Introduction
Bone remodeling is strongly regulated by hormones,
cytokines and other cellular interactions that affect communication
between osteoblasts and osteoclasts. Any disruption in this network
may result in abnormal bone mass, including osteoporosis (1).
It has previously been demonstrated that the nervous
system is an important regulator of bone formation. Due to the
thick innervation in bone tissue, chemical messengers are
transduced to bone and periosteum through the sensory and
sympathetic nerve fibers (2–4). In
addition, catecholaminergic positive axons may be visualized near
osteoblasts in vivo and damaged or missing peripheral nerve
fibers may result in abnormal bone formation (5,6).
Furthermore, the existence of β-adrenergic receptors in osteoblasts
and osteoclasts verifies the role of the central nervous system in
the regulation of bone formation (7). However, the molecular mechanisms
through which neurons and nerve fibers reach their targets and
function during osteogenesis are still poorly understood.
In the central nervous system, several protein
families involved in wiring, location, and migration of axons,
affecting the length and branching of dendrites were previously
reported. One of the most prevalent neural signaling molecular
groups are the Semaphorins, a verified set of neuro-immune
molecules, which exhibit a predominant role in cardiac and skeletal
development, epithelial morphogenesis, angiogenesis and tumor
regression (8–12). Semaphorin (Sema) 3A, the first
protein discovered in this large protein family, is a
chemorepellent for the nervous system that serves to induce the
retraction and collapse of the structure of axonal growth cone, and
to affect fiber plasticity in adults (13–15).
Sema3A completes its biological function via binding to a receptor
complex encompassing ligand-binding component Neuropilin (Nrp) 1
and class A Plexins (Plx) a 1, 2, 3 and 4, the latter of which is
necessary for proper signal transduction (16). Previously, Sema3A was reported to
have a significant role in bone remodeling (17–19).
In a previous study, Sema3A-deficient mice and neuro-specific
Sema3A-deficient mice were generated and both exhibited low bone
mass, low expression of sensory-nerve markers and their positive
nerve fibers, and defective innervations. Conversely,
osteoblast-specific Sema3A-deficient mice and wild-type mice did
not exhibit any of these malfunctions or abnormalities (20). These results suggested that the
absence of Sema3A in osteoblasts was not the only factor resulting
in bone defects and that Sema3A modulated sensory nerve innervation
during bone remodeling. However, the specific role of Sema3A
through which bone formation is affected is still unclear.
Previous studies have exposed the key role of
Schwann cells in repair and reconstruction following peripheral
nerve injury. Injury-induced Schwann cells undergo a series of
alterations including downregulation of myelin genes, upregulation
of trophic factors and cytokines, activation of myelin autophagy
and invasion of macrophages. Altogether, these alterations lead to
increased regeneration of axons, remyelination of nerve fibers,
avoidance of tissue loss and improved function (21–23).
Furthermore, Schwann cell migration is significantly impaired
following the loss of Nrp1 (24)
and binding between Sema3A and Nrp1 has been described as a key
interaction in bone cell differentiation (18,19).
It was therefore hypothesized that during Sema3A-associated
osteogenesis, neural cells, including Schwann cells, may exhibit a
pivotal role by interacting with Sema3A and osteoblastic cells.
The present study investigated the role of neural
cells in bone remodeling. Sema3A was added to a single cell culture
of MG63, and to a co-culture of MG63 and Schwann cells. Osteogenic
differentiation was assessed to determine if Sema3A osteogenic
induction is influenced by the addition of Schwann cells.
Materials and methods
Materials
Recombinant human Sema3A protein was purchased from
Sino Biological Inc. (Beijing, China). The human osteoblast cell
line MG63 and the human immortalized Schwann cell line sNF96.2
(SCs) were provided by the School of Stomatology of Jilin
University (Changchun, China). Dulbecco's modified Eagle's medium
(DMEM), fetal bovine serum (FBS), and penicillin/streptomycin were
purchased from Invitrogen; Thermo Fisher Scientific, Inc.,
(Waltham, MA, USA). Trypsin and Dulbecco's phosphate buffered
saline (PBS) were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Cell Counting Kit-8 (CCK8) was purchased from
7Sea Pharmatech (Shanghai, China). In Situ Cell Apoptosis Detection
kit (AP kit) was purchased from Boster Biological Technology
(Pleasanton, CA, USA). Total RNA was extracted using the
TRIzol® method (Invitrogen; Thermo Fisher Scientific,
Inc.). All absorbance represented by optical density (OD) value was
measured using Synergy HT spectrophotometer (BioTek Instruments,
Inc., Winooski, VT, USA).
Single and co-cell culture
systems
The MG63 and SCs were cultured in DMEM supplemented
with 10% FBS, 1% penicillin, and 1% streptomycin, according to
previously reported culturing conditions (25,26).
The medium was replaced every 3 days. Cells were trypsinized and
centrifuged at 183 × g, 37°C for 5 min when the confluence reached
80%. Following centrifugation, each cell was re-suspended. A total
of 500 cells were seeded in each well of 96-well plates at various
MG63 to SCs proportions (1:0, 1:1, 1:2, 2:1 and 0:1) to establish
single and co-culture systems. Cells were cultured at 37°C in a
humidified atmosphere containing 5% CO2.
Cell proliferation and apoptosis
assay
MG63 and SCs single-cell cultures and co-cultures
were seeded onto 96-well plates and cultured for 5 days to test
their level of proliferation. At days 1, 3 and 5, 10 µl of CCK8
reagent was added to each well. Cells were incubated at 37°C for 40
min for interaction with the reagent. Then the plate was examined
by spectrophotometer at a wavelength of 450 nm to assess OD. The
same test was conducted on the two single-cell cultures and the
co-culture system of MG63 to SCs at 1:1 (1:1 co-culture), with
addition of Sema3A to investigate if Sema3A affected proliferation.
Briefly, Sema3A (dissolved in PBS) was added to each well of the
96-well plates to reach final concentration of 25, 50 and 100
ng/ml. At days 1, 3 and 5, cell proliferation was measured
following CCK8 treatment by spectrophotometer (450 nm). Controls (0
ng/ml Sema3A) for both single-cell cultures and 1:1 co-culture,
were created using the same volume of PBS. The apoptosis assay was
carried out to investigate apoptosis in the 1:1 co-culture treated
with Sema3A using 6-well plates at days 1, 3 and 5 by AP kit
according to the manufacturer's protocol. The principle of this kit
is that cells in apoptosis produce DNA breakpoints with 3′-OH
terminals. Terminal Deoxynucleotidyl Transferase (TdT) tags
digoxigenin (Dig)-marked dUTP to 3′-OH terminals of broken DNAs.
Then by reaction with the anti-Dig-biotin and streptavidin-biotin
complex/alkaline phosphatase (SABC/AP), broken DNA will become
colored following the addition of a chromogenic substrate. Briefly,
cells were first fixed in 4% paraformaldehyde at room temperature
for 30 min, then went through reactions with TdT/Dig-dUTP,
anti-Dig-biotin and SABC/AP respectively, finally were stained by
20X 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium at
room temperature for 20 min and washed by 0.01 M Tris buffered
saline adequately to remove the excess dye. Under microscope,
nuclei of apoptotic cells were stained dark blue. For each group,
three individual visual fields of 500 cells each were observed and
the total amount of dead cells were counted.
Cytotoxicity assay
To assess the cytotoxicity of Sema3A, Sema3A was
added to the culture medium to reach the final concentrations of
25, 50 and 100 ng/ml. Medium with the same volume of PBS was used
as the control group (0 ng/ml Sema3A). 1:1 co-culture was
maintained in each medium for 6, 12 and 24 h and then tested by
CCK8 at each timepoint. OD values were measured by
spectrophotometer (450 nm) following incubation for 40 min.
Expression of Sema3A receptors
Gene expression of Sema3A receptors in MG63 and SCs
was analyzed using reverse transcription-semi quantitative
polymerase chain reaction (RT-sqPCR). Total RNA was extracted from
each cell group using the TRIzol method according to manufacturer's
protocol. Total RNA density was measured using NanoDrop 2000c
Spectrophotometer (Thermo Fisher Scientific, Inc.). Subsequently,
complementary DNA (cDNA) was synthesized from 1 µg total RNA using
the PrimeScript™ RT kit (Takara Bio, Inc., Otsu, Japan)
following the manufacturer's protocol. RT-sqPCR amplification was
carried out on cDNA using the Sapphire Amp Fast PCR Master Mix
(Takara Bio, Inc.). Synthesis and amplification were conducted in
an Alpha Thermal Cycler (Bibby Scientific, Ltd., Staffordshire,
UK). Primers for the house-keeping gene β-actin as well as Sema3A
receptors (Nrp1, Nrp2, Plxa1, Plxa2, Plxa3, and Plxa4) were used
for amplification. Sequences of these primers, as previously
described (27), are listed in
Table I. Each 50 µl PCR reaction
system consisted of 25 µl SapphireAmp Fast PCR Master Mix, 1 µl 10
µM forward primer, 1 µl 10 µM reverse primer, 2 µl 50 ng/µl cDNA,
and 21 µl dH2O. The PCR cycle reaction was performed
under the following conditions: Polymerase activation step at 94°C
for 1 min followed by 30 cycles (98°C for 5 sec, 55°C for 5 sec,
72°C for 10 sec), and final extension step at 72°C for 10 min. The
PCR products were then loaded onto a 2% agarose gel (Invitrogen;
Thermo Fisher Scientific, Inc.) containing 0.5 µg/ml ethidium
bromide (Invitrogen; Thermo Fisher Scientific, Inc.). The size of
the amplified bands was assessed against a 1,000 bp DNA ladder
(Takara Bio, Inc.). Bands were visualized and captured using
Molecular Imager Gel Doc XR+ (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) and Image Lab (v4.0; Bio-Rad Laboratories, Inc.).
 | Table I.Primer sequences used for RT-sqPCR
and RT-qPCR. |
Table I.
Primer sequences used for RT-sqPCR
and RT-qPCR.
| Gene | Forward
sequence | Reverse
sequence |
|---|
| β-actin |
TGGCACCCAGCACAATGAA |
CTAAGTCATAGTCCGCCTAGAAGCA |
| Nrp1 |
CTCCTGTTGTGTCTTCAGG |
CCCGATGAGGATCGGATTC |
| Nrp2 |
CATGCACTATGACACCCCTG |
ATGGGTTCCATGCAGTTCTC |
| Plxa1 |
GACTTCCTGCTGACCCTGAG |
GACTTCAACCTGAAGCCAGC |
| Plxa2 |
GCTACAAGAGCTGGGTGGAG |
CTCTCGGCTTGAAGAACCAC |
| Plxa3 |
CAGCAGATCGACTACAAGAC |
GCCGTGTCAGGTAGATCTC |
| Plxa4 |
TGTCAGGGTGTCAACGAGAGC |
ATACACCTGCTCCTTGGTGG |
| RUNX2 |
CACTGGCGCTGCAACAAGA |
CATTCCGGAGCTCAGCAGAATAA |
| Osteocalcin
(OCN) |
CCCAGGCGCTACCTGTATCAA |
GGTCAGCCAACTCGTCACAGTC |
Osteogenic mRNA expression
The expression of osteogenic genes was analyzed by
RT-qPCR. MG63 and 1:1 co-culture were divided into four groups with
the addition of Sema3A or PBS to the final concentration of 0, 25,
50, 100 ng/ml. The medium was replaced every 2 days. At days 3 and
7, total RNA of cells was obtained and cDNA was synthetized using
the aforementioned procedure. Then, primers for Runt-Related
Transcription Factor 2 (RUNX2) and Osteocalcin (OCN) were used for
RT-qPCR amplification using SYBR Premix EX™ Taq II
RT-PCR kit (Takara Bio, Inc.) in Stratagene Mx3005P (Agilent
Technologies, Inc., Santa Clara, CA, USA). The primer sequences
used for RUNX2 and OCN amplification were as previously described
(26) and listed in Table I. Briefly, each 25 µl RT-qPCR
mixture consisted of 12.5 µl SYBR Fast qPCR Mix, 1 µl 10 µM forward
and reverse primers, 0.5 µl Rox Reference Dye (50×), 2 µl 10 ng/µl
cDNA and 8 µl dH2O. Each mixture was programmed to go
through pre-denaturation at 95°C for 30 sec, then 40 cycles of 5
sec at 95°C followed by 30 sec at 60°C. β-actin was used as the
reference gene in RT-qPCR analysis and 2−ΔΔCq method was
applied to normalize and analyze the data as previously described
(27).
Alkaline phosphatase (ALP) assay
The MG63 single culture and 1:1 co-culture under
different concentrations of Sema3A were assessed by the ALP assay.
At days 7 and 14, ALP activity was measured using an Alkaline
phosphatase assay kit (Beyotime Institute of Biotechnology,
Shanghai, China) according to manufacturer's protocol. Briefly,
cells were washed with PBS three times and then lysed using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology). Cells were then centrifuged at 183 × g, 37°C for 5
min. For the ALP assay, 50 µl supernatant and 50 µl reaction
reagent was added to each well of the 96-well plate. A total of 100
µl stop buffer was finally added to each well following a 10 min
incubation at 37°C. Then, OD value at a wavelength of 405 nm was
measured.
Extracellular matrix (ECM)
mineralization
The single culture MG63 and 1:1 co-culture was
cultured 14 days for mineralization staining. Cells were washed
twice with PBS and fixed in 95% ethanol for 15 min at room
temperature. Then, the Alizarin Red solution (Sigma-Aldrich; Merck
KGaA) was used to dye the calcium nodules at room temperature for 5
min. Cells were rinsed adequately with distilled water to remove
excessive dye. Then, the dyed nodules were dissolved in 10% (w/v)
cetylpyridinium chloride (Shanghai Yuan Ye Biotechnology Co., Ltd.,
Shanghai, China) solution and OD was measured at a wavelength of
620 nm.
Statistical analysis
For data analysis of cell proliferation, apoptosis,
cytotoxicity assays, calcium nodule staining and alkaline
phosphatase assay, one-way analysis of variance followed by
post-hoc Tukey's test was applied using SPSS software, version 17.0
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard error of the mean. All statistical graphs were produced by
graphing software OriginLab 8 (v8.0725; OriginLab, Northampton, MA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Establishment of cell culture
systems
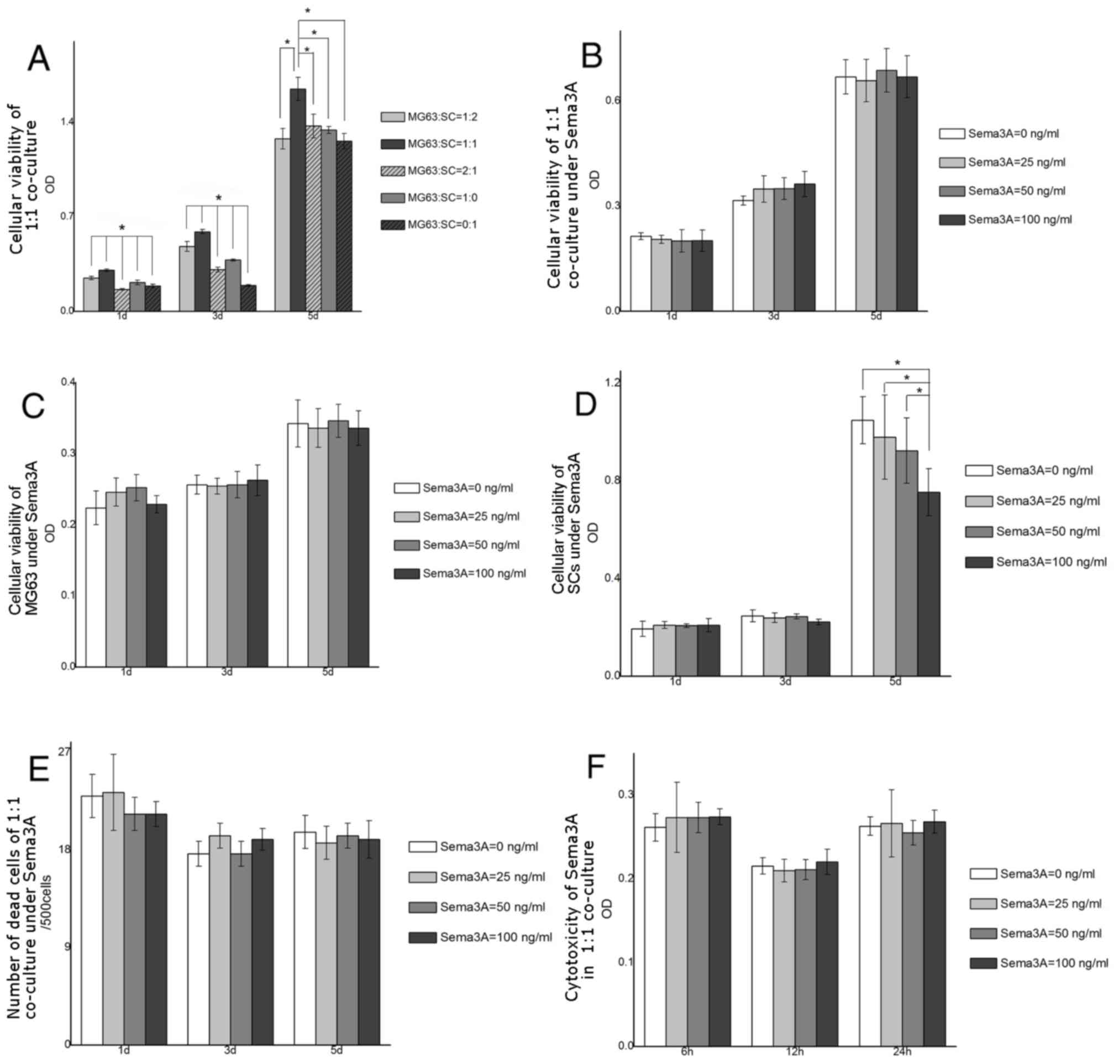
The three co-culture groups demonstrated different
cell viabilities. The 1:1 co-culture was the group with the most
increased cell proliferation at days 1, 3 and 5 (Fig. 1A). Other groups demonstrated
inferior cell proliferation (P<0.05) compared with this group,
at all time points examined. Cell proliferation of single MG63 and
SCs were not increased compared with the 1:1 group. Hence, 1:1 of
MG63 to SCs was the ratio selected for the follow-up
experiments.
Effect of Sema3A on cell
proliferation, apoptosis, and cytotoxicity
Cell proliferation, cell death, and cytotoxicity
following Sema3A addition were assessed. There was no difference in
cell proliferation among cell groups treated with different
concentrations of Sema3A (0, 25, 50 and 100 ng/ml) at days 1, 3 and
5 (Fig. 1B). Similarly, the
addition of Sema3A did not result in a significant alteration in
cell proliferation of MG63 single culture during the testing period
(Fig. 1C). The proliferation of
SCs was also not affected by the addition of Sema3A at days 1 and
3. However, at day 5, OD value in 100 ng/ml Sema3A group was
significantly decreased compared with 0, 25 and 50 ng/ml groups
(P<0.05; Fig. 1D). This trend
of reduction in the proliferation rate at day 5 may be observed
with increasing concentrations of Sema3A (though not reaching
statistical significance in other groups). For the apoptosis assay,
there was no difference in levels of apoptosis in 1:1 co-culture,
MG63 and SCs under different Sema3A concentrations at days 1, 3 and
5 (Fig. 1E). Similarly, there was
no difference in levels of cytotoxicity in the 1:1 co-culture under
different Sema3A concentrations (Fig.
1F).
Expression of Sema3A receptors
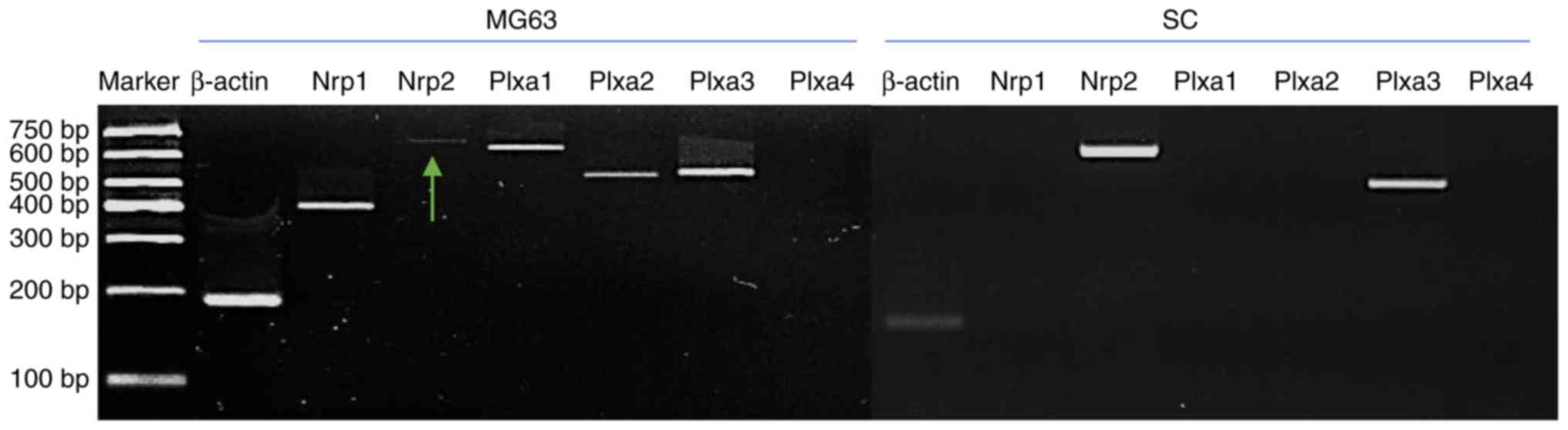
RT-sqPCR was performed to examine the expression of
Sema3A receptors. Nrp1 was only expressed in MG63. Nrp2 expression
was observed in SCs and was visible however weak in MG63. Gene
expression of Plxa1 and Plxa2 was only detected in MG63. Plxa3 was
the only Plexin receptor expressed in SCs (Fig. 2).
ALP assay and calcium nodule
staining
An ALP assay was undertaken to assess the level of
osteogenic differentiation. ALP activity in MG63 single culture
indicated a consistent decline at days 7 and 14 (Fig. 3A). At each time point, only 50 and
100 ng/ml Sema3A groups suggested statistical difference from
control group (0 ng/ml Sema3A; P<0.05). Furthermore, in MG63,
ALP activity decreased gradually with increasing concentrations of
Sema3A. Conversely, ALP activity in the 1:1 co-culture increased
gradually as concentrations of Sema3A increased at days 7 and 14
(Fig. 3B). ALP assay results in
1:1 the co-culture revealed that all Sema3A groups (25, 50 and 100
ng/ml) were markedly increased compared with the control group (0
ng/ml;) at days 7 and 14, however, there was no difference among
Sema3A treatment groups (Fig. 3B;
P>0.05). The osteogenic differentiation was further investigated
by calcium nodule staining. Differences in staining were not
visually detectable (data not shown), however OD values indicated
differences between groups. In MG63 single culture, OD value
declined as concentration of Sema3A increased, and the lowest OD
appeared at 100 ng/ml (Fig. 3C;
P<0.05). No meaningful difference was observed between 25 and 50
ng/ml groups (Fig. 3C; P>0.05).
In 1:1 co-culture, OD value demonstrated a steady increase as
Sema3A concentration increased, with all groups statistically
different compared with each other (P<0.05; Fig. 3C).
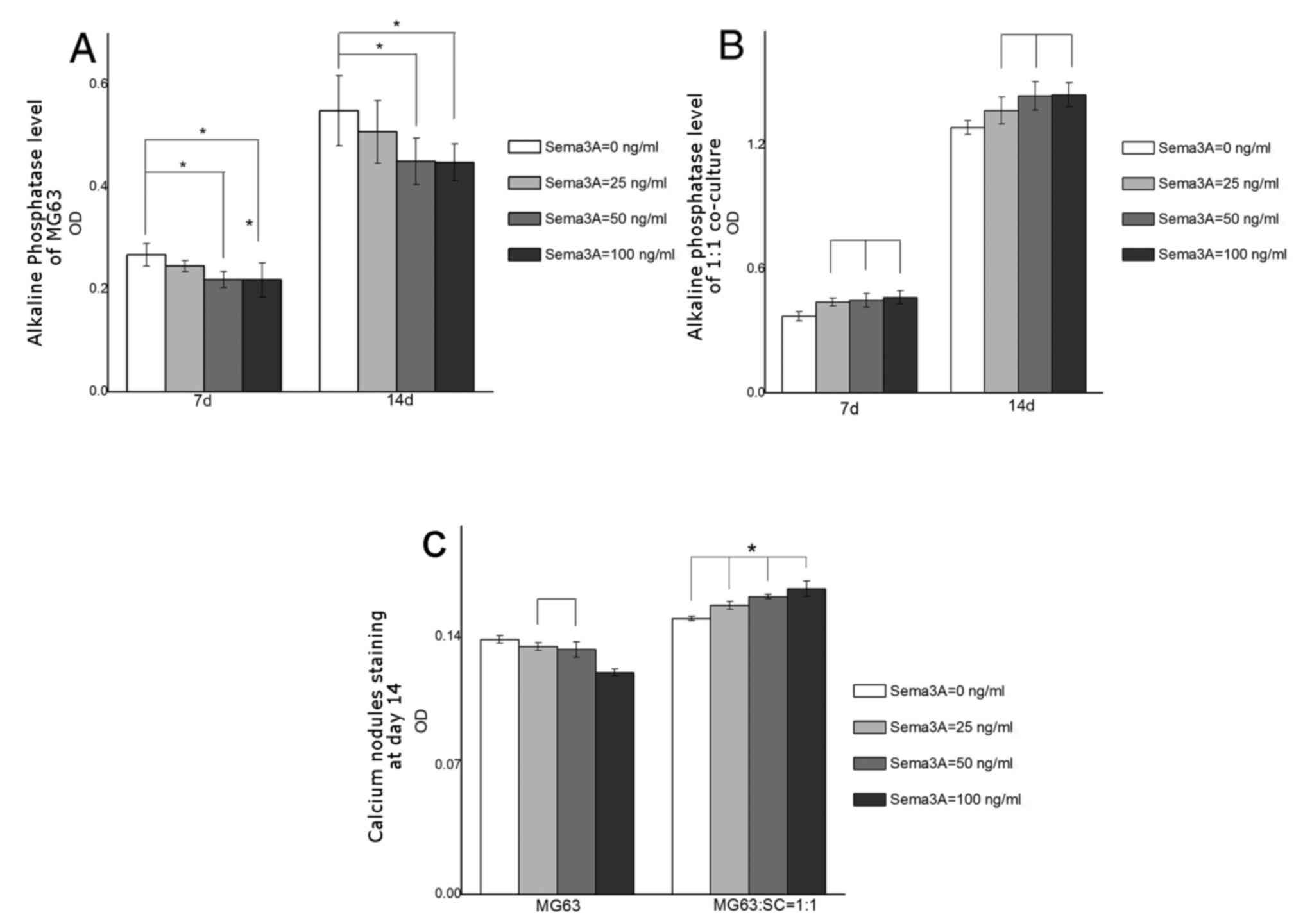 | Figure 3.Alkaline phosphatase and
extracellular matrix mineralization assay. (A) ALP activity of MG63
single culture at days 7 and 14. There were no statistical
differences between the 0 and 25 ng/ml Sema3A treatment groups. (B)
ALP activity of 1:1 co-culture at days 7 and 14. At all time
points, 25, 50 and 100 ng/ml Sema3A treatment groups had increased
ALP activity compared with the 0 ng/ml Sema3A control group.
However, no statistical difference was observed among Sema3A
treatment groups. (C) Absorbance values (620 nm) of calcium modules
stained by Alizarin Red. All groups were statistically different
from each other, with the exception being the 25 and 50 ng/ml
Sema3A groups in MG63. In both alkaline phosphatase and
extracellular matrix mineralization assays, three Sema3A treatment
groups (25, 50, and 100 ng/ml) and the control group (0 ng/ml) were
compared with each other within every time point for statistical
analysis. Data are presented as the mean ± standard error of the
mean. Lines above the bars without an asterisk in the histogram
indicate groups with no statistical difference (P>0.05);
*P<0.05. Sema3A, Semaphorin 3A; SC, Schwann cells; OD, optical
density. |
Expression of osteogenic genes
RT-qPCR was performed to detect alterations in
expression levels of osteogenic genes under different
concentrations of Sema3A. The pattern of alteration in mRNA
expression of osteogenic genes was in accordance with the ALP
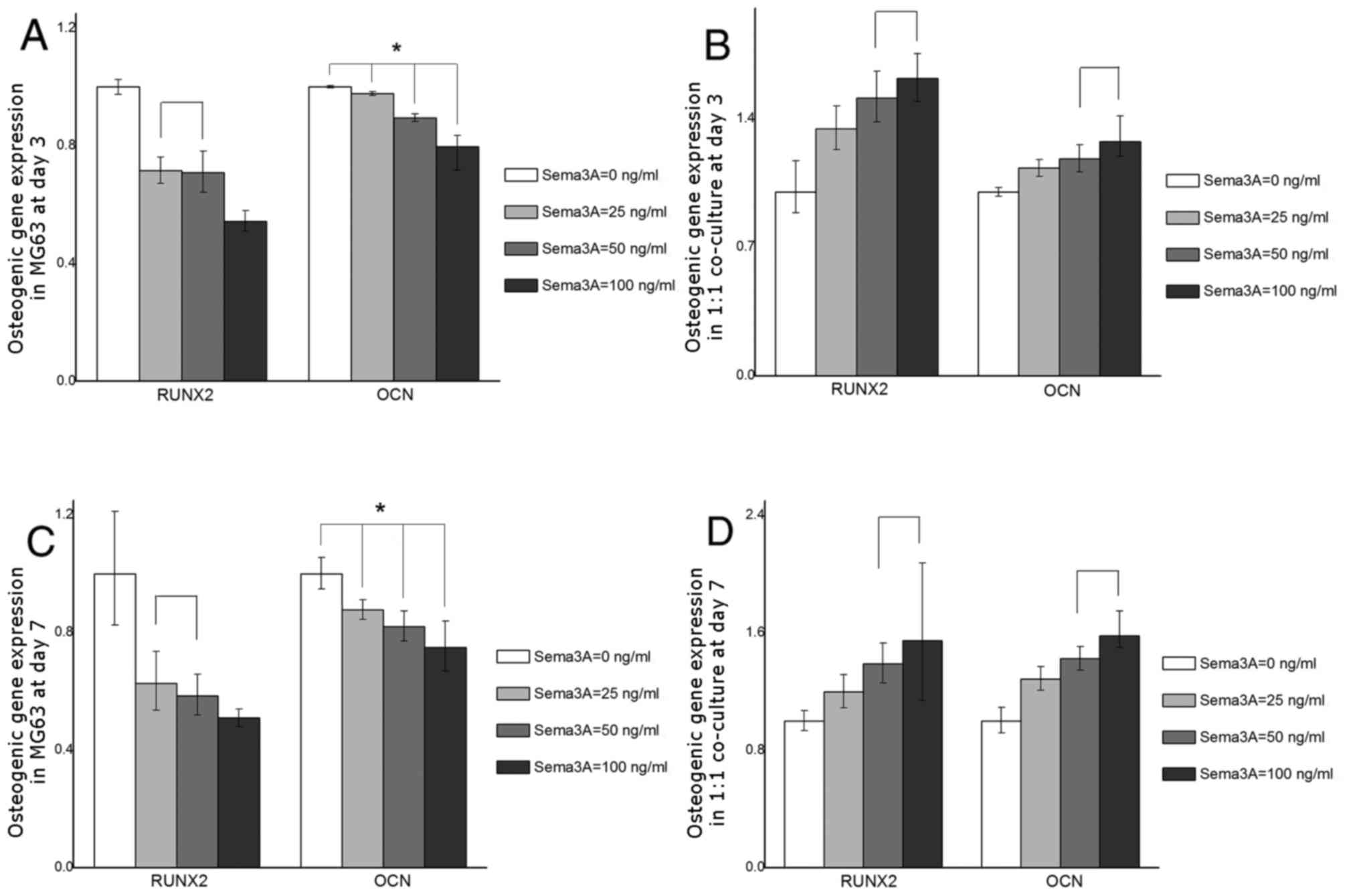
activity and calcium nodule mineralization results. At day 3, RUNX2
and OCN expression in MG63 cells declined when Sema3A was added,
with the lowest gene expression detected at 100 ng/ml Sema3A
compared with control group (0 ng/ml). On the contrary, the gene
expression levels of RUNX2 and OCN were increased in 1:1 co-culture
with the highest expression detected at 100 ng/ml Sema3A (Fig. 4A and B). Similarly, at day 7, RUNX2
and OCN indicated the same pattern of expression as described at
day 3 in both MG63 single culture and 1:1 co-culture. As
concentration of Sema3A increased, MG63 demonstrated a decline in
mRNA levels of both genes, whereas 1:1 co-culture demonstrated
increased expression levels of the genes (Fig. 4C and D). All Sema3A treatment
groups (25, 50 and 100 ng/ml) in MG63 and 1:1 co-culture were
statistically different (P<0.05) from the control group (0
ng/ml) at every time point.
Discussion
The present study established a co-culture system of
MG63 and Schwann cells to investigate the role of Sema3A and the
mechanisms underlying bone formation. Although co-culture systems
have been used in numerous studies, to the best of the author's
knowledge, the present study was the first to use a co-culture
system to dissect Sema3A function on osteogenesis. First, a
co-culture system was generated and optimal cell proliferation
determined at 1:1 of MG63:SCs. Cell proliferation in the 1:1
co-culture was consistently increased compared with MG63 and SCs
single cultures. This is in accordance with a previous study, in
which proliferation in a co-cultured system of Schwann cells and
osteoblasts has been demonstrated to be consistently increased
compared with single cell cultures (28). Then, the MG63 and SCs single
cultures and 1:1 co-culture were treated with different
concentrations of Sema3A (0, 25, 50 and 100 ng/ml) to observe any
effect of Sema3A on cell proliferation. The results demonstrated
that no statistical difference was present among groups in cell
proliferation of MG63 and 1:1 co-culture, indicating no influence
of Sema3A on cell growth. However, a decreased OD value in the 100
ng/ml Sema3A group of SCs was observed at day 5, suggesting that a
high concentration of Sema3A inhibited proliferation of SCs. The
apoptosis and cytotoxicity results for MG63 and SCs single culture
and 1:1 co-culture also suggested that Sema3A at applied
concentrations was non-cytotoxic and exerted no effect among
treatment groups on apoptosis.
RT-sqPCR was then performed to investigate gene
expression of Sema3A receptors in MG63 and SCs. Results verified
that MG63 expressed Nrp1, 2 and Plxa1, a2 and a3. Nrp2 and Plxa3
expression was detected in SCs. Previous studies reported that Nrp1
is the primary binding ligand for Sema3A (29) and Nrp1-Sema3A binding is involved
in regulation of the neural and immune system (24,30–32).
However, Nrp2, sharing 45% similarity in protein sequence with Nrp1
(33), has previously been
implicated in Sema3A signaling. Glioma cell migration is regulated
by Sema3A via Nrp2 (34), and Nrp2
improves Sema3A-dependent axonal targeting of the vomeronasal
nerves in Nrp1 knockout mice (35). This contradicts with the previous
view that Nrp1 bound preferentially with Sema3A and suggests a
potential role for Nrp2 in Sema3A signaling. The present study
observed low expression levels of Nrp2 in MG63 and high gene
expression of Nrp2 in SCs. It was therefore proposed that Nrp2 of
SCs may act as a principle receptor in Sema3A-mediated osteogenesis
in the co-culture system. However, this still requires further
investigation.
To dissect the role of Sema3A in osteogenesis and
its interaction with neural cells, level of ALP, extracellular
matrix mineralization and expression of osteogenic genes were
assessed. Sema3A was first applied to MG63 single cell culture.
However, contrary to previous findings on osteoblasts (36), ALP level, extracellular matrix
mineralization and the expression of osteogenic genes suggested
that Sema3A inhibited MG63 osteogenic differentiation compared with
the control group (0 ng/ml Sema3A), with inhibitory levels rising
with concentrations of Sema3A. Due to the lack of osteogenic
medium, and low concentrations of Sema3A applied in the present
study, 25 and 50 ng/ml Sema3A groups in calcium nodule staining and
RUNX2 expression in RT-qPCR did not indicate a statistical
difference. However, all Sema3A treatment groups, except the 25
ng/ml group of MG63 single culture in ALP assay, exhibited
statistically significant differences compared with control group
(0 ng/ml Sema3A) in all three assays. Furthermore, a trend of
decline in osteogenic differentiation of MG63 was observed and
verified in the present study. The authors present the following
explanation to describe the decline in osteogenic expression in
MG63 following Sema3A treatment. Firstly, MG63, alongside Saos-2
and U-2 OS cell lines, is a malignant bone tumor cell line acquired
from osteosarcoma. Although several osteoblastic features are
shared between these cell lines (37), cellular and molecular functions
including intercellular communication may vary due to chromosome
alterations (38). For instance,
MG63 expresses collagen-II and -IX whereas no expression of these
collagens is observed in human osteoblasts (39). The expression level of ALP in human
osteoblasts is decreased compared with osteosarcoma cells and is
dependent on the rate of confluence, which is different from MG63
(40). In addition, unlike
osteoblasts, bone marrow mesenchymal stem cells do not express Nrp1
(41), whereas MG63 was verified
to strongly express Nrp1 in the present study. Therefore, an
altered chromosome expression of MG63 from human osteoblasts may
exist, which may contribute to the deviated results of Sema3A
function on osteogenesis from previous studies. Secondly, Sema3A
has a complex role in cancer. Sema3A is downregulated in several
cancers (42) and serves as an
endogenous factor to inhibit tumor migration and progression
(43). In addition, its binding
with Nrp1 may inhibit cancer cell proliferation (44,45).
In the present study, MG63 cells did not demonstrate any regression
in proliferation under Sema3A. This may be due to the low
concentrations applied. Sema3A may still exhibit an inhibitory role
on the osteogenic differentiation of osteosarcoma-derived MG63,
however to the best of the author's knowledge, no report has been
discovered investigating the effect of Sema3A on osteosarcoma, and
thus, its specific role in these cells requires further
clarification. Furthermore, a previous study demonstrated that a
low concentration of Sema3A may prevent Plexin receptors from
performing signal transduction effectively due to insufficient
binding with Sema3A, whereas other receptors including PlexinA3 may
function properly at such concentrations (46). Therefore, instead of classic
receptors including Nrp1, other ligands including Nrp2 and Plxa3,
expression levels of which were verified in MG63 in the present
study, may be involved in osteogenesis along with Sema3A under low
concentrations. Sema3A may have engendered its osteogenic features
on a dose-dependent basis due to receptor preference. However, the
present study was rather limited in its ability to further
illustrate the exact role of Sema3A in MG63 differentiation.
To explore the potential interaction between Sema3A
and SCs in bone formation, the present study then added Sema3A at
different concentrations to the 1:1 co-culture to test possible
alterations in osteogenesis. The results indicated that ALP level,
extracellular matrix mineralization, and osteogenic gene expression
of 1:1 co-culture were gradually increased following addition of
Sema3A, with the highest level observed at 100 ng/ml in all three
tests, suggesting a dose-dependent pattern in skeletal function of
Sema3A. However, 50 and 100 ng/ml groups in RT-qPCR did not reveal
any statistical difference, indicating a more complicated role of
concentration in Sema3A-induced osteogenesis. However, results
revealed a positive role for SCs in bone formation. Schwann cells
are of vital importance in nerve repair. Following peripheral nerve
injury, class 3 Semaphorins including Sema3A are secreted by
Schwann cells, and Nrp1/Nrp2 expression is upregulated in Schwann
cells (47,48). This suggests the prominent
involvement that Sema3A and SCs share in nerve regeneration. In the
present study, the function of Sema3A in osteogenic expression in
the 1:1 co-culture was completely reversed to that observed in the
MG63 single culture. Firstly, according to a previous study
(49), SCs excrete various numbers
of neurotrophins including the brain-derived neurotrophic factor
and the nerve growth factor (NGF) following injury. These
neurotrophins, particularly NGF, have already been reported to have
a role in osteoblastic survival (50), and they bind receptors on multiple
non-neural cells including osteoblasts to inflict trophic features
(51). Therefore, the addition of
injury-associated Sema3A into the co-culture system may have
promoted secretion of trophic factors in SCs, which then promoted
MG63 differentiation. Secondly, since SCs were demonstrated to
express Nrp2 and Plxa3 in the present study, the link between
osteogenesis and Nrp2/Plxa3 was investigated. Verlinden et
al (52) demonstrated that
Nrp2 knock out mice have an insufficient number of osteoblasts, an
increased level of osteoclasts, and low bone mass. Notably, it has
previously been suggested that Nrp2, along with Plxa3, are
receptors of Sema3A that only partially and subordinately
participate in the signaling pathway in comparison to Nrp1 or
Plxa1/2 (32,53). However, Nrp2 is required for SCs
normal assembly into functional units for repair activities
(54), and Plxa3 is necessary for
nerve fibers to be precisely aimed at their targets (46). Therefore, whether this promotion in
osteogenesis is in part or entirely due to the binding of Sema3A to
Nrp2/Plxa3 or to other receptors, and semaphorins expressed and
secreted by SCs to form functional complexes, including the
bone-associated Semaphorin 6D-Plxa1[17] or the nerve-associated
Semaphorin 3F-Nrp2 (52,55), remains unclear.
Following the loss of a tooth, a lot of periodontal
exteroceptors and nerve fibers are lost. It is now well established
that oral implants gain stability and function through
osseointegration. However, numerous patients have reported having
felt a sensory difference following implant restoration compared
with their natural tooth, which may sometimes lead to occlusal
overload. Klineberg et al (56) suggested the term ‘osseoperception’
in 2005 to describe these alterations in sensation.
Neurophysiological and psychological explanations have been
proposed recently, however how oral sensations may be
re-transduced, or nerves be regenerated, is still unclear. In the
present study, Sema3A promoted osteogenesis of MG63 via interaction
with SCs, suggesting the potential of Sema3A in both the bone and
nervous system, which may be applied in periodontal rehabilitation,
helping to restore not only masticatory however also sensory, thus
completing oral function.
In conclusion, the present study established a
co-culture system to explore the potential role of Sema3A in
osteogenesis. Results revealed that in vitro, low
concentrations of Sema3A inhibited MG63 osteogenic expression.
Conversely, by interaction with Schwann cells, osteogenic
expression was elevated with increasing concentrations of Sema3A.
The molecular mechanisms underlying the function of Sema3A in
osteogenesis have not yet been fully elucidated, however
assumptions have been put forward to sustain the hypothesis that
Sema3A mediates bone cells via function of neural cells. Further
investigations will help to better understand the exact role that
the nervous system exhibit bone formation.
References
|
1
|
Nakahama K: Cellular communications in
bone homeostasis and repair. Cell Mol Life Sci. 67:4001–4009. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takeda S, Elefteriou F, Levasseur R, Liu
X, Zhao L, Parker KL, Armstrong D, Ducy P and Karsenty G: Leptin
regulates bone formation via the sympathetic nervous system. Cell.
111:305–317. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elefteriou F, Ahn JD, Takeda S, Starbuck
M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, et al:
Leptin regulation of bone resorption by the sympathetic nervous
system and CART. Nature. 434:514–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Serre CM, Farlay D, Delmas PD and Chenu C:
Evidence for a dense and intimate innervation of the bone tissue,
including glutamate-containing fibers. Bone. 25:623–629. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lerner UH, Persson E and Lundberg P:
Kinins and neuro-osteogenic factors. Principles Bone Biol.
2:1025–1057. 2008. View Article : Google Scholar
|
|
6
|
Chenu C and Marenzana M: Sympathetic
nervous system and bone remodeling. Joint Bone Spine. 72:481–483.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Togari A: Adrenergic regulation of bone
metabolism: Possible involvement of sympathetic innervation of
osteoblastic and osteoclastic cells. Microsc Res Tech. 58:77–84.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Toyofuku T, Zhang H, Kumanogoh A,
Takegahara N, Yabuki M, Harada K, Hori M and Kikutani H: Guidance
of myocardial patterning in cardiac development by Sema6D reverse
signaling. Nat Cell Biol. 6:1204–1211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Serini G, Valdembri D, Zanivan S, Morterra
G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L,
Logan M, et al: Class 3 semaphorins control vascular morphogenesis
by inhibiting integrin function. Nature. 424:391–397. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sierra JR, Corso S, Caione L, Cepero V,
Conrotto P, Cignetti A, Piacibello W, Kumanogoh A, Kikutani H,
Comoglio PM, et al: Tumor angiogenesis and progression are enhanced
by Sema4D produced by tumor-associated macrophages. J Exp Med.
205:1673–1685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maione F, Molla F, Meda C, Latini R,
Zentilin L, Giacca M, Seano G, Serini G, Bussolino F and Giraudo E:
Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks
tumor growth and normalizes tumor vasculature in transgenic mouse
models. J Clin Invest. 119:3356–3372. 2009.PubMed/NCBI
|
|
12
|
Jongbloets BC and Pasterkamp RJ:
Semaphorin signaling during development. Development.
141:3292–3297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo Y, Raible D and Raper JA: Collapsin: A
protein in brain that induces the collapse and paralysis of
neuronal growth cones. Cell. 75:217–227. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tamagnone L and Comoglio PM: To move or
not to move? Semaphorin signaling in cell migration. EBMO Rep.
5:356–361. 2004.
|
|
15
|
Roth L, Koncina E, Satkauskas S, Crémel G,
Aunis D and Bagnard D: The many faces of semaphorins: From
development to pathology. Cell Mol Life Sci. 66:649–666. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Narazaki M and Tosato G: Ligand-induced
internalization selects use of common receptor neuropilin-1 by
VEGF-165 and Semaphorin 3A. Blood. 107:3892–3901. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takegahara N, Takamatsu H, Toyofuku T,
Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DV,
Suzuki K, et al: Plexin-A1 and its interaction with DAP12 in immune
responses and bone homeostasis. Nat Cell Biol. 8:615–622. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayashi M, Nakashima T, Tanigushi M,
Kodama T, Kumanogoh A and Takayanagi H: Osteoprotection by
Semaphorin 3A. Nature. 485:69–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Negishi-Koga T, Shinohara M, Komatsu N,
Bito H, Kodama T, Friedel RH and Takayanagi H: Suppression of bone
formation by osteoblastic expression of semaphorin 4D. Nature Med.
17:1473–1480. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukuda T, Takeda S, Xu R, Ochi H, Sunamura
S, Sato T, Shibata S, Yoshida Y, Gu Z, Kimura A, et al: Sema3A
regulates bone-mass accrual through sensory innervations. Nature.
497:490–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barrette B, Hébert MA, Filali M, Lafortune
K, Vallières N, Gowing G, Julien JP and Lacroix S: Requirement of
myeloid cells for axon regeneration. J Neurosci. 28:9363–9376.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arthur-Farraj PJ, Latouche M, Wilton DK,
Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M,
Turmaine M, et al: c-Jun reprograms Schwann cells of injured nerves
to generate a repair cell essential for regeneration. Neuron.
75:633–647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jessen KR and Mirsky R: The repair Schwann
cell and its function in regenerating nerves. J Physiol.
594:3521–3531. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huttle RE and Huber AB: Cranial nerve
fasciculation and Schwann cell migration are impaired after loss of
Npn-1. Dev Biol. 359:230–241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tao Y: Isolation and culture of schwann
cells. Methods Mol Biol. 1018:93–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang K, Song W, Wang L, Jia S, Wei H, Ren
S, Xu X and Song Y: Immobilization of chitosan film containing
Semaphorin 3A onto a microarc oxidized titanium implant surface via
silane reaction to improve MG63 osteogenic differentiation. Int J
Nanomedicine. 9:4649–4657. 2014.PubMed/NCBI
|
|
27
|
Saad S, Dharmapatni AASSK, Crotti TN,
Cantley MD, Algate K, Findlay DM, Atkins GJ and Haynes DR:
Semaphorin-3a, neuropilin-1 and plexin-A1 in prosthetic-particle
induced bone loss. Acta Biomater. 30:311–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai XX, Luo E and Yuan Q: Interaction
between Schwann cells and osteoblasts in vitro. Int J Oral Sci.
2:74–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He Z and Tessier-Lavigne M: Neuropilin is
a receptor for the axonal chemorepellent Semaphorin III. Cell.
90:739–751. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huettl RE, Soellner H, Bianchi E, Novitch
BG and Huber AB: Npn-1 contributes to axon-axon interactions that
differentially control sensory and motor innervation of the limb.
PLoS Biol. 9:e10010202011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bouvrée K, Brunet I, Del Toro R, Gordon E,
Prahst C, Cristofaro B, Mathivet T, Xu Y, Soueid J, Fortuna V, et
al: Semaphorin3A, neuropilin-1 and plexinA1 are required for
lymphatic valve formation. Circ Res. 111:437–445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawasaki T, Kitsukawa T, Bekku Y, Matsuda
Y, Sanbo M, Yagi T and Fujisawa H: A requirement for neuropilin-1
in embryonic vessel formation. Development. 126:4895–4902.
1999.PubMed/NCBI
|
|
33
|
Cao Y, Hoeppner LH, Bach SEG, Guo Y, Wang
E, Wu J, Cowley MJ, Chang DK, Waddell N, et al: Neuropilin-2
promotes extravasation and metastasis by interacting with
endothelial α5 intergrin. Cancer Res. 73:4579–4590. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nasarre C, Koncina E, Labourdette G,
Cremel G, Roussel G, Aunis D and Bagnard D: Neuropilin-2 acts as a
modulator of Sema3A-dependent glioma cell migration. Cell Adh Migr.
3:383–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cariboni A, Davidson K, Rakic S, Maggi R,
Parnavelas JG and Ruhrberg C: Defective gonadotropin-releasing
hormone neuron migration in mice lacking SEMA3A signalling through
NRP1 and NRP2: Implications for the aetiology of hypogonadotropic
hypogonadism. Hum Mol Genet. 20:336–344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wan Q, Cho E, Yokota H and Na S: Rac1 and
Cdc42 GTPases regulate shear stress-driven β-catenin signaling in
osteoblasts. Biochem Biophys Res Commun. 433:502–207. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Declercq H, Van den Vreken N, De Maeyer E,
Verbeeck R, Schacht E, De Ridder L and Cornelissen M: Isolation,
proliferation and differentiation of osteoblastic cells to study
cell/biomaterial interactions: Comparison of different isolation
techniques and source. Biomaterials. 25:757–768. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Al-Romaih K, Bayani J, Vorobyova J,
Karaskova J, Park PC, Zielenska M and Squire JA: Chromosomal
instability in osteosarcoma and its association with centrosome
abnormalities. Cancer Genet Cytogenet. 144:91–99. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pautke C, Schieker M, Tischer T, Kolk A,
Neth P, Mutschler W and Milz S: Characterization of osteosarcoma
cell lines MG-63, Saos-2 and U-2 OS in comparison to human
osteoblasts. Anticancer Res. 24:3743–3748. 2004.PubMed/NCBI
|
|
40
|
Voegele TJ, Voegele-Kadletz M, Esposito V,
Macfelda K, Oberndorfer U, Vecsei V and Schabus R: The effect of
different isolation techniques on human osteoblast-like cell
growth. Anticancer Res. 20:3575–3581. 2000.PubMed/NCBI
|
|
41
|
Haixia D, Jingsong Z, Lei J, Hairong D,
Jun W, Hang X and Weixian C: Gene expression of neuropilin-1 and
its receptors, VEGF/Semaphorin 3A, in normal and cancer cells. Cell
Biochem Biophys. 59:39–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rehman M and Tamagnone L: Semaphorins in
cancer: Biological mechanisms and therapeutic approaches. Semin
Cell Dev Biol. 24:179–189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vacca A, Scavelli C, Serini G, Di Pietro
G, Cirulli T, Merchionne F, Ribatti D, Bussolino F, Guidolin D,
Piaggio G, et al: Loss of inhibitory Semaphorin 3A (SEMA3A)
autocrine loops in bone marrow endothelial cells of patient with
multiple myeloma. Blood. 108:1661–1667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Catalano A, Caprari P, Rodilossi S, Betta
P, Castellucci M, Casazza A, Tamagnone L and Procopio A: Cross-talk
between vascular endothelial growth factor and semaphorin-3A
pathway in the regulation of normal and malignant mesothelial cell
proliferation. FASEB J. 18:358–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Herman JG and Meadows GG: Increased class
3 semaphorin expression modulates the invasive and adhesive
properties of prostate cancer cells. Int J Oncol. 30:1231–1238.
2007.PubMed/NCBI
|
|
46
|
Cheng HJ, Bagri A, Yaron A, Stein E,
Pleasure SJ and Tessier-Lavigne M: Plexin-A3 mediates semaphorin
signaling and regulates the development of hippocampal axonal
projections. Neuron. 32:249–263. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ara J, Bannerman P, Hahn A, Ramirez S and
Pleasure D: Modulation of sciatic nerve expression of class 3
semaphorins by nerve injury. Neurochem Res. 29:1153–1159. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Scarlato M, Ara J, Bannerman P, Scherer S
and Pleasure D: Induction of neuropilins-1 and −2 and their
ligands, Sema3A, Sema3F and VEGF, during Wallerian degeneration in
the peripheral nervous system. Exp Neurol. 183:489–498. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alkhamarah BA, Hoshino N, Kawano Y, Harada
F, Hanada K and Maeda T: The periodontal Ruffini endings in brain
derived neurotrophic factor (BDNF) deficient mice. Arch Histol
Cytol. 66:73–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mogi M, Kondo A, Kinpara K and Togari A:
Anti-apoptotic action of nerve growth factor in mouse osteoblastic
cell line. Life Sci. 67:1197–1206. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mizuno N, Shiba H, Xu WP, Inui T, Fujita
T, Kajiya M, Takeda K, Hasegawa N, Kawaguchi H and Kurihara H:
Effect of neurotrophins on differentiation, calcification and
proliferation in cultures of human pulp cells. Cell Biol Int.
31:1462–1469. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Verlinden L, Kriebitzsch C, Beullens I,
Yan BK, Carmeliet G and Verstuyf A: Nrp2 deficiency leads to
trabecular bone loss and is accompanied by enhanced osteoclast and
reduced osteoblast numbers. Bone. 55:465–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yuan L, Moyon D, Pardanaud L, Bréant C,
Karkkainen MJ, Alitalo K and Eichmann A: Abnormal lymphatic vessel
development in neuropilin 2 mutant mice. Development.
129:4797–4806. 2002.PubMed/NCBI
|
|
54
|
Bannerman P, Ara J, Hahn A, Hong L,
McCauley E, Friesen K and Pleasure D: Peripheral nerve regeneration
is delayed in neuropilin 2-deficient mice. J Neurosci Res.
86:3163–3169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ara J, Bannerman P, Shaheen F and Pleasure
DE: Schwann cell-autonomous role of neuropilin-2. J Neurosci Res.
79:468–475. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Klineberg I, Calford MB, Dreher B, Henry
P, Macefield V, Miles T, Rowe M, Sessle B and Trulsson M: A
consensus statement on osseoperception. Clin Exp Pharmacol Physiol.
32:145–146. 2005. View Article : Google Scholar : PubMed/NCBI
|