Introduction
Mesenchymal stem cells (MSCs) differentiate along
several lineages, including osteoblasts, adipocytes and myoblasts,
in response to various environmental stimuli (1). MSC differentiation is tightly
regulated at transcriptional and post-transcriptional levels
(2). A number of genes are
important for the regulation of MSC differentiation, including bone
morphogenetic protein and runt-related transcription factor 2
(Runx2) (3,4). Canonical Wnt signaling is also
critical for the control of bone formation (5) as it accelerates the differentiation
of osteochondral progenitors towards the osteoblastic lineage,
while promoting early MSC proliferation (6). In addition, Wnt signaling functions
as an adipogenic switch, whereby loss of Wnt/β-catenin signaling
drives the shift of preosteoblasts from osteoblast to adipocyte
differentiation (5).
The canonical Wnt pathway requires binding of Wnt to
low-density lipoprotein receptor-related proteins 5 and 6, Frizzled
receptor and Dishevelled. In the absence of Wnt stimulation,
cytoplasmic β-catenin is recruited by a multiprotein destruction
complex consisting of two scaffold proteins, adenomatous polyposis
coli (APC) and axin and two kinases, glycogen synthase kinase 3β
(GSK3β) and casein kinase 1a (CKI). This complex leads to
constitutive phosphorylation of β-catenin and its subsequent
proteasomal degradation. Wnt receptor binding and activation
inhibits GSK3β activity, leading to accumulation and nuclear
translocation of β-catenin, which functions as a transcriptional
coactivator and drives the transcription of genes essential for
osteoblast differentiation. Numerous factors regulate osteoblast
differentiation through the Wnt-mediated pathway (7).
MicroRNAs (miRNAs/miRs) negatively regulate
translation or induce degradation of target mRNAs by binding to
their 3′ untranslated regions (UTRs). miRNAs are potent regulators
of diverse physiological processes, including cell proliferation,
differentiation and apoptosis (8).
It was recently reported that miRNAs are essential for the
regulation of osteoblastic differentiation (2). Huang et al demonstrated that
miR-204 inhibits osteoblast differentiation by targeting Runx2 in
bone marrow-derived MSC (4).
miR-138 represses osteoblast differentiation by inhibiting FAK
(9). By contrast, miR-29b promotes
osteoblast differentiation by targeting inhibitors of this process
(10). A number of miRNAs regulate
osteoblast differentiation through the Wnt signaling pathway.
miR-29a potentiates Wnt signaling and regulates osteoblast
differentiation (6). miR-27
positively regulates osteoblast differentiation by targeting APC
(11). miR-142-3p regulates
myeloid and erythroid differentiation (12,13),
however, the role of miR-142-3p in regulating osteoblast
differentiation remains unknown.
In the present study, the molecular mechanism by
which miR-142-3p promotes osteoblastic differentiation was
investigated. Results showed that miR-142-3p activated Wnt
signaling and promoted osteoblast differentiation by repressing APC
expression.
Materials and methods
Cell culture
The human fetal osteoblastic 1.19 (hFOB1.19) cell
line was obtained from the American Type Culture Collection
(Manassas, VA, USA) and cultured at 33.5°C in medium composed of
1:1 Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium without
phenol red, supplemented with 10% FBS (Atlas Biologicals, Fort
Collins, CO, USA), 1X penicillin/streptomycin (Invitrogen Life
Technologies, Carlsbad, CA, USA) and 0.3 mg/ml G418/geneticin
(Calbiochem, La Jolla, CA, USA). To induce osteoblastic
differentiation, confluent cultures of hFOB1.19 cells were
maintained in complete medium containing the following
differentiation cocktail: 100 μg/μl ascorbic acid, 5 mM β-glycerol
phosphate, 10−8 M menadione and 10−7 M
1,25(OH)2D3 (all from Sigma, St. Louis, MO, USA).
Plasmid constructs
Sequences containing the predicted binding sites in
the 3′ UTR of APC mRNA were amplified and cloned into the
Dual-Luciferase reporter vector, psiCHECK2 (Promega Corporation,
Madison, WI, USA). Binding site mutations were introduced by
whole-plasmid amplification in the seed region of miR-142-3p (New
England Biolabs, Ipswich, MA, USA). Pre-miR-142 and anti-miR-142
were obtained from GeneCopoeia (Rockville, MD, USA).
Luciferase activity assay
HEK293 cells were plated in 96-well plates and
transfected with 0.02 μg wild-type or mutated psiCHECK2-APC 3′ UTR,
with 0.08 μg pre-miR-142 (MI0000167) or vector (GeneCopoeia). Each
transfection was repeated twice in triplicate. Following 48 h of
transfection, firefly and Renilla luciferase activities were
measured with the Dual-Luciferase® Reporter 1000 Assay
System (Promega Corporation).
Real-time quantitative PCR
Total RNA was extracted with TRIzol (Invitrogen Life
Technologies). cDNA was synthesized using 1 μg RNA with a reverse
transcription kit (Tiangen Biotech, Beijing, China). miRNAs and the
synthesized cDNAs were prepared with a microRNA extraction kit
(Tiangen Biotech). Real-time PCR was performed with a standard
SYBR-Green PCR kit from Toyobo (Osaka, Japan). Primers used
included: β-actin F: 5′-AGCCATGTACGTTGCTA-3′, R:
5′-AGTCCGCCTAGAAGCA-3′; Runx2 F: 5′-TCTTCACAAATCCTCCCC-3′, R:
5′-TGGATTAAAAGGACTTGG-3′; osteocalcin (OC) F:
5′-CATGAGAGCCCTCACA-3′, R: 5′-AGA GCGACACCCTAGAC-3′; and alkaline
phosphatase (ALP) F: 5′-ACGTGGCTAAGAATGTCATC-3′, R:
5′-CTGGTAGGCGATGTCCTTA-3′. Primers for miR-142-3p and U6 snRNA were
obtained from GeneCopoeia. Runx2, OC and ALP mRNA levels were
normalized to β-actin and the expression of miR-142-3p was
normalized to U6 snRNA.
Measurement of cell differentiation
Osteoblast marker transcripts (Runx2, OC and ALP)
were detected by real-time PCR as described. ALP activity was
measured as previously described (14).
Western blot analysis
Western blot analysis to measure APC and β-catenin
protein levels was performed as previously described (15). Anti-APC and anti-β-catenin primary
antibodies were purchased from Cell Signaling Technology (Beverly,
MA, USA). Blots were incubated with anti-mouse or anti-rabbit IgG
conjugated to IRDye™ (800CW) for 1 h at 37°C and visualized using
the Odyssey Infrared Imaging System (Licor Biosciences, Lincoln,
NE, USA).
Statistical analysis
Data were expressed as the mean ± SE. Data were
analyzed using the Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Upregulated miR-142-3p promotes
osteoblastic differentiation
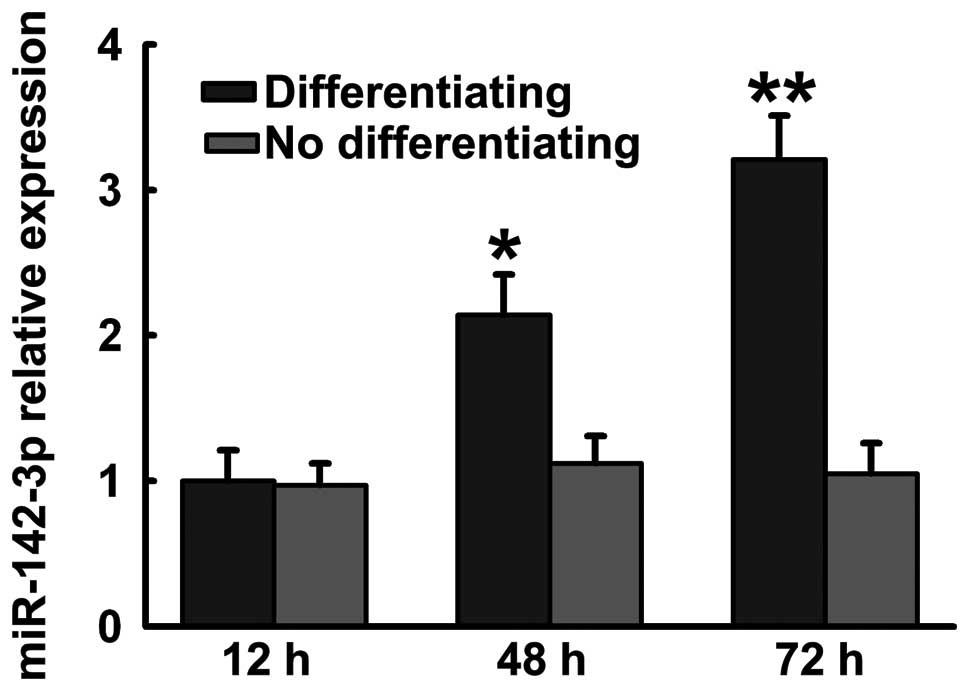
Previous studies have demonstrated that miR-142-3p
regulates myeloid and erythroid differentiation. We detected
miR-142-3p expression during hFOB1.19 cell differentiation.
miR-142-3p increased in hFOB1.19 cells maintained in osteoblastic
differentiation medium (Fig.
1).
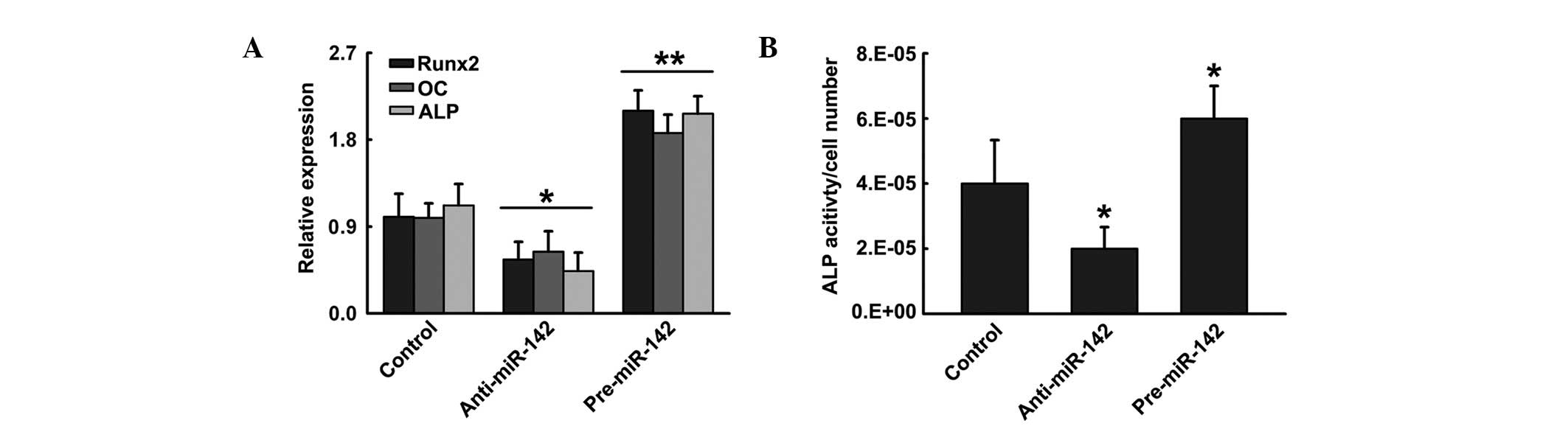
To investigate the potential function of miR-142-3p
in hFOB1.19 differentiation, cells were transfected with control
vector, pre-miR-142 or anti-miR-124. Pre-miR-142 enhanced
osteoblastic differentiation, which was indicated by higher
expression of the osteoblast marker genes Runx2, OC and ALP
(16), accompanied by increased
ALP activity in comparison to cells transfected with control
vector. By contrast, ALP activity and osteoblast-specific genes
were identified to be significantly decreased when hFOB1.19 cells
were transfected with anti-miR-142, a blocker of miR-142 (Fig. 2). These data indicate that
upregulation of miR-142-3p positively regulated hFOB1.19
differentiation, whereas inhibition of miR-142-3p negatively
regulated hFOB1.19 differentiation.
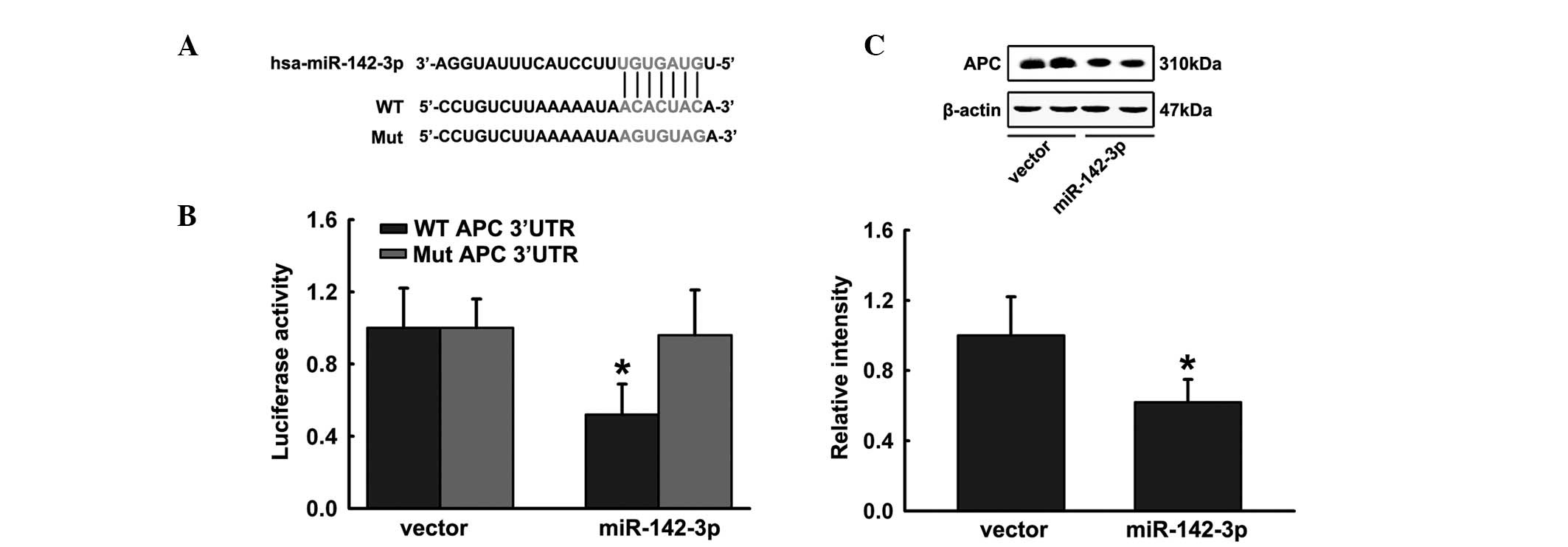
miR-142-3p targets APC via predicted
binding sites
Prediction Algorithms miRbase and Targetscan were
used to select potential targets of miR-142-3p in osteoblastic
differentiation. miR-142-3p targets APC, which acts as a regulatory
factor in Wnt signaling (Fig. 3A).
In addition, luciferase reporter constructs containing possible
binding sites and specific mutations within the 3′ UTR of APC mRNA
were generated. Wild-type and mutated APC 3′ UTR luciferase
reporter constructs were cotransfected into HEK293 cells with the
miR-142-3p overexpression plasmid or a control vector. Significant
inhibition was observed when miR-142-3p was cotransfected with the
wild-type APC 3′ UTR in HEK293 cells, whereas mutated APC 3′ UTR
luciferase activity was altered marginally in comparison to the
vector control (Fig. 3B). The
reporter assay demonstrated that miR-142-3p significantly repressed
luciferase expression and mutation of four nucleotides in the
binding sites led to complete abrogation of this suppressive
effect. Moreover, the ectopic expression of miR-142-3p
significantly reduced APC protein levels (Fig. 3C). These observations indicate that
miR-142-3p may inhibit endogenous APC expression in differentiating
hFOB1.19 cells.
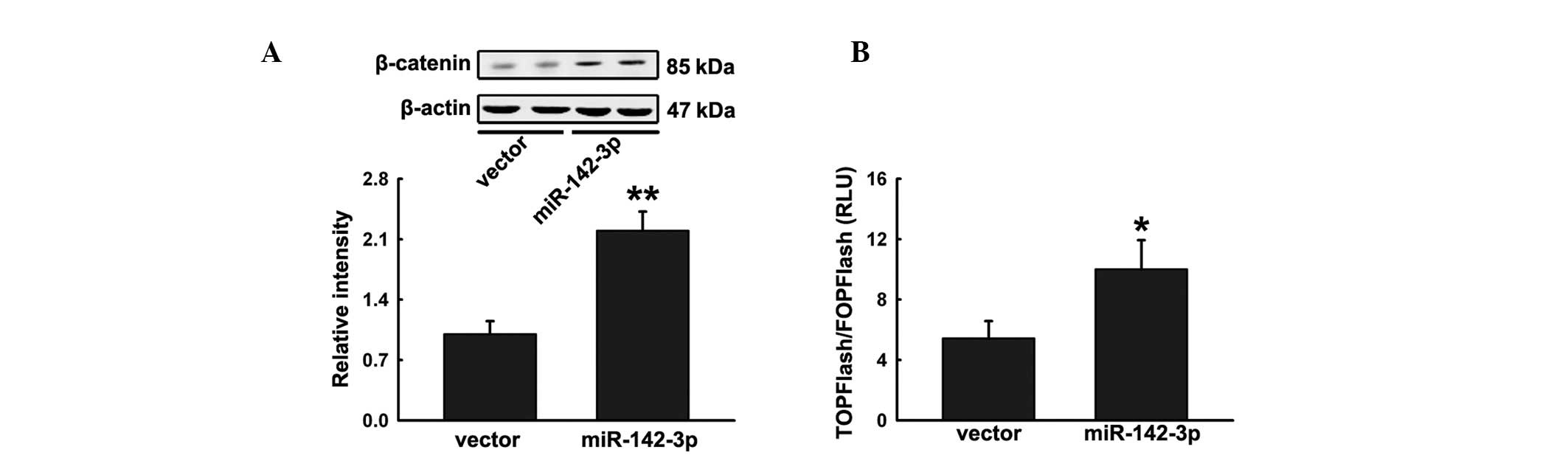
miR-142-3p promotes Wnt signaling by APC
inhibition
Previous studies have revealed that APC negatively
regulates Wnt signaling by interacting with β-catenin, a key
component of the pathway (17).
Ectopic expression of miR-142-3p in hFOB1.19 cells significantly
increased accumulation of β-catenin (Fig. 4A). To confirm the effect on Wnt
signaling, hFOB1.19 cells were cotransfected with pre-miR-142 or
miRNA control with the Wnt signaling reporter TOPFlash or negative
control FOPFlash and then treated with Wnt3a. Cells transfected
with pre-miR-142 were observed to significantly increase TOPFlash
activity (Fig. 4B). These
observations indicate that miR-142-3p positively regulates hFOB1.19
differentiation by activating Wnt signaling.
Discussion
miRNAs are small non-coding RNA molecules that
post-transcriptionally regulate gene expression by targeting
sequences within the 3′ UTR of target mRNAs. miRNAs are involved in
several essential biological processes, including tumorigenesis,
hormone signaling and differentiation (8) and are critical for the control of
adipogenesis, myeloblast differentiation and osteoblastogenesis
(2). In the present study,
miR-142-3p was identified to positively regulate osteoblastic
differentiation by promoting Wnt signaling.
miR-142-3p is specifically associated with the
regulation of hematopoietic lineage specification, obesity and
periodontitis by targeting diverse molecules, including
glucocorticoid receptor-α and IL-6 (18,19).
Kim et al reported that miR-142-3p is involved in
TGF-β3-mediated region-dependent chondrogenesis through regulation
of ADAM9 (20). miR-142-3p is an
essential regulator of myeloid differentiation and downregulation
of miR-142-3p is involved in the development of acute myeloid
leukemia (12,18). miR-142-3p is also involved in
erythroid differentiation of human embryonic stem cells (13). However, the potential function of
miR-142-3p in the regulation of osteoblastic differentiation
remains unclear. Our results showed that miR-142-3p levels
increased in differentiating hFOB1.19 cells and overexpression
promoted differentiation. When miR-142-3p function was inhibited,
Runx2, OC and ALP mRNA levels were significantly reduced,
accompanied by decreased ALP activity. In addition, miR-142-3p was
observed to target APC, an important Wnt signaling molecule. The
luciferase reporter assay revealed that miR-142-3p significantly
inhibited APC 3′ UTR expression. Western blot analysis also
confirmed that miR-142-3p significantly suppressed APC protein
levels in differentiating hFOB1.19 cells.
APC is a scaffold protein for GSK3β/CKI-mediated
phosphorylation and subsequent degradation of β-catenin, the Wnt
signaling effector. Without Wnt stimulation, β-catenin interacts
with a multiprotein complex of APC, axin, GSK3β and CKI, known as
the destruction complex, leading to phosphorylation of β-catenin by
GSK3β and CKI. Once phosphorylated, β-catenin is ubiquitinated and
degraded by the proteasome. Activation of Wnt signaling may inhibit
GSK3β activity and result in the accumulation of non-phosphorylated
β-catenin in the cytoplasm and nuclear translocation. Binding of
the Tcf/Lef family of transcription factors promotes target gene
expression (17). Blocking
β-catenin binding sites on APC inhibits β-catenin degradation and
leads to unchecked Wnt signaling (21). Therefore, APC inhibition by
miR-142-3p may disrupt the destruction complex, induce nuclear
accumulation of β-catenin and activate Wnt signaling.
The present study has demonstrated that miR-142-3p
positively regulates osteoblast differentiation by targeting APC,
indicating that miR-142-3p may be an important candidate
therapeutic agent for treatment of osteogenesis disorders.
Abbreviations:
|
MSCs
|
mesenchymal stem cells
|
|
Runx2
|
runt-related transcription factor
2
|
|
APC
|
adenomatous polyposis coli
|
|
GSK3β
|
glycogen synthase kinase 3β
|
|
CKI
|
casein kinase 1a
|
|
hFOB
|
human fetal osteoblastic
|
|
OC
|
osteocalcin
|
|
ALP
|
alkaline phosphatase
|
References
|
1
|
Kang SK, Shin IS, Ko MS, Jo JY and Ra JC:
Journey of mesenchymal stem cells for homing: strategies to enhance
efficacy and safety of stem cell therapy. Stem Cells Int.
2012:3429682012.PubMed/NCBI
|
|
2
|
Lian JB, Stein GS, van Wijnen AJ, et al:
MicroRNA control of bone formation and homeostasis. Nat Rev
Endocrinol. 8:212–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki D, Yamada A, Aizawa R, et al: BMP2
differentially regulates the expression of Gremlin1 and Gremlin2,
the negative regulators of BMP function, during osteoblast
differentiation. Calcif Tissue Int. 91:88–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang J, Zhao L, Xing L and Chen D:
MicroRNA-204 regulates Runx2 protein expression and mesenchymal
progenitor cell differentiation. Stem Cells. 28:357–364.
2009.PubMed/NCBI
|
|
5
|
Song L, Liu M, Ono N, Bringhurst FR,
Kronenberg HM and Guo J: Loss of wnt/β-catenin signaling causes
cell fate shift of preosteoblasts from osteoblasts to adipocytes. J
Bone Miner Res. Jun 22–2012.(Epub ahead of print).
|
|
6
|
Kapinas K, Kessler C, Ricks T, Gronowicz G
and Delany AM: miR-29 modulates Wnt signaling in human osteoblasts
through a positive feedback loop. J Biol Chem. 285:25221–25231.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herr P, Hausmann G and Basler K: WNT
secretion and signalling in human disease. Trends Mol Med.
18:483–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pritchard CC, Cheng HH and Tewari M:
MicroRNA profiling: approaches and considerations. Nat Rev Genet.
13:358–369. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eskildsen T, Taipaleenmaki H, Stenvang J,
et al: MicroRNA-138 regulates osteogenic differentiation of human
stromal (mesenchymal) stem cells in vivo. Proc Natl Acad Sci USA.
108:6139–6144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Hassan MQ, Jafferji M, et al:
Biological functions of miR-29b contribute to positive regulation
of osteoblast differentiation. J Biol Chem. 284:15676–15684. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang T and Xu Z: miR-27 promotes
osteoblast differentiation by modulating Wnt signaling. Biochem
Biophys Res Commun. 402:186–189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang XS, Gong JN, Yu J, et al:
MicroRNA-29a and microRNA- 142-3p are regulators of myeloid
differentiation and acute myeloid leukemia. Blood. 119:4992–5004.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin HL, Kim JS, Kim YJ, Kim SJ, Broxmeyer
HE and Kim KS: Dynamic expression of specific miRNAs during
erythroid differentiation of human embryonic stem cells. Mol Cells.
34:177–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu W, Hu Y, Andersen TE, et al: Tumor
necrosis factor receptor superfamily member 19 (TNFRSF19) regulates
differentiation fate of human mesenchymal (stromal) stem cells
through canonical Wnt signaling and C/EBP. J Biol Chem.
285:14438–14449. 2010. View Article : Google Scholar
|
|
15
|
Zhu HC, Wang LM, Wang M, et al:
MicroRNA-195 downregulates Alzheimer’s disease amyloid-β production
by targeting BACE1. Brain Res Bull. 88:596–601. 2012.PubMed/NCBI
|
|
16
|
Abdallah BM, Haack-Sorensen M, Burns JS,
et al: Maintenance of differentiation potential of human bone
marrow mesenchymal stem cells immortalized by human telomerase
reverse transcriptase gene despite [corrected] extensive
proliferation. Biochem Biophys Res Commun. 326:527–538.
2005.PubMed/NCBI
|
|
17
|
Akiyama T: Wnt/beta-catenin signaling.
Cytokine Growth Factor Rev. 11:273–282. 2000. View Article : Google Scholar
|
|
18
|
Wang F, Wang XS, Yang GH, et al: miR-29a
and miR-142-3p downregulation and diagnostic implication in human
acute myeloid leukemia. Mol Biol Rep. 39:2713–2722. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perri R, Nares S, Zhang S, Barros SP and
Offenbacher S: MicroRNA modulation in obesity and periodontitis. J
Dent Res. 91:33–38. 2011. View Article : Google Scholar
|
|
20
|
Kim D, Song J, Kim S, Kang SS and Jin EJ:
MicroRNA-142-3p regulates TGF-β3-mediated region-dependent
chondrogenesis by regulating ADAM9. Biochem Biophys Res Commun.
414:653–659. 2011.PubMed/NCBI
|
|
21
|
Waaler J, Machon O, Tumova L, et al: A
novel tankyrase inhibitor decreases canonical Wnt signaling in
colon carcinoma cells and reduces tumor growth in conditional APC
mutant mice. Cancer Res. 72:2822–2832. 2012. View Article : Google Scholar : PubMed/NCBI
|