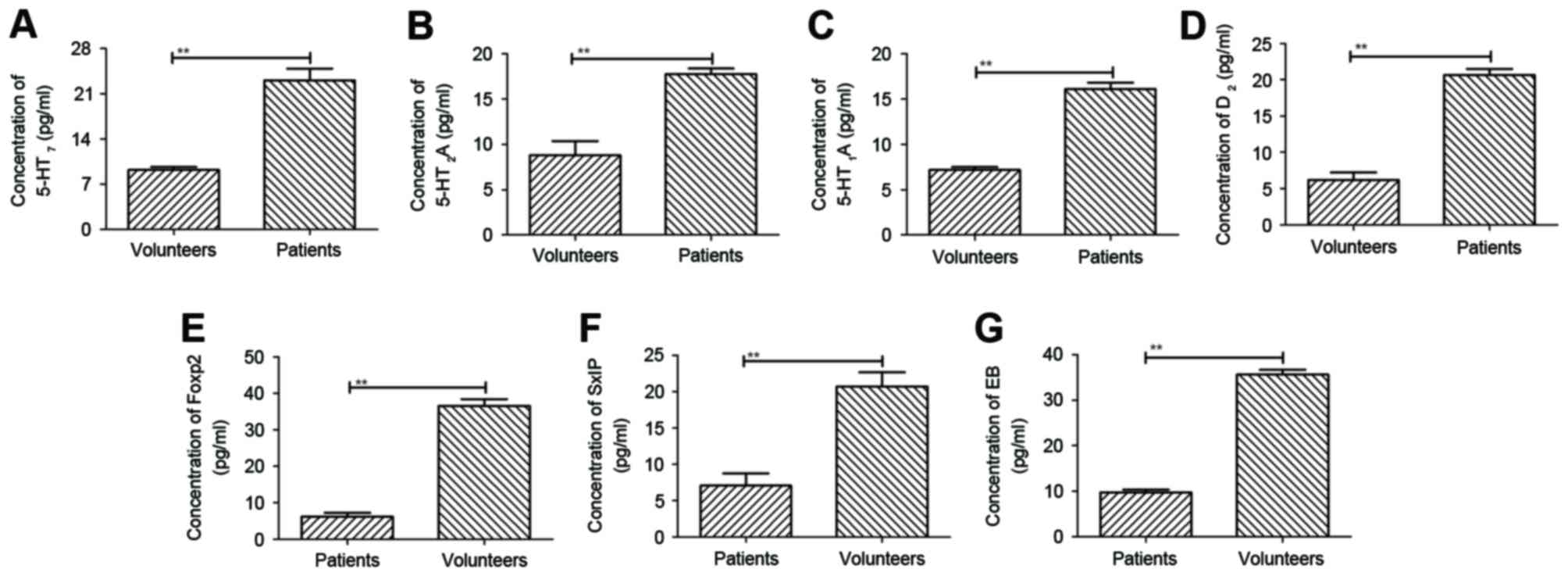
Detection of serum histamine and
neuroprotective proteins in patients with cranial nerve
involvement
A previous study indicated that histamine chemical
compounds were upregulated in patients with cranial nerve
involvement (23). In the present
study, histamine chemical compounds, including serotonin 5-HT7,
serotonin 5-HT2A, serotonin 5-HT1A, serotonin
5-HT6, and the neuroprotective protein, Foxp2, SxIP
motif and EB protein, were detected in sera of patients with
schizophrenia. The concentrations of histamine chemical compound in
the serum were elevated in patients with cranial nerve involvement,
compared with healthy volunteers. The results, as shown in Fig. 1A-D, showed that plasma
concentration levels of serotonin 5-HT7, serotonin
5-HT2A, serotonin 5-HT1A and serotonin
5-HT6 were downregulated, as determined using ELISA,
compared with those of healthy volunteers. In addition, plasma
concentration levels of three important neuroprotective protein,
Foxp2, SxIP motifi and EB protein, were decreased in patients with
cranial nerve involvement, compared with healthy volunteers
(Fig. 1E-G). These results
suggested that the expression levels of histamine were
downregulated and may be associated with the expression of
neuroprotective proteins.
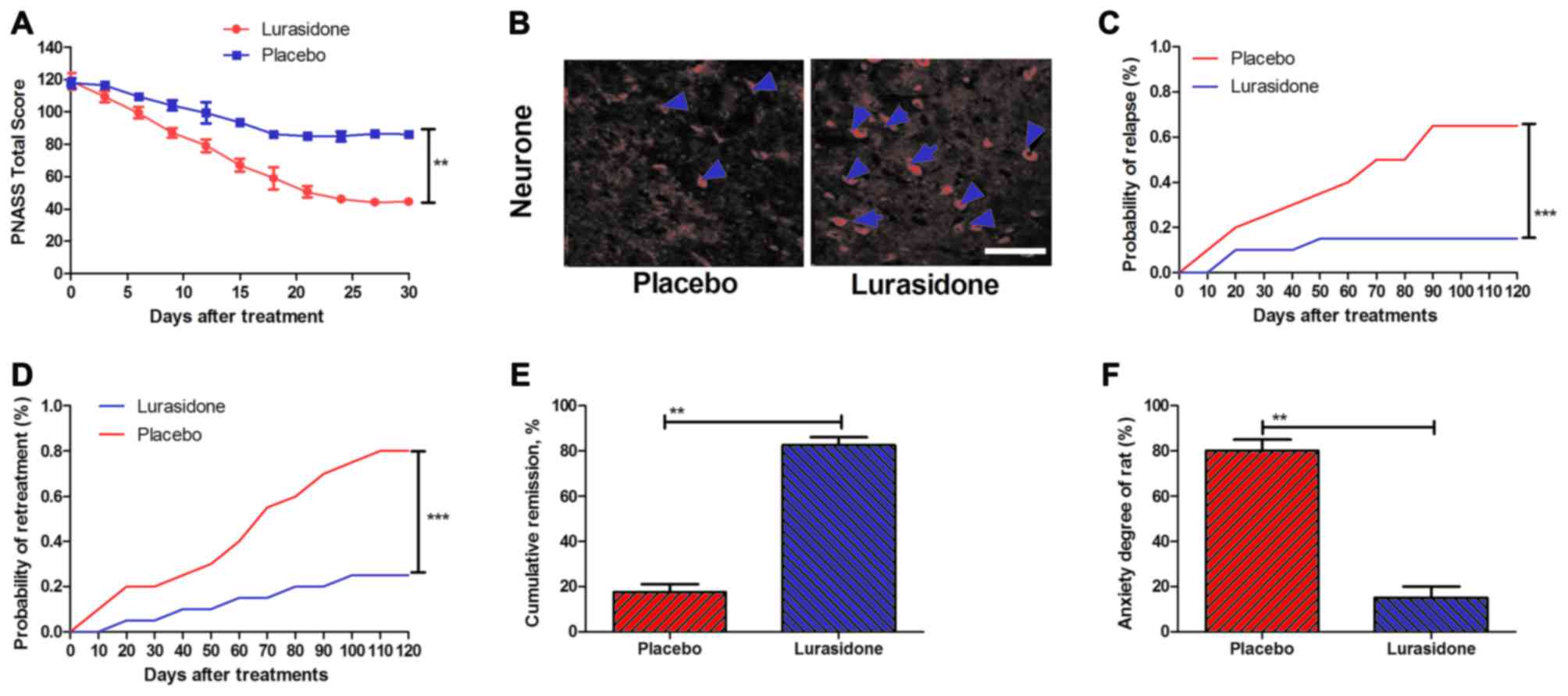
Therapeutic effects of lurasidone in
the cranial nerve involvement rat model
To investigate the beneficial preclinical outcomes
of lurasidone, a rat model of cranial nerve involvement model was
established. The results (Fig. 2A)
indicated that the total PANSS score was significantly reduced in
the rats with cranial nerve involvement treated with lurasidone
compared with those treated with placebo. In addition, neuron
impairment was analyzed on day 30 following lurasidone or placebo
treatment. The results (Fig. 2B)
showed that neuron impairment was significantly reduced in the
hippocampus of lurasidone-treated mice. In addition, the risks of
relapse and re-treatment in a 180-day observation period were
analyzed following treatment with lurasidone, compared with the
placebo. P-values were 0.00063 and 0.00048 for relapse rate and
re-treatment, respectively (Fig. 2C
and D). Furthermore, as shown in Fig. 2E and F, the lurasidone-treated mice
had significantly higher rates of remission and reduced anxiety,
compared with the placebo (P=0.0075 and P=0.0086, vs. placebo,
respectively).
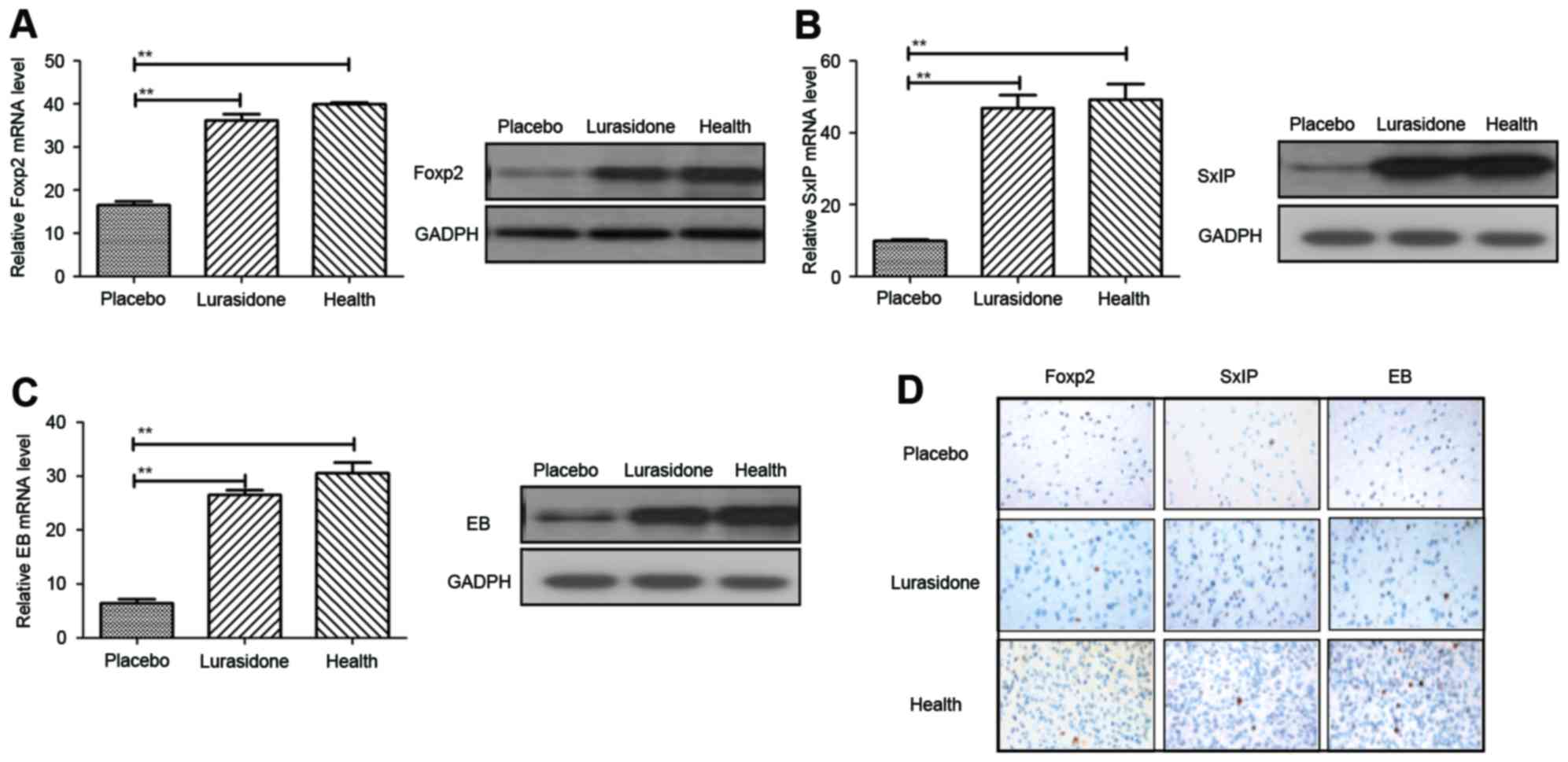
Neuroprotective effect of lurasidone
in rats with cranial nerve involvement
The short-term and long-term effects of lurasidone
were apparent in mice with cranial nerve involvement. In order to
obtain further insight into the primary mechanism of lurasidone
efficacy, the expression levels of Foxp2, SxIP and EB, and the
distribution of neurons in the hippocampus were analyzed using
RT-qPCR and immunohistochemcal analyses. The results (Fig. 3A) showed that the Foxp2 gene,
associated with language competence, was increased in the
hippocampus of the cranial nerve involvement rats following
treatment with lurasidone (P=0.00044), compared with those in the
placebo-treated group. In addition, the expression levels of
neuroprotective SxIP motif and EB proteins were also upregulated
following treatment with lurasidone, which led to neuroprotection
and synaptic plasticity (Fig. 3B and
C; P=0.0052 and P=0.0066, vs. placebo, respectively). As shown
in Fig. 3D, the immunohistology
revealed that neuron distributions were homogeneous in the
hippocampus following treatment with lurasidone, compared with the
placebo.
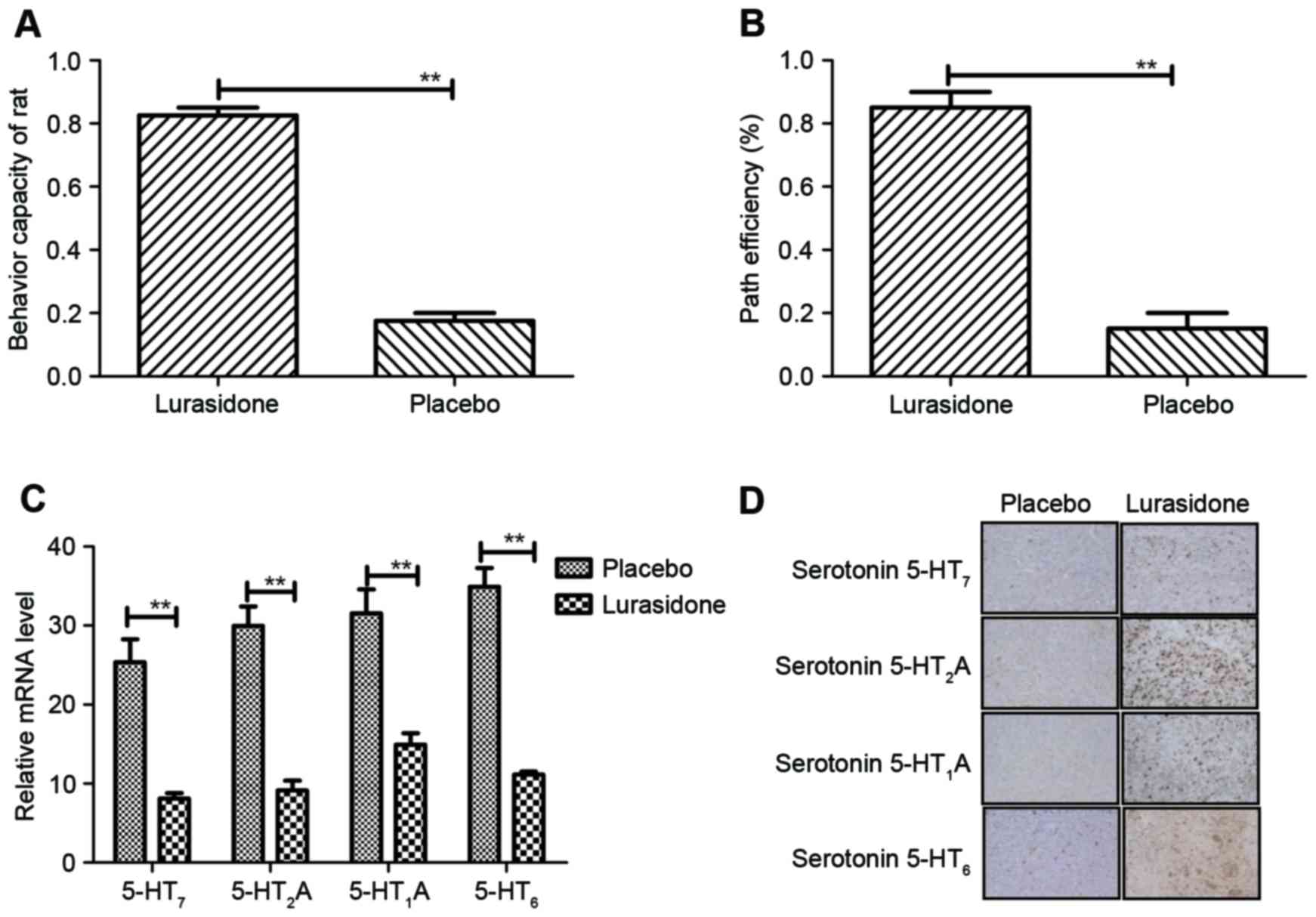
Antagonistic effect of lurasidone on
histamine in rats with cranial nerve involvement
An important characteristic of cranial nerve
involvement is anxiety. It was concluded that anxiety was in
remission according to the experimental data. The behavior of rats
with cranial nerve involvement was assessed using an improved water
maze and recognition test. The results (Fig. 4A) indicated that the behavior of
the rats with cranial nerve involvement improved following
treatment with lurasidone, compared with the placebo (P=0.0059, vs.
placebo). In addition, the data showed that lurasidone
significantly improved cognitive impairment in rats with cranial
nerve involvement (P=0.0059, vs. placebo; (Fig. 4B). Changes of histamine, including
serotonin 5-HT7, serotonin 5-HT2A, serotonin
5-HT1A, and serotonin 5-HT6 in the serotonin
systematic signaling pathway were also examined. The results
(Fig. 4C) showed that the
concentration levels of serotonin were downregulated in the
cerebrospinal fluid of the lurasidone-treated rats, compared with
the placebo-treated rats. The same observations were found in the
hippocampus of cranial nerve involvement mice between the
lurasidone and placebo groups (Fig.
4D). These data indicated that the serotonin systematic
signaling pathway was inhibited by the antagonistic effect of
lurasidone, which led to beneficial outcomes in behavior and
cognitive improvements in the rats with cranial nerve
involvement.
Discussion
Currently, although several factors have been
reported to exhibit correlation with the initiation and development
of cranial nerve involvement, the mechanism of interactions between
cranial nerve involvement and affecting factors remain to be fully
elucidated (24). Several
potentially neuroprotective agents function to upregulate gene
transcription at the onset of brain ischemia, which may be an
effective approach for limiting brain tissue damage (25). Studies on the mechanisms of cranial
nerve involvement have shown that the serotonin systematic
signaling pathway shows high correlation with the initiation,
development, treatment and prognosis of cranial nerve involvement
(26,27). In addition, an increasing number of
studies have reported that the overexpression of histamine in the
cerebrospinal fluid is a potential therapeutic target in patients
with cranial nerve involvement and has been found be important in
the pathogenesis of cranial nerve involvement (28–31).
Targeted therapy for cranial nerve involvement is a
novel concept and the rationale for the trial in a mouse model of
cranial nerve involvement, which was performed in a previous study
(32). Serotonin is one of the
most important predisposing and aggravating factors, which
orchestrate the responses to neuron impairment and other exogenous
insults (33). A previous study
reported that serotonin was significantly correlated with neuron
loss and continuation of non-motor symptoms in patients with
Parkinson's disease treated with dopamine grafts (34). Serotonin has been shown to
stimulate GnRH neuron excitability to exert biphasic actions
through 5-HT1 and 5-HT2 receptors in the mouse indicated that 5-HT1
and 5-HT2 receptors were indicated as potential targets in the
treatment of neuron impairment (31). Serotonergic neuron dysfunction in
the hippocampal area is regulated by extracellular serotonin
receptors, leading to hyperexcitability and discharge in the
neurons (35). These findings
suggest that the inhibition activity of the serotonin serotonergic
neurons signaling pathway may benefit patients with cranial nerve
involvement in clinical treatment.
A second antipsychotic agent, lurasidone, has been
approved for the treatment of patients with schizophrenia and was
identified as an adjunctive therapy with lithium or valproate for
the treatment of bipolar I depression (15). In the present study, lurasidone was
selected to investigate therapeutic efficacy in mice with cranial
nerve involvement. Lurasidone is similar to the majority of
antipsychotic drugs, in that it possesses an antagonist at
serotonin 5-HT7, serotonin 5-HT2A, serotonin
5-HT1A and serotonin 5-HT6, respectively
(12). However, reports on the
clinical use of antipsychotic treatment for cranial nerve
involvement are limited and primary mechanism of lurasidone as a
long-term medicine remains to be fully elucidated.
In the present study, the efficacy of lurasidone in
a rat model of cranial nerve involvement was evaluated. The
antagonist of lurasidone for serotonin receptors was confirmed and
demonstrated promising pharmacological function for the treatment
of patients with cranial nerve involvement. The data demonstrated
that 0.32 mg of lurasidone once a day was beneficial in the
treatment of cranial nerve involvement. The body weight of the rats
was minimally affected, and hypertension and heart rate parameters
were low risk in terms of clinically meaningful alterations. Of
note, cognitive competence was significantly improved following
treatment with lurasidone, and lurasidone downregulated histamine
receptors via the serotonin serotonergic neuron signaling pathway
and increased the expression of neuroprotective proteins by
impaired neurons. In conclusion, lurasidone showed efficacy in a
rat model of cranial nerve involvement, and indicated that
lurasidone may offer potential for application in the treatment of
cranial nerve involvement.
References
|
1
|
Sakakibara Y, Mori M, Kuwabara S, Katayama
K, Hattori T, Koga M and Yuki N: Unilateral cranial and phrenic
nerve involvement in axonal Guillain-Barré syndrome. Muscle Nerve.
25:297–299. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sahin E, Yilmaz A, Ersöz G, Uğuz M and
Kaya A: Multiple cranial nerve involvement caused by Brucella
melitensis. South Med J. 102:855–857. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki T: A further consideration on
long-acting injectable versus oral antipsychotics in the treatment
of schizophrenia: A narrative review and critical appraisal. Expert
Opin Drug Deliv. 13:253–264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bortolato B, Miskowiak KW, Köhler CA,
Vieta E and Carvalho AF: Cognitive dysfunction in bipolar disorder
and schizophrenia: A systematic review of meta-analyses.
Neuropsychiatr Dis Treat. 11:3111–3125. 2015.PubMed/NCBI
|
|
5
|
Ushio M, Iwasaki S, Sugasawa K and
Murofushi T: Superficial siderosis causing retrolabyrinthine
involvement in both cochlear and vestibular branches of the eighth
cranial nerve. Acta Otolaryngol. 126:997–1000. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bahi A, Schwed JS, Walter M, Stark H and
Sadek B: Anxiolytic and antidepressant-like activities of the novel
and potent non-imidazole histamine H3 receptor
antagonist ST-1283. Drug Des Devel Ther. 8:627–637. 2014.PubMed/NCBI
|
|
7
|
Borella L, Russell J, Rimele TJ, Grimes D,
Failli A and Mir GN: Antisecretory and antiulcer activities of a
potent new histamine H2-receptor antagonist with an intermediate
duration of action. Arzneimittelforschung. 38:366–372.
1988.PubMed/NCBI
|
|
8
|
Kent JS, Bolbecker AR, O'Donnell BF and
Hetrick WP: Eyeblink conditioning in schizophrenia: A critical
review. Front Psychiatry. 6:1462015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McEvoy J and Citrome L: Brexpiprazole for
the treatment of schizophrenia: A review of this novel
serotonin-dopamine activity modulator. Clin Schizophr Relat
Psychoses. 9:177–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naito Y, Yoshikawa T, Matsuyama K, Yagi N,
Nakamura Y, Nishimura S, Kaneko T, Yoshida N and Kondo M: Effect of
the histamine H2-receptor antagonist
(+/−)-(E)-1-[2-hydroxy-2-(4-hydroxyphenyl)ethyl]-3′-[2-[[[5-methylamino)methyl-2-furyl]methyl]thio]ethyl]-2′-(methylsulfonyl)guanidine
on acute gastric mucosal injury in rats and its free-radical
scavenging activities. Arzneimittelforschung. 47:845–848.
1997.PubMed/NCBI
|
|
11
|
Ishikawa H, Ito H, Higaki M, Higaki M,
Matsumoto Y, Kamimura T, Katsura Y, Tomishi T, Inoue Y, Takasugi H,
et al: FR145715, a novel histamine H2 receptor antagonist, with
specific anti-Helicobacter pylori activities. Eur J Pharmacol.
378:299–310. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishibashi T, Horisawa T, Tokuda K,
Ishiyama T, Ogasa M, Tagashira R, Matsumoto K, Nishikawa H, Ueda Y,
Toma S, et al: Pharmacological profile of lurasidone, a novel
antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and
5-HT1A receptor activity. J Pharmacol Exp Ther. 334:171–181. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loebel A and Citrome L: Lurasidone: A
novel antipsychotic agent for the treatment of schizophrenia and
bipolar depression. BJPsych Bull. 39:237–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Citrome L: Lurasidone for schizophrenia: A
review of the efficacy and safety profile for this newly approved
second-generation antipsychotic. Int J Clin Pract. 65:189–210.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Loebel A, Cucchiaro J, Silva R, Kroger H,
Sarma K, Xu J and Calabrese JR: Lurasidone as adjunctive therapy
with lithium or valproate for the treatment of bipolar I
depression: A randomized, double-blind, placebo-controlled study.
Am J Psychiatry. 171:169–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Citrome L: Lurasidone in schizophrenia:
New information about dosage and place in therapy. Adv Ther.
29:815–825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nunes PM, Wright AJ, Veltien A, van Asten
JJ, Tack CJ, Jones JG and Heerschap A: Dietary lipids do not
contribute to the higher hepatic triglyceride levels of
fructose-compared to glucose-fed mice. FASEB J. 28:1988–1997. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wai-Hoe L, Wing-Seng L, Ismail Z and
Lay-Harn G: SDS-PAGE-Based quantitative assay for screening of
kidney stone disease. Biol Proced Online. 11:145–160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dirani M, Nasreddine W, Abdulla F and
Beydoun A: Seizure control and improvement of neurological
dysfunction in Lafora disease with perampanel. Epilepsy Behav Case
Rep. 2:164–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vaisburd S, Shemer Z, Yeheskel A, Giladi E
and Gozes I: Risperidone and NAP protect cognition and normalize
gene expression in a schizophrenia mouse model. Sci Rep.
5:163002015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berezova IV, Shishkina GT, Kalinina TS and
Dygalo NN: Behavior in the forced-swimming test and expression of
BDNF and Bcl-xl genes in the rat brain. Zh Vyssh Nerv Deiat Im I P
Pavlova. 61:332–339. 2011.(In Russian). PubMed/NCBI
|
|
23
|
Garcia-Calero E, Botella-Lopez A,
Bahamonde O, Perez-Balaguer A and Martinez S: FoxP2 protein levels
regulate cell morphology changes and migration patterns in the
vertebrate developing telencephalon. Brain Struct Funct.
221:2905–2917. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chebel S, Letaief L, Boughammoura-Bouatay
A, Dachraoui F, Ouanes I, Ouanes-Besbes L, Abroug F and Frih-Ayed
M: Multiple cranial nerve involvement: Consider the diagnosis of
cephalic tetanus. A case report and review of the literature. Rev
Neurol (Paris). 166:948–950. 2010.(In French).
|
|
25
|
Mabray MC, Glastonbury CM, Mamlouk MD,
Punch GE, Solomon DA and Cha S: Direct cranial nerve involvement by
gliomas: Case series and review of the literature. AJNR Am J
Neuroradiol. 36:1349–1354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mijajlovic M, Mirkovic M,
Mihailovic-Vucinic V, Aleksic V and Covickovic-Sternic N:
Neurosarcoidosis: Two case reports with multiple cranial nerve
involvement and review of the literature. Biomed Pap Med Fac Univ
Palacky Olomouc Czech Repub. 158:662–667. 2014.PubMed/NCBI
|
|
27
|
Galassi G, Albertini G, Valzania F and
Barbieri A: Cranial nerve involvement as presenting sign of
multifocal motor neuropathy. J Clin Neurosci. 19:1733–1735. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Watanabe Y, Someya T and Nawa H: Cytokine
hypothesis of schizophrenia pathogenesis: Evidence from human
studies and animal models. Psychiatry Clin Neurosci. 64:217–230.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Witte L, Tomasik J, Schwarz E, Guest
PC, Rahmoune H, Kahn RS and Bahn S: Cytokine alterations in
first-episode schizophrenia patients before and after antipsychotic
treatment. Schizophr Res. 154:23–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Debnath M and Chaudhuri TK: The role of
HLA-G in cytokine homeostasis during early pregnancy complicated
with maternal infections: A novel etiopathological approach to the
neurodevelopmental understanding of schizophrenia. Med Hypotheses.
66:286–293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bhattarai JP, Roa J, Herbison AE and Han
SK: Serotonin acts through 5-HT1 and 5-HT2 receptors to exert
biphasic actions on GnRH neuron excitability in the mouse.
Endocrinology. 155:513–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Girgis RR, Kumar SS and Brown AS: The
cytokine model of schizophrenia: Emerging therapeutic strategies.
Biol Psychiatry. 75:292–299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Andrade R and Haj-Dahmane S: Serotonin
neuron diversity in the dorsal raphe. ACS Chem Neurosci. 4:22–25.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Politis M, Wu K, Loane C, Quinn NP, Brooks
DJ, Oertel WH, Björklund A, Lindvall O and Piccini P: Serotonin
neuron loss and nonmotor symptoms continue in Parkinson's patients
treated with dopamine grafts. Sci Transl Med. 4:128ra412012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mlinar B, Montalbano A, Baccini G, Tatini
F, Berlinguer Palmini R and Corradetti R: Nonexocytotic serotonin
release tonically suppresses serotonergic neuron activity. J Gen
Physiol. 145:225–251. 2015. View Article : Google Scholar : PubMed/NCBI
|