Introduction
Osteoarthritis (OA) is a common degenerative
disorder of human articular cartilage characterized by the
destruction of articular cartilage and osteophyte formation
(1). Chondrocytes are the only
cell type present in articular cartilage and show little metabolic
activity. Recent studies have suggested that chondrocyte apoptosis
is related to extracellular matrix remodeling (2). In the progression of OA, the
imbalance between apoptosis and the proliferation of chondrocytes
causes chondrocyte cytokine production and matrix degeneration
(3). Therefore, one method to
prevent articular cartilage degeneration is to inhibit
apoptosis-related signaling molecules.
Paeoniflorin, a major pharmacological pinane
monoterpene glucoside, was first isolated from the Ranunculaceae
plant in 1963. It is widely accepted that paeoniflorin has
antioxidant, anti-inflammation, hepatoprotective and
neuroprotective effects (4–7). In
an adjuvant-induced arthritis model, paeoniflorin inhibited the
expression of IL-1β, IL-6, IL-17, and TNF-α and upregulated the
production of TGF-β1 (8). In other
musculoskeletal systems, Chen et al (9) demonstrated that paeoniflorin could
block the apoptosis of fiber ring cells by reducing the expression
of Fas and caspase-3 proteins via regulation of Fas-FasL signaling.
Moreover, in internal disc disruption disease, paeoniflorin was
also reported to decrease the percentage of dead nucleus pulposus
cells by inhibiting the activation of caspase-3 and −9 and
increasing Bcl-2 family protein expression (10).
We previously reported that treatment with
paeoniflorin downregulated the expression of matrix
metalloproteinase (MMP)-1, −3 and −13, and increased the expression
of TIMP-1 at both the mRNA and protein levels in a dose-dependent
manner in rat articular chondrocytes stimulated by IL-1β. Hui et
al (11) found that increased
levels of MMP-13 were closely related to the destruction of
cartilage matrix and chondrocyte apoptosis. Nevertheless, little is
known about the effect of paeoniflorin in chondrocyte apoptosis
(11). Therefore, the present
study evaluated the effects of paeoniflorin on IL-1β-induced
chondrocyte apoptosis and determined the associated mechanism by
examining Bcl-2, Bax and caspase-3.
Materials and methods
Reagents
Paeoniflorin (purity ≥98%) was obtained from
Sigma-Aldrich (St. Louis, MO, USA). Recombinant IL-1β was purchased
from PeproTech (Rocky Hill, NJ, USA). Dulbecco's modified Eagles
medium (DMEM), penicillin and streptomycin, fetal bovine serum
(FBS), 0.05% trypsin, and collagenase II were obtained from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA).
Primary cultures of normal rat
articular chondrocytes
Rat articular chondrocytes for primary culture were
obtained from the tibial plateau and femoral condyle of a
4-week-old Sprague-Dawley rat (The Animal Center of Zhejiang
University, Hangzhou, China). In brief, cartilage was rinsed in
phosphate-buffered saline (PBS) three times and finely cut into
pieces of 1–3 mm3, digested with 0.2% pronase for 0.5 h,
and then digested with 0.1% collagenase for 4 h at 37°C. Cells were
cultured in complete DMEM containing antibiotic-antimycotic
solution and 10% FBS at 37°C under a humidified 5% CO2
atmosphere. The medium was replaced every 2 days. The animal
experiments performed in the present study were approved by the
University of Zhejiang Institutional Animal Care and Use Committee,
Hang Zhou, China.
Lactate dehydrogenase cytotoxicity
assay
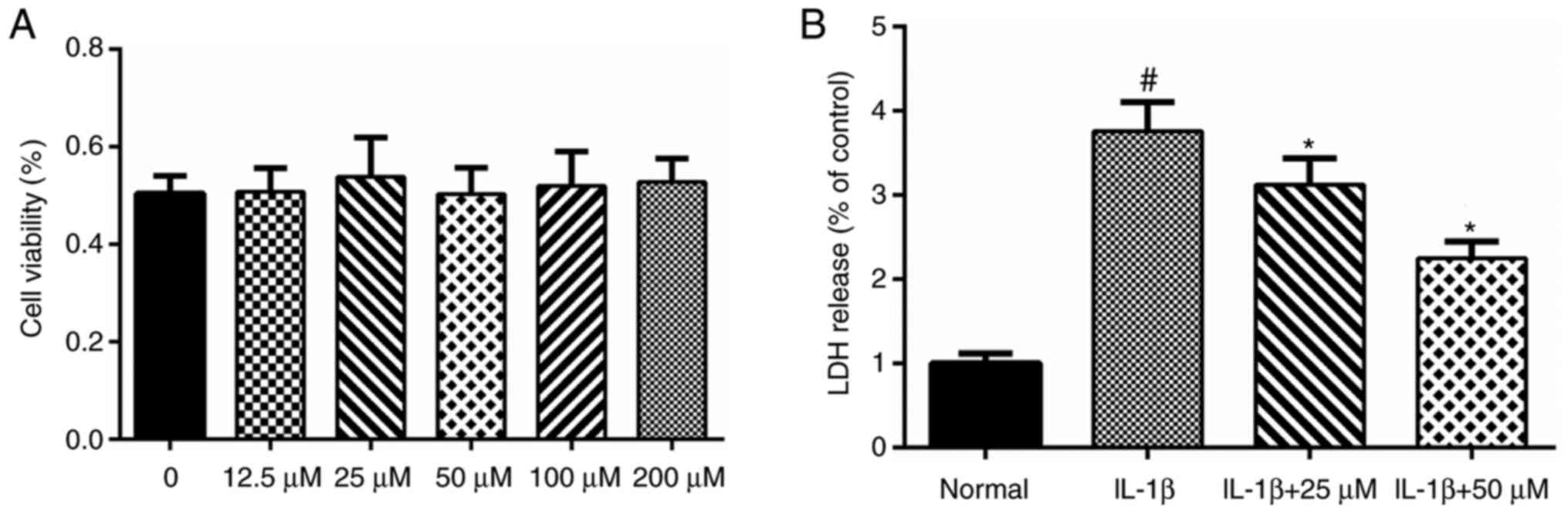
According to our previous MTT assay, paeoniflorin
concentrations ranging from 12.5 to 100 µM did not show any
significant toxicity to chondrocytes (Fig. 1A). Therefore, concentrations from
25 to 50 µM were used in subsequent experiments. The lactate
dehydrogenase (LDH) cytotoxicity assay was performed using the LDH
Cytotoxicity Assay Kit (Beyotime Institute of Biotechnology,
Jiangsu, China) (12). In brief,
chondrocytes were cultured in 96-well plates. Cells were pretreated
with 25 or 50 µM paeoniflorin for 3 h and then incubated with IL-1β
(10 ng/ml) for 24 h. The release of LDH was measured according to
the manufacturer's instructions.
Analysis of apoptotic cells by flow
cytometry
Annexin V-fluorescein isothiocyanate (FITC) antibody
immunofluorescence combined with propidium iodide (PI)/DNA binding
was used to analyze apoptosis. Chondrocytes were pretreated in
growth medium supplemented with paeoniflorin for 3 h and then
incubated in the absence or presence of rat recombinant IL-1β (10
ng/ml) for 24 h. Next, according to the instructions with the
Annexin V-FITC Apoptosis Detection Kit (Beyotime Institute of
Biotechnology), 1×105 cells were treated with Annexin
V-FITC and PI in the provided binding buffer for 0.5 h in the dark
at 4°C. Cells were then subjected to flow cytometry at the emission
wavelength of 488 nm.
Caspase-3 activity
Caspase-3 activity was determined using a Caspase-3
Cellular Activity Assay Kit (Cell Signaling Technology, Inc.,
Danvers, MA, USA). During the assay, activated caspase-3 cleaves
the fluorogenic substrate
(N-Acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin [Ac-DEVDAMC])
between DEVD and AMC. Thus, we can determine highly fluorescent AMC
concentrations using a fluorescence reader with excitation at 380
nm. Cells were pre-incubated in growth medium supplemented with
different concentrations of paeoniflorin for 3 h, and then
incubated with rat recombinant IL-1β (10 ng/ml) for 24 h. According
to the manufacturer's protocol, chondrocytes were collected and
lysed using cell lysis buffer in the presence or absence of 5 µl
DEVD-pNA for 1 h at 37°C. Caspase-3 activity was measured at 405 nm
on a microplate reader. Experiments were performed in
triplicate.
Paeoniflorin treatment and mRNA
expression analysis of Bcl-2 and Bax by reverse
transcription-quantitative polymerase chain reaction (PCR)
Chondrocytes were incubated in growth medium
supplemented with 25 or 50 µM paeoniflorin for 3 h and then
incubated in the absence or presence of rat recombinant IL-1β (10
ng/ml) for 24 h. Total RNA was isolated using TRIzol reagent
(Sigma-Aldrich). Briefly, 1 µg of total RNA after genomic DNA
deletion by DNase I was reverse transcribed in 10 pmol of random
hexanucleotide primers (Promega, Madison, WI, USA), 0.5 mM dNTPs,
and 200 U of Moloney murine leukemia virus reverse transcriptase
(Promega). Then, the Bcl-2 and Bax mRNA levels were quantified by
RT-qPCR, using the iQ™ SYBR-Green SuperMix PCR Kit (Bio-Rad,
Hercules, CA, USA) according to the manufacturer's protocol with 5
ng of template cDNA, 45 cycles: 95°C/15 sec, 60°C/15 sec with the
primers listed in Table I.
Rat-GAPDH (NM_017008) was used as a parallel amplification to
normalize the expression data of the targeted genes. The relative
gene expression was calculated using the formula: n = 100 × 2
− (ΔCq targeted gene - ΔCq GAPDH).
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primers and cycling conditions. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primers and cycling conditions.
| Gene | GenBank accession
no. | Primer sequence
(5′-3′) | Size (bp) | Annealing temperature
(°C) |
|---|
| Bax | NM_017059 | F:
CATGGGCTGGACACTGGACTT | 152 | 60 |
|
|
| R:
TGGTGAGTGAGGCAGTGAGGA |
|
|
| Bcl-2 | L14680 | F:
GGATTGTGGCCTTCTTTGAGTTCG | 155 | 60 |
|
|
| R:
GGCATCCCAGCCTCCGTTATC |
|
|
| GAPDH | NM_017008.4 | F:
GAAGGTCGGTGTGAACGGATTTG | 127 | 60 |
|
|
| R:
CATGTAGACCATGTAGTTGAGGTCA |
|
|
Western blot analyses of Bcl-2, Bax,
Akt and phosphorylated Akt
Rat articular chondrocytes were plated onto 6-well
plates at a density of 5×104 cells/cm2. Then,
the cells were treated using the same settings for RT-qPCR. After
rinsing with ice-cold PBS, the cells were lysed using cell lysis
buffer and boiled at 100°C for 10 min. Western blotting was carried
out following our reported protocol. Targeted protein was probed
with primary antibodies against Bax (Cell Signaling Technology,
Inc.), Bcl-2, protein kinase B (Akt), and phosphorylated Akt
(p-Akt; Abcam, Cambridge, UK). After incubation with horse radish
peroxidase (HRP)-labeled secondary antibodies, the blots were
detected using enhanced chemiluminescent (ECL) substrate and
exposure to Kodak X-Omat film.
Statistical analysis
All experiments were performed in triplicate.
Results are expressed as the mean ± standard deviation (SD) of
three experiments. The data were evaluated using one-way ANOVA and
followed by Dunnett's analysis. Statistical significance was set at
P<0.05. The statistical analyses were performed with SPSS 19.0
for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Effects of paeoniflorin on LDH
release
In our previous study of paeoniflorin and
chondrocytes (unpublished data), we found that paeoniflorin
concentrations from 12.5 to 100 µM caused no significant toxicity
to chondrocytes (Fig. 1A).
Therefore, in the present study, we used paeoniflorin
concentrations from 25 to 50 µM. According to the LDH release
assay, IL-1β significantly increased the levels of LDH release.
Paeoniflorin (25–50 µM) suppressed the LDH release induced by IL-1β
in a dose-dependent manner and showed a protective effect in
vitro (Fig. 1B).
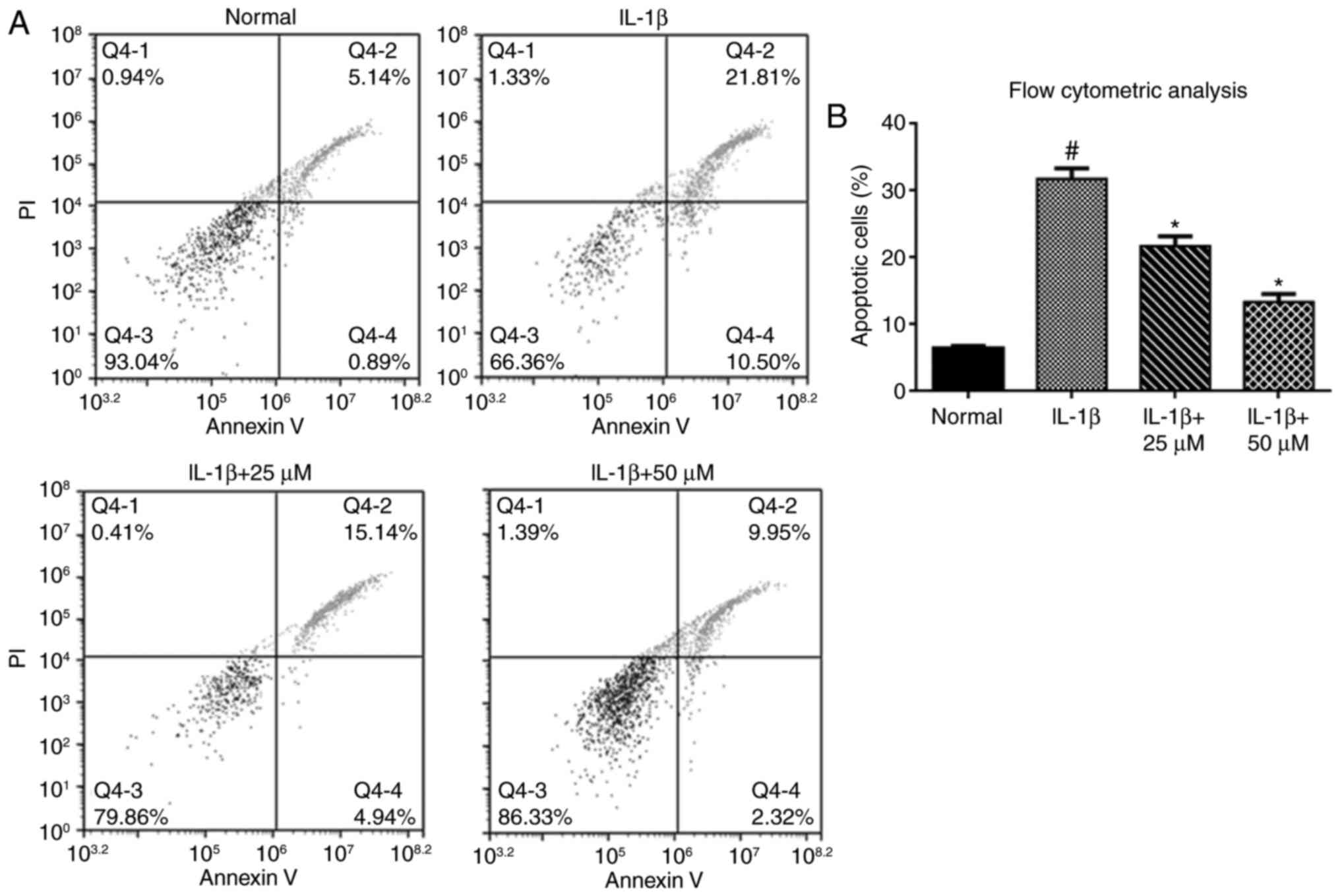
Paeoniflorin suppresses IL-1β-induced
chondrocyte apoptosis
We assessed chondrocyte apoptosis by flow cytometric
analysis. The percentage of apoptotic chondrocytes was
significantly increased in the IL-1β group compared with the
controls. When chondrocytes were pretreated with paeoniflorin for 3
h, a decrease in the percentage of apoptotic chondrocytes was
observed compared with the IL-1β alone group (Fig. 2A and B).
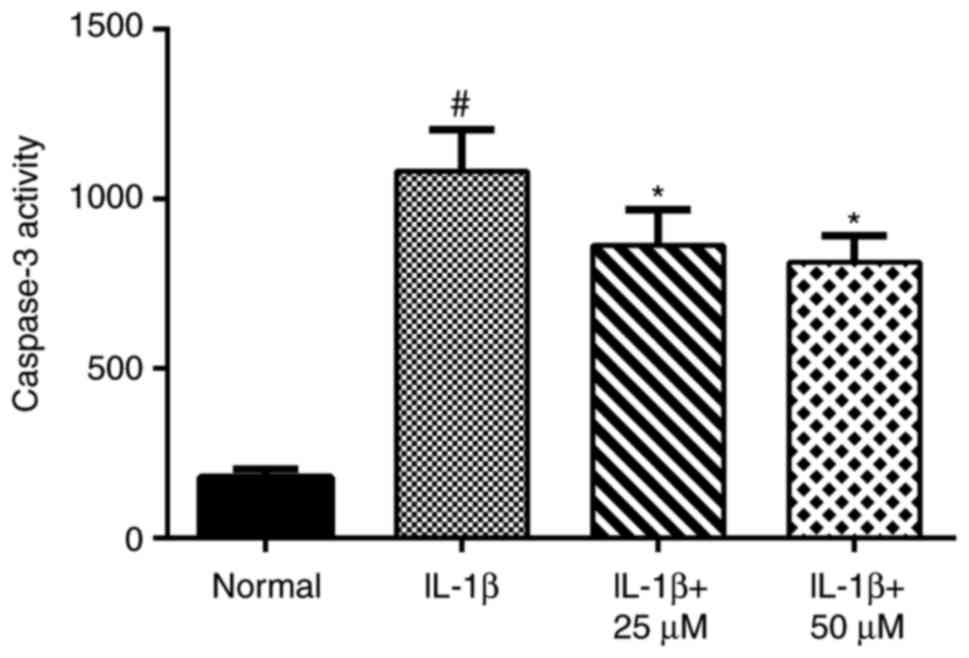
Paeoniflorin inhibits IL-1β-induced
apoptosis by suppressing caspase-3 activity
After treatment of chondrocytes with IL-1β for 24 h,
caspase-3 activity increased significantly. However, chondrocytes
treated with paeoniflorin exhibited markedly decreased caspase-3
activity caused by IL-1β stimulation (Fig. 3).
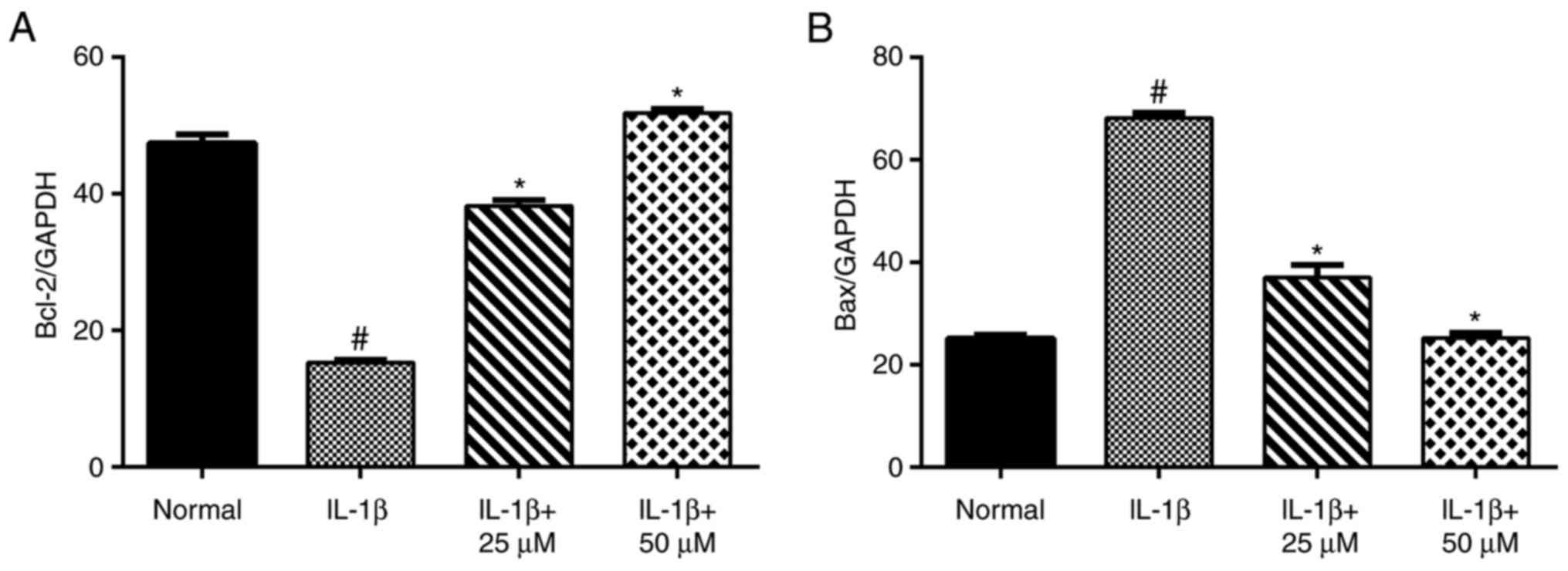
Paeoniflorin suppresses the apoptotic
pathway mediated by Bcl-2 and Bax
Using RT-qPCR (Fig.
4) and western blot analyses (Fig.
5), IL-1β-stimulation alone significantly increased the level
of Bax and decreased the level of Bcl-2. Moreover, the production
of Bax in the low- and high-dose paeoniflorin pretreated groups was
significantly decreased compared with the IL-1β group (P<0.05).
However, the transcript and protein levels of Bcl-2 in the
paeoniflorin groups were significantly increased compared with the
IL-1β group (P<0.05).
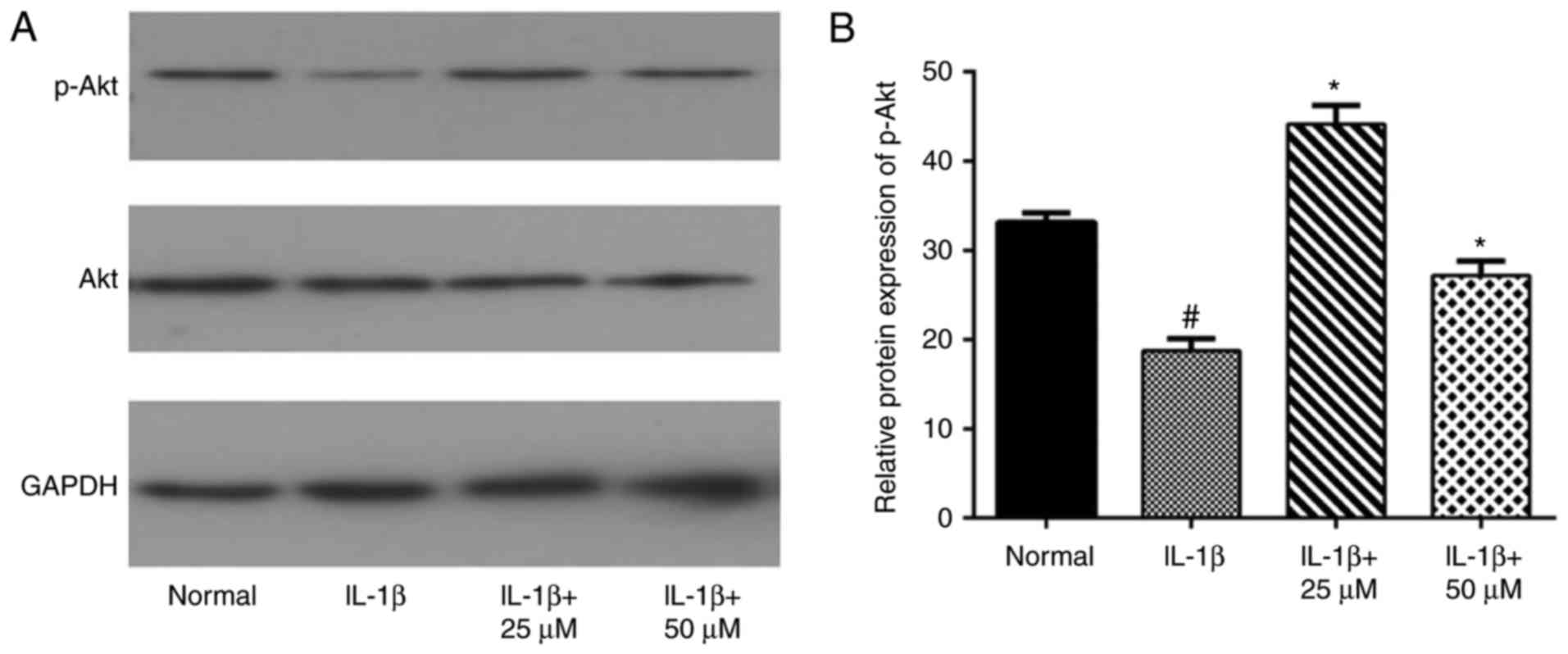
Effects of paeoniflorin on the Akt
pathway in IL-1β-treated chondrocytes
In the present study, we also examined Akt and p-Akt
to evaluate the involvement of the Akt pathway. As expected, the
western blot assay showed that IL-1β-stimulation alone decreased
the phosphorylation level of Akt, which was consistent with another
study (24286347). Different concentrations of paeoniflorin
increased the IL-1β-induced activation of Akt, as shown in Fig. 6 (*P<0.05 vs. IL-1β cells).
Discussion
Paeoniflorin not only has various pharmacological
effects, it also exhibits low toxicity and few side-effects against
different cell types (13). In an
experimental model of intervertebral disc degeneration,
paeoniflorin was shown to hinder the Bcl-2/caspase-9 pathway, which
resulted in the inhibition of nucleus pulposus cell apoptosis. This
demonstrates the close link between paeoniflorin and the
musculoskeletal system. To date, little has been reported on the
effects of paeoniflorin on chondrocytes (10). In the present study, we
investigated the anti-apoptotic effects of paeoniflorin in
vitro. Treatment of IL-1β-induced rat articular chondrocytes
with paeoniflorin decreased the rate of apoptosis by regulating the
production of Bcl-2 family proteins. Moreover, IL-1β-induced
caspase-3 activity was abolished by high-dose paeoniflorin.
Apoptosis is a normal physiological process that is
a critical step in the progression of osteoarthritis (OA) (14). In several immunohistochemical
studies of cartilage specimens obtained from OA patients, the
results indicated that apoptosis-positive cells were closely
related to the process of OA (15). Bcl-2 family proteins are key
factors in the apoptotic process (16). Bcl-2 family proteins can be divided
into an anti-apoptotic group (e.g., Bcl-2 and Bcl-2-like 1 protein
extra-large) and a pro-apoptotic group (e.g., Bax, and Bcl-2-like
protein 11) (17). As a classical
anti-apoptotic protein, Bcl-2 mainly inhibits the release of
cytochromec and blocks the activation of caspase-9 (18). Bax, a bcl-2-like protein 4, was
found in the cytosol and is involved in the initiation of apoptosis
(19). The ratio of Bax/Bcl-2
protein determines whether the cell will survive or undergo
apoptosis (20). Karaliotas et
al (21) found that the level
of Bax transcripts in the OA group was significantly higher than
that in the control group, while the Bcl-2/Bax was significantly
decreased in the OA group. Studies have also shown that IL-1β
induces chondrocyte apoptosis by regulating the expression of
Bcl-2/Bax (22). In the present
study, we found that IL-1β (10 ng/ml) significantly inhibited both
the protein and gene expression of Bcl-2 and increased the level of
Bax, which was consistent with the previous results.
The caspase family also plays an essential role in
chondrocyte apoptosis (23).
Sharif et al (24)
demonstrated that the expression of apoptosis-related mediators
such as caspase-3 was higher in OA cartilage compared with
non-arthritic controls, as analyzed with TUNEL assay and
immunohistochemistry. Without pretreatment with paeoniflorin, IL-1β
significantly increased caspase-3 activity compared with the normal
group. Treatment with 25 or 50 µM paeoniflorin decreased caspase-3
activity, demonstrating that paeoniflorin exerts an anti-apoptotic
effect by blocking the activation of caspase-3 (50 µM paeoniflorin
was the optimum concentration).
Akt, also called protein kinase B, has several
important physiological functions and is involved in cell survival
(25). Specifically, the activated
PI3K/Akt pathway has been implicated in chondrocyte survival
(26). A previous study that
focused on the role of Akt in paeoniflorin-induced gastric
carcinoma suggested that paeoniflorin induces apoptosis by
suppressing PI3K/Akt signaling (27). In our research, it was clear that
Akt activated by paeoniflorin was involved in the chondroprotective
effect of paeoniflorin on IL-1β-induced apoptosis. However, the
precise mechanism by which Akt controls this process is not
entirely understood and further studies are needed.
In summary, we determined that paeoniflorin blocked
IL-1β-induced LDH release and decreased the percentage of apoptotic
cells. Paeoniflorin also exhibited a chondroprotective effect by
downregulating both the mRNA and protein expression of Bax and
increasing the level of Bcl-2. Paeoniflorin also reduced the
activity of caspase-3 in chondrocytes. Furthermore, paeoniflorin
regulates the Akt signaling pathway by increasing the
phosphorylation of Akt. These results demonstrate that paeoniflorin
plays an anti-apoptotic role in the progression of OA and may be
useful in the treatment of OA.
Acknowledgements
The authors would like to thank Mr. Jun Liu (School
of Life Sciences, China Jiliang University, Hangzhou, Zhejiang,
China) for providing excellent technical assistance.
Funding
The present study was supported by a grant from The
National Natural Science Foundation of China (grant no.
81301584).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PFH and LDW conceived and designed the study. PFH,
WPC and JPB performed the experiments, and PFH and LDW wrote the
present study. PFH, WPC, JPB and LDW reviewed and edited the
manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
The animal experiments performed in this study were
approved by the University of Zhejiang Institutional Animal Care
and Use Committee (Hangzhou, Zhejiang, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kamimura M, Nakamura Y, Uchiyama S,
Ikegami S, Mukaiyama K and Kato H: The pathophysiology and
progression of hip osteoarthritis accompanied with joint pain are
potentially due to bone alterations-follow-up study of hip OA
patients. Open Rheumatol J. 8:46–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hashimoto S, Ochs RL, Komiya S and Lotz M:
Linkage of chondrocyte apoptosis and cartilage degradation in human
osteoarthritis. Arthritis Rheum. 41:1632–1638. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim HA, Suh DI and Song YW: Relationship
between chondrocyte apoptosis and matrix depletion in human
articular cartilage. J Rheumatol. 28:2038–2045. 2001.PubMed/NCBI
|
|
4
|
Gu X, Cai Z, Cai M, Liu K, Liu D, Zhang Q,
Tan J and Ma Q: Protective effect of paeoniflorin on inflammation
and apoptosis in the cerebral cortex of a transgenic mouse model of
Alzheimer's disease. Mol Med Rep. 13:2247–2252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen Z, Zhu Y, Zhao Y, Ma X, Niu M, Wang
J, Su H, Wang R, Li J, Liu L, et al: Serum metabolomic profiling in
a rat model reveals protective function of paeoniflorin against
ANIT induced cholestasis. Phytother Res. 30:654–662. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li P and Li Z: Neuroprotective effect of
paeoniflorin on H2O2-induced apoptosis in
PC12 cells by modulation of reactive oxygen species and the
inflammatory response. Exp Ther Med. 9:1768–1772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu H, Li W, Wang T, Shu Y and Liu P:
Paeoniflorin suppress NF-kappaB activation through modulation of I
kappaB alpha and enhances 5-fluorouracil-induced apoptosis in human
gastric carcinoma cells. Biomed Pharmacother. 62:659–666. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang Y, Jia X, Wei F, Wang C, Sun X, Xu
S, Yang X, Zhao Y, Chen J, Wu H, et al: CP-25, a novel compound,
protects against autoimmune arthritis by modulating immune
mediators of inflammation and bone damage. Sci Rep. 6:262392016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen SQ, Lin JP, Zheng QK, Chen SJ, Li M,
Lin XZ and Wang SZ: Protective effects of paeoniflorin against
FasL-induced apoptosis of intervertebral disc annulus fibrosus
cells via Fas-FasL signalling pathway. Exp Ther Med. 10:2351–2355.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi L, Teng H, Zhu M, Li C, Huang K, Chen
BI, Dai Y and Wang J: Paeoniflorin inhibits nucleus pulposus cell
apoptosis by regulating the expression of Bcl-2 family proteins and
caspase-9 in a rabbit model of intervertebral disc degeneration.
Exp Ther Med. 10:257–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hui W, Young DA, Rowan AD, Xu X, Cawston
TE and Proctor CJ: Oxidative changes and signalling pathways are
pivotal in initiating age-related changes in articular cartilage.
Ann Rheum Dis. 75:449–458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu M, Xiao Y, Yin J, Hou W, Yu X, Shen L,
Liu F, Wei L and Jia W: Berberine promotes glucose consumption
independently of AMP-activated protein kinase activation. PLoS One.
9:e1037022014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi EM, Suh KS, Rhee SY and Kim YS:
Inhibitory effect of paeoniflorin on methylglyoxal-mediated
oxidative stress in osteoblastic MC3T3-E1 cells. Phytomedicine.
21:1170–1177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aigner T, Kim HA and Roach HI: Apoptosis
in osteoarthritis. Rheum Dis Clin North Am. 30:639–653. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Musumeci G, Loreto C, Carnazza ML and
Martinez G: Characterization of apoptosis in articular cartilage
derived from the knee joints of patients with osteoarthritis. Knee
Surg Sports Traumatol Arthrosc. 19:307–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Häcker G: The morphology of apoptosis.
Cell Tissue Res. 301:5–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Delbridge AR, Grabow S, Strasser A and
Vaux DL: Thirty years of BCL-2: Translating cell death discoveries
into novel cancer therapies. Nat Rev Cancer. 16:99–109. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Skulachev VP: Cytochrome c in the
apoptotic and antioxidant cascades. FEBS Lett. 423:275–280. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pena-Blanco A and García-Sáez AJ: Bax Bak
and beyond-mitochondrial performance in apoptosis. FEBS J.
2017.(Epub ahead of print). PubMed/NCBI
|
|
20
|
Yoon O and Roh J: Downregulation of KLF4
and the Bcl-2/Bax ratio in advanced epithelial ovarian cancer.
Oncol Lett. 4:1033–1036. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karaliotas GI, Mavridis K, Scorilas A and
Babis GC: Quantitative analysis of the mRNA expression levels of
BCL2 and BAX genes in human osteoarthritis and normal articular
cartilage: An investigation into their differential expression. Mol
Med Rep. 12:4514–4521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Gai P, Xu R, Zheng Y, Lv S, Li Y
and Liu S: Shikonin protects chondrocytes from
interleukin-1beta-induced apoptosis by regulating PI3K/Akt
signaling pathway. Int J Clin Exp Pathol. 8:298–308.
2015.PubMed/NCBI
|
|
23
|
Lo MY and Kim HT: Chondrocyte apoptosis
induced by hydrogen peroxide requires caspase activation but not
mitochondrial pore transition. J Orthop Res. 22:1120–1125. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sharif M, Whitehouse A, Sharman P, Perry M
and Adams M: Increased apoptosis in human osteoarthritic cartilage
corresponds to reduced cell density and expression of caspase-3.
Arthritis Rheum. 50:507–515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng X, Xia C, Chen Z, Huang J, Gao F, Li
G and Zhang B: Requirement of the phosphatidylinositol 3-kinase/Akt
signaling pathway for the effect of nicotine on
interleukin-1beta-induced chondrocyte apoptosis in a rat model of
osteoarthritis. Biochem Biophys Res Commun. 423:606–612. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng YB, Xiao GC, Tong SL, Ding Y, Wang
QS, Li SB and Hao ZN: Paeoniflorin inhibits human gastric carcinoma
cell proliferation through up-regulation of microRNA-124 and
suppression of PI3K/Akt and STAT3 signaling. World J Gastroenterol.
21:7197–7207. 2015. View Article : Google Scholar : PubMed/NCBI
|