Introduction
Ovarian cancer is the leading cause of mortality
among malignant gynecological tumors worldwide (1). In the US alone, >20,000 women are
diagnosed ovarian cancer and there are >14,000 ovarian
cancer-associated mortalities each year (2). The high mortality rate of ovarian
cancer is attributed to its typically advanced stage at detection,
and high invasive and metastatic malignancy. Despite efforts to
develop approaches for ovarian cancer treatment, no treatment
option has been proven to reduce the mortality of ovarian cancers
(3). Therefore, it is urgent to
develop novel therapeutic treatments for better control of ovarian
cancers, and thus, understanding of the mechanisms responsible for
ovarian cancer invasion and metastasis may provide potential
therapeutic targets.
Recently, AMPK-related protein kinase-5 (ARK5), a
crucial member of the human AMP-activated protein kinases (AMPKs)
family, has been identified to be associated with metastasis of
cancer cells (4). Metastasis is a
complex multistep process involving cell migration and invasion.
ARK5 has been reported to be an important participant in mediating
cancer cell migration activity and its activation is caused by
protein kinase B (Akt)-dependent phosphorylation (5). In addition, it has been demonstrated
that high expression of ARK5 can accelerate tumor invasion and
metastasis (6). However,
mechanisms of ARK5 involved in ovarian cancer invasion and
metastasis has not been fully ascertained.
The present study aimed to clarify the involvement
of ARK5 in ovarian cancer invasion. The expression of ARK5 in
ovarian cancer cell lines and tissues, and the biological impact of
ARK5 on the invasive capabilities of ovarian cancer cells were
investigated. In addition, mechanistic targets of ARK5 in
modulating ovarian cancer invasion were explored in the current
study.
Patients and methods
Patients and tissue specimens
This study was conducted using 20 pairs of selected
frozen (liquid nitrogen) ovarian cancer tissues (T) and adjacent
non-tumor ovarian tissues (N). Details of clinical pathological
features of the patients with ovarian cancer are presented in
Table SI. Neither radiotherapy
nor preoperative chemotherapy was received prior to tissue
collection. All primary ovarian cancer tissues and corresponding
adjacent nontumor ovarian tissues were identified by routine
pathological observation. Institutional research ethics committee
approval of Weifang Medical University (Shandong, China) and
patient consent were obtained in advance of the use of these
clinical specimens for research purposes.
Cell culture and reagents
Human ovarian cancer cell lines SKOV3 and OVCAR-3
were obtained from the American Type Culture Collection (Manassas,
VA, USA). Ovarian cancer cell line. A2780 and normal ovarian
epithelial cell line IOSE80 were obtained from Shanghai Huiying
Biotechnology Co., Ltd. (Shanghai, China). All cells were cultured
in RPMI-1640 (Hyclone, GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal calf serum from Zhejiang Tianhang
Biotechnology Co., Ltd. (Zhejiang, China) and were maintained at
37°C in 5% CO2 incubator. Membranes and chemotaxis
chambers were from Neuro Probe, Inc. (Gaithersburg, MD, USA). The
human epidermal growth factor-1 (EGF-1) was from R&D Systems,
Inc. (Minneapolis, MN, USA).
Plasmid construction, small
interfering RNA (siRNA) and plasmid transfection
The cells (2×105 cells) were cultured in
a 35 mm dish with serum-free medium for 24 h and then moved into
complete medium for transfection. Two expression plasmids (200
ng/µl) containing a scrambled sequence (5′-CTCAACTTGATCCTGTGAG-3′)
(pGCsilencerU6/GFP/Neo-RNAi-Scr) and a target sequence
(5′-GAAGTTATGCTTTATTCAC-3′; pGCsilencerU6/GFP/Neo-RNAi-ARK5) were
obtained from Shanghai GeneChem Co., Ltd. (Shanghai, China). The
transfection was performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Stable transfection was selected using 500 µg/ml neomycin for at
least 6 months (7).
Reverse transcription polymerase chain
reaction (RT-PCR)
ARK5 mRNA expression was determined by RT-PCR. The
total RNA from SKOV3 cells was extracted using TRIzol (Thermo
Fisher Scientific, Inc.). One Step RNA PCR kit (AMV) from Takara
Biotechnology Co., Ltd. (Dalian, China) was used to reverse
transcribe RNA to cDNA at 50°C for 30 min and 94°C for 2 min. The
β-actin primers (forward, 5′-ATGTTTGAGACCTTCAACAC-3′ and reverse,
5′-CACGTCACACTTCATGATGG-3′) and ARK5 (forward,
5′-GACATGGTTCACATCAGACGA-3′ and reverse,
5′-CAATAGTGCACAGCAGAGACG-3′) were synthesized using a Takara
PrimeScript RT kit (Takara Biotechnology Co., Ltd) with reaction at
50°C for 30 min, pre-denaturation at 94°C for 2 min, 30 cycles of
denaturation at 94°C for 30 sec, annealing at 60°C for 30 sec and
extension at 72°C for 1 min in order to produce amplification
products which were then electrophoresed on 1.5% the agarose
gel.
Western blotting
For western blot analysis, the whole-cell extracts
were prepared in radioimmunoprecipitation assay buffer (40 mM NaF,
20 mM Tris, 2.5 mM EDTA, 1% deoxycholate, 1% Triton X-100, 0.1%
SDS, 10 mM Na4P2O7 and 1 mM
phenylmethylsulfonyl fluoride). Protein concentration was
determined with BCA and 25 µl per well of protein samples were
separated by 10% SDS-PAGE, then transferred onto polyvinylidene
difluoride membranes, immunoblotted with appropriate primary
antibodies at 4°C overnight and secondary antibodies at room
temperature for 1 h. (goat anti-mouse IgG, cat. no. SA00001-1;
1:2,000 and goat anti-rabbit IgG, cat. no. SA00001-2, 1:2,000;
Shanghai Biyuntian Biotechnology Co., Ltd), The chemiluminescent
signals were detected using ECL Plus (WBKLS0100; EMD Millipore,
Billerica, MA, USA) and finally visualized by Image Quant LAS 500
(GE Healthcare). Western blot analysis is representative of at
least three independent repeated experiments. Densitometric
analysis was used to quantify the protein bands and β-actin was
used as normal control, with the protein bands being quantified
using ImageJ software version 1.4.3.67 (National Institutes of
Health, Bethesda, MD, USA). The following primary antibodies were
used in this study: mTOR (cat. no. 2983; 1:1,000 dilution), phospho
(p)-mTOR (cat. no. 2971; 1:1,000 dilution), ARK5 (ab71814; 1:500;
Abcam, Cambridge, MA, USA), MMP-2 (cat. no. 4022; 1:1,000
dilution), MMP-9 (cat. no. 3852; 1:1,000 dilution), E-cadherin
(cat. no. 3195; 1:1,000 dilution), N-cadherin (cat. no. 4061S;
1:1,000 dilution; all Cell Signaling Technology, Inc., Danvers, MA,
USA) and β-actin (cat. no. sc-47778; 1:1,000 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA).
Chemotaxis assay
A chemotaxis assay was performed as described
previously (8). Briefly, the
chemotaxis ability of cells was measured using Transwell inserts
with 8.0 mm pore polycarbonate membrane. The chemoattractant EGF-1
(10 ng/ml) was loaded into the lower chemotaxis chamber with serum
free RPMI 1640 medium and 5×105 cells/ml cells suspended
in the binding medium [RPMI-1640, 0.1% bovine serum albumin (BSA)
and 25 mM HEPES] were added into the upper chambers. After 3 h, the
non-migrating cells were removed by wiping the upper side of the
membrane, and the migrating cells were fixed in 4% paraformaldehyde
for 10 min and then stained with 0.1% crystal violet at room
temperature for 15 min. The number of cells on the lower surface,
which had migrated through the membrane, was counted under a light
microscope in at least five random fields at a magnification of
×400. Chemotaxis index (CI) was defined by the ratio of the number
of cells on the lower surface of the membrane in the experimental
group to the number of corresponding cells in the control group.
All assays were repeated at least three times independently.
Matrigel invasion assay
The invasion of SKOV3 ovarian cells in vitro
was evaluated using Matrigel-coated Transwell inserts (Corning
Incorporated, Corning, NY, USA) as described previously (9). Briefly, the Transwell inserts with 8
mm pore size were coated with a final concentration of 1.5 mg/ml
Matrigel. The Matrigel contains collagen type IV, heparin sulfate
proteoglycan, entactin and laminin. Cell suspension
(5×105 cells/ml in 200 µl) were plated on the surface of
top chamber and 300 µl binding medium (RPMI-1640, 0.1% BSA and 25
mM HEPES) with 10 ng/ml of EGF-1 was added into the lower well. The
assembly was incubated for 24 h at 37°C in humidified 5%
CO2. The invasive cells on the lower surface of the
membrane were fixed in 4% paraformaldehyde for 10 min and stained
with 0.1% crystal violet at room temperature for 30 min. The number
of invading cells was counted under a light microscope in five
random fields at a magnification of ×400. All assays were repeated
at least three times independently.
Statistical analysis
The results are presented as the mean ± standard
deviation. Each experiment was performed at least three times. The
statistical difference of data between multiple groups was analyzed
by one-way analysis of variance. The Fisher's LSD test was used to
determine significance among the multiple groups. All statistical
analyses were carried out using the SPSS 17.0 statistical software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Increased expression of ARK5 in
ovarian cancer cell lines and tissues
To reveal the role of ARK5 in human ovarian cancer,
western blot analysis was performed to evaluate ARK5 expression in
human ovarian cancer cell lines and primary cancer tissues. The
results demonstrated that the protein levels of ARK5 in ovarian
cancer cell lines, OVCAR-3, A2780 and SKOV3 were significantly
increased compared with the normal ovarian epithelial cells line,
IOSE80. SKOV3 cells exhibited the highest ARK5 protein expression,
thus, SKOV3 cells were selected for used in further experiments
(Fig. 1A and B). Meanwhile, ARK5
was also strongly up regulated in ovarian cancer tissues compared
with paired adjacent non-cancerous tissues (Fig. 1C and D). Taken together, the
results demonstrated that the expression of ARK5 was markedly
upregulated in human ovarian cancer cells and clinical primary
human ovarian cancer tissues.
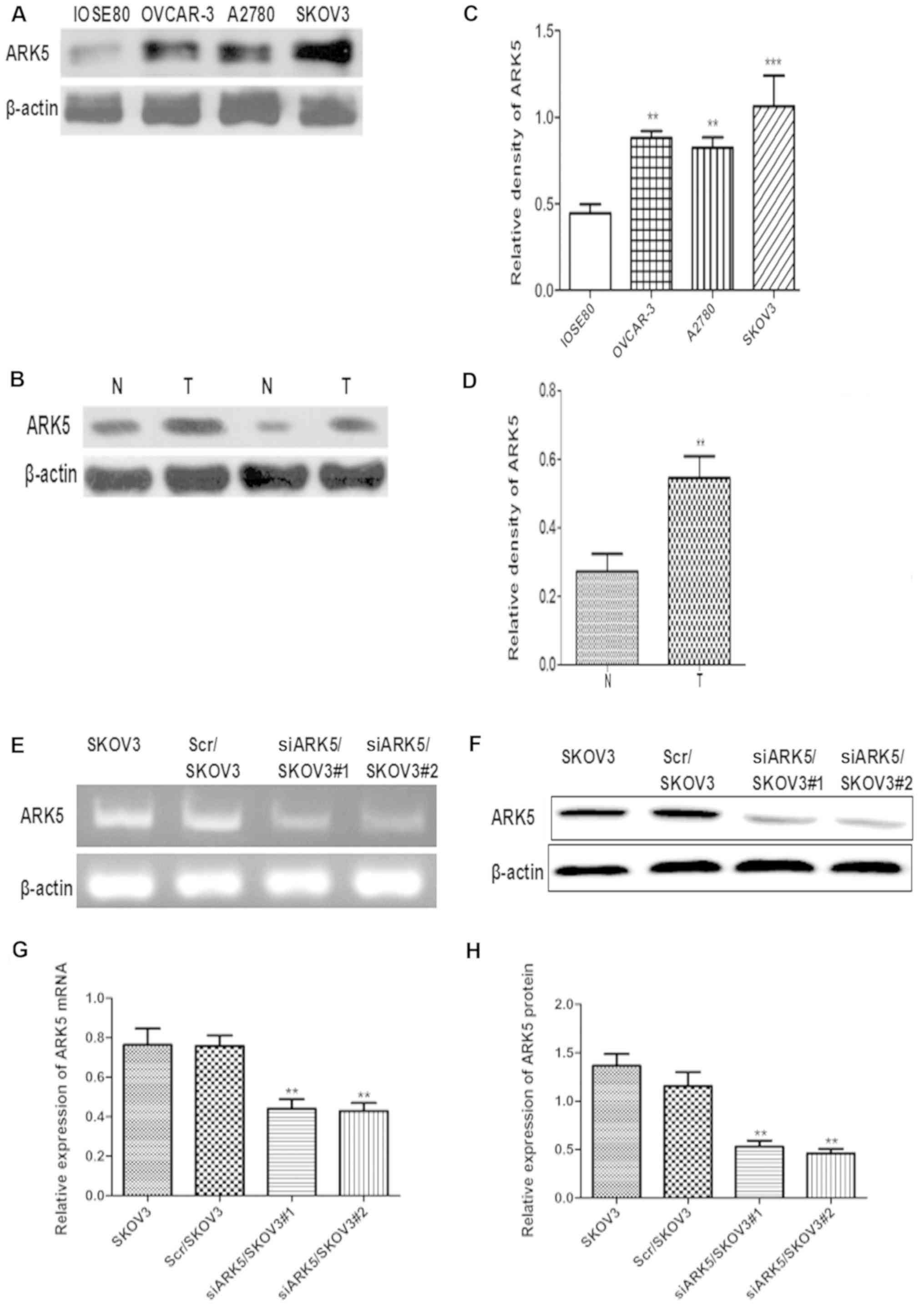 | Figure 1.Increased ARK5 expression in ovarian
cancer cell lines and ovarian cancer tissues, and ARK5 knockdown in
SKOV3 cells. (A) Western blot analysis of ARK5 in ovarian cancer
cell lines, OVCAR-3, A2780 and SKOV3, and normal ovarian epithelial
cells line, IOSE80. (B) Western blot analysis of ARK5 in resected
ovarian cancer tissues and adjacent normal ovarian tissues. (C)
Densitometry analysis of ARK5 in ovarian cancer cell lines,
OVCAR-3, A2780 and SKOV3, and normal ovarian epithelial cells line,
ISOE80, **P<0.01, ***P<0.001 vs. IOSE80. (D) Densitometry
analysis of ARK5 expression levels in resected ovarian cancer
tissues and adjacent normal ovarian tissues. **P<0.01 vs. N. (E)
Reverse transcription-polymerase chain reaction analysis of ARK5
mRNA expression in Scr/SKOV3 and siARK5/SKOV3 cells. (F) Western
blot analysis of ARK5 protein expression in Scr/SKOV3 and
siARK5/SKOV3 cells. (G) Statistical analysis of ARK5 mRNA
expression in Scr/SKOV3 and siARK5/SKOV3 cells. **P<0.01 vs.
SKOV3. (H) Densitometry analysis of ARK5 protein expression in
Scr/SKOV3 and siARK5/SKOV3 cells. **P<0.01 vs. SKOV3. ARK5,
AMPK-related protein kinase-5; N, adjacent normal tissue; T,
ovarian cancer tissue; Scr, scramble control; siARK, ARK small
interfering RNA. |
Knockdown of ARK5 in ovarian cancer
SKOV3 cells
To confirm the role of ARK5 in ovarian cancer cell
metastasis, an RNA interference (RNAi) expression vector was
designed to target human ARK5 in SKOV3 cells, with a scrambled
sequence vector used as a control. RNAi-mediated ARK5 knockdown
SKOV3 (siARK5/SKOV3#1 and siARK5/SKOV3#2) and the RNAi control
SKOV3 (Scr/SKOV3) cell lines were produced. PCR and western
analysis tests demonstrated that ARK5 was markedly reduced in
siARK5/SKOV3#1 and siARK5/SKOV3#2 cells. siARK5/SKOV3#2 was named
‘siARK5/SKOV3’ cells and used in the subsequent assays (Fig. 1E-H).
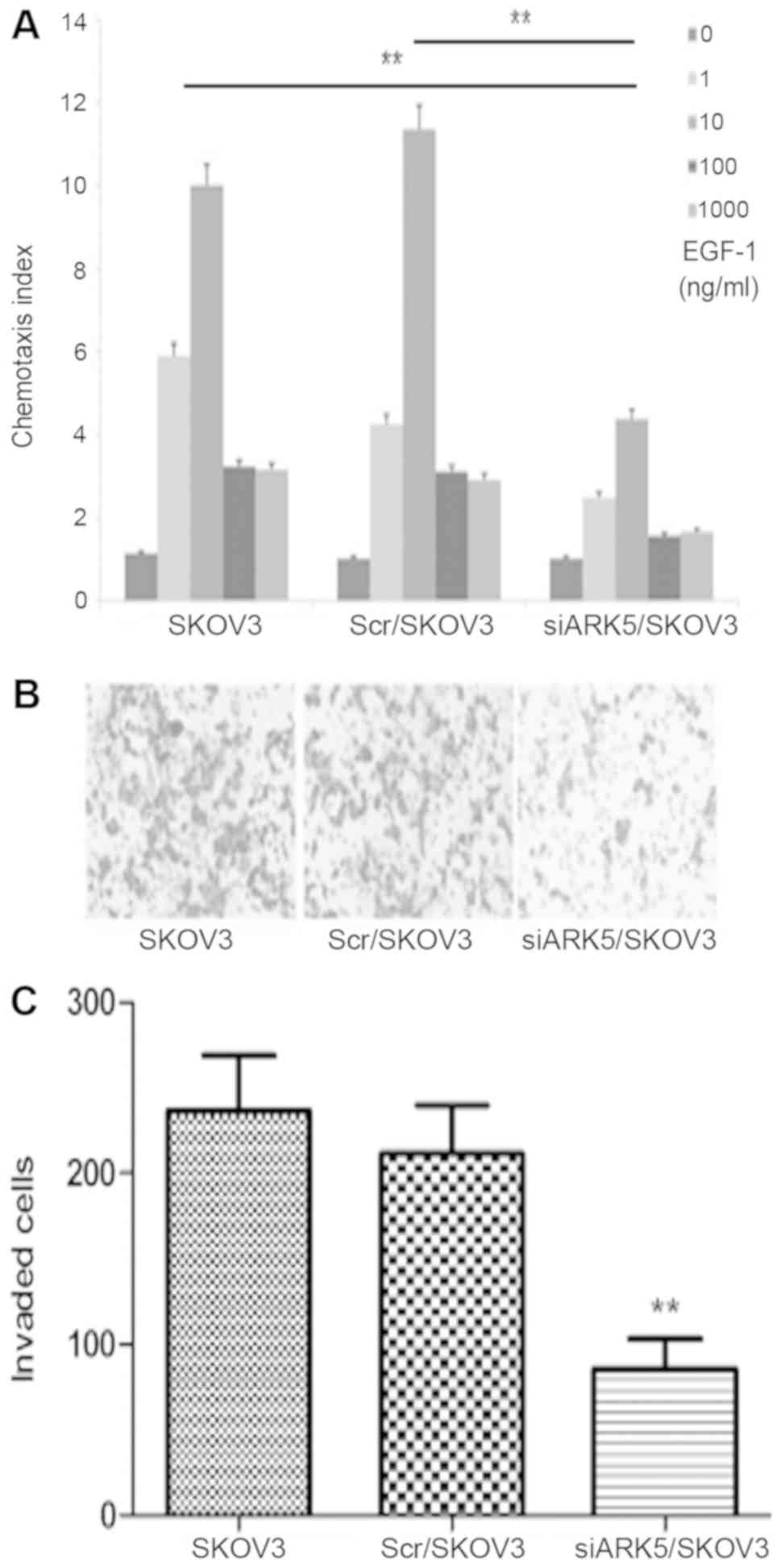
ARK5 knockdown impairs migration of
ovarian cancer cells
Cell migration is essential for cancer metastasis
(10). A chemotaxis assay was
performed to identify whether ARK5 influences SKOV3 cells
migration. The results revealed similar chemotaxis in parental
SKOV3 and Scr/SKOV3 cells, and EGF-1 induced the robust chemotaxis
of parental SKOV3 and Scr/SKOV3 cells with typical bell-shaped
response curves with increasing doses. Chemotaxis was decreased in
siARK5/SKOV3 cells compared with Scr/SKOV3 cells (Fig. 2A). The chemotaxis results indicated
that ARK5 has a role in the chemotaxis of SKOV3 cells and
downregulation of ARK5 impaired cell migration. The materials and
methods states that the change in cell proliferation, however, did
not interfere with the chemotaxis of SKOV3 cells in the present
study, because it took <3 h to finish the chemotaxis assay,
which is far shorter than the cell doubling time.
ARK5 knockdown impairs SKOV3 cell
invasion
Cell migration is crucial in tumor invasion, this
whether ARK5 can influence ovarian cancer cell invasion was
investigated in the current study (11,12).
EGF-1 (10 ng/ml) was used as a chemoattractant to stimulate the
cells to penetrate through Matrigel and filters. Compared with the
Scr/SKOV3 cells, the number of invading siARK5/SKOV3 cells was
dramatically decreased. This result indicates that ARK5 has a role
in SKOV3 cell invasion, and ARK5 knockdown results in reduced
invasive ability (Fig. 2B and
C).
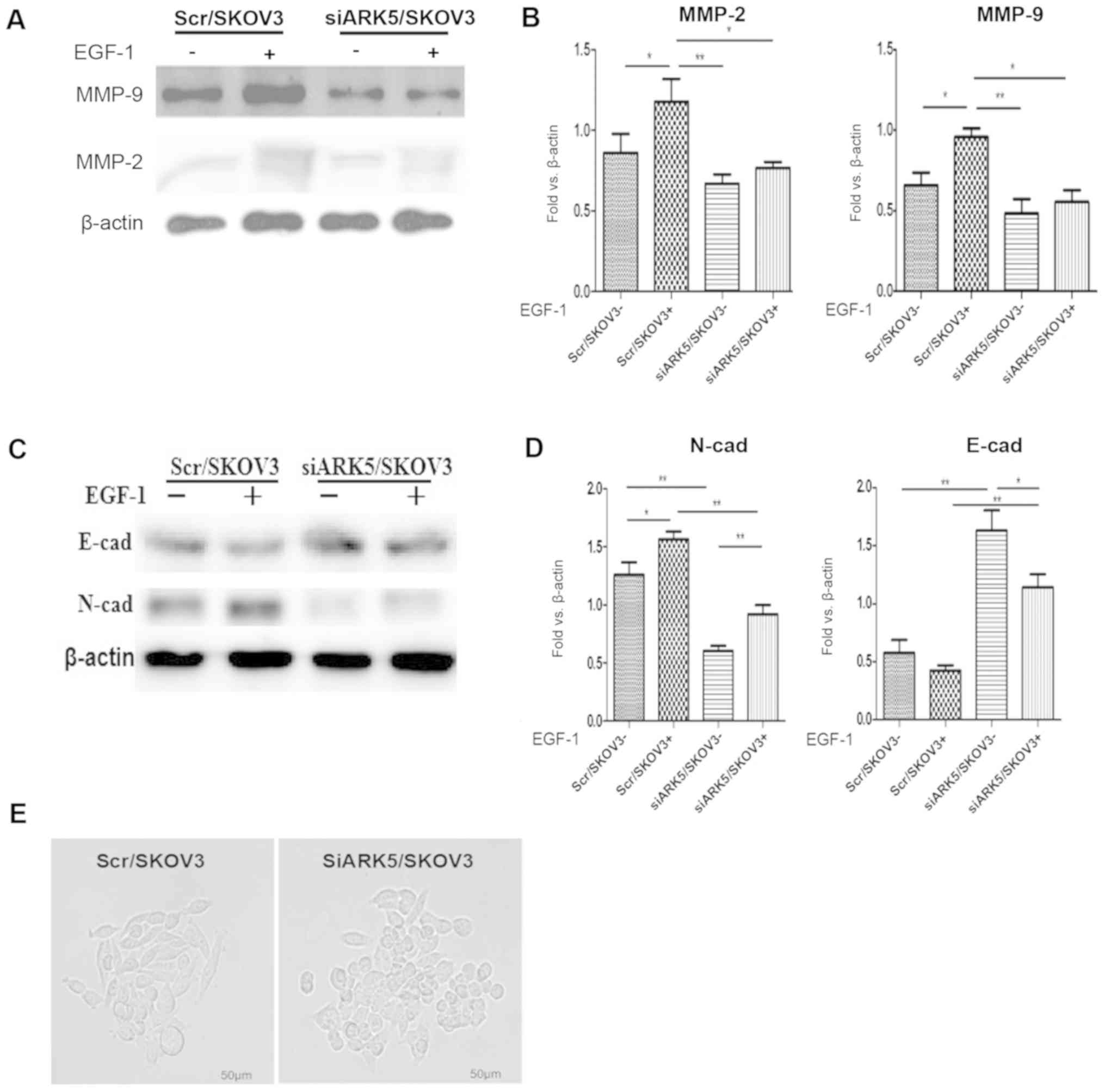
ARK5 knockdown reduces the expression
of MMP-2 and MMP-9 in SKOV3 cells
It is established that MMPs are involved in tumor
invasion (13), therefore, MMP-2
and MMP-9 expression was assessed in siARK5/SKOV3 and control cells
(14). Western blot analysis
demonstrated that MMP-2 and MMP-9 expression was lower in
siARK5/SKOV3 cells than in Scr/SKOV3 cells. In addition, EGF-1 (10
ng/ml) stimulation induced increased expression of MMP-2 and MMP-9
in Scr/SKOV3 cells; however, there was no obvious increase of MMP-2
and MMP-9 in siARK5/SKOV3 cells (Fig.
3A and B). These results suggest that downregulation of ARK5
was associated with reduced expression of MMP-2 and MMP-9 in
ovarian cancer SKOV3 cells, indicating that MMP-2 and MMP-9, at
least partially, have important roles in the invasiveness of
ovarian cancer cells induced by ARK5.
Knockdown of ARK5 inhibited cell
mesenchymal properties and reversed EMT
EMT is a vital process for metastasis of cancers,
during which epithelial tumor cells acquire a more motile and
invasive phenotype (15). In the
current study, the expression of EMT markers was analyzed to
address the mechanism of ARK5-facilitated ovarian cancer invasion.
As shown in, the expression of mesenchymal marker N-cadherin was
much higher in Scr/SKOV3 cells than that in SiARK5/SKOV3 cells
whereas the expression of epithelial marker E-cadherin was much
higher in SiARK5/SKOV3 cells than that in Scr/SKOV3 cells (Fig. 3C and D). Additionally, there were
changes in cell morphology that are associated with decreased
mesenchymal properties (Fig. 3E).
siARK5/SKOV3 cells changed their morphology from an elongated,
fibroblastic-like appearance to a cobblestone-like epithelial
shape. Furthermore, EGF-1 (10 ng/ml) can increased the expression
of N-cadherin in Scr/SKOV3 cells, while there was only a marginal
increase in N-cadherin expression in siARK5/SKOV3 cells. These
findings indicate that siARK5 reduces the expression of N-cadherin
and increases the expression of E-cadherin, which indicates that
ARK5 may promote EMT of ovarian cancer cell and knockdown ARK5 may
reverse the EMT process (16).
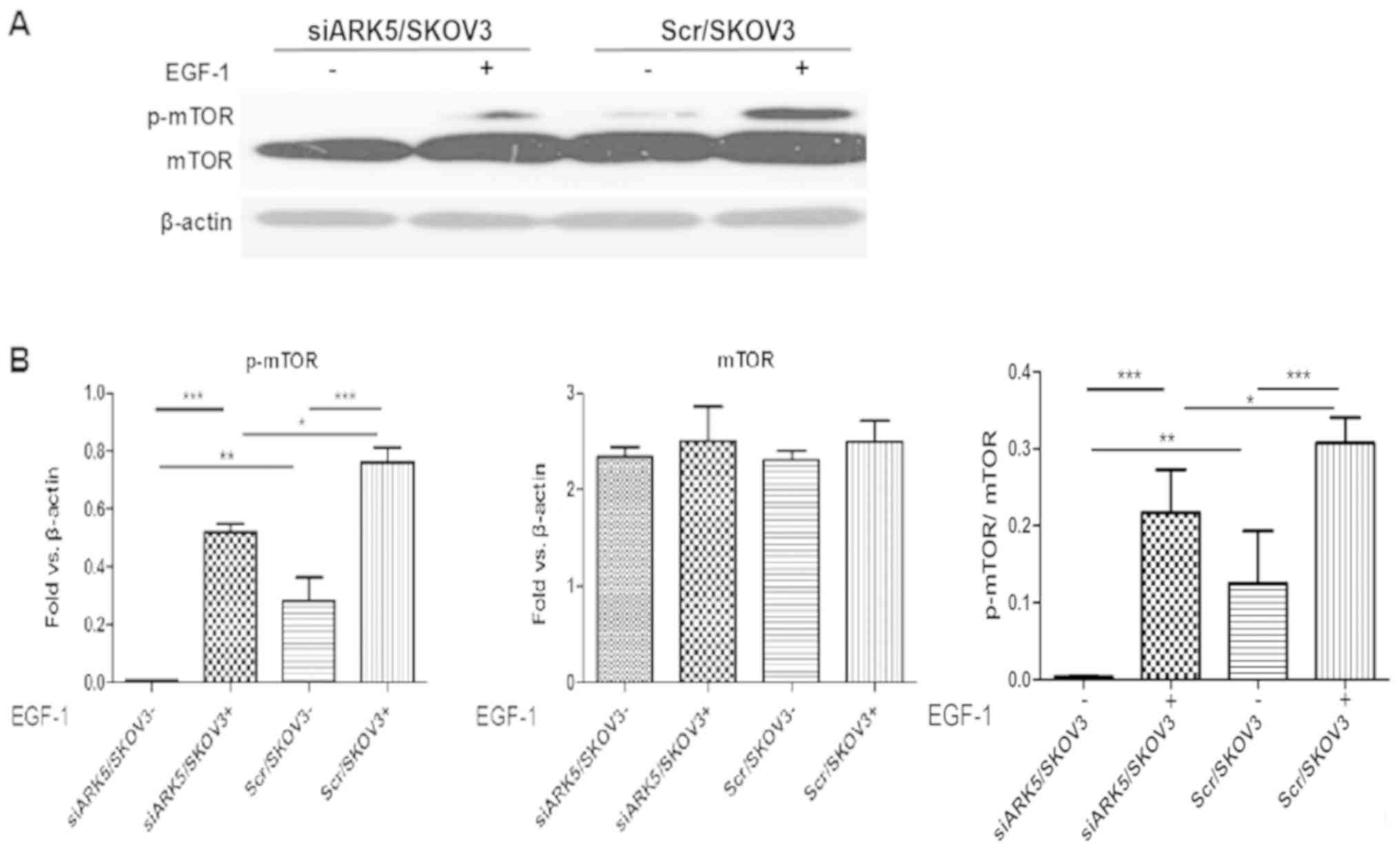
ARK5 knockdown stabilized Akt-mTOR
signaling pathway
Furthermore, it is well established that Akt
activation can increase tumor invasion and metastasis in ovarian
cancer (17). Multiple
extracellular stimuli can induce the phosphorylation of Akt and
mTOR downstream. The activation of the Akt-mTOR signaling pathway
can promote the expression of MMPs and the EMT process (18). However, the function of ARK5 in
ovarian cancer invasion is not fully understood. To determine
whether ARK5 is an upstream regulator of mTOR during ovarian cancer
cell invasion, the effect of ARK5 on EGF-1-induced activation of
mTOR was investigated in SKOV3 cells. In Scr/SKOV3 cells,
phosphorylated mTOR was higher than in siARK5/SKOV3 cells (Fig. 4A and B). Notably, EGF-1 induced
increased expression of p-mTOR in Scr/SKOV3 cells and siARK5/SKOV3
cells, whereas there was markedly lower increase in p-mTOR in
siARK5/SKOV3 cells compared with Scr/SKOV3 cells. These results
indicated that EGF-1-induced mTOR phosphorylation was inhibited in
siARK5/SKOV3 cells and ARK5 may be a key mediator of ovarian cell
invasion via activation of the mTOR signaling pathway.
Discussion
ARK5 is a novel member of the AMPK family that has
been reported to be crucial in mediating malignant activity of
various cancers (19). ARK5 is
directly activated by Akt and has critical roles in tumorigenesis,
cancer invasion and metastasis (20,21).
Similar results have also been reported in ovarian cancer, with
ARK5 upregulated in ovarian cancer tissues and is associated with
poor prognosis (22,23). However, the mechanism of ARK5 in
facilitating ovarian cancer invasion has not been fully
elucidated.
Understanding tumor invasion is an important issue
for novel therapeutic applications and, thus, improving the
clinical outcome of patients with ovarian cancer. In the current
study, it was initially identified that ARK5 was highly expressed
in ovarian cancer cell lines and primary ovarian cancer tissues,
which corroborates a recent report by Phippen et al
(23). Polarized cell migration is
a well-regulated process that is closely associated with the
infiltration and invasion of tumors. Knockdown of ARK5 markedly
impaired the chemotaxis and invasion ability of SKOV3 ovarian
cancer cells. This result suggests that ARK5 may be a vital factor
involved in the migration and invasion of ovarian cancer cells.
MMPs regulate invasion by degrading the
extracellular matrix and basement membrane to promote invasion in
various cancer types. In human pancreatic cancer, ARK5 has been
reported to activate MMP-2 and MMP-9 via rapamycin-sensitive
signaling (24). The findings of
the current study demonstrated that ARK5 knockdown leads to
repression of MMP-2 and MMP-9 expression, which is consistent with
the observations in pancreatic cancer cells (25). Furthermore, EGF-1-induced
expression of MMP-2 and MMP-9 was also markedly inhibited in ARK5
knockdown siARK5/SKOV3 cells. These results suggest that ARK5
functions upstream of MMP-2 and MMP-9, and directly regulates
EGF-1-induced MMP expression.
The loss of epithelial markers and gain of
mesenchymal markers are hallmarks of EMT. Numerous studies have
indicated that EMT is a potential mechanism by which tumor cells
acquire a more invasive and metastatic phenotype (26). Phippen et al (23) reported that elevated ARK5 is
significantly associated with the mesenchymal subtype in high-grade
serous ovarian cancer. The findings of the current study
demonstrated that the expression of ARK5 is associated with EMT in
SKOV3 cells. Knockdown of ARK5 repressed N-cadherin levels and
increased E-cadherin expression. In addition, EGF-1 induced EMT
features in Scr/SKOV3 cells, which was blocked by ARK5 knockdown.
These data indicated that ARK5 knockdown promotes epithelial
characteristics and reduces mesenchymal features. These findings
are in accordance with the cellular migration and invasion
properties of Scr/ARK5 and SiARK5/SKOV3 cells, which indicate that
the hallmarks of EMT can be altered by ARK5.
This switch in cell epithelial and mesenchymal
features is controlled by certain signaling pathways involved in
cell invasion and metastasis (27). Recently, Zhang et al
(22) demonstrated that ARK5
promoted EMT in ovarian cancer by inhibiting a
microRNA-1181/homeobox A10 axis. In the current study, the function
of Akt/mTOR signaling in regulating invasion of SKOV3 ovarian
cancer cells was emphasized. As a member of the human AMPK family,
ARK5 is directly activated by Akt dependent phosphorylation at
serine residue on the regulatory domain (28). Akt/mTOR signaling is very important
in promoting invasion and metastasis cancer cells via EMT and the
expression of MMPs (29–31). ARK5 is a critical downstream
effector of Akt and has critical roles in facilitating the invasion
and metastasis of human malignant cancer cells (32). The findings of the current study
demonstrated that ARK5 knockdown inhibited mTOR phosphorylation,
regardless of EGF-1 stimulation. Considering that mTOR is directly
phosphorylated by Akt (33), and
the complexity and importance of the Akt/mTOR pathway, it is
uncertain whether mTOR phosphorylation is directly modulated by
ARK5 or indirectly by other factors that are regulated by ARK5.
Collectively, the effects of ARK5 on SKOV3 cells invasion and
metastasis may be attributed to its activation of the Akt/mTOR
pathway (34).
In summary, human ovarian cancer exhibits high
expression of ARK5 and knockdown of ARK5 significantly inhibits the
invasive features of SKOV3 cells. In addition, the findings of the
current study also indicate that ARK5 may mediate invasion of SKOV3
cells by increasing MMP-2 and MMP-9 expression and promoting EMT
process via Akt/mTOR signaling. Thus, the findings have important
implications for developing novel therapeutic targets for ovarian
cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Scientific
Foundation of Shandong (grant no. ZR2015HL119), Scientific
Foundation of Weifang Medical University (grant nos. 2017BSQD33 and
2017BSQD12), Scientific Foundation of Gansu Province (grant no.
2014GS02292), National Natural Scientific Foundation of China
(grant nos. 81872163, 81672631, 81503108, 81072068 and 81001001),
Shandong Province Study Abroad Program Foundation to LS and Weifang
Medical University Study Abroad Program Foundation to XS and
Domestic Visiting Scholar Foundation To RL. Scientific Foundation
Of Shandong Administration Of Traditional Chinese Medicine (grant
no. 2015-229). Scientific Foundation Of Shandong Education
Administration (grant no. J17KA255).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW, BZ and LS were involved in the conceptual design
of the project and were major contributors in writing the
manuscript. SW, SL and HW performed the western blot analysis,
RT-PCR and Transwell assay, and were also major contributors in
writing the manuscript. WL, YG, XW and CF performed substantive
revision of the important content of the manuscript and made
analysis and interpretation of data. CF and XS performed the
chemotaxis index of cells under different concentrations of EGF-1.
MC, RL and WS drafted the article and contributed to cell culture
and cell transfection. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the ethical standards in the Declaration of Helsinki (1975) and was
approved by the Institutional Ethics Committee at Weifang Medical
University All donors provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bowtell DD: The genesis and evolution of
high-grade serous ovarian cancer. Nat Rev Cancer. 10:803–808. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ledermann JA, Embleton AC, Raja F, Perren
TJ, Jayson GC, Rustin GJS, Kaye SB, Hirte H, Eisenhauer E, Vaughan
M, et al: Cediranib in patients with relapsed platinum-sensitive
ovarian cancer (ICON6): A randomised, double-blind,
placebo-controlled phase 3 trial. Lancet. 387:1066–1074. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shevade A, Strogolova V, Orlova M, Yeo CT
and Kuchin S: Mitochondrial voltage-dependent anion channel protein
Por1 positively regulates the nuclear localization of saccharomyces
cerevisiae AMP-activated protein kinase. mSphere. 3(pii):
e00482–17. 2018.PubMed/NCBI
|
|
5
|
Zhang X, Lv H, Zhou Q, Elkholi R, Chipuk
JE, Reddy MV, Reddy EP and Gallo JM: Preclinical pharmacological
evaluation of a novel multiple kinase inhibitor, ON123300, in brain
tumor models. Mol Cancer Ther. 13:1105–1116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao D, Li D, Huang Y, Ma Y, Zhang B, Zhao
C, Deng S, Luo M, Yin T, Wei YQ and Wang W: 5-azacytidine promotes
invadopodia formation and tumor metastasis through the upregulation
of PI3K in ovarian cancer cells. Oncotarget. 8:60173–60187.
2017.PubMed/NCBI
|
|
7
|
Nehate C, Moothedathu Raynold AA and Koul
V: ATRP fabricated and short chain polyethylenimine grafted redox
sensitive polymeric nanoparticles for codelivery of anticancer drug
and siRNA in cancer therapy. ACS Appl Mater Interfaces.
9:39672–39687. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stubelius A, Andersson A, Islander U and
Carlsten H: Ovarian hormones in innate inflammation. Immunobiology.
222:878–883. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Gong M, Zhao Y, Zhao X and Li Q:
FOXK1 facilitates cell proliferation through regulating the
expression of p21, and promotes metastasis in ovarian cancer.
Oncotarget. 8:70441–70451. 2017.PubMed/NCBI
|
|
10
|
Peng F, Zhong Y, Liu Y, Zhang Y, Xie Y, Lu
Y, Zhang X and Li D: SPARC suppresses lymph node metastasis by
regulating the expression of VEGFs in ovarian carcinoma. Int J
Oncol. 51:1920–1928. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mu QJ, Li HL, Yao Y, Liu SC, Yin CG and Ma
XZ: Chromodomain helicase/ATPase DNA-binding protein 1-like gene
(CHD1L) expression and implications for invasion and metastasis of
breast cancer. PLoS One. 10:e01430302015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki A, Lu J, Kusakai G, Kishimoto A,
Ogura T and Esumi H: ARK5 is a tumor invasion-associated factor
downstream of Akt signaling. Mol Cell Biol. 24:3526–3535. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi L, Sun X, Zhang J, Zhao C, Li H, Liu
Z, Fang C, Wang X, Zhao C, Zhang X, et al: Gab2 expression in
glioma and its implications for tumor invasion. Acta Oncol.
52:1739–1750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang J, Qin Z, Han P, Wang W, Yang C, Xu
Z, Li R, Liu B, Qin C, Wang Z, et al: High Annexin A5 expression
promotes tumor progression and poor prognosis in renal cell
carcinoma. Int J Oncol. 50:1839–1847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y and Zhou BP: Epithelial-mesenchymal
transition in breast cancer progression and metastasis. Chin J
Cancer. 30:603–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu T, Zhang J, Chen W, Pan S, Zhi X, Wen
L, Zhou Y, Chen BW, Qiu J, Zhang Y, et al: ARK5 promotes
doxorubicin resistance in hepatocellular carcinoma via
epithelial-mesenchymal transition. Cancer Lett. 377:140–148. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lue H, Thiele M, Franz J, Dahl E,
Speckgens S, Leng L, Fingerle-Rowson G, Bucala R, Lüscher B and
Bernhagen J: Macrophage migration inhibitory factor (MIF) promotes
cell survival by activation of the Akt pathway and role for
CSN5/JAB1 in the control of autocrine MIF activity. Oncogene.
26:5046–5059. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lamouille S, Connolly E, Smyth JW, Akhurst
RJ and Derynck R: TGF-β-induced activation of mTOR complex 2 drives
epithelial-mesenchymal transition and cell invasion. J Cell Sci.
125:1259–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li M, Zheng C, Xu H, He W, Ruan Y, Ma J,
Zheng J, Ye C and Li W: Inhibition of AMPK-related kinase 5 (ARK5)
enhances cisplatin cytotoxicity in non-small cell lung cancer cells
through regulation of epithelial-mesenchymal transition. Am J
Transl Res. 9:1708–1719. 2017.PubMed/NCBI
|
|
20
|
Kusakai G, Suzuki A, Ogura T, Kaminishi M
and Esumi H: Strong association of ARK5 with tumor invasion and
metastasis. J Exp Clin Cancer Res. 23:263–268. 2004.PubMed/NCBI
|
|
21
|
Lu S, Niu N, Guo H, Tang J, Guo W, Liu Z,
Shi L, Sun T, Zhou F, Li H, et al: ARK5 promotes glioma cell
invasion, and its elevated expression is correlated with poor
clinical outcome. Eur J Cancer. 49:752–763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang HY, Li JH, Li G and Wang SR:
Activation of ARK5/miR-1181/HOXA10 axis promotes
epithelial-mesenchymal transition in ovarian cancer. Oncol Rep.
34:1193–1202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Phippen NT, Bateman NW, Wang G, Conrads
KA, Ao W, Teng PN, Litzi TA, Oliver J, Maxwell GL, Hamilton CA, et
al: NUAK1 (ARK5) is associated with poor prognosis in ovarian
cancer. Front Oncol. 6:2132016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roh SA, Choi EY, Cho DH, Jang SJ, Kim SY,
Kim YS and Kim JC: Growth and invasion of sporadic colorectal
adenocarcinomas in terms of genetic change. J Korean Med Sci.
25:353–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Long H, Xie R, Xiang T, Zhao Z, Lin S,
Liang Z, Chen Z and Zhu B: Autocrine CCL5 signaling promotes
invasion and migration of CD133+ ovarian cancer stem-like cells via
NF-κB-mediated MMP-9 upregulation. Stem Cells. 30:2309–2319. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao TT and Yang MH: Revisiting
epithelial-mesenchymal transition in cancer metastasis: The
connection between epithelial plasticity and stemness. Mol Oncol.
11:792–804. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi L, Zhang B, Sun X, Lu S, Liu Z, Liu Y,
Li H, Wang L, Wang X and Zhao C: MiR-204 inhibits human NSCLC
metastasis through suppression of NUAK1. Br J Cancer.
111:2316–2327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki A, Kusakai G, Kishimoto A, Lu J,
Ogura T, Lavin MF and Esumi H: Identification of a novel protein
kinase mediating Akt survival signaling to the ATM protein. J Biol
Chem. 278:48–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu J, Tao S, Hu P, Wang R, Fang C, Xu Y,
Qi D, Wei Z, Zhang J and Tan Q: CCR7 promote lymph node metastasis
via regulating VEGF-C/D-R3 pathway in lung adenocarcinoma. J
Cancer. 8:2060–2068. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martin TA and Jiang WG: Anti-cancer agents
in medicinal chemistry (formerly current medicinal
chemistry-anti-cancer agents). Anticancer Agents Med Chem.
10:12010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng K and Hao M: Metformin inhibits
TGF-β1-induced epithelial-to-mesenchymal transition via PKM2
relative-mTOR/p70s6k signaling pathway in cervical carcinoma cells.
Int J Mol Sci. 17(pii): E20002016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suzuki A, Kusakai G, Kishimoto A, Shimojo
Y, Miyamoto S, Ogura T, Ochiai A and Esumi H: Regulation of
caspase-6 and FLIP by the AMPK family member ARK5. Oncogene.
23:7067–7075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo
P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen D, Liu G, Xu N, You X, Zhou H, Zhao X
and Liu Q: Knockdown of ARK5 expression suppresses invasion and
metastasis of gastric cancer. Cell Physiol Biochem. 42:1025–1036.
2017. View Article : Google Scholar : PubMed/NCBI
|