Introduction
Warfarin is the most commonly used oral
anti-coagulant in clinical practice and is widely used in the
prevention and treatment of various types of deep venous
thrombosis, pulmonary embolism, prosthetic heart valves and atrial
fibrillation. Warfarin is characterized by its narrow therapeutic
window, which means that a small change in concentration can cause
serious side effects (1,2). Due to differences in the metabolism
of warfarin in individuals, inadequate or excessive use of warfarin
can lead to thrombus or hemorrhage (1,2).
Genetic and non-genetic factors, including age, sex, race, weight
and drug interactions can account for the better patient responses
to specific doses (2–5). So the genetic polymorphisms
contribute to the individual differences in warfarin dose response
(3–5). A number of clinical research studies
have revealed that a predictive equation based on pharmacogenomics
is reliable for determining the dosage that is affected by
individuals' genetic characteristics (6–11).
Genetic polymorphisms of cytochrome P450 family 2 subfamily C
member 9 (CYP2C9), vitamin K epoxide reductase complex
subunit 1 (VKORC1) and cytochrome P450 family 4 subfamily F
member 2 (CYP4F2) may result in 30–50% of individual
warfarin dose variability (12–14).
Lane et al (15) reported
that CYP2C19 and CYP3A4 genotypes had a profound
effect on R-warfarin clearance. Rieder et al (16) found that the γ-glutamyl carboxylase
(GGCX)-12970 SNP was associated with the warfarin
maintenance dose. Chung et al (17) confirmed that polymorphisms of
microsomal epoxide hydrolase 1 (EPHX1) and VKORC1-like 1 (VKORC1L1)
could contribute to the warfarin dose variability. A number of
studies and clinical trials regarding the pharmacogenomics of
warfarin have been conducted worldwide, and they have yielded a
number of different drug equations (4,5,10,18,19).
However, the value of each equation in the clinical application
between different races in different regions is quite different
(3–5,7,13). A
study published by Lou et al (5) in May 2014 identified the stable dose
of warfarin by evaluating Han Chinese patients and the results were
of particular interest. The Lou type warfarin pharmacokinetic
dosing algorithm equation was based on the genetic polymorphisms of
CYP2C9, VKORC1, and CYP4F2 and other non-genetic variables. In the
present study, the Lou type warfarin pharmacokinetic dosing
algorithm equation was applied to verify the efficacy of warfarin
in clinical treatments via a randomized, controlled prospective
study of Han Chinese patients in Zhejiang, which supported the
implementation of medicine stemming from clinical studies.
Materials and methods
Patients
The present study was approved by the Ethics
Committee of the Jinhua Hospital of TCM Affiliated to Zhejiang
University of Traditional Chinese Medicine (Zhejiang, China).
Patients (n=87; Table I) who were
admitted to Jinhua Hospital of TCM Affiliated to Zhejiang
University of Traditional Chinese Medicine (Zhejiang, China), Yiwu
Central Hospital (Zhejiang, China), and Zhejiang Jinhua Guangfu
Hospital (Zhejiang, China) and Rehabilitation Hospital of Yiwu
(Zhejiang, China) and required warfarin treatment were recruited to
the present study between June 2014 to 2016. Written informed
consent was obtained from all participants.
 | Table I.Comparison of clinical parameters and
genotypes between the experimental and control groups. |
Table I.
Comparison of clinical parameters and
genotypes between the experimental and control groups.
| Clinical
parameters | Experimental group
(n=42) | Control group
(n=45) | P-value |
|---|
| Age (years) | 62.5±12.8 | 61.4±13.2 | 0.628 |
| Sex
(female/male) | 17/25 | 17/28 | 0.797 |
| Height (cm) | 160.2±8.2 | 159.5±8.6 | 0.649 |
| Weight (kg) | 65.2±10.9 | 66.8±11.3 | 0.458 |
| Anticoagulation
indications |
|
| 0.876 |
| Atrial
fibrillation (n) | 29 | 32 |
|
| Venous
thrombosis (n) | 5 | 7 |
|
|
Pulmonary embolism (n) | 3 | 2 |
|
| Valve
replacement (n) | 5 | 4 |
|
| Combined use of
drugs |
|
| 0.905 |
|
Amiodarone (n) | 2 | 2 |
|
| Digoxin
(n) | 8 | 7 |
|
| CYP2C9 |
|
| 0.808 |
| *1/*1
(n) | 38 | 40 |
|
| *1/*3
(n) | 4 | 5 |
|
| *3/*3
(n) | 0 | 0 |
|
| VKORC1 |
|
| 0.563 |
| AA
(n) | 35 | 35 |
|
| AG
(n) | 7 | 9 |
|
| GG
(n) | 0 | 1 |
|
| CYP4F2 |
|
| 0.849 |
| CC
(n) | 22 | 22 |
|
| CT
(n) | 16 | 17 |
|
| TT
(n) | 4 | 6 |
|
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) Patients
were >18 years and were able to behave independently; ii)
patients with sufficient medical data available; and iii) patients
consented to a 50 day experimental period following treatment. The
exclusion criteria were as follows: i) Patients with no serious
bleeding within the last 6 months; ii) patients who rejected
long-term anti-coagulant therapy; and iii) patients were unable to
complete the study.
Lou type warfarin pharmacokinetic
dosing algorithm equation
The present study selected the Lou type warfarin
pharmacokinetic dosing algorithm equation following comparisons
between the equations from multiple studies (1,5,7,18).
The warfarin stable dosage equation was as follows: Daily dose of
warfarin (mg) = 1.087 + 2.226 × VKORC1(1639AG)# + 3.844
× VKORC1(1639GG)$ - 1.284 ×
CYP2C9(*1/*3)& - 2.182 × CYP2C9(*3/*3)α +
0.221 × CYP4F2(CT)β + 0.336 × CYP4F2(TT)γ -
0.018 × age (years) + 0.015 × weight (kg) + 0.013 × height (cm) -
0.777 × Amiodaroneλ - 0.379 × digoxinσ. Where
the following have been applied: #VKORC1(1639AA) = 0,
VKORC1(1639AG) = 1; $VKORC1(1639AA) = 0, VKORC1(1639GG)
= 1; &CYP2C9(*1/*1) = 0, CYP2C9(*1/*3) = 1;
αCYP2C9(*1/*1) = 0, CYP2C9(*3/*3) = 1;
βCYP4F2(CC) = 0, CYP4F2(CT) = 1; γCYP4F2(CC)
= 0, CYP4F2(TT) = 1; λused amiodarone = 1, did not use
amiodarone = 0; σused digoxin = 1, did not use digoxin =
0.
Information collection
According to Table
I, 87 patients prescribed with anti-clotting warfarin were
randomly divided into the experimental and control groups. Clinical
data and blood samples were then collected. The personal data of
each participant, including age, sex, height, weight, medical
history, hemorrhage history and medication history were recorded. A
total of 5 ml blood was obtained from each patient for the present
study.
Polymerase chain reaction-restriction
fragment length polymorphism (PCR-RFLP) and sequencing
The polymorphism of CYP2C9, VKORC1, and CYP4F2 were
detected by PCR-RFLP and DNA sequencing. Peripheral white blood
cell genomic DNA were extracted from blood samples using the
PAXgene Blood DNA kit (Qiagen, GmbH, Hilden, Germany) (the Japanese
supplier being Cosmo Bio Co., Ltd., Tokyo, Japan), according to the
manufacturer's instructions. According to the method described
previously (20,21), CYP2C9*3 (1075A/C, rs1057910),
VKORC1 (1639G/A, rs9923231) and CYP4F2 V433M (rs2108622) were
amplified by PCR. Then, the PCR products were digested for 1.5 h at
37°C with a restriction endonuclease, and analyzed by 2% agarose
gel electrophoresis. The DNA sequences of all samples were
visualized by ethidium bromide under ultraviolet light using the
Molecular Imager Chemi Doc XRS+ System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and analyzed with Quantity One 1D Analysis
software (version 4.6.6; Bio-Rad Laboratories, Inc.). Then, all
samples were analyzed by DNA sequencing in Sangon Biotech
(Shanghai) Co., Ltd. (Shanghai, China).
Study strategy
To evaluate the equation in a single-blind manner,
the patients were divided into 2 groups without any knowledge of
which group they were assigned to. The polymorphism of the 3 genes
was determined and the predicted dose of warfarin was calculated
according to the Lou type equation, as aforementioned. For the
first 3 administrations, all patients in the control group were
treated according to the dosages prescribed by an experienced
doctor, while all participants in the experimental group were
treated using the dosages calculated by the Lou type equation.
Then, the dose of warfarin administrated to all patients was
gradually adjusted to a stable dose according to the changes in the
international standardized ratio (INR). The stable dose means that
after the same patient received the same dose, the two consecutive
INR values were between 2.0–3.0, and the interval time was >7
days. Following the 50 day experimental period following the first
administration, the stable dose, the time to achieve a stable dose
and adverse reactions were recorded. In addition, the physician in
charge was informed of the study's results and appropriate measures
were applied if adverse reactions, including INR >3.5, bleeding,
or new thrombosis occurred.
Statistical analysis
All analyses were performed using Stata 12.0
software (StataCorp LP, College Station, TX, USA). Quantitative
data are presented as the mean ± standard error of the mean.
Measurement data were analyzed using a paired t-test and the
enumeration data were analyzed with an χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Sequencing analysis
PCR-RFLP was used to detect the genotypes of
CYP2C9*3 (1075A/C, rs1057910), VKORC1 (1639G/A, rs9923231) and
CYP4F2 V433M (rs2108622). The amplification products of CYP2C9,
VKORC1 and CYP4F2 (V433M) were 200, 423 and 491 bp, respectively.
Furthermore, the mutated amplification product of CYP2C9*3
(1075A/C) was digested by KpnI into 2 fragments of 180 and 20 bp;
the mutated amplification product of VKORC1 (1639G/A) can be
digested by MspI into 2 fragments of 207 and 216 bp; and the
mutated amplification product of CYP4F2 V433M can be digested by
PvuII into 2 fragments of 319 and 178 bp. According to the results
of enzyme digestion, there were 3 genotypes of each gene: Mutant
homozygote, heterozygote and wild-type (Fig. 1; Table
I). All samples were analyzed by DNA sequencing and the results
were consistent with those of PCR-RFLP.
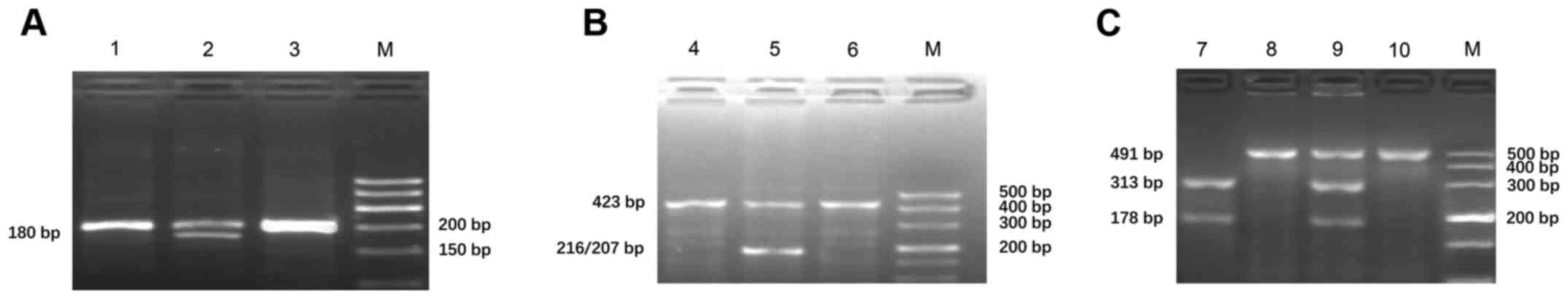 | Figure 1.Genotype analysis of (A) CYP2C9*3
(1075A/C, rs1057910), (B) VKORC1 (1639G/A, rs9923231) and (C)
CYP4F2 V433M (rs2108622). Lane M, DNA marker; lane 1, wild-type of
CYP2C9 (*1/*1); lane 2, heterozygote of CYP2C9 (*1/*3); lane 3,
amplification product of CYP2C9 by PCR; lane 4, wild-type of
VKORC1(1639A/A); lane 5, heterozygote of VKORC1(1639A/G); lane 6,
amplification product of VKORC1 by PCR; lane 7, mutant homozygote
of CYP4F2 V433M (TT); lane 8, wild-type of CYP4F2 V433M (CC); lane
9, heterozygote of CYP4F2 V433M (CT); lane 10, amplification
product of CYP4F2 V433M by PCR; PCR, polymerase chain reaction;
CYP2C9, cytochrome P450 family 2 subfamily C member 9; VKORC1,
vitamin K epoxide reductase complex subunit 1; CYP4F2, cytochrome
P450 family 4 subfamily F member 2. |
Comparison of the clinical
parameters
The 87 patients were divided into 2 groups, with 42
participants in the experimental group and 45 participants in the
control group. No significant differences were identified in the
distribution of the CYP2C9*3, VKORC1 (1639G/A) and CYP4F2 V433M
genotypes, age, sex, height, weight and history of disease
(Table I) between the 2
groups.
Comparisons of the number of patients
exhibited a stable dose, the time for patients to obtain a stable
dose and the adverse reactions
According to the obtained statistics, there was a
significant difference in the number of patients reaching a stable
dose between the 2 groups, with 35 patients (83.3%) in the
experimental group and 29 patients (64.4%) in the control group
(P=0.046). In addition, it took 18.2±1.7 days for the participants
in the experimental group to achieve a stable dose, which was less
than that of the control group (27.3±2.0 days). The median time to
achieve a stable dose was 11.7±1.1 days for the experimental group
and 20.5±1.8 days for the control group, which indicated a
statistically significant difference between the two groups
(P<0.001). Furthermore, the incidence of adverse reactions in
the experimental group was 9.5% (4 cases), which was significantly
lower than that of the control group (26.7%; 12 cases; P=0.039). In
addition, the average time of developing adverse reactions in the
experimental group (43.9±1.6 days) was significantly longer than
that of the control groups (38.6±1.5 days; P=0.046).
Comparisons between the predictive
dose and actual dose
At the end of the study, 64 participants received
the stable dose aggregately. The average dose of 3.4±1.1 mg/day,
which was predicted using the Lou type equation, was lower than the
actual average dose of 3.5±1.4 mg/day; however, no significant
difference was observed between the predicted dose and the actual
dose (P=0.313).
Discussion
Individualized treatment refers to the use of
individual drugs and also the use of individualized drug dosages
based on pharmacogenomics, which has become a popular method for
treating cardiovascular illnesses (22). Warfarin is one of the preferred
anti-coagulant drugs for the anti-coagulant therapy in patients
with atrial fibrillation, deep vein thrombosis, pulmonary embolism,
cerebral infarction, radiofrequency ablation, and multiple types of
cardiomyopathy at present (23).
Previous studies have demonstrated that non-genetic factors
including sex, body weight, body surface area, age, number of
increasing INR drugs, smoking habit, preoperative stroke history
and hypertension were minor determinants of warfarin stable dosage
(6,19,24).
Thus, the problem of how to determine the stable dosage and how to
maintain the dose accurately and conveniently should be
addressed.
Some previous studies focusing on pharmacogenomics
have indicated that the single nucleotide polymorphism (SNP) of
CYP2C9*3 and VKORC1(1639G/A) may affect the pharmacodynamics and
pharmacokinetics of warfarin (3,4,8–11,14,22,
24,25). In addition, other previous studies
have suggested that the SNP of CYP4F2 V433M may be a determinant of
warfarin's pharmacodynamic and pharmacokinetic properties (21).
Although numerous equations for predicting a stable
dose of warfarin have been certificated, their practical
application value has differed (4,5,7,19,22,26).
A number of verification tests performed for these equations did
not produce perfect results (27–29).
However, the results were worse still in clinics, as many studies
from China were only based on a single disease, including atrial
fibrillation, pulmonary embolism or valve replacement; particularly
in the cardiovascular departments of primary hospitals, where
warfarin was often used for atrial fibrillation, and in larger
hospitals, where warfarin was used for valve replacements (30,31).
In some cases, combining warfarin with digoxin treatment has
produced better results in patients with rheumatic heart disease or
chronic heart failure; however, patients who also had valve
replacement may require the combination of warfarin with amiodarone
(32–35). Therefore, the warfarin stable dose
prediction equation derived by taking into account a variety of
factors that affect the stability of warfarin dose may be the best
option for patients and doctors. Following comparison with other
equations, it was demonstrated that the Lou type equation used in
the present study may have greater practical value in the Chinese
Han population (1,5,7,21,26–28).
In the present study, the value of the Lou type
warfarin pharmacokinetic dosing algorithm equation was verified
through a randomized controlled prospective study of Han Chinese
patients in Zhejiang. The results indicated that the experimental
group yielded a greater number of cases reaching a stable dose and
took less time to achieve a stable dosage than the control group.
Therefore, using the Lou type equation may significantly shorten
the dosage adjusting time, facilitate an effective and stable drug
concentration, reduce detection by INR and even decrease
therapeutic costs.
Furthermore, the incidence of adverse reactions in
the control group was 26.7% (12 cases), which was significantly
higher than that of the 9.5% in the experimental group (4 cases).
In addition, the experimental group took 43.9±1.6 days to develop
adverse reactions, while these reactions were observed in the
control group following 38.6±1.5 days. Thus, using the Lou type
equation may reduce the incidence of side effects and delay the
occurrence of adverse reactions, resulting in a safer clinical
application of warfarin.
In the present study, 65 participants received the
stable dose aggregately. The average dose of 3.5±1.1 mg/day, which
was predicted by the Lou type equation, was lower than the actual
average dose of 3.5±1.4 mg/day, however, no significant difference
was found between the two groups. The results of the present study
are indicative of the strong application value of the Lou type
warfarin pharmacokinetic dosing algorithm equation for treating Han
Chinese patients.
Although the present study yielded favorable
results, it did have some limitations. For individuals, the
predicted stable dose was an estimate that may have a large
discrepancy with the actual value, likely leading to an unnecessary
clinical risk. Therefore, the predicted stable dose should be
considered as a reference in the process of adjusting dosages.
Doctors or physicians should be more concerned with the monitored
INR. In terms of estimating eating habits, drug use and health
should not be underestimated. As the present study did not adjust
dosages for factors including eating habits, socioeconomic status
and patients' cognition regarding warfarin, a local large-scale
study is required to yield more accurate data.
In conclusion, the application of Lou type warfarin
pharmacokinetic dosing algorithm equation markedly shortened the
adjustment time and reduced the occurrence of adverse reactions,
which suggested that it may have great value in clinical drug
application.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JGJ designed the study, performed the experiments,
analyzed the data and wrote the manuscript. NNJ, JLL, XPG and XMD
collected the cases. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Jinhua Hospital of TCM Affiliated to Zhejiang
University of Traditional Chinese Medicine (Jinhua, Zhejiang,
China). Written informed consent was obtained from all
participants.
Consent for publication
Written informed consent was obtained from all
participants.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Yu Z, Ding YL, Lu F, Miao LY, Shen ZY and
Ye WX: Warfarin dosage adjustment strategy in Chinese population.
Int J Clin Exp Med. 8:9904–9910. 2015.PubMed/NCBI
|
|
2
|
Baglin TP, Keeling DM and Watson HG;
British Committee for Standards in Haematology, : Guidelines on
oral anticoagulation (warfarin): Third edition-2005 update. Br J
Haematol. 132:277–285. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park SM, Lee JK, Chun SI, Lee HI, Kwon SU,
Kang DW and Kim JS: VKORC1 and CYP2C9 genotype variations in
relation to warfarin dosing in Korean stroke patients. J Stroke.
15:115–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krishna Kumar D, Shewade DG, Loriot MA,
Beaune P, Balachander J, Sai Chandran BV and Adithan C: Effect of
CYP2C9, VKORC1, CYP4F2 and GGCX genetic variants on Warfarin
maintenance dose and explicating a new pharmacogenetic algorithm in
South Indian population. Eur J Clin Pharmacol. 70:47–56. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lou Y, Hua L, Han L, Li Y, Zhang X, Tang
M, Yu H, Liu Z, Wang W, Xu J, et al: Establishment and preliminary
validation of Warfarin maintenance dose algorithm in Chinese Han
Population. Zhonghua Xin Xue Guan Bing Za Zhi. 42:384–388. 2014.(In
Chinese). PubMed/NCBI
|
|
6
|
Routledge PA, Davies DM, Bell SM, Cavanagh
JS and Rawlins MD: Predicting patients' warfarin requirements.
Lancet. 2:854–855. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Liu R, Luo ZY, Yan H, Huang WH, Yin
JY, Mao XY, Chen XP, Liu ZQ, Zhou HH and Zhang W: Comparison of the
predictive abilities of pharmacogenetics-based warfarin dosing
algorithms using seven mathematical models in Chinese patients.
Pharmacogenomics. 16:583–590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aithal GP, Day CP, Kesteven PJ and Daly
AK: Association of polymorphisms in the cytochrome P450 CYP2C9 with
warfarin dose requirement and risk of bleeding complications.
Lancet. 353:717–719. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bodin L, Verstuyft C, Tregouet DA, Robert
A, Dubert L, Funck-Brentano C, Jaillon P, Beaune P, Laurent-Puig P,
Becquemont L and Loriot MA: Cytochrome P450 2C9 (CYP2C9) and
vitamin K epoxide reductase (VKORC1) genotypes as determinants of
acenocoumarol sensitivity. Blood. 106:135–140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sconce EA, Khan TI, Wynne HA, Avery P,
Monkhouse L, King BP, Wood P, Kesteven P, Daly AK and Kamali F: The
impact of CYP2C9 and VKORC1 genetic polymorphism and patient
characteristics up-on warfarin dose requirements: Proposal for a
new dosing regiment. Blood. 106:2329–2333. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rost S, Fregin A, Ivaskevicius V,
Conzelmann E, Hörtnagel K, Pelz HJ, Lappegard K, Seifried E,
Scharrer I, Tuddenham EG, et al: Mutations in VKORC1 cause warfarin
resistance and multiple coagulation factor deficiency type 2.
Nature. 427:537–541. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou SF, Zhou ZW and Huang M:
Polymorphisms of human cytochrome P450 2C9 and the functional
relevance. Toxicology. 278:165–188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Namazi S, Azarpira N, Hendijani F,
Khorshid MB, Vessal G and Mehdipour AR: The impact of genetic
polymorphisms and patient characteristics on warfarin dose
requirements: A cross-sectional study in Iran. Clin Ther.
32:1050–1060. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Higashi MK, Veenstra DL, Kondo LM,
Wittkowsky AK, Srinouanprachanh SL, Farin FM and Rettie AE:
Association between CYP2C9 genetic variants and anticoagulation
related outcomes during warfarin therapy. JAMA. 287:1690–1698.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lane S, Al-Zubiedi S, Hatch E, Matthews I,
Jorgensen AL, Deloukas P, Daly AK, Park BK, Aarons L, Ogungbenro K,
et al: The population pharmacokinetics of R- and S-warfarin: Effect
of genetic and clinical factors. Br J Clin Pharmacol. 73:66–76.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rieder MJ, Reiner AP and Rettie AE:
Gamma-glutamyl carboxylase (GGCX) tagSNPs have limited utility for
predicting warfarin maintenance dose. J Thromb Haemost.
5:2227–2234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung JE, Lee KE, Chang BC and Gwak HS:
Polymorphisms of vitamin K-related genes (EPHX1 and VKORC1L1) and
stable warfarin doses. Gene. 641:68–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hamberg AK, Hellman J, Dahlberg J, Jonsson
EN and Wadelius M: A Bayesian decision support tool for efficient
dose individualization of warfarin in adults and children. BMC Med
Inform Decis Mak. 15:72015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
International Warfarin Pharmacogenetics
Consortium, ; Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE,
Lee MT, Limdi NA, Page D, Roden DM, et al: Estimation of the
warfarin dose with clinical and pharmacogenetic data. N Engl J Med.
360:753–764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui GL, Ding H, Xu YJ, et al: High
resolution melting curves (HRM) in the prevention of Warfarin
initiation guide measurement genotyping and clinical application of
various genotyping methods. Chin J Cardiol. 40:477–481. 2012.
|
|
21
|
Deng S, Zhu G, Liu F, Zhang H, Qin X, Li L
and Zhiyi H: CYP4F2 gene V433M polymorphism is associated with
ischemic stroke in the male Northern Chinese Han population. Prog
Neuropsychopharmacol Biol Psychiatry. 34:664–668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fung E, Patsopoulos NA, Belknap SM,
O'Rourke DJ, Robb JF, Anderson JL, Shworak NW and Moore JH: Effect
of genetic variants, especially CYP2C9 and VKORC1, on the
pharmacology of Warfarin. Semin Thromb Hemost. 38:893–904. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirsh J, Dalen JE, Anderson DR, Poller L,
Bussey H, Ansell J, Deykin D and Brandt JT: Oral anticoagulants:
Mechanism of action, clinical effectiveness, and optimal
therapeutic range. Chest. 114 5 Suppl:445S–469S. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shahin MH, Cavallari LH, Perera MA,
Khalifa SI, Misher A, Langaee T, Patel S, Perry K, Meltzer DO,
McLeod HL and Johnson JA: VKORC1 Asp36Tyr geographic distribution
and its impact on warfarin dose requirements in Egyptians. Thromb
Haemost. 109:1045–1050. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mushiroda T, Ohnishi Y, Saito S, Takahashi
A, Kikuchi Y, Saito S, Shimomura H, Wanibuchi Y, Suzuki T, Kamatani
N and Nakamura Y: Association of VKORC1 and CYP2C9 polymorphisms
with warfarin dose requirements in Japanese patients. J Hum Genet.
51:249–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J, Shao L, Gong L, Luo F, Wang J, Shi
Y, Tan Y, Chen Q, Zhang Y, Hui R and Wang Y: A
pharmacogenetics-based Warfarin maintenance dosing algorithm from
Northern Chinese patients. PLoS One. 9:e1052502014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhuang WF, Chen YH, Luo RP, et al:
Evaluation on the stable dose prediction accuracy of Warfarin
anticoagulant therapy by pharmacogenetics among Shanghai patients.
Lab Med. 30:697–702. 2015.
|
|
28
|
Yu LP, Song HT, Zeng ZY, Wang QM and Qiu
HF: Validation and comparison of pharmacogenetics-based warfarin
dosing algorithms in Han Chinese Patients. Zhonghua Xin Xue Guan
Bing Za Zhi. 40:614–619. 2012.(In Chinese). PubMed/NCBI
|
|
29
|
Roper N, Storer B, Bona R and Fang M:
Validation and comparison of pharmacogenetics-based warfarin dosing
algorithms for application of pharmacogenetic testing. J Mol Diagn.
12:283–291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao S, Zhao H, Wang X, Gao C, Qin Y, Cai
H, Chen B and Cao J: Factors influencing medication knowledge and
beliefs on warfarin adherence among patients with atrial
fibrillation in China. Patient Prefer Adherence. 11:213–220. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han ZH, Ren XJ and Wang Y: Anticoagulation
management of patients with long-term warfarin therapy after valve
replacement during the perioperative period of pacemaker
implantation. Int J Clin Exp Med. 6:594–598. 2013.PubMed/NCBI
|
|
32
|
Hudson M, Richard H and Pilote L:
Differences in outcomes of patients with congestive heart failure
prescribed celecoxib, rofecoxib, or non-steroidal anti-inflammatory
drugs: Population based study. BMJ. 330:13702005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cleland JG, Findlay I, Jafri S, Sutton G,
Falk R, Bulpitt C, Prentice C, Ford I, Trainer A and Poole-Wilson
PA: The Warfarin/Aspirin Study in Heart failure (WASH): A
randomized trial comparing antithrombotic strategies for patients
with heart failure. Am Heart J. 148:157–164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu Y, Won KA, Nelson BJ, Qi D, Rausch DJ
and Asinger RW: Characteristics of the amiodarone-warfarin
interaction during long-term follow-up. Am J Health Syst Pharm.
65:947–952. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhong SL, Liu Y, Yu XY, Xu D, Tan HH, Lin
QX, Yang M, Lao HY and Lin SG: The influence of genetic
polymorphisms and interacting drugs on initial response to warfarin
in Chinese patients with heart valve replacement. Eur J Clin
Pharmacol. 67:581–590. 2011. View Article : Google Scholar : PubMed/NCBI
|















