Introduction
Diabetic nephropathy (DN), as a diabetic
microvascular complication, is mainly responsible for chronic renal
failure in diabetic patients worldwide (1,2).
Mesangial cell abnormalities and the deposition of extracellular
matrix (ECM) proteins, such as fibronectin and collagen, are the
main pathological hallmarks of DN (3). It has been reported that the
proliferation of mesangial cells serves a vital role in the
initiation and development of DN (4). Under high glucose (HG) conditions,
glomerular mesangial cell dysfunction, followed by imbalances in
ECM protein secretion and degradation, result in the deposition of
ECM proteins in the mesangium and basement membrane regions, which
leads to pathological changes in glomerular morphology, structure
and function, and the development of glomerulosclerosis (5,6). At
present, various factors have been identified to be important in
the development of DN; however, the underlying mechanisms remain
unclear.
Betaine, a neutral zwitterionic compound, is a
naturally occurring byproduct of sugar beet refinement, which is
extracted from molasses. Betaine has been detected in
microorganisms, animals and plants, including wheat, spinach,
shellfish and shrimp (7). This
compound serves dual roles in human physiology, functioning as an
osmolyte and as a methyl donor in transmethylation. As an osmolyte,
in order to maintain fluid balance, betaine can protect cells,
enzymes and proteins from environmental stresses, including high
salinity and extreme temperatures. As a methyl donor, betaine is
involved in the methionine cycle in the kidneys and liver in humans
(8). In addition, betaine
participates in a variety of biological processes. Betaine was
reported to suppress prostaglandin synthesis in rat liver
macrophages, thus modulating tumor necrosis factor-α secretion and
reversing the inhibitory effects of acetaldehyde on the interferon
signaling pathway (9,10). Additionally, as a natural food
additive, betaine can induce an autoimmune response to regulate the
fat:lean mass ratio and the neuro-endocrine system (11). Patients with inflammatory bowel
disease exhibit notable declines of betaine in urine, which
suggests that betaine may be involved in the modulation of immune
responses (12). Furthermore, it
has been shown that betaine decreased serum glucose and renal
oxidative stress in diabetic rats (13). Thus, we speculated that betaine may
be an effective agent for the treatment of diabetes and its
associated complications. The present study aimed to investigate
the effects of betaine on the development of DN, and to determine
the underlying potential mechanisms.
Materials and methods
Cell culture
Kidneys from mice were removed in a sterile manner
in accordance with the guidelines set by the National Institutes of
Health Guide for the Care and Use of Laboratory Animals (14). Briefly, 10 mice aged 5–6 weeks old
and weighing 18–20 g were purchased form the Experimental Animal
Center of Shanxi Medical University. These mice were maintained
under standard conditions (temperature 22°C, 12-h light-dark cycle)
and given free access to water and a standard diet. The present
study was approved by Institutional Animal Care and Use Committee
of Tianjin Third Central Hospital. Mouse mesangial cells (MMCs)
were extracted from kidneys and cultured as previously described
(15). MMCs were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) containing
HG (30 mM D-glucose) or with normal glucose levels (5.5 mM
D-glucose), 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), and a 1% penicillin and streptomycin solution
(Sigma-Aldrich; Merck KGaA) for 48 h in a humidified incubator with
5% CO2 at 37°C.
Cell treatment
MMCs were plated at a density of 5×104
cells/well 24 h prior to treatment. Betaine (1, 5 and 10 µM) and
100 mM metformin (Squibb Pharmaceutical Co., Ltd.) were
respectively added to the cells and incubated for 48 h at 37°C
under HG conditions (30 mM D-glucose). Cells without any treatment
were regarded as the normal control group, while cells treated with
metformin alone were regarded as the positive control group.
MTT assay
Cell proliferation was determined by an MTT assay.
Briefly, cells at a density of 1.0×106 were seeded into
a 96-well culture plate. Following various treatment for 48 h at
37°C, cells were incubated in 0.2 mg/ml MTT solution (Amresco LLC)
for 4 h at 37°C. Then, dimethyl sulfoxide was added to each well to
dissolve the formazan crystals and the optical density at 490 nm
was detected using a Synergy™ Multi-Mode Microplate Reader (Bio-Tek
Instruments, Inc.).
Cell cycle assay
For cell cycle analysis, cells were harvested after
treatment for 48 h at a initial density of 6.0×105
cells/well in 6-well plates, washed with PBS, and then fixed with
70% ethanol at 4°C overnight. Subsequently, MMCs were incubated
with RNase A (50 µg/ml; Sigma-Aldrich; Merck KGaA) and propidium
iodide (50 µg/ml; Sigma-Aldrich; Merck KGaA) at 4°C for 30 min.
Finally, the cell cycle was analyzed with a flow cytometer
(FACSCanto II; BD Biosciences) and CellQuest software (BD
Biosciences).
Western blot analysis
For western blot analysis, cells were lysed using
lysis buffer (Cell Signaling Technology, Inc.). Total protein was
extracted from cells and its concentration was measured with a BCA
protein assay kit (Thermo Fisher Scientific, Inc.). Samples were
subjected to 11% SDS-PAGE and then transferred to polyvinylidene
difluoride membranes. The membranes were incubated with primary
antibodies (1:1,000) against fibronectin (ab2413, Abcam), type IV
collagen (ab6586, Abcam), p21 (cat. no. 2947, Cell Signaling
Technology, Inc.), p27 (cat. no. 3686, Cell Signaling Technology,
Inc.), phosphorylated (p)-Akt (cat. no. 9614, Cell Signaling
Technology, Inc.), Akt (cat. no. 9272, Cell Signaling Technology,
Inc.), p-extracellular-signal-regulated kinase (Erk)1/2 (cat. no.
3510, Cell Signaling Technology, Inc.), Erk1/2 (cat. no. 4695, Cell
Signaling Technology, Inc.), p-p38 mitogen-activated protein kinase
(MAPK; cat. no. 4511, Cell Signaling Technology, Inc.), p38 MAPK
(cat. no. 8690, Cell Signaling Technology Inc.) and GAPDH (cat. no.
5174, Cell Signaling Technology, Inc.) overnight at 4°C after
blocking with 5% non-fat milk at room temperature for 2 h.
Subsequently, the membranes were incubated with corresponding
horseradish peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology) at room temperature for 2 h. An enhanced
chemiluminescence detection system (SuperSignal West Dura Extended
Duration Substrate, Pierce; Thermo Fisher Scientific, Inc.) was
used to determine protein expression and the Quantity One analysis
system version 4.6 (Bio-Rad Laboratories, Inc.) was used for the
quantification of protein expression.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from MMCs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. Complementary DNA
was synthetized at 37°C for 15 min and 85°C for 5 sec using a
PrimeScript RT Reagent kit (Takara Biotechnology Co., Ltd.) and
analyzed with a TaqMan Universal PCR Master Mix kit (Thermo Fisher
Scientific, Inc.) under the thermocycling conditions: initial
denaturation at 95°C for 5 min, followed by 40 cycles of 95°C for
10 sec and 60°C for 30 sec. The following primer pairs were used
for PCR amplification: Fibronectin, forward
5′-GCAGTGACCACCATTCCTG-3′, reverse, 5′-GGTAGCCAGTGAGCTGAACAC-3′;
type IV collagen, forward 5′-TCCTTGTGACCAGGCATAGT-3′, reverse,
5′-TTGAACATCTCGCTCCTCTC-3′; and GAPDH, forward:
5′-ATCCCATCACCATCTTCCAG-3′, reverse, 5′-CCATCACGCACAGTTTCC-3′.
GAPDH was used as an internal control. For relative gene expression
quantification, the 2−ΔΔCq method was employed (16).
Statistical analysis
All experiments were repeated three times. Data were
expressed as the mean ± standard deviation. SPSS 17.0 statistical
software (SPSS, Inc.) was used for all statistical analyses.
One-way analysis of variance followed by a Tukey's test was used
for comparisons between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
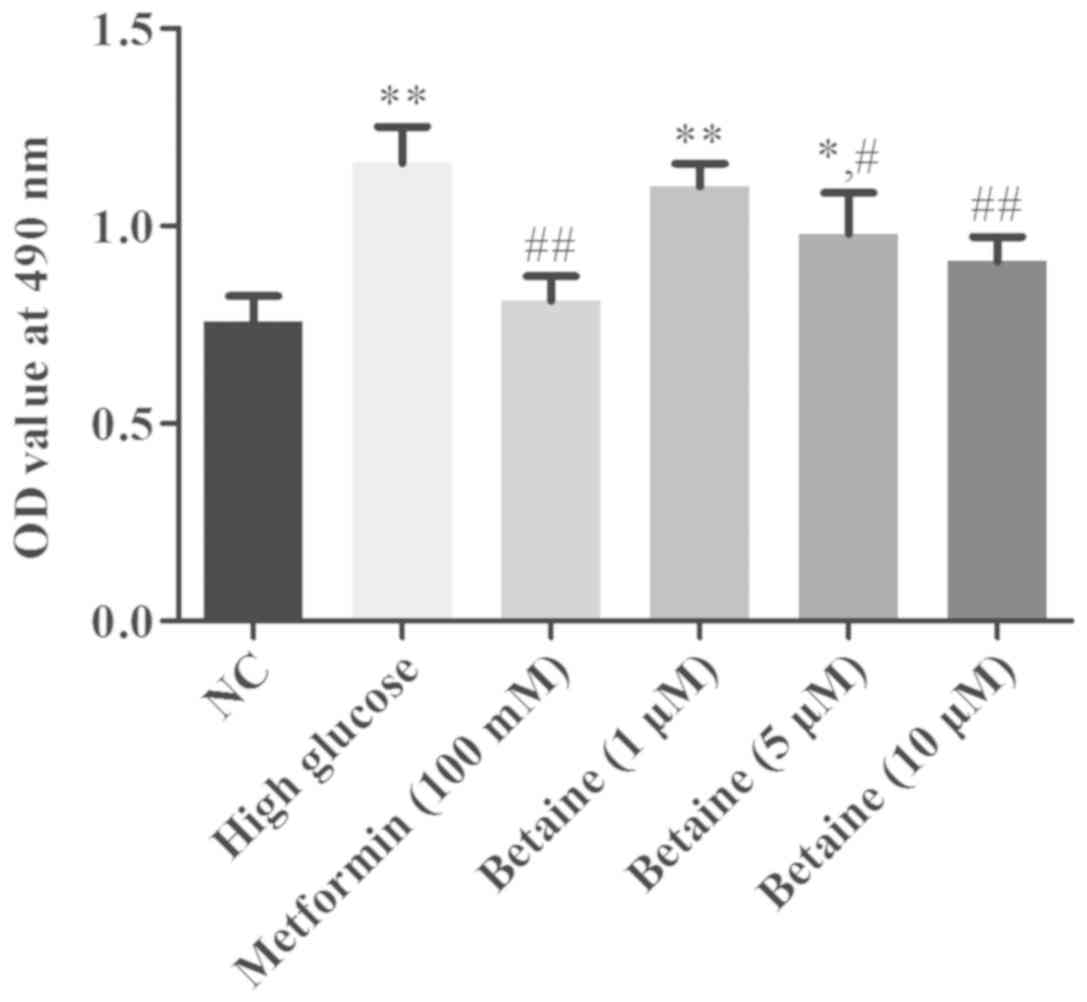
Betaine inhibits the proliferative
ability of MMCs via G1-phase arrest
The effects of betaine on the growth of MMCs was
determined by an MTT assay. Compared with the control group, the
proliferative ability of MMCs was significantly enhanced under HG
conditions. Betaine treatment inhibited MMC proliferation in a
dose-dependent manner. Metformin significantly repressed MMCs
proliferation compared with HG treatment (Fig. 1).
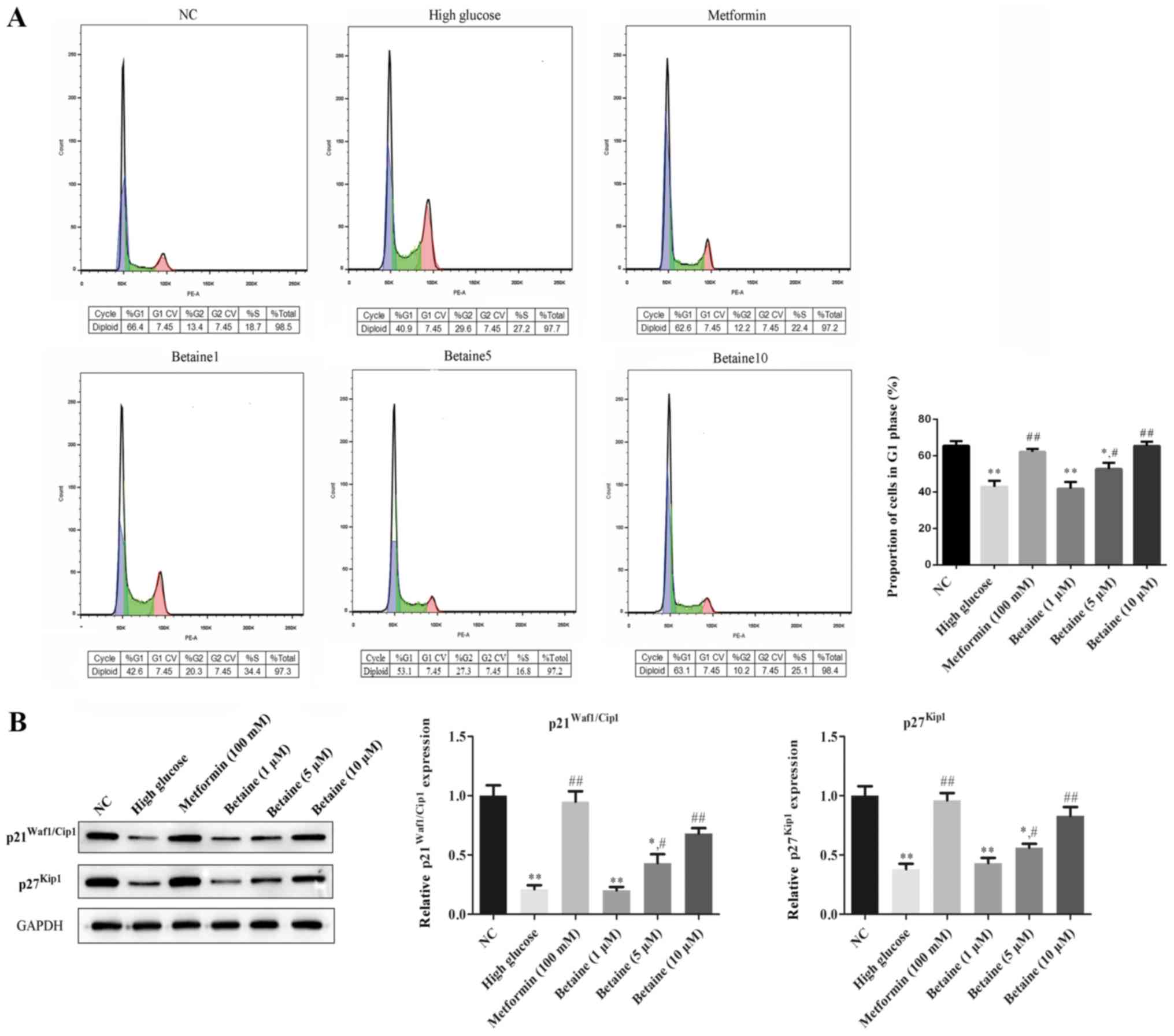
As presented in Fig.
2A, compared with the control group, HG significantly reduced
the proportion of cells in G1 phase, while betaine treatment
induced G1-phase arrest of MMCs in a dose-dependent manner.
Compared with the cells treated with HG, the abundance of G1 phase
cells significantly increased in MMCs treated with metformin. In
addition, the protein expression levels of p21 and p27 were
significantly decreased in MMCs treated with HG compared with
control cells, while betaine treatment increased protein p21 and
p27 protein expression in MMCs in a dose-dependent manner.
Furthermore, a significant increase in the expression of the
aforementioned proteins was reported following treatment with
metformin compared with the HG conditions (Fig. 2B).
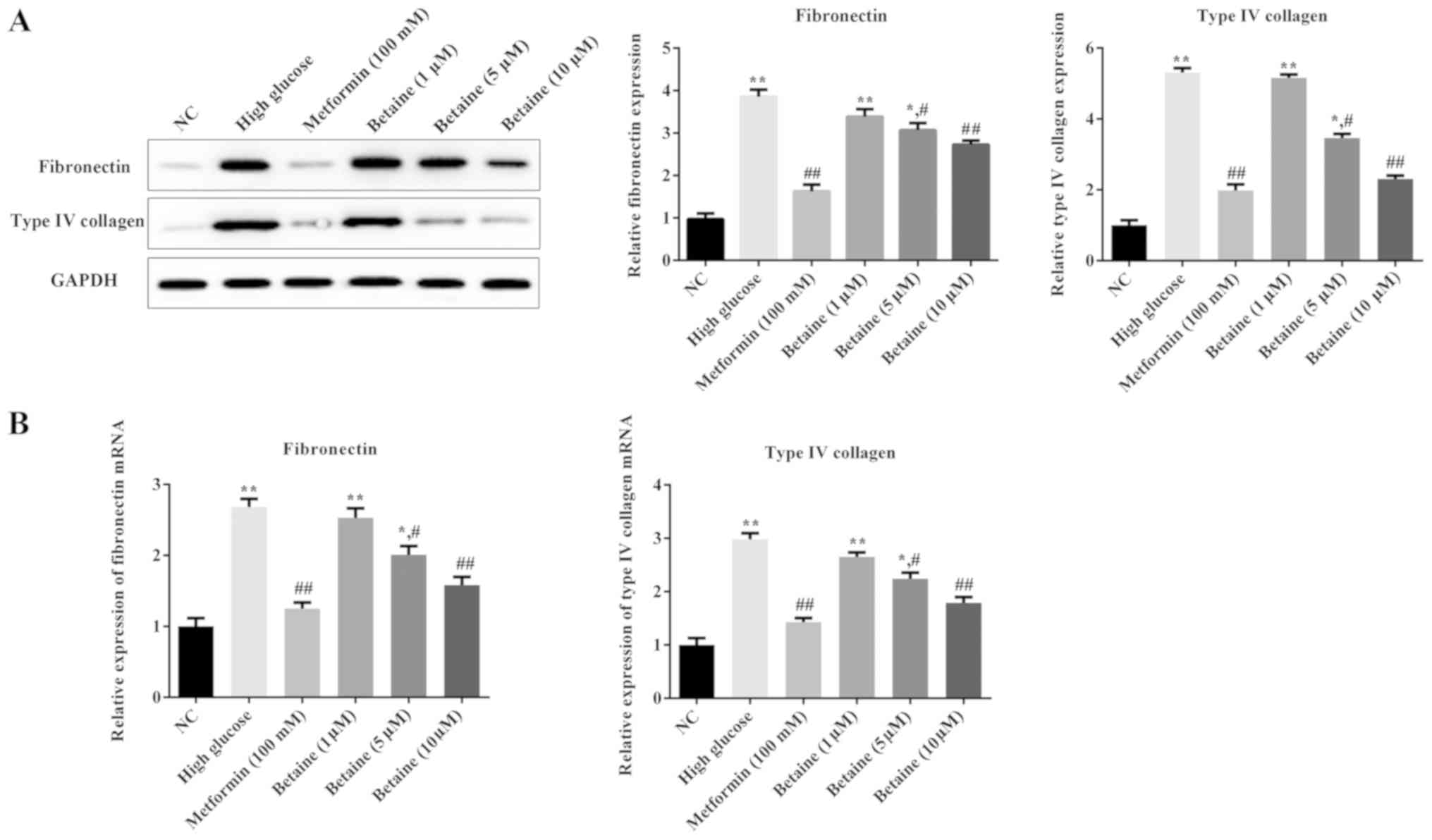
Betaine prevents ECM deposition in
MMCs
To investigate the effects of betaine on ECM
deposition in MMCs, the expression levels of ECM proteins,
including fibronectin and type IV collagen, were determined. As
presented in Fig. 3, the protein
and mRNA expression levels of fibronectin and type IV collagen were
significantly increased in MMCs treated with HG compared with the
control cells; betaine treatment decreased the levels of
fibronectin and type IV collagen in MMCs in a dose-dependent
manner. Additionally, metformin significantly inhibited fibronectin
and type IV collagen expression in MMCs compared with the HG
conditions (Fig. 3). These results
suggested that betaine could prevent ECM deposition induced by
HG.
Betaine prevents the activation of
Akt, Erk1/2 and p38
To explore the underlying mechanism of the effects
of betaine on MMCs, the Akt and MAPK signaling pathways were
analyzed. As presented in Fig. 4,
the protein expression levels of p-Akt, p-Erk1/2 and p-p38 were
significantly increased in MMCs treated with HG compared with
control cells. Betaine treatment decreased the levels of p-Akt,
p-Erk1/2 and p-p38 in MMCs in a dose-dependent manner. On the
contrary, metformin significantly inhibited p-Akt, p-Erk1/2 and
p-p38 protein expression in MMCs compared with the HG conditions
(Fig. 4). These results indicated
that betaine might exert its functions through the Akt and MAPK
signaling pathway.
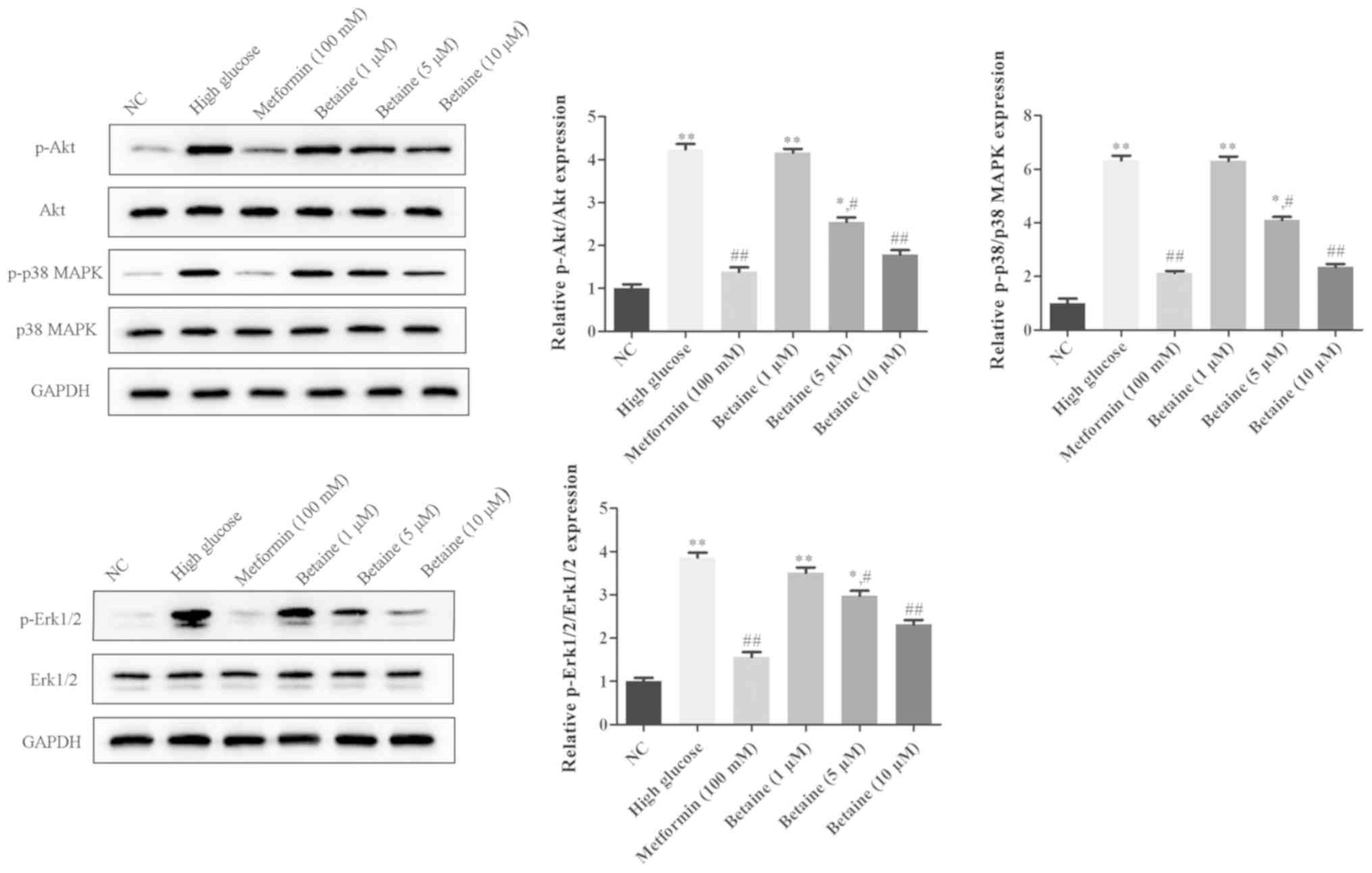 | Figure 4.Effects of betaine on the activation
of Akt, Erk1/2 and p38 in mouse mesangial cells. Following
treatment, the protein expression levels of Akt, Erk1/2, p38 MAPK,
p-AKT, p-Erk1/2 and p-p38 MAPK was measured via western blotting.
*P<0.05, **P<0.01 vs. NC; #P<0.05,
##P<0.01 vs. High glucose. AKT, protein kinase B;
Erk, extracellular-signal-regulated kinase; MAPK, mitogen-activated
protein kinase; NC, negative control; p, phosphorylated. |
Discussion
In the present study, betaine inhibited cell
proliferation, induced G1-phase arrest and reduced ECM deposition
in MMCs, possibly via suppression of the Akt/Erk1/2/p38 MAPK
signaling pathway. The results revealed that betaine may be a
promising therapeutic agent for the treatment of DN.
DN is considered as one of the major microvascular
complication of diabetes; ~50% of diabetes cases exhibit DN, which
is mainly responsible for end-stage renal disease (17). As DN poses great social and
economic burden to individuals, families and society, it is a major
public health problem worldwide (18). In China, the proportion of patients
with end-stage renal disease caused by DN is increasing every year
(19); however, the pathogenesis
of DN is markedly complicated and its mechanism has not yet been
fully elucidated. As the pathogenesis of DN involves in various
bioactive compounds and several signaling pathways, effective
preventative and treatment measures are required. Thus, exploring
the pathogenesis of DN and identifying potential treatment methods
to delay the progression of DN have important social and economic
value.
Betaine, a methyl donor, has been reported to
possess various physiological and pharmacological functions
(20,21). Betaine hydrochloride can be used
for the prevention and therapy of atherosclerosis, liver disease
gastric acid deficiency and rheumatism (22–27).
Betaine possesses notable medicinal value and has broad
applications; however, few studies have investigated the effects of
betaine on DN. Thus, the current study aimed to investigate the
effects and possible mechanism of betaine on HG-induced MMCs.
Mesangial cell abnormalities and ECM deposition are pathological
hallmarks of DN (6). Various
studies have demonstrated that mesangial cell proliferation is
crucial in the occurrence and evolvement of DN (6,28).
Our findings demonstrated that betaine and metformin inhibited cell
proliferation, induced G1-phase arrest and prevented ECM deposition
in MMCs.
In addition, the Akt, Erk1/2 and p38 MAPK signaling
pathways were determined to be involved in the mechanism underlying
the effects of betaine on MMCs. Akt, is a serine/threonine protein
kinase reported to be anti-apoptotic and one of the main downstream
targets of the phosphatidylinositol (3,4,5)-trisphosphate signaling pathway
(29). Inactivation of Akt, a key
regulator of cell viability, is involved in degenerative diseases
and stress-induced pathological cell death (30,31).
It has been reported that the Akt signaling pathway is associated
with DN (29); an Akt inhibitor
was able to attenuate HG-induced cell proliferation, inflammation
and ECM expression in mesangial cells (32). Compounds such as daphnetin and
zeaxanthin, could ameliorate HG-induced mesangial cell apoptosis
via the Akt signaling pathway (32,33).
Our results indicated that betaine inhibited MMC proliferation and
ECM deposition via the Akt signaling pathway, which is in
consistent with previous studies. The Erk1/2 signaling pathway is
also involved in DN (34). Erk has
been implicated in cell proliferation and differentiation, as it
can induce the expression of certain genes (35). As mesangial cell proliferation is
facilitated by the activation of Erk1/2, its inhibition protected
mesangial cells under HG conditions by suppressing cell
proliferation and ECM deposition (36,37).
In addition, p38 MAPK, which is associated with cell apoptosis
initiation and cell cycle arrest, has been demonstrated to be
activated in glomerular mesangial cells under HG conditions
(38,39). In the present study, it was
demonstrated that Akt, Erk1/2 and p38 MAPK were activated in MMCs
under HG conditions, and betaine was proposed to exert its
protective effects via the Akt/Erk/p38 MAPK signaling pathway.
However, there are certain limitations to the
present study. There are three isoforms of Akt in mammalian cells,
namely Akt1, Akt2 and Akt3. Though it has been reported that Akt2
was strongly associated with the regulation of glucose homoeostasis
and is the predominant Akt isoform expressed in insulin-responsive
tissues (40), the specific
binding sites for betaine on Akt were not determined. Additionally,
the specific targets activated downstream of the Akt/Erk1/2/p38
MAPK signaling pathway should be investigated in subsequent
studies. Furthermore, HG in culture cannot completely mimic
diabetic conditions in vivo; experiments using diabetic
mouse models should be performed to validate these preliminary
data. The present study reported the protective effects of betaine
in vitro; the effects of betaine treatment in vivo
should be determined in the future.
Collectively, the findings of the current study
indicated that betaine exerted a protective effect on MMCs under HG
conditions by inhibiting MMC proliferation and ECM deposition via
regulation of the Akt/Erk1/2/p38 MAPK signaling pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Clinical
Evaluation of the Traditional Chinese and Western Medicine Research
Project of Tianjin Municipal Health Bureau ‘Clinical Evaluation of
the Quantitative Effect Relationship of Pancreatic Live Dispersion
in the Treatment of Type 2 Diabetes’ (grant no. 07025).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL made substantial contributions to the design of
the present study. LW and HM performed the experiments. XL and HM
analyzed the data. XL and LW wrote the manuscript. XL revised the
manuscript. All authors reviewed the manuscript.
Ethics approval and consent to
participate
The present study was approved by Institutional
Animal Care and Use Committee of Tianjin Third Central Hospital and
conducted in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals (14).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fineberg D, Jandeleit-Dahm KA and Cooper
ME: Diabetic nephropathy: Diagnosis and treatment. Nat Rev
Endocrinol. 9:713–723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jha V, Garcia-Garcia G, Iseki K, Li Z,
Naicker S, Plattner B, Saran R, Wang AY and Yang CW: Chronic kidney
disease: Global dimension and perspectives. Lancet. 382:260–272.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gross JL, de Azevedo MJ, Silveiro SP,
Canani LH, Caramori ML and Zelmanovitz T: Diabetic nephropathy:
Diagnosis, prevention, and treatment. Diabetes Care. 28:164–176.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu L, Hu X, Cai GY, Lv Y, Zhuo L, Gao JJ,
Cui SY, Feng Z, Fu B and Chen XM: High glucose-induced hypertrophy
of mesangial cells is reversed by connexin43 overexpression via
PTEN/Akt/mTOR signaling. Nephrol Dial Transplant. 27:90–100. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mason RM and Wahab NA: Extracellular
matrix metabolism in diabetic nephropathy. J Am Soc Nephrol.
14:1358–1373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Zhong HB, Lin Y, Yao W and Huang
JY: KLF15 suppresses cell proliferation and extracellular matrix
expression in mesangial cells under high glucose. Int J Clin Exp
Med. 8:20330–20336. 2015.PubMed/NCBI
|
|
7
|
Craig SA: Betaine in human nutrition. Am J
Clin Nutr. 80:539–549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cabezón FA, Stewart KR, Schinckel AP,
Barnes W, Boyd RD, Wilcock P and Woodliff J: Effect of natural
betaine on estimates of semen quality in mature AI boars during
summer heat stress. Anim Reprod Sci. 170:25–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang F, Warskulat U and Häussinger D:
Modulation of tumor necrosis factor-alpha release by
anisoosmolarity and betaine in rat liver macrophages (Kupffer
cells). FEBS Lett. 391:293–296. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ganesan M, Zhang J, Bronich T, Poluektova
L, Donohue TM Jr, Tuma DJ, Kharbanda KK and Osna NA: Acetaldehyde
accelerates HCV-induced impairment of innate immunity by
suppressing methylation reactions in liver cells. Am J Physiol
Gastrointest Liver Physiol. 309:G566–G577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sillence MN: Technologies for the control
of fat and lean deposition in livestock. Vet J. 167:242–257. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schicho R, Shaykhutdinov R, Ngo J,
Nazyrova A, Schneider C, Panaccione R, Kaplan GG, Vogel HJ and
Storr M: Quantitative metabolomic profiling of serum, plasma, and
urine by (1)H NMR spectroscopy discriminates between patients with
inflammatory bowel disease and healthy individuals. J Proteome Res.
11:3344–3357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Evran B, Aydın AF, Uğuralp B, Sar M,
Doğru-Abbasoğlu S and Uysal M: Betaine treatment decreased serum
glucose and lipid levels, hepatic and renal oxidative stress in
streptozotocin-induced diabetic rats. Turk J Biochem. 43:2017.
|
|
14
|
Guide for the Care and Use of Laboratory
Animals. Washington (DC): National Academies Press (US); 2011
|
|
15
|
Kim YS, Reddy MA, Lanting L, Adler SG and
Natarajan R: Differential behavior of mesangial cells derived from
12/15-lipoxygenase knockout mice relative to control mice. Kidney
Int. 64:1702–1714. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He F, Xia X, Wu XF, Yu XQ and Huang FX:
Diabetic retinopathy in predicting diabetic nephropathy in patients
with type 2 diabetes and renal disease: A meta-analysis.
Diabetologia. 56:457–466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schieppati A and Remuzzi G: Chronic renal
diseases as a public health problem: Epidemiology, social, and
economic implications. Kidney Int Suppl. 98:S7–S10. 2005.
View Article : Google Scholar
|
|
19
|
Zhuo L, Zou G, Li W, Lu J and Ren W:
Prevalence of diabetic nephropathy complicating non-diabetic renal
disease among Chinese patients with type 2 diabetes mellitus. Eur J
Med Res. 18:42013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cholewa JM, Guimarães-Ferreira L and
Zanchi NE: Effects of betaine on performance and body composition:
A review of recent findings and potential mechanisms. Amino Acids.
46:1785–1793. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hagar H and Al Malki W: Betaine
supplementation protects against renal injury induced by cadmium
intoxication in rats: Role of oxidative stress and caspase-3.
Environ Toxicol Pharmacol. 37:803–811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ananth CV, Elsasser DA, Kinzler WL,
Peltier MR, Getahun D, Leclerc D and Rozen RR; New Jersey Placental
Abruption Study Investigators, : Polymorphisms in methionine
synthase reductase and betaine-homocysteine Smethyl-transferase
genes: Risk of placental abruption. Mol Genet Metab. 91:104–110.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kempson SA, Vovor-Dassu K and Day C:
Betaine transport in kidney and liver: Use of betaine in liver
injury. Cell Physiol Biochem. 32:32–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonig H, Daublin G, Schwahn B and Wendel
U: Psychotic symptoms insevere MTHFR deficiency and their
successful treatment with betaine. Eur J Pediatr. 162:200–201.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Patrick L: Nonalcoholic fatty liver
disease: Relationship to insulin sensitivity and oxidative stress.
Treatment approaches using vitamin E, magnesium, and betaine.
Altern Med Rev. 7:276–291. 2002.PubMed/NCBI
|
|
26
|
Hammer MA and Baltz JM: Betaine is a
highly effective organic osmolyte but does not appear to be
transported by established organic osmolyte transporters in mouse
embryos. Mol Reprod Dev. 62:195–202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abdelmalek MF, Angulo P, Jorgensen RA,
Sylvestre PB and Lindor KD: Betaine, a nonalcoholic
steatohepatitis: Results of a pilot promising new study. Am J
Gastroenterol. 96:2711–2717. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma Y, Chen F, Yang S, Chen B and Shi J:
Protocatechuic acid ameliorates high glucose-induced extracellular
matrix accumulation in diabetic nephropathy. Biomed Pharmacother.
98:18–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ying C, Mao Y, Chen L, Wang S, Ling H, Li
W and Zhou X: Bamboo leaf extract ameliorates diabetic nephropathy
through activating the AKT signaling pathway in rats. Int J Biol
Macromol. 105:1587–1594. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang S, Chen X, Huang Z, Chen D, Yu B,
Chen H, Luo J, He J, Zheng P and Yu J: Leucine promotes
differentiation of porcine myoblasts through the protein kinase B
(Akt)/Forkhead box O1 signalling pathway. Br J Nutr. 119:727–733.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heath JM, Sun Y, Yuan K, Bradley WE,
Litovsky S, Dell'Italia LJ, Chatham JC, Wu H and Chen Y: Activation
of AKT by O-linked N-acetylglucosamine induces vascular
calcification in diabetes mellitus. Circ Res. 114:1094–1102. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu K, Guo L, Bu H and Wang H: Daphnetin
inhibits high glucose-induced extracellular matrix accumulation,
oxidative stress and inflammation in human glomerular mesangial
cells. J Pharmacol Sci. 139:91–97. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ying C, Chen L, Wang S, Mao Y, Ling H, Li
W and Zhou X: Zeaxanthin ameliorates high glucose-induced mesangial
cell apoptosis through inhibiting oxidative stress via activating
AKT signaling-pathway. Biomed Pharmacother. 90:796–805. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shang J, Zhang Y, Jiang Y, Li Z, Duan Y,
Wang L, Xiao J and Zhao Z: NOD2 promotes endothelial-to-mesenchymal
transition of glomerular endothelial cells via MEK/ERK signaling
pathway in diabetic nephropathy. Biochem Biophys Res Commun.
484:435–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Flores K and Seger R: Stimulated nuclear
import by β-like importins. F1000Prime Rep. 5:412013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Y, Wang M, Chen B and Shi J:
Scoparone attenuates high glucose-induced extracellular matrix
accumulation in rat mesangial cells. Eur J Pharmacol. 815:376–380.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suzaki Y, Yoshizumi M, Kagami S, Nishiyama
A, Ozawa Y, Kyaw M, Izawa Y, Kanematsu Y, Tsuchiya K and Tamaki T:
BMK1 is activated in glomeruli of diabetic rats and in mesangial
cells by high glucose conditions. Kidney Int. 65:1749–1760. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen L, Mayer JA, Krisko TI, Speers CW,
Wang T, Hilsenbeck SG and Brown PH: Inhibition of the p38 kinase
suppresses the proliferation of human ER-negative breast cancer
cells. Cancer Res. 69:8853–8861. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goldberg H, Whiteside C and Fantus IG:
O-linked β-N-acetylglucosamine supports p38 MAPK activation by high
glucose in glomerular mesangial cells. Am J Physiol Endocrine
Metab. 301:E713–E726. 2011. View Article : Google Scholar
|
|
40
|
Dummler B and Hemmings BA: Physiological
roles of PKB/Akt isoforms in development and disease. Biochem Soc
Trans. 35:231–235. 2007. View Article : Google Scholar : PubMed/NCBI
|