Introduction
Pulmonary arterial hypertension (PAH) shows
characteristics such as increased pulmonary artery resistance and
pulmonary vascular remodeling. It is a fatal disease that
eventually leads to death of the patient due to right heart failure
(1). A previous studies have
indicated that 74% of PAH patients are at a moderate- or high-risk
stage when they are diagnosed (2);
furthermore, the 1-, 3- and 5-year survival rates of patients with
moderate- and high-risk pulmonary arterial hypertension after
target therapy are only 90, 61 and 43%, respectively (3). Pulmonary artery remodeling serves a
critical role at the high-risk stage of PAH (4). The main effect of the current target
medicine agents for PAH, such as macitentan, tadalafil and
selexipag, is vasodilatation (5),
whichhas little impact on reversing pulmonary artery remodeling.
Therefore, discovery of safe and effective agents to alleviate
pulmonary artery remodeling is urgently needed.
Pulmonary artery remodeling is mainly caused by the
abnormal proliferation of pulmonary artery smooth muscle cells
(PASMCs) (6), pulmonary artery
endothelial cells (7) and
pulmonary artery fibroblasts (8).
Abnormal proliferation of PASMCs in the pulmonary artery media is
the main feature of vascular remodeling in PAH (9). A previous study reported that aerobic
glycolysis is involved in the abnormal proliferation of PASMCs
(10). Moreover, it has been
reported that inhibition of aerobic glycolysis reverses pulmonary
artery remodeling and reduces pulmonary circulatory resistance by
inhibition of the abnormal proliferation of PASMCs (11). In multiple cancer types, the
expression of pyruvate kinase M2 (PKM2) is upregulated, which
promotes the occurrence of aerobic glycolysis (12–14).
In human glioblastoma specimens, a previous study reported the
direct binding of EGFR-activated ERK2 to PKM2 through the ERK2
docking groove, which facilitated phosphorylation of PKM2 at Ser37.
Ser37-phosphorylated PKM2 promotes cis-trans isomerization of PKM2
by recruiting PIN1, and cis-trans isomerized PKM2 binds to importin
α5 and translocates to the nucleus. In the nucleus PKM2 induces
c-Myc expression as a coactivator of β-catenin, which results in
upregulation of glucose transporter 1 (GLUT1), lactate
dehydrogenase A (LDHA) and polypyrimidine tract-binding protein
(PTB)-dependent PKM2 expression (15).
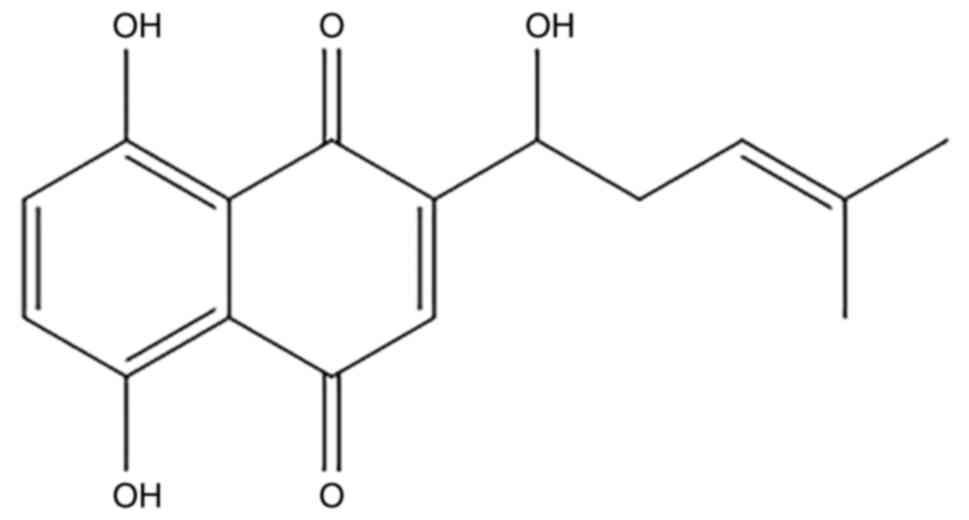
Shikonin (Fig. 1)
is a naphthoquinone substance extracted from the traditional
Chinese medicine ‘comfrey’. The main effect of comfrey has been
reported to be tumor suppression (16) via inhibition of the proliferation
and promotion of the apoptosis of tumor cells (17,18).
A previous study reported that the antitumor role of shikonin
depends on its specific inhibition of PKM2 (19). Furthermore, a previous study
reported that PKM2 exerts a critical effect on PAH, through
transformation of the cell metabolic state to aerobic glycolysis,
known as the Warburg affect that promotes cell proliferation.
Warburg metabolism and its epigenetic regulators are high-value
therapeutic targets in PAH (20).
Inhibition of PKM2 alleviates hypoxic PAH through attenuation of
the proliferation of endothelial cells and adventitial fibroblasts
(7,8); however, the mechanism by which it
ameliorates PAH has not been fully elucidated.
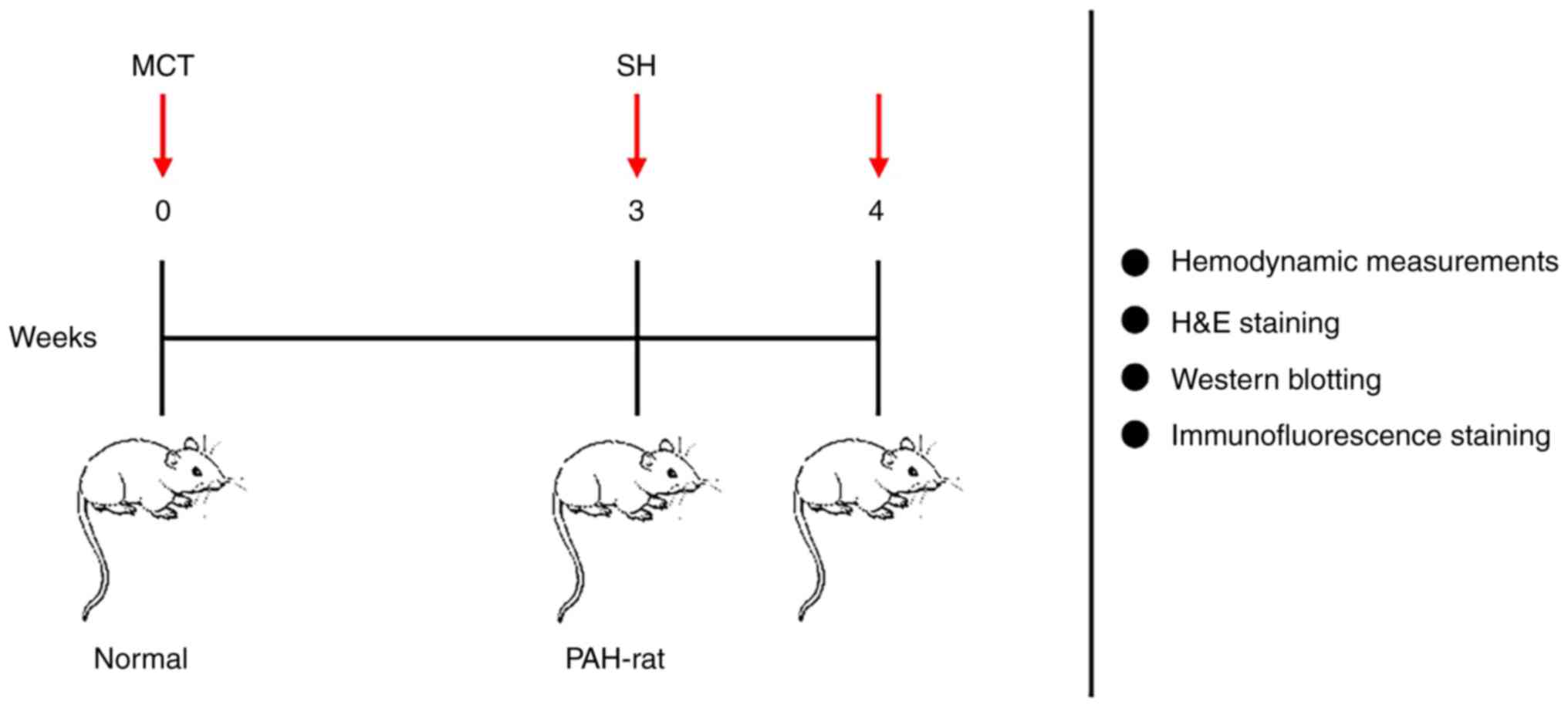
In the present study, the mechanisms leading to the
protective effect of shikonin in monocrotaline (MCT) induced PAH
(MCT-PAH) experimental rats were evaluated. A rat pulmonary artery
hypertension experimental model, was established 21 days after
intraperitoneal injection of MCT, and then treated with shikonin to
assess its efficacy. An in vitro pulmonary vascular smooth
muscle cell model of PAH, was used to assess the effect of shikonin
on glucose metabolism to elucidate the possible mechanism of
shikonin in the treatment of PAH.
Materials and methods
Animals and experimental design
A total of 24 specific pathogen-free male
Sprague-Dawley (SD) rats (weight, 180–200 g; age, 6–8 weeks) were
purchased from Hunan Changsha Tianqin Biotechnology Co., Ltd.). All
rats were housed under particular conditions (12 h light/dim cycle;
22±3°C; humidity, 30–60%) and given free access to food and water.
The SD rats were divided into three groups as follows: i) MCT
group, were subcutaneously injected with 60 mg/kg MCT (n=8; cat.
no. C2401; MilliporeSigma) (21);
ii) control group, were intraperitoneally injected with an
equivalent volume of saline (n=8); and iii) shikonin group, were
treated with 10 mg/kg/day shikonin (cat. no. C.I. 75535; Selleck
Chemicals) via intraperitoneal infusion once daily from day 21–28
(n=8) (22) (Fig. 2). The control and MCT groups were
treated with an equivalent volume of saline. The Hunan Children's
Hospital Ethics Committee approved the present study (approval no.
HCHLL-2020-44).
Cell culture
Primary murine PASMCs were collected from another
five normal, specific pathogen-free male SD rats (weight, 180–200
g; age, 6–8 weeks) following euthanasia using 1% sodium
pentobarbital (intraperitoneal injection; 130 mg/kg). PASMCs were
divided into a control group, a DMSO group, a model group [20 ng/ml
platelet derived growth factor-BB (PDGF-BB) (cat. no. 100-14B;
PeproTech, Inc.) with DMSO] and the shikonin treatment group (1.0
µM), all treatments were applied for 24 h before the growth media
was replaced with untreated media (23). All PASMCs were cultured at 37°C.
The primary murine PASMCs were cultured with Dulbecco's modified
Eagle's medium (DMEM) (cat no. C11995500BT; Gibco; Thermo Fisher
Scientific, Inc.) containing 1% penicillin, 15% fetal bovine serum
(SFBS) (cat. no. FS301; TransGen Biotech Co., Ltd.) and nutrient
mixture F-12 (DMEM/F12) (cat. no. C11330500BT; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 1% streptomycin in a 5%
CO2 incubator. The medium was replaced every 48 h, and
subculture was performed at 70–80% confluence. PASMCs were
identified by their morphologic appearance under phase-contrast
microscopy and immunofluorescence using an anti-α-smooth muscle
actin antibody (1:200; cat. no. GB11044; Wuhan Servicebio
Technology Co., Ltd.) incubated with the cells overnight at 4°C and
anti-rabbit Alexa Fluor 488 secondary antibody (1:500; cat. no.
GB25303; Wuhan Servicebio Technology Co., Ltd.) incubated with the
cells at room temperature for 50 min. The cells were used for
further experimentation at passages 3 to 5, synchronized with serum
starvation prior to intervention (24).
Hemodynamic measurements
After anesthetization with 1% sodium pentobarbital
(intraperitoneal injection, 40 mg/kg), the rats were placed in the
lateral decubitus position for echocardiography. Using
echocardiography, the pulmonary artery blood flow acceleration time
(PAAT), the inner diameter of the right ventricle (RVID) and the
tricuspid annular plane systolic excursion (TAPSE) were assessed.
After echocardiography, the rats were placed supine on an operating
table and a polyethene catheter filled with heparin was inserted
into the right ventricle via the right external jugular vein to
test the right ventricular systolic pressure (RVSP) with a BL-420
biological function experiment system (Chengdu Taimeng Software
Co., Ltd).
Evaluation of right ventricular
hypertrophy
After the pressure measurements, the rats were
euthanized using 1% sodium pentobarbital (intraperitoneal
injection, 130 mg/kg) and the heart and lungs were collected. The
hearts were divided to dissect the right ventricle (RV) free wall
from the left ventricle (LV) plus the interventricular septum (S),
and the portions were weighed separately. The right ventricular
hypertrophy index (RVHI) was determined from the RV/(LV + S)
proportion.
Histomorphometric analysis
The lung tissues of every group were placed in 4%
paraformaldehyde buffer overnight at room temperature, dehydrated
and embedded in paraffin. All lung tissue sections (5 µm) were
fixed on slides and baked until dry. Hematoxylin and eosin
(H&E) staining was performed according to the manufacturer's
instructions using a H&E Staining Kit (cat. no. C02-04004;
BIOSS). Then, the sections were immersed in xylene, an increasing
ethanol concentration gradient and sealed with resin. After drying,
the pulmonary vascular morphology was assessed and imaged using an
optical light microscope. Between 10–20 small pulmonary blood
vessels with a diameter of 50–150 µm were randomly selected for
analysis. The pulmonary artery wall thickness ratio (WT%) and
pulmonary artery wall area ratio (WA%) were used to evaluate
pulmonary artery remodeling. These measurements were calculated as
follows: WT%=(outer diameter-inner diameter)/outer diameter and
WA%=(transaction region of the walls-lumen region)/transaction area
of the walls.
Western blotting
Rat lung tissue protein was extracted by incubation
with RIPA lysis buffer (RIPA:PMSF, 100:1; cat. no. C5029; BIOSS),
followed by sonication (1 min) and centrifugation at 15,000 × g for
15 min at 4°C. All protein concentrations were measured using the
BCA assay (cat. no. CW0014; CoWin Biosciences). Sample loading
buffer (5×) was employed to dilute an equal amount of protein (20
µg/lane) from each sample, and the diluted proteins were boiled for
5 min. The proteins were separated using 8% SDS-PAGE, blotted onto
nitrocellulose membranes, and probed with a specific rabbit
monoclonal anti-PKM2 antibodies (1:500; cat. no. AF5234; Affinity
Biosciences), rabbit monoclonal anti-phosphorylated (p)-PKM2
(Ser37) antibodies (1:500; cat. no. AF7231; Affinity Biosciences),
rabbit monoclonal anti-ERK1 + ERK2 antibodies (1:1,000; cat. no.
ab17942; Abcam), rabbit monoclonal anti-LDHA antibody (1:500; cat.
no. ab101562; Abcam), mouse monoclonal anti-p-ERK antibody
(1:1,000; cat. no. sc-7383; Santa Cruz Biotechnology, Inc.) and
mouse monoclonal anti-GLUT1 antibody (1:500; cat. no. ab40084;
Abcam) overnight at 4°C. Subsequently, PBST was used to wash the
membranes, which were then incubated with goat anti-mouse or goat
anti-rabbit IgG antibodies (1:5,000; cat. no. SA00001-1 or
SA00001-2, respectively; Proteintech Group, Inc.) for 1 h at room
temperature. For confirmation of equal loading, the blots were
incubated with an anti-β-actin monoclonal antibody (1:1,000; cat.
no. BS6007M; Bioworld Technology, Inc.). TBST with 1% Tween 20
(cat. no. bs100; Biosharp Life Sciences) was used for washing. The
protein bands were assessed using ECL reagent (Applygen
Technologies, Inc.). Quantity One software 4.6.6 (Bio-Rad
Laboratories, Inc.) was used to semi-quantify the grayscale values
of all bands on the blots.
Immunofluorescence staining of
PKM2
Paraffin sections of lung tissues were dewaxed using
immersion in xylene, a decreasing ethanol concentration gradient
and distilled water and then placed in a repair box containing EDTA
antigen repair buffer (pH 9.0) (cat. no. C1034; Beijing Solarbio
Science & Technology Co., Ltd.), and repaired using a microwave
(400 watts (W) for 8 min and 100 W for 7 min). Incubation with a
rabbit monoclonal anti-PKM2 antibody (1:200; cat. no. AF5234;
Affinity Biosciences) at 4°C overnight was performed and incubation
with Alexa Fluro 488-conjugated secondary antibodies was then
performed (1:500; cat. no. 550037; Chengdu Zhengneng Biotechnology
Co., Ltd.) at room temperature for 50 min. The nuclei were stained
using DAPI at room temperature for 10 min, and after washing, an
anti-fade mounting solution (cat. no. P0126; Beyotime Institute of
Biotechnology) was used to seal the sections. The sections were
then imaged using fluorescence microscopy.
Glucose, lactic acid and ATP
evaluation
The levels of glucose, lactic acid and ATP in the
PASMCs were assessed using glucose (cat. no. BC2505; Beijing
Solarbio Science & Technology Co., Ltd.), lactic acid (cat. no.
BC2235; Beijing Solarbio Science & Technology Co., Ltd.) and
ATP (cat. no. BC0305; Beijing Solarbio Science & Technology
Co., Ltd.) assay kits, respectively, according to the
manufacturer's protocols. Briefly, lysis buffer extracted from the
PASMCs was added to a 96-well plate, and the absorbance values were
measured to assess the concentrations of glucose, lactic acid and
ATP. The rates of glucose consumption and lactic acid production
and the amount of cellular ATP were normalized against the protein
concentrations.
Statistical analysis
Quantitative data were presented as the mean ±
standard error of the mean. One-way ANOVA was used for comparisons
between multiple groups, and the multiple comparison least
significant difference and Bonferroni tests were used for pairwise
comparisons. P<0.05 was considered to indicate a statistically
significant difference. All experiments were repeated
independently. at least three times. GraphPad Prism version 8.0
(GraphPad Software; Dotmatics) was used to perform all
analyses.
Results
Shikonin improves hemodynamics in
experimental PAH rats
Echocardiography demonstrated that the PAAT and
TAPSE in MCT-PAH rats were significantly lower compared with those
in control rats and that shikonin treatment significantly increased
PAAT and TAPSE in MCT-PAH rats compared with those not treated with
shikonin (Fig. 3). Shikonin
significantly reduced the end-diastolic RVID of MCT-PAH
experimental rats compared with those not treated with shikonin
(Fig. 3). Right heart
catheterization showed that the RVSP of MCT-PAH experimental rats
was significantly higher compared with that of experimental rats in
the control group and that Shikonin significantly reduced RVSP in
MCT-PAH experimental rats compared with those not treated with
shikonin (Fig. 4A and B).
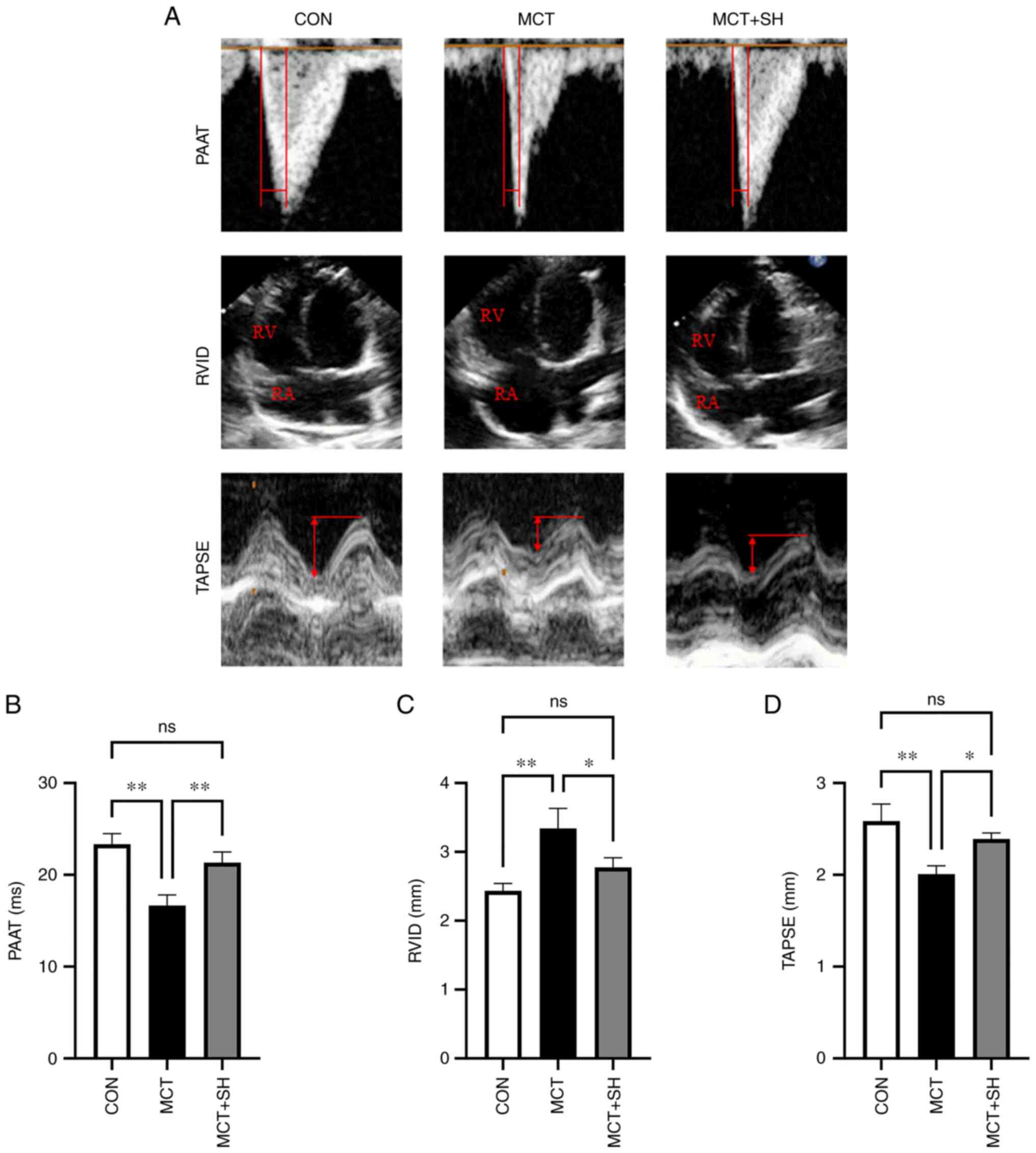 | Figure 3.Shikonin improves the echocardiogram
parameters of MCT-induced PAH in rats. (A) Representative
echocardiogram images of the three rat groups. (B) PAAT, (C) RVID
and (D) TAPSE in PAH-rats with shikonin treatment. Data are
presented as mean ± SD. Control, n=8; MCT, n=6; MCT + SH, n=7.
*P<0.05 and **P<0.01. CON, control; MCT, monocrotaline; SH,
shikonin; PAAT, pulmonary artery blood flow acceleration time;
RVID, inner diameter of the right ventricle; TAPSE, tricuspid
annular plane systolic excursion; RV, right ventricle; RA, right
atrium; ns, not significant. |
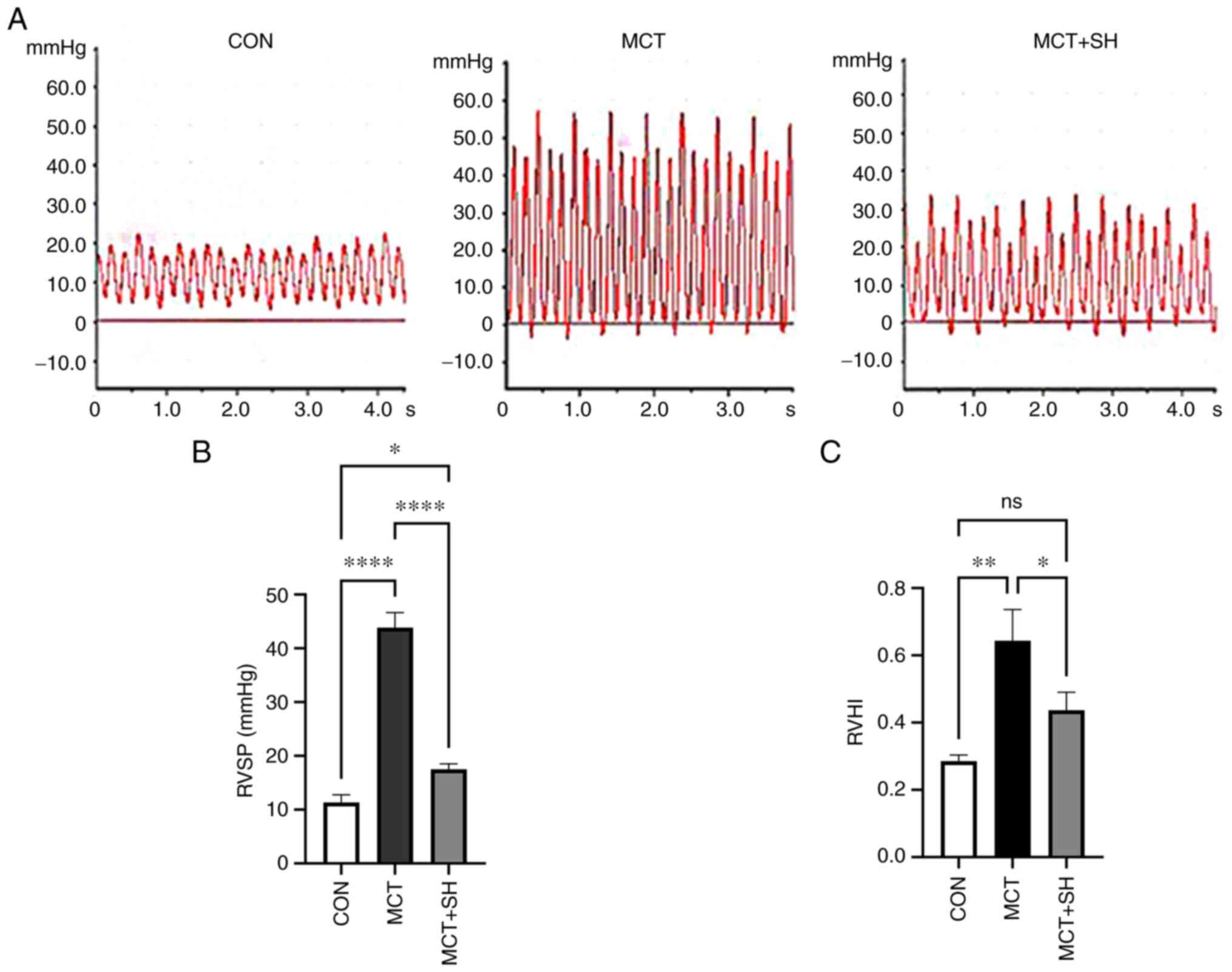 | Figure 4.Shikonin decreased RVSP of MCT-induced
pulmonary arterial hypertension in rats. (A) RVSP waveforms of the
three rat groups. (B) RVSP in PAH-rats with shikonin treatment. (C)
Shikonin reduced the RVHI of MCT-induced pulmonary arterial
hypertension in rats. Data are presented as mean ± SD. Control,
n=8; MCT, n=6; MCT + SH, n=7. *P<0.05, **P<0.01 and
****P<0.0001. CON, control; MCT, monocrotaline; SH, shikonin;
RVSP, right ventricular systolic pressure; RV/(LV + S), Right
ventricle free wall/left ventricle plus the interventricular
septum; RVHI, right ventricular hypertrophy index; ns, not
significant. |
Shikonin treatment improves the RVHI
in PAH rats
The RV/(LV+S) ratio was calculated to assess the
RVHI. A significant increase in RVHI was demonstrated in MCT-PAH
rats compared with control rats, which indicated possible right
ventricular hypertrophy. Administration of Shikonin by
intraperitoneal injection for seven consecutive days significantly
decreased RVHI in MCT-PAH rats compared with those not treated with
shikonin (Fig. 4C).
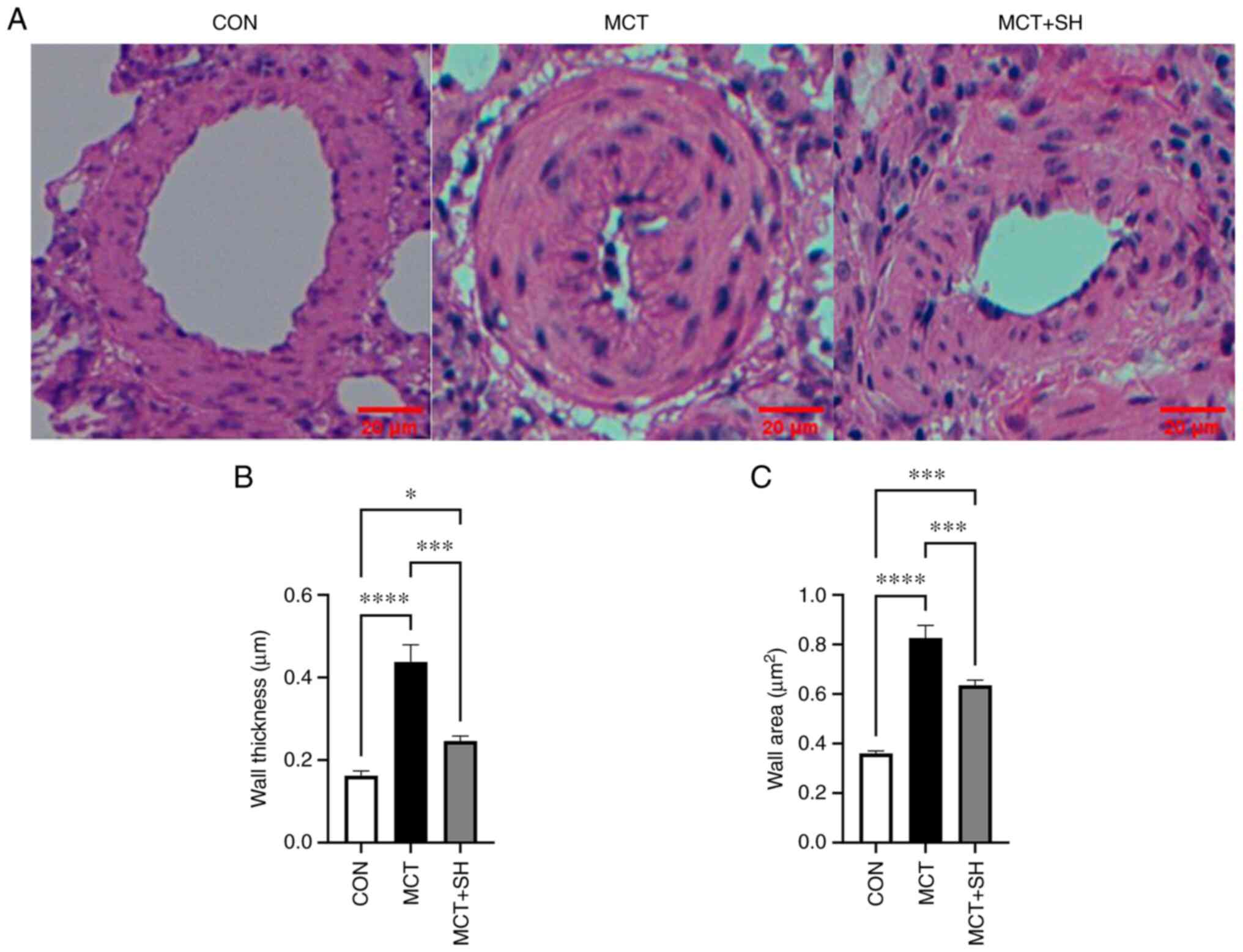
Shikonin improves pulmonary vascular
remodeling in MCT-induced PAH rats
Pathological changes in the small pulmonary arteries
(50–150 µm) were evaluated using H&E staining. The H&E
staining demonstrated that the pulmonary arteries of MCT-PAH rats
exhibited increased wall thickness and luminal stenosis compared
with the control group rats which had thin medial walls and large
lumen. Shikonin significantly relieved MCT-induced thickening of
the pulmonary artery wall compared with MCT-PAH rats not treated
with shikonin. These results indicated that Shikonin greatly
improved MCT-induced pulmonary vascular remodeling (Fig. 5).
Shikonin inhibits PKM2 and downstream
signaling protein expression
The expression of aerobic glycolysis enzymes in
MCT-PAH rats was assessed using western blotting. To further
investigate the connection between shikonin and aerobic glycolysis,
the effect of shikonin on the protein expression levels of PKM2 and
its downstream signaling proteins was evaluated, as these proteins
have been reported to serve key roles in aerobic glycolysis
(20). The results demonstrated,
that compared with the control rats, the MCT-PAH rats exhibited
significantly increased protein expression levels of PKM2, p-PKM2,
p-ERK, GLUT1 and LDHA in lung tissue, which were significantly
reversed by shikonin. However, significant changes in ERK1/2
protein expression levels were not detected in lung tissue from any
experimental rats (Fig. 6).
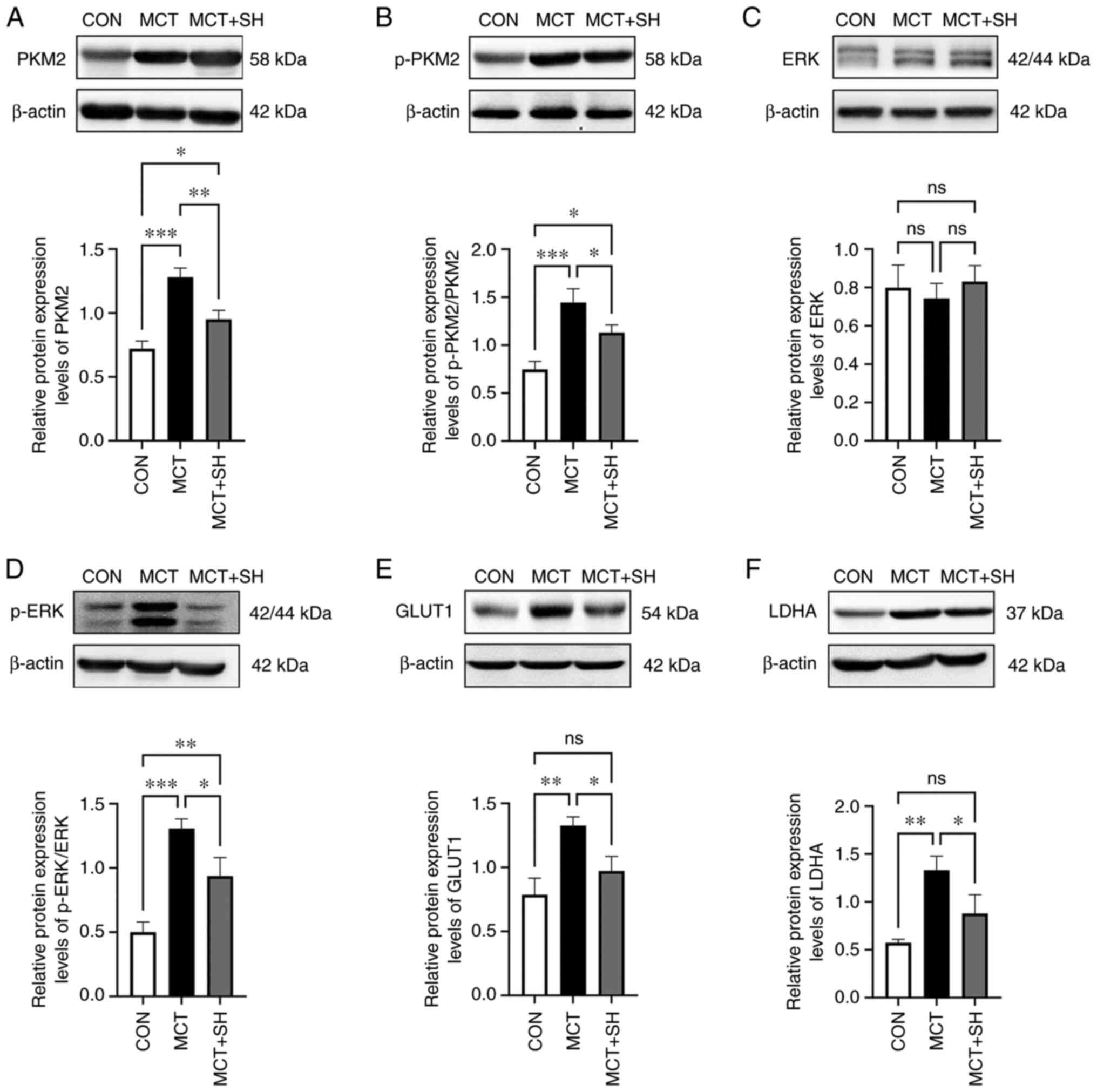 | Figure 6.Expression of PKM2 signal pathway in
the three groups of rat lung tissue. Representative western blot
and corresponding densitometric analysis of (A) PKM2, (B) p-PKM2,
(C) ERK, (D) p-ERK, (E) GLUT1 and (F) LDHA protein expression
levels in the three groups of rat lung tissue. Data are presented
as mean ± SD. Control, n=8; MCT, n=6; MCT + SH, n=7. *P<0.05,
**P<0.01 and ***P<0.001. CON, control; MCT, monocrotaline;
SH, shikonin; PKM2, pyruvate kinase M2; p, phosphorylated; GLUT1,
glucose transporter 1; LDHA, lactate dehydrogenase A; ns, not
significant. |
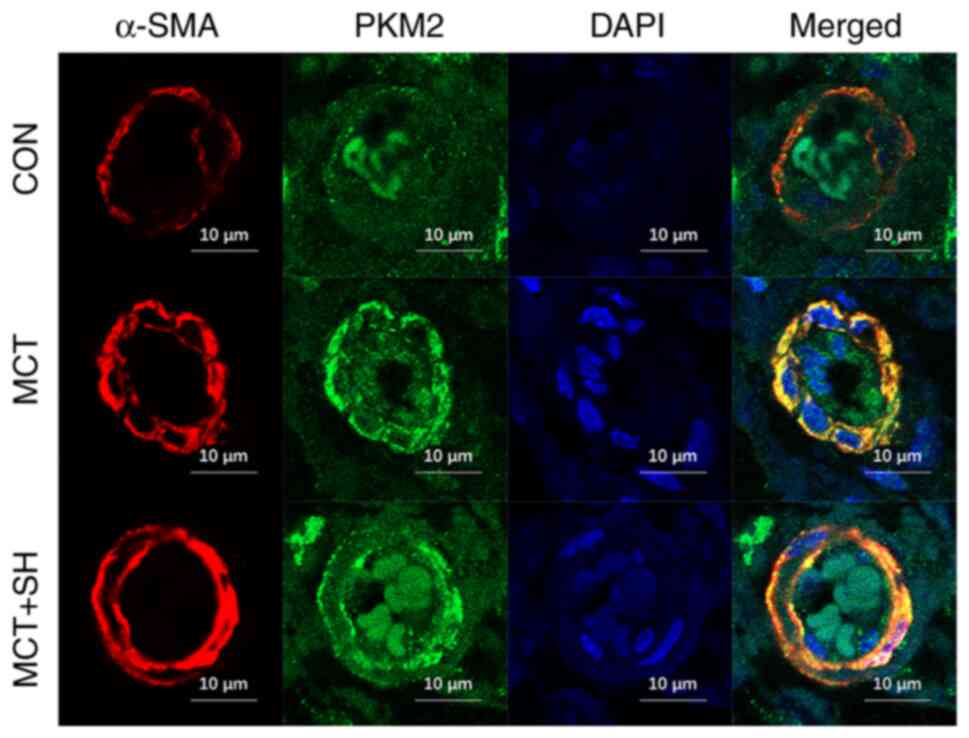
Shikonin inhibits PKM2 expression in
the pulmonary arteries of MCT-treated rats
Laser confocal microscopy was used to assess the
PKM2 fluorescence intensity in pulmonary arteries and demonstrated
that the fluorescence intensity was markedly increased in MCT-PAH
rats compared with the control. Shikonin reduced the PKM2
fluorescence intensity in the pulmonary arteries of MCT-PAH rats
compared with those not treated with shikonin (Fig. 7).
Shikonin decreases the Warburg effect
in PASMCs
Extracellular glucose consumption, lactic acid
production and cellular ATP levels were assessed to evaluate
whether shikonin improved PAH through inhibition of the Warburg
effect. The results demonstrated significant increases in glucose
consumption and lactic acid generation and a significant decrease
in ATP generation in PDGF-treated PASMCs compared with the control.
Shikonin significantly suppressed the PDGF-induced Warburg effect
in vitro (Fig. 8).
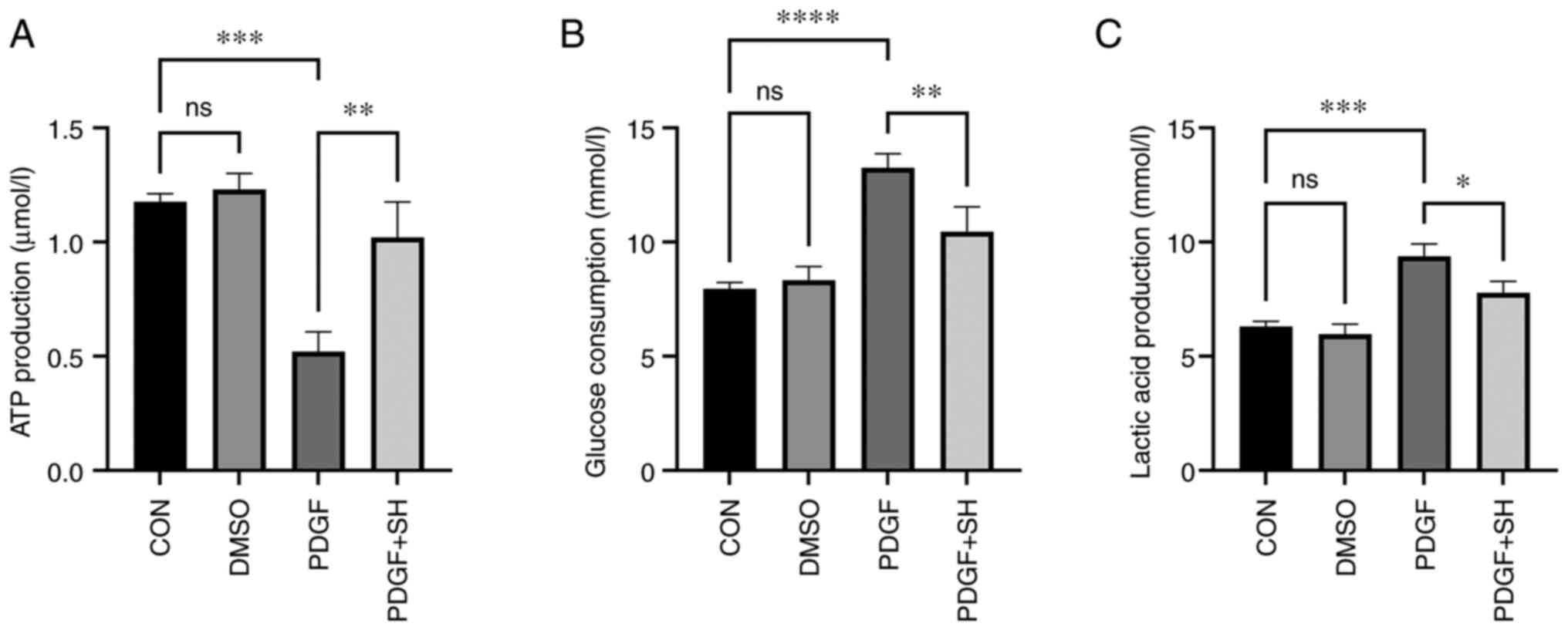 | Figure 8.The cellular ATP, glucose consumption
and lactic acid levels of primary pulmonary artery smooth muscle
cells in vitro. Statistical graph of (A) ATP production, (B)
glucose consumption and (C) lactic acid production. Data presented
as mean ± SD. Control, n=8; DMSO, n=8; PDGF, n=8; PDGF + SH, n=8.
*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. CON,
control; DMSO, dimethyl sulfoxide; PDGF, platelet derived growth
factor; SH, shikonin; ns, not significant. |
Discussion
The present study demonstrated that intraperitoneal
injection of shikonin for 7 consecutive days exerted protective
effects against MCT-induced PAH in rats. After shikonin treatment,
PAAT, RVID and TAPSE values were significantly improved in
MCT-induced PAH rats. Moreover, it was demonstrated that shikonin
alleviated pulmonary vascular remodeling and significantly improved
the RVSP and RVHI values of MCT-induced PAH rats. Western blotting
demonstrated that shikonin also significantly decreased the
upregulated protein expression levels of PKM2, p-PKM2, p-ERK, GLUT1
and LDHA in MCT-induced PAH rat lung tissues. Furthermore, aerobic
glycolysis was significantly inhibited by shikonin in PDGF-treated
PASMCs. This result indicated that shikonin might improve
MCT-induced PAH through downregulation of PKM2 expression and
decreasing aerobic glycolysis. In summary, the present study
demonstrated that shikonin exerted a protective effect against
MCT-induced experimental PAH, partly through the reduction of
aerobic glycolysis.
Pulmonary arterial hypertension has an insidious
onset, rapid progression and high mortality. Therefore, elucidation
of the pathogenic mechanism and improving pulmonary arterial
vascular remodeling have important theoretical significance and
application. The ‘metabolic theory’ of PAH pathogenesis centered on
aerobic glycolysis and metabolism has become a popular research
topic. It has been reported that aerobic glycolysis serves a core
role in PAH and that blocking aerobic glycolysis can attenuate the
proliferation of PASMCs in PAH (25).
Shikonin is the biologically active component of a
traditional Chinese medicine with marked antioxidant and
anti-inflammatory effects (26,27).
Shikonin depresses cancer by targeting certain aspects of this
devastating disease, such as inhibiting cell development, migration
and invasion and inducing cell death (28,29).
Shikonin can improve the proliferation of endothelial cells and
adventitial fibroblasts in a hypoxic pulmonary arterial
hypertension mouse model through the microRNA-124/PTBP1/PKM
signaling pathway and reduce pulmonary pressure in mice with PAH
(8). pyruvate kinase (PK),
especially PK type M2, generates pyruvate and ATP through control
of the final step of glycolysis, dephosphorylating
phosphoenolpyruvate and contributing to glycolytic flux in
PKM2-expressing cells. Shikonin can inhibit cancer cell
proliferation by modulation of the expression of PKM2. The present
study evaluated whether shikonin could ameliorate MCT-induced PAH
through the inhibition of PKM2 expression or activity and partly
elucidated the mechanism of this.
The results of echocardiography, right heart
catheterization and H&E staining demonstrated that shikonin
significantly improved RVSP, pulmonary vascular remodeling and the
RVHI in MCT-induced PAH rats. These results demonstrated the
therapeutic effect of shikonin on MCT-induced experimental PAH, but
the specific mechanism remained unclear.
After shikonin treatment, the protein expression
levels of PKM2, downstream signaling pathway proteins and aerobic
glycolysis-related proteins were assessed in rat lung tissue. A
proliferation model of pulmonary artery smooth muscle cells was
also developed in vitro with PDGF-BB and glucose
consumption, lactic acid production and ATP production were
assessed using assay kits. Shikonin significantly decreased the
protein expression levels of PKM2, which was accompanied by a
significant decrease in the ERK1/2 phosphorylation level and
significant decreases in the protein expression levels of LDHA and
GLUT1. These results indicated that the increase in PKM2 protein
expression levels in PAH could also promote ERK1/2 and PKM2
phosphorylation and further upregulate the expression of critical
enzymes in aerobic glycolysis, such as LDHA and GLUT1, thereby
participating in PAH vascular remodeling. Furthermore, in
vitro experiments demonstrated that after shikonin treatment,
glucose consumption and lactic acid generation were significantly
reduced and ATP production was significantly elevated, which
indicated that shikonin suppressed the aerobic glycolysis induced
by PDGF. These results provide preliminary evidence that there is
upregulation of PKM2 expression in PAH and that upregulation of
PKM2 promoted the phosphorylation and activation of ERK1/2,
resulting in upregulation of GLUT1 and LDHA and positive feedback
regulation of PKM2 expression, which enhances aerobic glycolysis
and pulmonary vascular remodeling. Shikonin ameliorates PAH and
pulmonary vascular remodeling in MCT-PAH rats. This mechanism may
be related to the inhibition of PKM2 expression, ERK1/2
phosphorylation and aerobic glycolysis.
There were certain limitations to the present study.
During the modeling process, 2 deaths occurred in the MCT group and
1 death occurred in the MCT + SH group. The two rats in the MCT
group died of severe pulmonary hypertension and the rat in the
MCT+SH group died of massive bloody ascites and severe peritonitis
due to accidental puncture of the intestinal tube by the needle tip
during intraperitoneal injection of shikonin. Moreover, in the
present study, specific regulatory mechanisms between PKM2 and
downstream signaling pathways were not evaluated. Therefore, the
effects of shikonin on aerobic glycolysis and downstream signaling
process require further evaluation in future studies.
In summary, shikonin treatment, in rats with PAH
induced by MCT, exerted a significant protective effect. Shikonin
treatment enhanced hemodynamics and right ventricular hypertrophy
and decreased pulmonary artery remodeling. The protective effect of
shikonin against PAH was associated with downregulation of PKM2,
p-PKM2, p-ERK, GLUT1 and LDHA protein expression levels and
inhibition of aerobic glycolysis. These results suggested that
shikonin may be a therapeutic option for patients with PAH.
Acknowledgments
Not applicable.
Funding
The present study was funded by the Hunan Provincial Health
Commission Project (grant no. 20200483), Hunan Provincial Research
on Chinese Medicine (grant no. 201914) and The Hunan Provincial Key
Laboratory of Emergency Medicine for Children (grant no.
2018TP1028).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Author's contributions
WL and YX conceived and designed the experiments.
WL, YZ and TH performed the experiments. WC, HP, ZX, JL, QS and XW
acquired, analyzed and interpreted the data. WL and YX wrote the
paper. WL and YX confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The animal protocols and experimental procedures
were approved by The Hunan Children's Hospital Ethics Committee.
(approval no. HCHLL-2020-44).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zelt JGE, Sugarman J, Weatherald J,
Partridge ACR, Liang JC, Swiston J, Brunner N, Chandy G, Stewart
DJ, Contreras-Dominguez V, et al: Mortality trends in pulmonary
arterial hypertension in Canada: A temporal analysis of survival
per ESC/ERS guideline era. Eur Respir J. 59:21015522022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boucly A, Weatherald J, Savale L, Jaïs X,
Cottin V, Prevot G, Picard F, de Groote P, Jevnikar M, Bergot E, et
al: Risk assessment, prognosis and guideline implementation in
pulmonary arterial hypertension. Eur Respir J. 50:17008892017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raina A and Humbert M: Risk assessment in
pulmonary arterial hypertension. Eur Respir Rev. 25:390–398. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galie N, Humbert M, Vachiery JL, Gibbs S,
Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A,
Beghetti M, et al: 2015 ESC/ERS Guidelines for the diagnosis and
treatment of pulmonary hypertension: The Joint Task Force for the
Diagnosis and Treatment of Pulmonary Hypertension of the European
Society of Cardiology (ESC) and the European Respiratory Society
(ERS): Endorsed by: Association for European Paediatric and
Congenital Cardiology (AEPC), International Society for Heart and
Lung Transplantation (ISHLT). Eur Heart J. 37:67–119. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chin KM, Sitbon O, Doelberg M, Feldman J,
Gibbs JSR, Grünig E, Hoeper MM, Martin N, Mathai SC, McLaughlin VV,
et al: Three-Versus two-drug therapy for patients with newly
diagnosed pulmonary arterial hypertension. J Am Coll Cardiol.
78:1393–1403. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dumas SJ, Bru-Mercier G, Courboulin A,
Quatredeniers M, Rücker-Martin C, Antigny F, Nakhleh MK, Ranchoux
B, Gouadon E, Vinhas MC, et al: NMDA-Type glutamate receptor
activation promotes vascular remodeling and pulmonary arterial
hypertension. Circulation. 137:2371–2389. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caruso P, Dunmore BJ, Schlosser K, Schoors
S, Dos Santos C, Perez-Iratxeta C, Lavoie JR, Zhang H, Long L,
Flockton AR, et al: Identification of MicroRNA-124 as a major
regulator of enhanced endothelial cell glycolysis in pulmonary
arterial hypertension via PTBP1 (Polypyrimidine Tract Binding
Protein) and pyruvate kinase M2. Circulation. 136:2451–2467. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Wang D, Li M, Plecitá-Hlavatá L,
D'Alessandro A, Tauber J, Riddle S, Kumar S, Flockton A, McKeon BA,
et al: Metabolic and proliferative state of vascular adventitial
fibroblasts in pulmonary hypertension is regulated through a
MicroRNA-124/PTBP1 (Polypyrimidine Tract Binding Protein
1)/pyruvate kinase muscle axis. Circulation. 136:2468–2485. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quatredeniers M, Nakhleh MK, Dumas SJ,
Courboulin A, Vinhas MC, Antigny F, Phan C, Guignabert C,
Bendifallah I, Vocelle M, et al: Functional interaction between
PDGFβ and GluN2B-containing NMDA receptors in smooth muscle cell
proliferation and migration in pulmonary arterial hypertension. Am
J Physiol Lung Cell Mol Physiol. 316:L445–L455. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Savai R, Al-Tamari HM, Sedding D,
Kojonazarov B, Muecke C, Teske R, Capecchi MR, Weissmann N,
Grimminger F, Seeger W, et al: Pro-proliferative and inflammatory
signaling converge on FoxO1 transcription factor in pulmonary
hypertension. Nat Med. 20:1289–1300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao Y, Peng H, Hong C, Chen Z, Deng X,
Wang A, Yang F, Yang L, Chen C and Qin X: PDGF promotes the Warburg
effect in pulmonary arterial smooth muscle cells via activation of
the PI3K/AKT/mTOR/HIF-1α signaling pathway. Cell Physiol Biochem.
42:1603–1613. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tamada M, Suematsu M and Saya H: Pyruvate
kinase M2: Multiple faces for conferring benefits on cancer cells.
Clin Cancer Res. 18:5554–5561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen M, Sheng XJ, Qin YY, Zhu S, Wu QX,
Jia L, Meng N, He YT and Yan GR: TBC1D8 amplification drives
tumorigenesis through metabolism reprogramming in ovarian cancer.
Theranostics. 9:676–690. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Deng X, Liu Y, Liu Y, Sun L and
Chen F: PKM2, function and expression and regulation. Cell Biosci.
9:522019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo
F, Lyssiotis CA, Aldape K, Cantley LC and Lu Z: ERK1/2-dependent
phosphorylation and nuclear translocation of PKM2 promotes the
Warburg effect. Nat Cell Biol. 14:1295–1304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gong K and Li W: Shikonin, a Chinese
plant-derived naphthoquinone, induces apoptosis in hepatocellular
carcinoma cells through reactive oxygen species: A potential new
treatment for hepatocellular carcinoma. Free Radic Biol Med.
51:2259–2271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia L, Zhu Z, Li H and Li Y: Shikonin
inhibits proliferation, migration, invasion and promotes apoptosis
in NCI-N87 cells via inhibition of PI3K/AKT signal pathway. Artif
Cells Nanomed Biotechnol. 47:2662–2669. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang F, Yao X, Zhang Y and Tang J:
Synthesis, biological function and evaluation of Shikonin in cancer
therapy. Fitoterapia. 134:329–339. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao X, Zhu Y, Hu J, Jiang L, Li L, Jia S
and Zen K: Shikonin inhibits tumor growth in mice by suppressing
pyruvate kinase M2-mediated aerobic glycolysis. Sci Rep.
8:145172018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Archer SL: Pyruvate kinase and Warburg
metabolism in pulmonary arterial hypertension: Uncoupled glycolysis
and the cancer-like phenotype of pulmonary arterial hypertension.
Circulation. 136:2486–2490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li XH, Peng J, Tan N, Wu WH, Li TT, Shi RZ
and Li YJ: Involvement of asymmetric dimethylarginine and Rho
kinase in the vascular remodeling in monocrotaline-induced
pulmonary hypertension. Vascul Pharmacol. 53:223–229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu D, Shang X, Ni Z and Shi G: Shikonin
inhibits inflammation and chondrocyte apoptosis by regulation of
the PI3K/Akt signaling pathway in a rat model of osteoarthritis.
Exp Ther Med. 12:2735–2740. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu D, Xiao Y, Zhou B, Gao S, Li L, Zhao
L, Chen W, Dai B, Li Q, Duan H, et al: PKM2-dependent glycolysis
promotes skeletal muscle cell pyroptosis by activating the NLRP3
inflammasome in dermatomyositis/polymyositis. Rheumatology
(Oxford). 60:2177–2189. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zuo W, Liu N, Zeng Y, Xiao Z, Wu K, Yang
F, Li B, Song Q, Xiao Y and Liu Q: Luteolin ameliorates
experimental pulmonary arterial hypertension via suppressing
Hippo-YAP/PI3K/AKT signaling pathway. Front Pharmacol.
12:6635512021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thenappan T, Ormiston ML, Ryan JJ and
Archer SL: Pulmonary arterial hypertension: Pathogenesis and
clinical management. BMJ. 360:j54922018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Imai K, Kato H, Taguchi Y and Umeda M:
Biological effects of Shikonin in human gingival fibroblasts via
ERK 1/2 signaling pathway. Molecules. 24:35422019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu B, Jin J, Zhang Z, Zuo L, Jiang M and
Xie C: Shikonin exerts antitumor activity by causing mitochondrial
dysfunction in hepatocellular carcinoma through PKM2-AMPK-PGC1α
signaling pathway. Biochem Cell Biol. 97:397–405. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim HJ, Hwang KE, Park DS, Oh SH, Jun HY,
Yoon KH, Jeong ET, Kim HR and Kim YS: Shikonin-induced necroptosis
is enhanced by the inhibition of autophagy in non-small cell lung
cancer cells. J Transl Med. 15:1232017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi S and Cao H: Shikonin promotes
autophagy in BXPC-3 human pancreatic cancer cells through the
PI3K/Akt signaling pathway. Oncol Lett. 8:1087–1089. 2014.
View Article : Google Scholar : PubMed/NCBI
|