Introduction
The lack of adult mammalian hair cell regeneration
after damage leads to permanent hearing loss, which for years has
attracted attention to manipulating stem/progenitor cells to
participate in hair cell regeneration. To date, cells dissociated
from the neonatal mammalian basilar membrane have been proved to
re-enter the cell cycle and have shown a limited ability to
proliferate and differentiate into hair cells in vitro
(1).
Typically, only a small fraction of the dissociated
cells is able to grow into colonies and ultimately give rise to
hair cells. Sinkkonen et al purified four different
non-sensory cell populations from neonatal mouse cochleae by
fluorescence-activated cell sorting (FACS). They found that
cochlear-supporting cells and cells of the lesser epithelial ridge
showed robust potential to proliferate and differentiate into hair
cells (2). Shi et al found
that Lgr5-expressing supporting cells, as sorted by flow cytometry,
displayed enhanced capacity for self-renewing neurosphere formation
in response to Wnt and were converted into hair cells at a higher
(>10-fold) rate than unsorted cells were. Lgr5-positive cells
had the capacity to act as cochlear progenitor cells, and lineage
tracing confirmed that Lgr5-expressing cells accounted for the
cells that formed neurospheres and differentiated into hair cells
(3). Furthermore, the
proliferative ability of dissociated cochlear cells is transient,
and it ceased after the second postnatal week in mice (4,5).
Several genes expressed in the inner ear during postnatal
development were proved to be involved in maintaining the
proliferative potential of progenitor cells, but the mechanism for
regulating the proliferation and differentiation of cochlear
progenitor cells remains relatively poorly understood.
Telomerase, a reverse transcriptase in eukaryocytes,
is active in most stem cells, tumor cells and embryonic tissues.
The main function of telomerase is to maintain the integrity of
chromosomes and the cell cycle. The telomerase complex is composed
of two main subunits that provide the enzymatic core function:
Telomerase reverse transcriptase (TERT) and an RNA component
(TERC). TERC is ubiquitously expressed in normal somatic cells,
which serve as a template for telomeric DNA synthesis. TERT
expression is limited to highly proliferative tissues; thus, the
presence of TERT is rate limiting for telomerase activity (6). The overexpression of TERT could
promote proliferation and immortalization in several series of
cells (7,8), but the role of TERT in the inner ear
remains unclear.
In this study, we evaluated the expression of TERT
in the cochlear basilar membrane during postnatal development (P0,
P3, P7, P14, P28). Further, the differential TERT levels in
cochlear progenitor cells of different culture times, in different
otospheres with distinct morphologies and in different generations
were evaluated by RT-qPCR. This study allowed us to evaluate for
the first time the role of TERT in the development of the cochlea
and the proliferation process of cochlear progenitors, thereby
providing evidence for the application of TERT in regulating
cochlear progenitor cell proliferation and hair cell
regeneration.
Materials and methods
Animals and cochlear dissection
Sprague-Dawley (SD) rats were provided by the
Laboratory Animal Center of the Air Force Military Medical
University. Experiments were conducted under protocols approved by
the Animal Care and Use Committee of the Air Force Military Medical
University.
P0 rats were anesthetized on ice, and the others
were deeply anesthetized by intraperitoneally administration of an
over-dose of choral hydrate (45 mg/kg). After anesthesia, rats at
different ages (P0, P3, P7, P14, P28) were sacrificed by cervical
dislocation, and the skulls were opened midsagitally. Using a
dissecting microscope (SZX10; Olympus, Tokyo, Japan), the cochleae
were dissected from the temporal bone followed by removal of the
otic bulla to visualize the otic capsule. For ex vivo
culture, the membranous labyrinth from P0-P3 rats was exposed, and
the cochlear duct was retrieved after excision of the cartilage.
The cochlear basilar membranes were micro-dissected from Reissner's
membrane, the spiral ligament, and the stria vascularis and were
then washed in ice-cold Hank's Balanced Salt Solution (HBSS;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
collected for further use.
For in vitro cell culturing, the harvested
sheets were inspected, rinsed in sterile, chilled Hanks' balanced
salt solution, and processed for cell dissociation.
Cell dissociation and sphere
generation
The basilar membranes were incubated in D-Hanks'
solution containing 0.5 mg/ml thermolysin (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) for 20 min at 37°C, and the digestive
enzymes were blocked by 10% fetal bovine serum in Dulbecco's
modified Eagle's medium/F12 medium (Gibco; Thermo Fisher
Scientific, Inc.). The tissue was triturated carefully 30–50 times
with glass pipette tips. The suspension was passed through a 70 µm
cell trainer to remove clumps and cellar aggregates. The collected
cells were cultured at a density of 1×104 cells/ml in
serum-free medium consisting of DMEM/F12 (Gibco; Thermo Fisher
Scientific, Inc.), supplemented with N2 and B27, bFGF (10 ng/ml),
and ampicillin (50 µg/ml) (all from Sigma-Aldrich; Merck KGaA) in
24-well suspension culture plates. The otospheres were collected at
days 2, 4, and 7 for study. The morphological description of sphere
formation was the same as previously reported (4). Briefly, the morphologies of the three
types of otospheres were distinguished, the numbers were counted
under a light microscope (5 wells) at days 2, 4, and 7, and the
ratio of each type of otosphere was calculated. For propagation,
otospheres were collected and dissociated from the cells
mechanically after treatment with 0.125% trypsin at 37°C for 5 min,
and the cells were reflated in sphere culture medium for 3–5 days
to obtain a second generation.
Cell proliferative capability
evaluation
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) solution (MTT assay kit; Sigma-Aldrich; Merck KGaA)
was applied to evaluate the cell proliferation rates in
vitro culture. Briefly, the cells dissociated from basilar
epithelial sheets and were plated in 96-well dishes at 3,000
cells/well. The time-points for MTT assay were from day 1 to 7
after culture. After the predetermined time-points of incubation,
10 µl of 10 mg/ml MTT solution was added and incubated for 4 h.
Then, the medium was removed, and 150 µl dimethyl sulfoxide (DMSO)
was added, followed by shaking for 10 min. The optical density of
the solutions in the wells was measured at 490 nm using a
photometer (MK3 Multilabel Plate Reader; Thermo Fisher Scientific,
Inc.).
Immunocytochemistry
The cochlear from P0, P7, P14, P28 were fixed with
4% paraformaldehyde in 0.1 M PBS for 6 h at room temperature and
then dehydrated by 30% sucrose overnight, then the slices were
prepared in 10 µm of thickness. The cells were fixed with 4%
paraformaldehyde in 0.1 M PBS for 15 min at room temperature and
were then washed in 0.01 M PBS for 5 min three times. For
immunofluorescent staining, the slices and fixed cells were treated
with 1% Triton X-100 (Sigma-Aldrich; Merck KGaA) on ice for 5 min.
Non-specific binding sites were blocked for 1 h in PBS containing
0.2% Triton X-100 and 5% bovine serum solution. Primary antibodies
of mouse anti-nestin (1:100; Millipore, Billerica, MA, USA) and
rabbit anti-TERT (1:50; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) were incubated in PBS with 5% BSA and 0.2% Triton X-100
overnight at 4°C. Then, the secondary antibodies conjugated with
Alexa Flour 594 or 488 (1:500; Invitrogen; Thermo Fisher
Scientific, Inc.) were added for 2 h at room temperature, and the
nuclei were stained by DAPI (4′,6-diamidino-2-phenylindole,
1:1,000; Sigma-Aldrich; Merck KGaA). Specimens were evaluated under
a confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Negative control experiments were performed as above by omitting
the primary antibody.
RT-qPCR
Total RNA was isolated from cultured otospheres with
RNeasy Micro kits, and 500 ng of total RNA was used for reverse
transcription (both from Qiagen, Valencia, CA, USA). Polymerase
chain reaction (PCR) analysis was performed with an ABI PRISM 7500
fast real-time PCR system (Applied Biosystems, Foster City, CA,
USA). The 20 µl reaction mixture contained 10 µl of 2× Green PCR
Master Mix, 1 µl of cDNA template, 1 µl of each primer (10 nm), and
7 µl of RNase-free water. The reaction was performed at 95°C for 5
min, followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec.
A sample without cDNA template was processed in parallel and served
as a negative control. All of the RT-qPCR reactions were performed
in triplicate, with the resultant values combined into an average
cycle threshold (cq). The Δcq method with GAPDH as the endogenous
reference was used to determine the relative levels of gene
expression.
The primers were as follows: PCNA sense,
5′-GCAACTTGGAATCCCAGAACAGG-3′ and antisense,
5′-CGCAGAAAACTTCACCCCGTC-3′. Other primers were provided by
GeneCopoeia, Rockville, MD, USA, including TERT (cat. no.
RQP051634), nestin (cat. no. RPQ049303), and GAPDH (cat. no.
RQP049537).
Western blot analysis
Isolated basilar membrane sheets were collected and
homogenized in ice-cold protein lysis buffer (Sigma-Aldrich; Merck
KGaA) containing NaCl 150 mM, Tris 50 mM, NP-40 1%, DOC 0.5%, SDS
0.1% and protein inhibitor cocktail (1 mM). After incubation for 30
min on ice, the samples were centrifuged at 14,000 rpm for 30 min
at 4°C. The supernatant was transferred to a new tube. Protein was
then collected for further use.
Protein samples were denatured in gel sample buffer
[100 mM Tris-Cl (pH 6.8), 20% glycerol, 4% SDS, 200 mM DTT, and
0.2% bromophenol blue] by boiling for 5 min at 100°C. An equivalent
quantity of protein (35 µg/lane) was separated on 10%
SDS-polyacrylamide gels and was transferred to PVDF membranes
(Roche Diagnostics, Basel, Switzerland). After blocking in 5%
bovine serum albumin, the membranes were incubated for 1 h at room
temperature and then overnight at 4°C with the following
antibodies: Rabbit anti-TERT polyclonal antibody (1:200; Santa Cruz
Biotechnology, Inc.) and rabbit anti-GAPDH polyclonal antibody
(1:00; Proteintech Group, Inc., Chicago, IL, USA). The membranes
were then incubated with the peroxidase-labeled secondary
antibodies (1:2,000; Santa Cruz Biotechnology, Inc.) at room
temperature for 2 h. The membranes were detected by enhanced
chemiluminescence (ECL; Millipore). The band intensity was
measured, and the value was normalized with regard to GAPDH. All
experiments were repeated 5 times to ensure the reproducibility of
the results.
Statistical analysis
Quantitative data are expressed as the means ± SD.
The statistical process was examined by one-way analysis of
variance (ANOVA), followed by the least significant difference
(LSD) post hoc test. Analysis was performed using the Statistical
Package for the Social Sciences (SPSS software, version 21.0; SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
TERT expression in the cochlear
basilar membrane during postnatal development
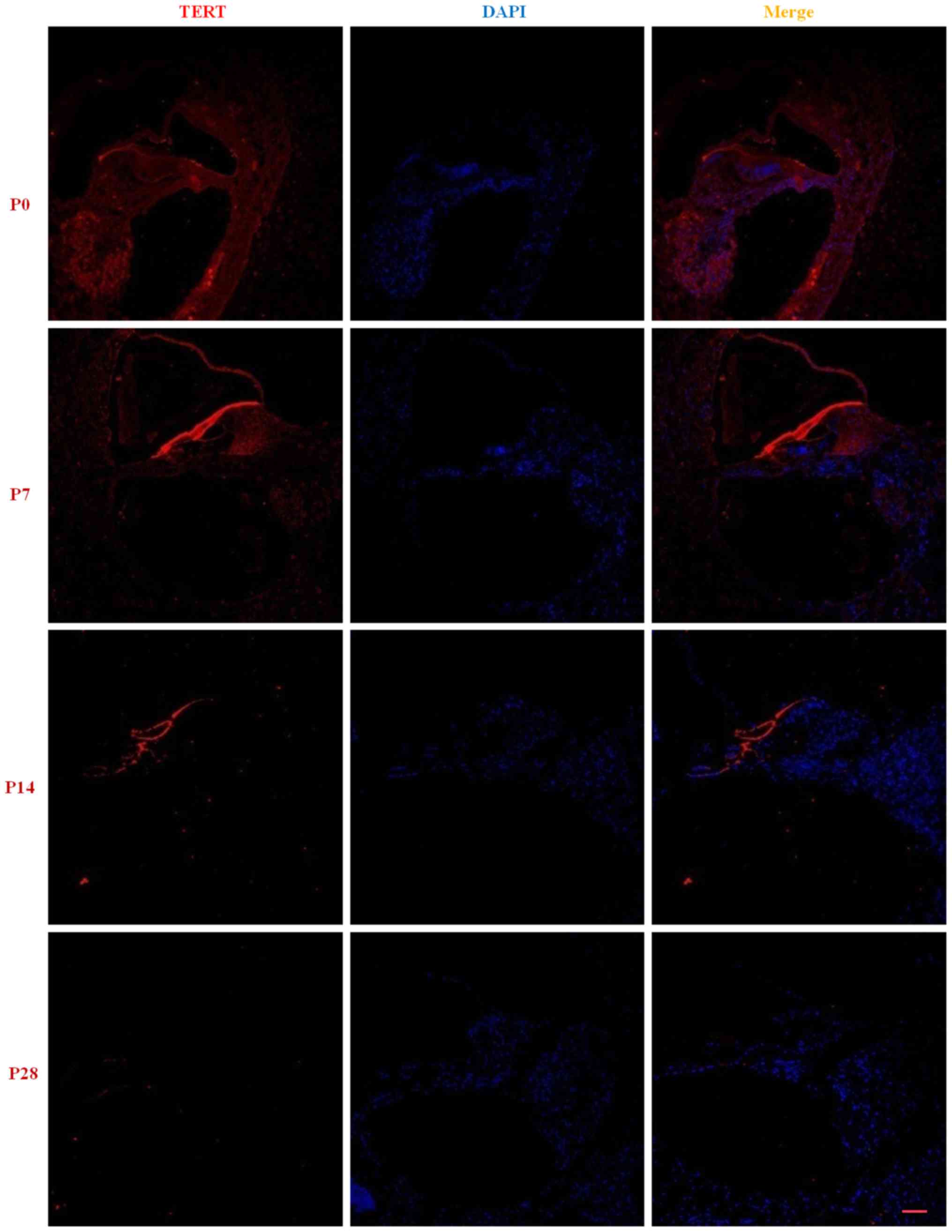
There was low expression of TERT in basilar
membrane, Spiral ganglion and lateral wall of cochlea at P0 and the
fluorescence gradually decreased as cochlear development (red). At
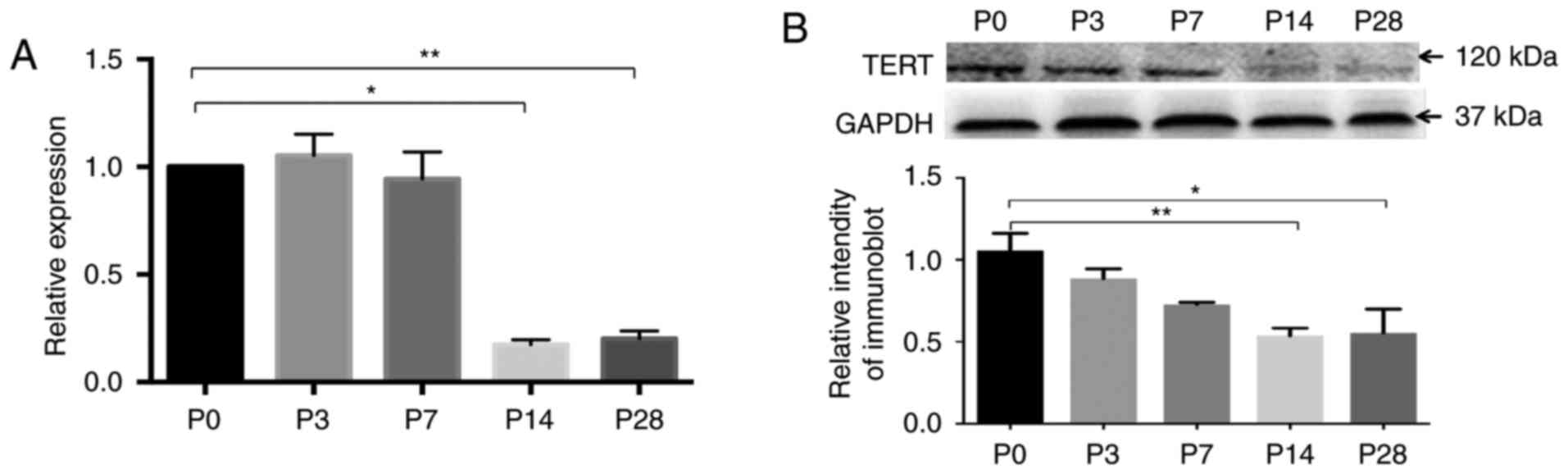
P28, almost no gradually were observed (Fig. 1). The RT-qPCR results showed that
relatively high levels of TERT mRNA in the basilar membrane were
observed at P0, P3, and P7 and gradually decreased to a minimum
level at P14 and P28 (Fig. 2A;
P<0.05, n=3). The western blot results showed that the principal
immunoreactive band of 120 kDa was detected with TERT antibody. The
antibody GAPDH was used as a loading control. The TERT protein was
expressed at a maximum level after birth, but it gradually
decreased during postnatal development, and the levels of TERT at
P14 and P28 were significantly lower than that at P0 (Fig. 2B; P<0.05, n=3).
TERT expression in cochlear progenitor
cells in vitro
The proliferative rate of cochlear
progenitor cells decreased as the culture time increased
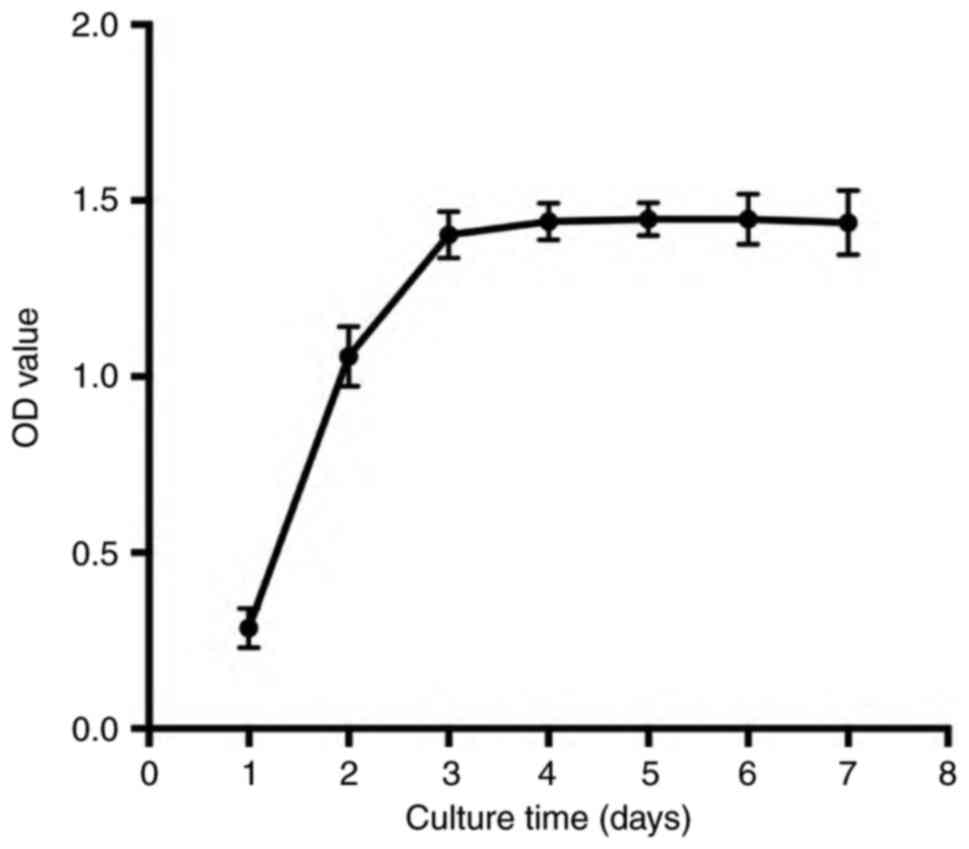
We collected cultured cochlear otospheres at
different times to evaluate the cell proliferation rate by MTT
assay. We observed a significantly enhanced proliferation of cells
from day 1 to 4 and found the maximum effect at day 4. Then, the
growth of cochlear progenitor cells remained stable from day 5 to 7
(Fig. 3; n=3), which indicated
that the proliferative rate of the cochlear progenitor cells was
decreased.
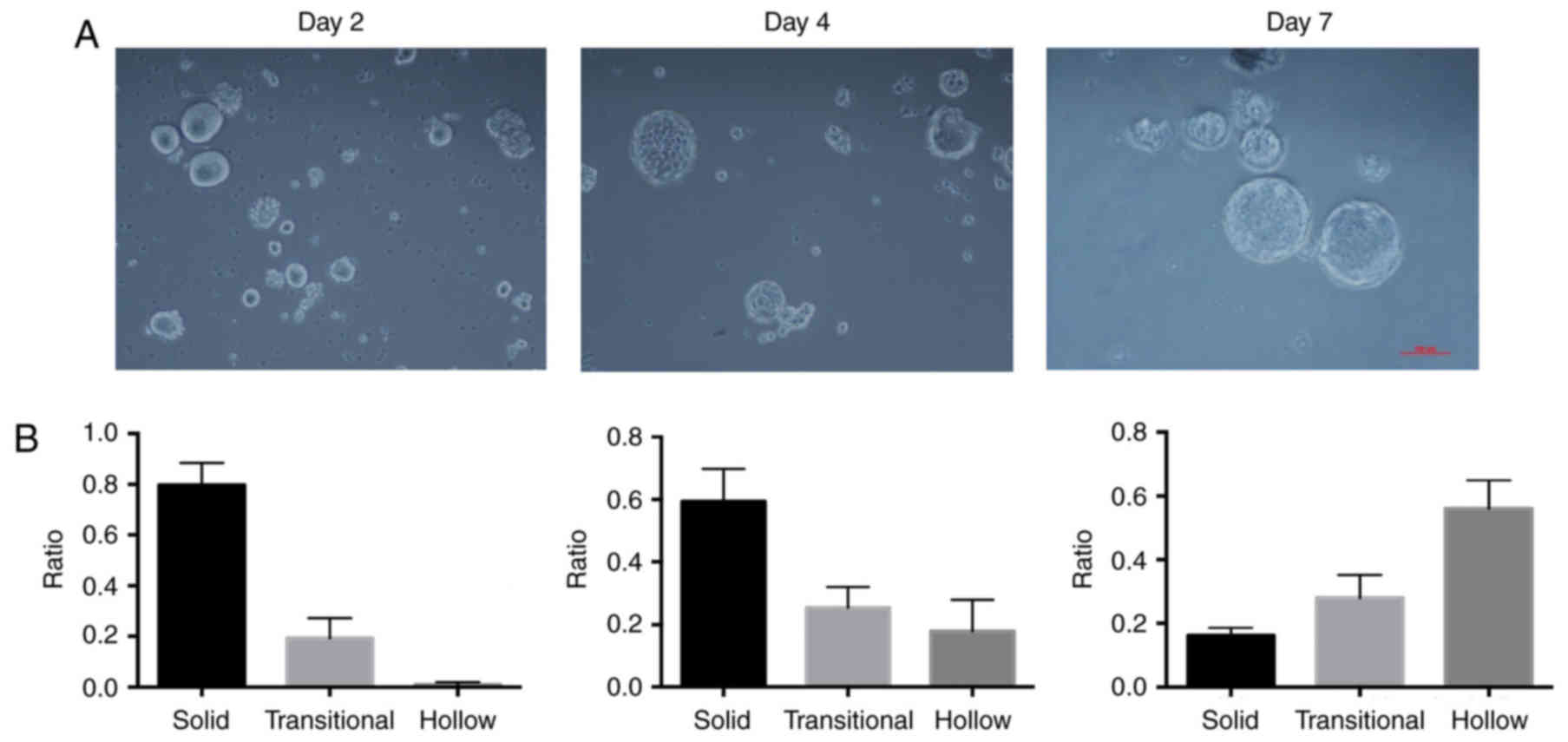
The harvested cells could give rise to three
different types of morphologically distinct spheres when cultured
in vitro, and the ratio of each type of morphologically
distinct sphere varied as the culture times prolonged. We compared
the ratios of the three different otospheres at days 2, 4, and 7.
As shown in Fig. 4A, the volume of
otospheres gradually increased as the culture time increased. The
ratio of solid otospheres was maximal at day 2 and then decreased,
whereas the ratio of hollow otospheres increased and reached the
maximum ratio at day 7 (Fig.
4B).
The expression of TERT in cochlear
progenitor cells cultured in vitro
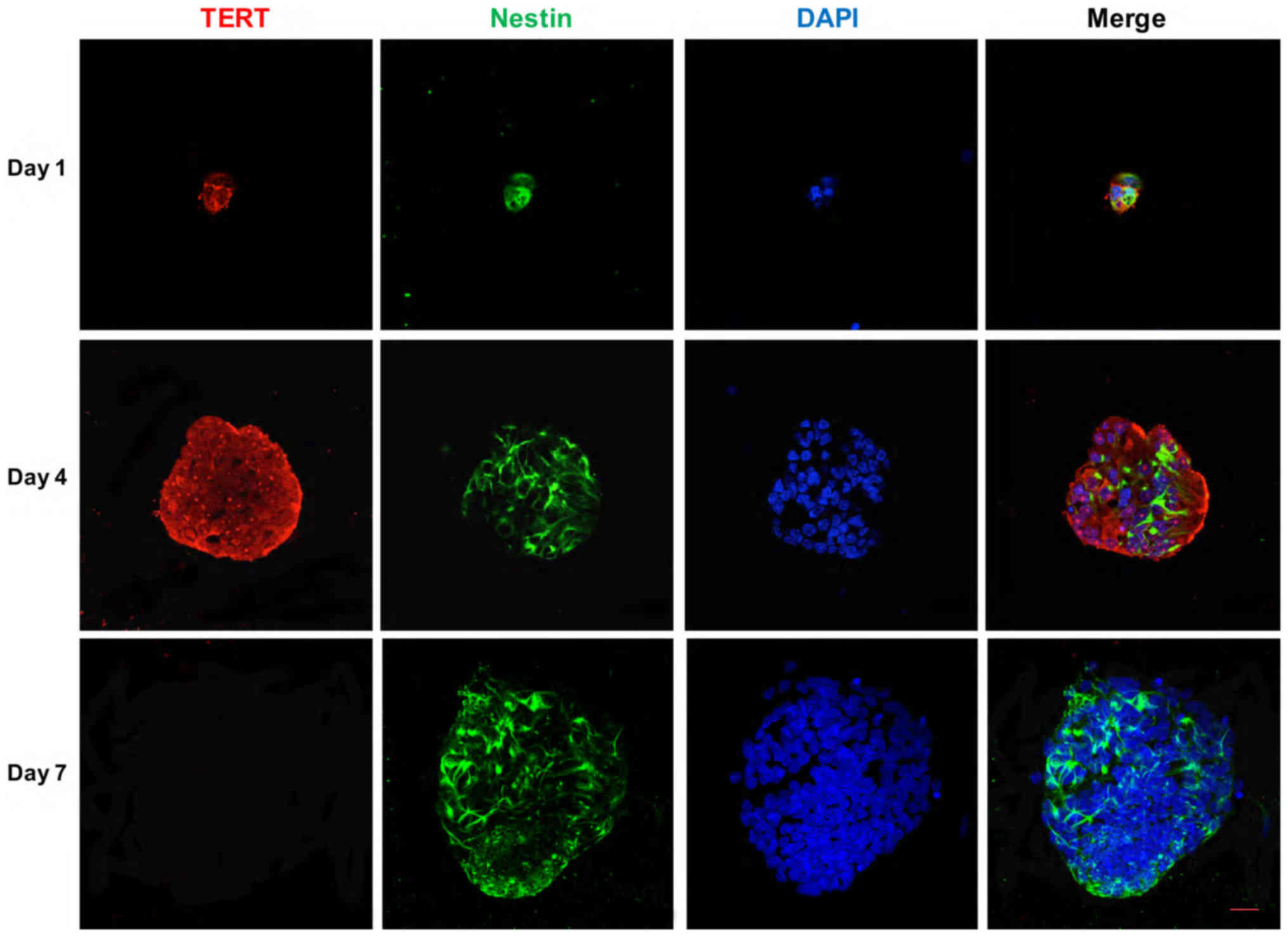
Immunocytochemical studies showed that cochlear
progenitor cells were positive for TERT expression when cultured
in vitro. The fluorescence intensity became stronger from
day 2 to 4, and the strongest fluorescence was observed at day 4,
but almost no fluorescence was observed at day 7 (Fig. 5). RT-qPCR showed that the TERT mRNA
expression was at the minimum level at day 2, gradually increasing
and reaching at the maximum level at day 4, coincident with the
expression of nestin and PCNA (P<0.05, n=3) (Fig. 6A).
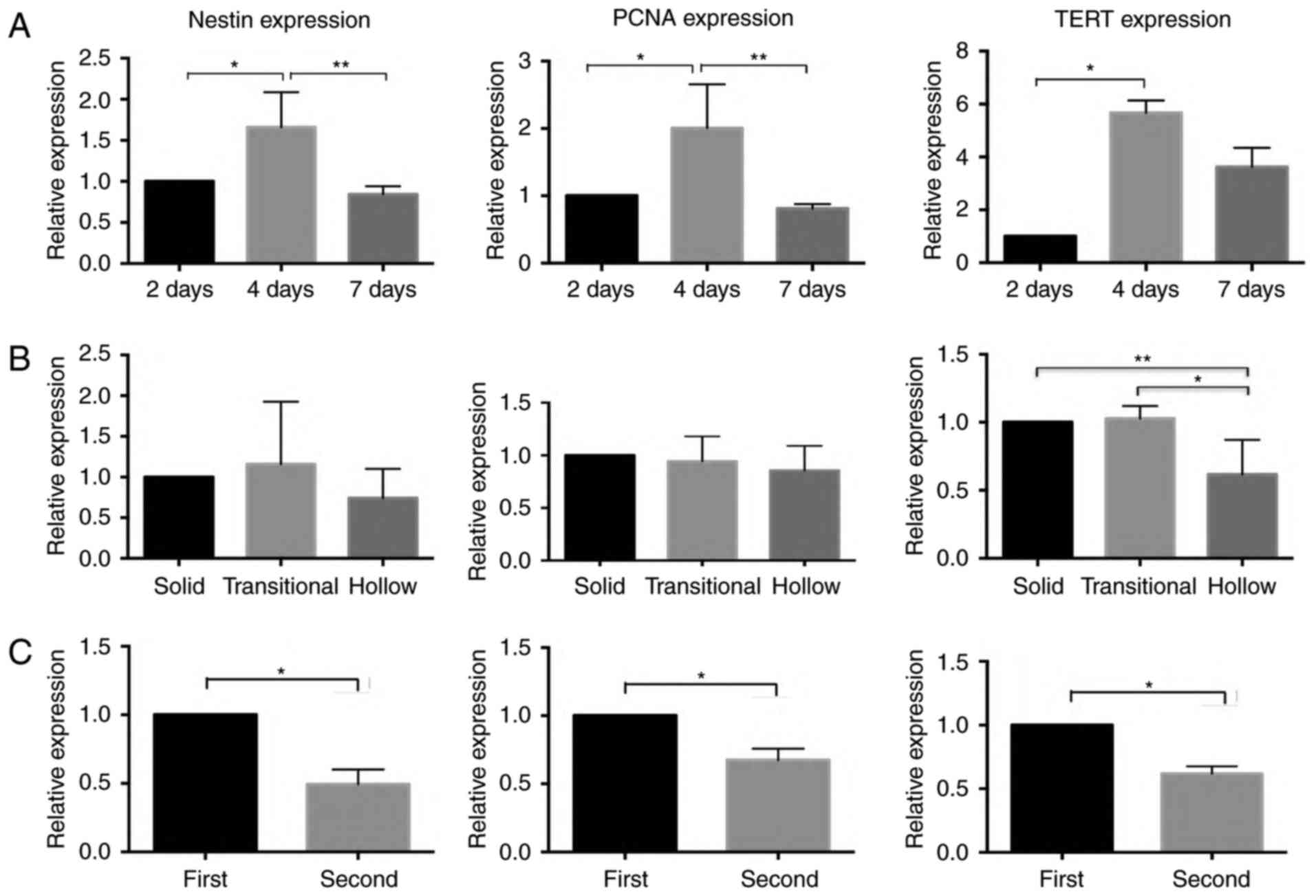 | Figure 6.RT-qPCR results for nestin, PCNA, and
TERT expression in progenitor cells at days 2, 4, and 7. The
expression of nestin and PCNA attained the highest level at day 4
(***P<0.05, n=3); the expression of TERT was significantly
higher at day 4 than at days 2 and 7 (A) (*,**P<0.05, n=3).
RT-qPCR results of nestin, PCNA and TERT mRNA in three different
types of morphological otospheres. The expression of nestin and
PCNA did not show any significant differences among the three types
of otospheres. The expression of TERT was significantly higher in
solid and transitional otospheres than in hollow otospheres (B)
(*,**P<0.05, n=3). Differential expression of nestin, PCNA and
TERT mRNA in the first and second generations of progenitor cells.
The expressions of nestin, PCNA and TERT were significant higher in
the first generation than in the second generation (C) (*P<0.05,
n=3). TERT, telomerase reverse transcriptase. |
The otospheres with different morphologies were
identified under a microscope on day 7 to evaluate the levels of
nestin, PCNA and TERT mRNA among the three types of otospheres. The
results showed that the level of TERT mRNA was significantly higher
in solid otospheres than in the hollow otospheres (P<0.05, n=3),
whereas the expression of nestin and PCNA did not show any
significant differences among the three types of otospheres
(P>0.05, n=3) (Fig. 6B).
As is known, the cochlear progenitor cells obtained
limited proliferative capabilities when cultured in vitro,
and the proliferation capabilities decreased after cell passage. In
our study, we collected the first and second generations of
progenitor cells. The expression of TERT, nestin and PCNA was
evaluated by RT-qPCR. The expression of TERT significantly
decreased when the progenitor cells were passaged, coincident with
the expression of nestin and PCNA (P<0.05, n=3) (Fig. 6C).
Discussion
Cochlear progenitor cells are a subset of distinct
cells between stem cells and terminally differentiated cells, and
they obtain limited proliferative capability and exit the cell
cycle after mitosis (9). Several
genes involved in cochlear development have been proved to promote
the proliferation of cochlear progenitor cells. Chai et al
demonstrated that upregulating the level of β-catenin in progenitor
cells could significantly promote cell proliferation (10), but that inhibiting the Notch
pathway could promote progenitor cell differentiation into hair
cells (11). However, the
mechanism for the regulation of cochlear progenitor cell
proliferation remains poorly understood.
In this study, we evaluated the expression of TERT
in inner ear cells. First, we evaluated the differential expression
levels of TERT in the basilar membrane during postnatal development
by RT-qPCR and western blot analysis. Then, the TERT expression in
the progenitor cells was evaluated by immunocytochemistry and
RT-qPCR. The aim of this study was to evaluate the expression level
and potential functions of TERT in postnatal cochlear development
and in cochlear progenitor cells cultured in vitro.
Collectively, our results could provide evidence for the presence
of TERT in the postnatal cochlea in vivo and progenitor
cells in vitro, suggesting a possible role of TERT in the
regulation of postnatal cochlear development and progenitor cell
proliferation.
TERT is the main component of telomerase complex,
the presentation of which is critical to maintaining the activity
of telomerase (12–17). Most eukaryocyte somatic cells have
undetectable telomerase activity due to transcriptional repression
of the catalytic subunit TERT during the early stage of embryo
development, but its expression is limited to highly proliferative
tissues, such as germ cells and stem cells after birth. Several
studies have proved that TERT is critical for maintaining the
capability of stem cells and promoting cell proliferation (18–24).
TERT was expressed in the basilar membrane during
the first 7 days after birth, which indicated that TERT may be
related to the postnatal development of the cochlea. As previously
reported, the MTT assay results showed that progenitor cells
proliferated significantly from day 1 to 4 and reached the maximum
effect at day 4. Then, the growth of cochlear progenitor cells
remained stable from day 5 to 7. The RT-qPCR results showed that
nestin and PCNA were expressed at the highest levels at day 4. All
of these results suggested that the proliferation capability of
progenitor cells increased gradually and reached the maximum at day
4. The TERT expression in the cochlear progenitor cells reached the
maximum level at day 4, coincident with the variety of
proliferation abilities of progenitor cells, provided evidence that
the expression of TERT might be involved in maintaining the
proliferation capability of cochlear progenitor cells.
The progenitor cells cultured in vitro were
observed to give rise to three different types of morphologies,
that is, solid otospheres, transitional otospheres and hollow
otospheres. Each type of otosphere obtained distinct features. The
solid otospheres showed the smallest volume, and they were composed
of small densely packed cells without any features of epithelial
origin. In contrast, the hollow otospheres obtained the largest
volume, and the cells were larger, with the classic characteristics
of epithelial cells, coincident with previous reports. Whitlon
reported their observation that the progenitor cells initially
formed solid otospheres and that these otospheres became larger and
transformed into hollow otospheres 48 h after culture, with the
increasing volume of single cells. The cells in hollow otospheres
formed a single layer organization, with increased expression of
E-cadherin, which was localized to cellular junctions (25). All of these findings indicated a
mature epithelial morphology. The cells from hollow otospheres
could not form new spheres after they were digested into single
cells, whereas cells from solid otospheres could give rise to new
otospheres after passage, which indicated that the progenitor cells
gradually lost the features and proliferative ability of stem cells
as they transformed into hollow otospheres (4,5,26).
The RT-qPCR results showed that the nestin and PCNA mRNA levels
were significantly higher in the solid otospheres and decreased
after passage, indicating that the proliferative capacity of
progenitor cells decreased as culture time and passage number
increased, but the expression of TERT significantly decreased when
the otospheres transformed from solid otospheres into hollow
otospheres, and progenitor cells were passaged.
In conclusion, we demonstrated that TERT was
expressed in the basilar membrane during the first 7 days after
birth. The expression of TERT in progenitor cells reached the
maximum at day 4 and decreased as both the culture time and number
of passages increased, coincident with the proliferation variety of
cochlear progenitor cells. All of these results led us to
hypothesize that TERT might be involved in the development of the
cochlea and in maintaining the proliferative capability of
progenitor cells. Further investigation is needed to explore the
potential role of TERT in progenitor cell proliferation.
Understanding the mechanism of the regulation of TERT expression
could provide valuable insights into cochlear hair cell
regeneration and the treatment of hearing impairment.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81271069) and the National
Natural Science Foundation of Major Projects Overseas of China
(grant no. 81120108008).
References
|
1
|
White PM, Doetzlhofer A, Lee YS, Groves AK
and Segil N: Mammalian cochlear supporting cells can divide and
trans-differentiate into hair cells. Nature. 441:984–987. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sinkkonen ST, Chai R, Jan TA, Hartman BH,
Laske RD, Gahlen F, Sinkkonen W, Cheng AG, Oshima K and Heller S:
Intrinsic regenerative potential of murine cochlear supporting
cells. Sci Rep. 1:262011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi F, Kempfle JS and Edge AS:
Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors
in the cochlea. J Neurosci. 32:9639–9648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diensthuber M, Oshima K and Heller S:
Stem/progenitor cells derived from the cochlear sensory epithelium
give rise to spheres with distinct morphologies and features. J
Assoc Res Otolaryngol. 10:173–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oshima K, Grimm CM, Corrales CE, Senn P,
Martinez Monedero R, Géléoc GS, Edge A, Holt JR and Heller S:
Differential distribution of stem cells in the auditory and
vestibular organs of the inner ear. J Assoc Res Otolaryngol.
8:18–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Collins K and Mitchell JR: Telomerase in
the human organism. Oncogene. 21:564–579. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi J, Southworth LK, Sarin KY,
Venteicher AS, Ma W, Chang W, Cheung P, Jun S, Artandi MK, Shah N,
et al: TERT promotes epithelial proliferation through
transcriptional control of a Myc- and Wnt-related developmental
program. PLoS Genet. 4:e102008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarin KY, Cheung P, Gilison D, Lee E,
Tennen RI, Wang E, Artandi MK, Oro AE and Artandi SE: Conditional
telomerase induction causes proliferation of hair follicle stem
cells. Nature. 436:1048–1052. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gage FH: Mammalian neural stem cells.
Science. 287:1433–1438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan
TA, Liu Z, Taketo MM, Oghalai JS, Nusse R, et al: Wnt signaling
induces proliferation of sensory precursors in the postnatal mouse
cochlea. Proc Natl Acad Sci USA. 109:pp. 8167–8172. 2012;
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li W, Wu J, Yang J, Sun S, Chai R, Chen ZY
and Li H: Notch inhibition induces mitotically generated hair cells
in mammalian cochleae via activating the Wnt pathway. Proc Natl
Acad Sci USA. 112:pp. 166–171. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harley CB and Villeponteau B: Telomeres
and telomerase in aging and cancer. Curr Opin Genet Dev. 5:249–255.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huffman KE, Levene SD, Tesmer VM, Shay JW
and Wright WE: Telomere shortening is proportional to the size of
the G-rich telomeric 3′-overhang. J Biol Chem. 275:19719–19722.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schneider RP, Garrobo I, Foronda M,
Palacios JA, Marion RM, Flores I, Ortega S and Blasco MA: TRF1 is a
stem cell marker and is essential for the generation of induced
pluripotent stem cells. Nat Commun. 4:19462013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okamoto K, Bartocci C, Ouzounov I,
Diedrich JK, Yates JR III and Denchi EL: A two-step mechanism for
TRF2-mediated chromosome-end protection. Nature. 494:502–505. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miller AS, Balakrishnan L, Buncher NA,
Opresko PL and Bambara RA: Telomere proteins POT1, TRF1 and TRF2
augment long-patch base excision repair in vitro. Cell Cycle.
11:998–1007. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McKerlie M, Lin S and Zhu XD: ATM
regulates proteasome-dependent subnuclear localization of TRF1,
which is important for telomere maintenance. Nucleic Acids Res.
40:3975–3989. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie Y, Zhao X, Jia H and Ma B: Derivation
and characterization of goat fetal fibroblast cells induced with
human telomerase reverse transcriptase. In Vitro Cell Dev Biol
Anim. 49:8–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu XQ, Huang C, He X, Tian YY, Zhou DX, He
Y, Liu XH and Li J: Feedback regulation of telomerase reverse
transcriptase: New insight into the evolving field of telomerase in
cancer. Cell Signal. 25:2462–2468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singhapol C, Pal D, Czapiewski R, Porika
M, Nelson G and Saretzki GC: Mitochondrial telomerase protects
cancer cells from nuclear DNA damage and apoptosis. PLoS One.
8:e529892013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Plantinga MJ, Pascarelli KM, Merkel AS,
Lazar AJ, von Mehren M, Lev D and Broccoli D: Telomerase suppresses
formation of ALT-associated single-stranded telomeric C-circles.
Mol Cancer Res. 11:557–567. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cong Y and Shay JW: Actions of human
telomerase beyond telomeres. Cell Res. 18:725–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahmed S, Passos JF, Birket MJ, Beckmann T,
Brings S, Peters H, Birch-Machin MA, von Zglinicki T and Saretzki
G: Telomerase does not counteract telomere shortening but protects
mitochondrial function under oxidative stress. J Cell Sci.
121:1046–1053. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hao LY, Armanios M, Strong MA, Karim B,
Feldser DM, Huso D and Greider CW: Short telomeres, even in the
presence of telomerase, limit tissue renewal capacity. Cell.
123:1121–1131. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Whitlon DS: E-cadherin in the mature and
developing organ of Corti of the mouse. J Neurocytol. 22:1030–1038.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Senn P, Oshima K, Teo D, Grimm C and
Heller S: Robust postmortem survival of murine vestibular and
cochlear stem cells. J Assoc Res Otolaryngol. 8:194–204. 2007.
View Article : Google Scholar : PubMed/NCBI
|