Introduction
Inflammation is the most common cause of clinical
pain resulting from tissue injury. The primary function of pain is
to protect the organism from potential tissue-damaging stimuli. The
aim of drug treatment for inflammation is to normalize pain
sensitivity; however, drugs that are currently available are
associated with critical side-effects and low efficacy.
Non-steroidal anti-inflammatory drugs (NSAIDs) have been shown to
be effective in the treatment of various disorders with
inflammation and have fewer side-effects compared with steroids
(1). NSAIDs function through
binding to the cyclooxygenase (COX) enzymes to inhibit the
production of prostaglandins from arachidonic acid. Two isoforms of
the COX protein, COX-1 and -2, exist; COX-1 is constitutively
expressed in the majority of organs and cells, whereas COX-2 is
generally absent and is induced upon stimulation by inflammatory
stimuli, including endotoxin lipopolysaccharides (LPS). This
suggests that COX-2 is an inflammation-specific isoform (1–3).
NSAIDs and COX-2 selective inhibitors are currently used to treat
several inflammatory disorders (4–6).
However, selective inhibitors of COX-2 have been associated with a
risk of myocardial infarction and stroke. The recent withdrawal of
conventional NSAIDs and selective COX-2 inhibitors due to their
adverse cardiovascular and gastric side-effects has led to the
development of alternative anti-inflammatory agents (7–9).
Therefore, plant-derived natural agents, such as essential oil, are
considered to be useful sources for the next generation of
anti-inflammatory drugs.
Essential oils are concentrated hydrophobic liquids
containing volatile aromatic compounds from plants. They are
extracted by distillation, expression or solvent extracts, and are
used in perfumes, cosmetics, soaps and other products, for adding
scents to incense and household products and for flavoring food and
drink. Chamaecyparis obtusa (C. obtusa), a tropical
tree species found in Japan and the southern region of South Korea,
is used for construction and furniture due to its advantage in
structural properties and natural scent. Its essential oil is
extracted from pruned leaves and twigs of the C. obtusa
tree, and has been commercially used in soap, toothpaste and
cosmetics as a functional additive. The C. obtusa essential
oil contains several types of terpenes that have been shown to
exert anti-oxidative and anti-inflammatory effects, including
sabinene, limonene, bornyl acetate, borneol, α-terpineol and
elemol, while essential oils from fruits contain myrcene,
γ-terpinene, p-cymene, borneol, α-terpineol and β-caryophyllene
(10,11).
In the present study, the anti-inflammatory property
of the essential oil from C. obtusa was investigated in
vivo and in vitro, and the ability of C. obtusa
to serve as a source of novel pharmaceuticals was determined.
Materials and methods
Animals and treatment
Male Sprague-Dawley (SD) rats (9 weeks old) were
obtained from Koatech Co., Ltd. (Pyeongtaek-si, Korea). All the
animals were housed in polycarbonate cages and acclimated in an
environmentally controlled room (23±2°C; relative humidity, 50±10%;
frequent ventilation; and a 12-h light/dark cycle) prior to use as
previously described (12). C.
obtusa essential oil used in this study was provided by Enbita
Co., Ltd. (Seoul, Korea) and was produced through steam
distillation of pruned twigs and leaves of the C. obtusa
tree, according to previously described methods (13). The rats were treated with C.
obtusa essential oil to investigate its effects. A total of 50
ml tap water or essential oil solution (25, 50 or 100%) was
administered to the rats for 2 weeks. Rats in the control group
were treated with a saline solution (0.9% NaCl), and the remaining
rats (treatment group) were treated with 0, 25, 50 or 100%
solutions of C. obtusa essential oil in the presence or
absence of LPS (Sigma-Aldrich, St. Louis, MO, USA). Rats in the
LPS-treated group (LPS-positive group) were administered 1 μg/kg
LPS via an intraperitoneal (i.p.) injection 8 h prior to sacrifice;
rats in the control group for LPS (LPS-negative group) were
injected with an equivalent volume of saline solution at the same
time-point with LPS. All the experimental procedures were approved
by the Ethics Committee of the Chungbuk National University
(Cheongju, Korea).
Peripheral blood mononuclear cell (PMNC)
isolation
PMNCs were isolated from rat peripheral blood
vessels using a double density gradient centrifugation method as
previously described (14,15). Briefly, heparinized blood samples
were overlaid on Histopaque-1077 solution (specific gravity, 1.077;
Sigma-Aldrich) and Histopaque-1119 solution (specific gravity,
1.199; Sigma-Aldrich), centrifuged for 40 min at 700 × g and washed
three times with cold phosphate-buffered saline (PBS). The
viability of PMNCs, determined by trypan blue dye exclusion, was
>97%. The PMNCs were resuspended in RPMI-1640 medium
(Sigma-Aldrich) supplemented with 2 mM L-glutamine, 0.02 mg/ml
gentamicin and 5% heat-inactivated fetal bovine serum (FBS;
Invitrogen, Grand Island, NY, USA) for use in subsequent
experiments.
Prostaglandin E2
(PGE2) ELISA
For the in vivo experiment, rats were treated
with various solutions of C. obtusa essential oil in the
presence or absence of LPS. Following treatment, serum was
collected from peripheral blood vessels and subjected to a
PGE2 ELISA assay. For the in vitro assay, rats
were treated with the essential oil solutions for 2 weeks. PMNCs
were harvested from rat peripheral blood vessels and plated on
24-well plates at a density of 0.5×105. The PMNCs were
then stimulated with 10 ng/ml LPS for the indicated times, and the
supernatant was collected. For the PGE2 ELISA assay, the
collected serum and supernatant were analyzed using a monoclonal
PGE2 ELISA kit (Cayman Chemical, Ann Arbor, MI,
USA).
Quantification of mRNA by RT-PCR
Total cellular RNA was extracted using TRIzol
reagent (Invitrogen), and the concentration of RNA was determined
at 260 nm. RT-PCR was performed as previously described (13). Briefly, total RNA (1 μg) was
reverse transcribed into complementary DNA (cDNA) using M-MLV
reverse transcriptase (Invitrogen) and a random primer (9-mer;
Takara Bio, Inc., Shiga, Japan). The cDNA (1 μl) was used for PCR
under standard conditions (13,16);
denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec and
extension at 72°C for 1 min. The primer sequences used were as
follows: COX-2 sense, 5′-TACCCGGACTGGATTCTACG-3′ and antisense,
5′-AAGTTGGTGGGCTGTCAATC-3′; TNFα sense, 5′-CTGAGCTCAAGCCCTGGTAT-3′
and antisense, 5′-GGTCAGAGTAATGGGGGTCA-3′; and cytochrome c
oxidase subunit 1 (1A) sense, 5′-CCAGGGTTTGGAATTATTTC-3′ and
antisense, 5′-GAAGATAAACC CTAAGGCTC-3′, which was used as an
internal control. The PCR products (10 μl) were separated on 2%
agarose gel and stained with ethidium bromide (EtBr). The gel image
was captured and analyzed using Quantity One software (Gel Doc EQ;
Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data were analyzed by non-parametric one-way
analysis of variance using the Kruskal-Wallis test, followed by
Dunnett’s test for multiple comparisons. Data values were converted
to ranks for these tests. All the statistical analyses were
performed with SPSS for Windows (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of essential oil from C. obtusa on
serum PGE2 concentration
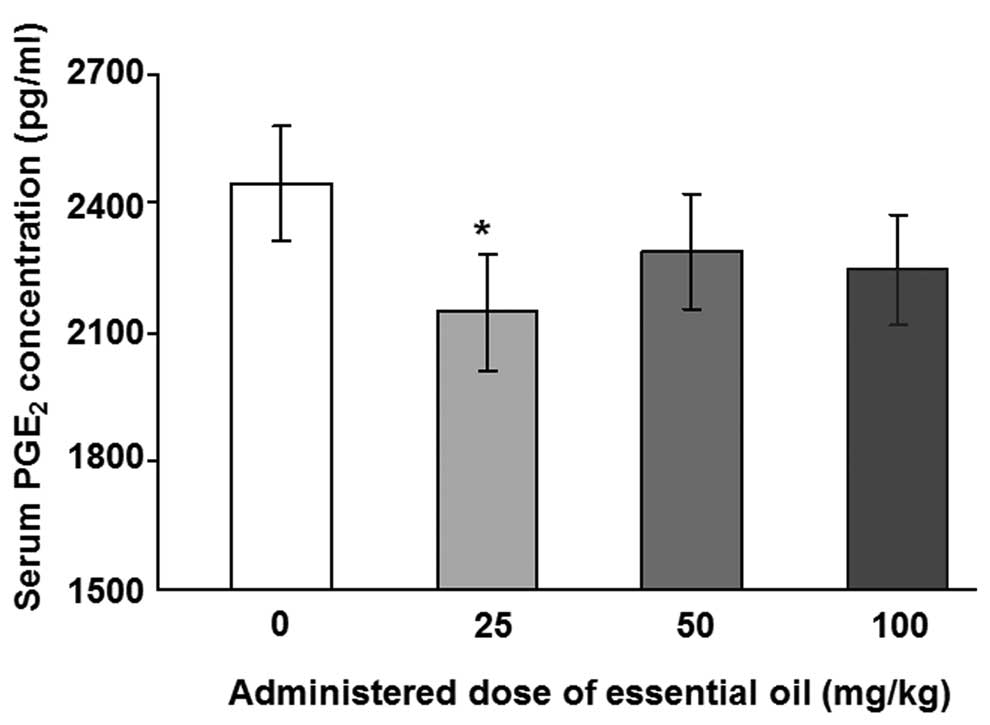
The regulation of serum PGE2 by essential
oils from C. obtusa was investigated. Rats were treated with
various concentrations of the essential oil for 2 weeks and 1 μg/kg
LPS was injected to induce an inflammatory reaction following
treatment with the essential oil.
To analyze the levels of serum PGE2,
ELISA with a specific antibody for PGE2 was performed.
The levels of LPS-induced serum PGE2 were significantly
reduced in animals treated with essential oil at a dose of 25 mg/kg
(Fig. 1). Other concentrations of
C. obtusa essential oil (50 and 100 mg/kg) also reduced the
levels of serum PGE2; however, these results were not
statistically significant. Next, the effects of essential oil were
evaluated, using PMNCs to verify the increase in serum
PGE2 in the rats. PMNCs were isolated following
treatment with various concentrations of the essential oil for 2
weeks and administration of 10 ng/ml LPS for 12 (Fig. 2A) or 24 h (Fig. 2B). The essential oil was shown to
have protective effects on LPS-induced inflammation by reducing
PGE2 secretion in the PMNCs. The 12-h LPS-induced
PGE2 secretion level was inhibited by treatment with the
essential oil at all the doses examined, and the maximum inhibition
was observed at a dose of 50 mg/kg. The inhibition of
PGE2 production induced by LPS treatment for 24 h was
shown to be significant only when 100 mg/kg of the essential oil
was applied.
Effect of essential oils from C. obtusa
on expression of the inflammation-related genes TNFα and COX-2 in
lung tissue
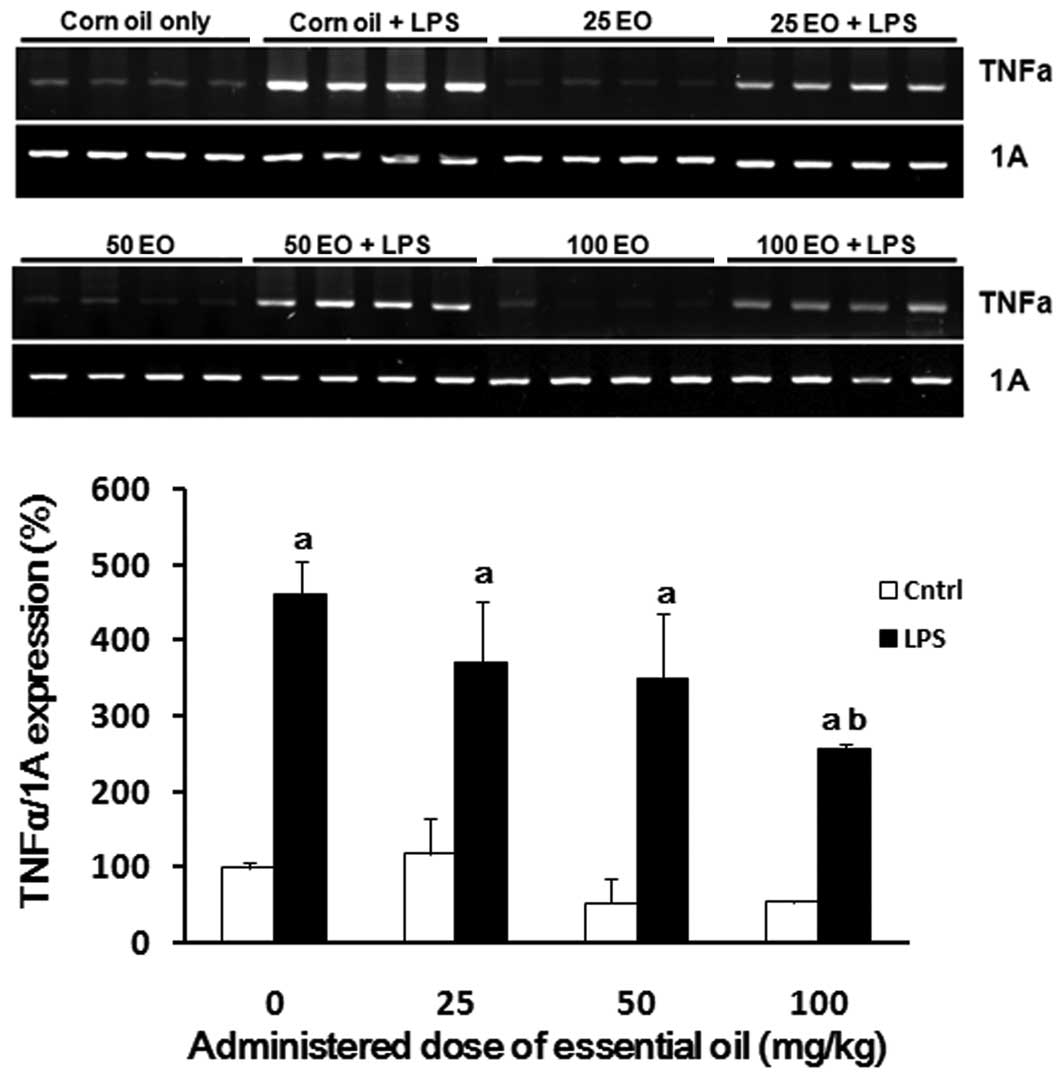
To examine the possible mechanisms of action
underlying the anti-inflammatory effects of the essential oil, we
analyzed the expression of inflammation-associated genes in the
lungs of rats following treatment with the essential oil in the
presence or absence of LPS. As expected, LPS enhanced the
expression of TNFα (Fig. 3).
LPS-induced TNFα expression in the lung was reduced by
administration of the essential oil in a dose-dependent manner,
where the most potent effect was observed at a dose of 100 mg/kg
(56% reduction).
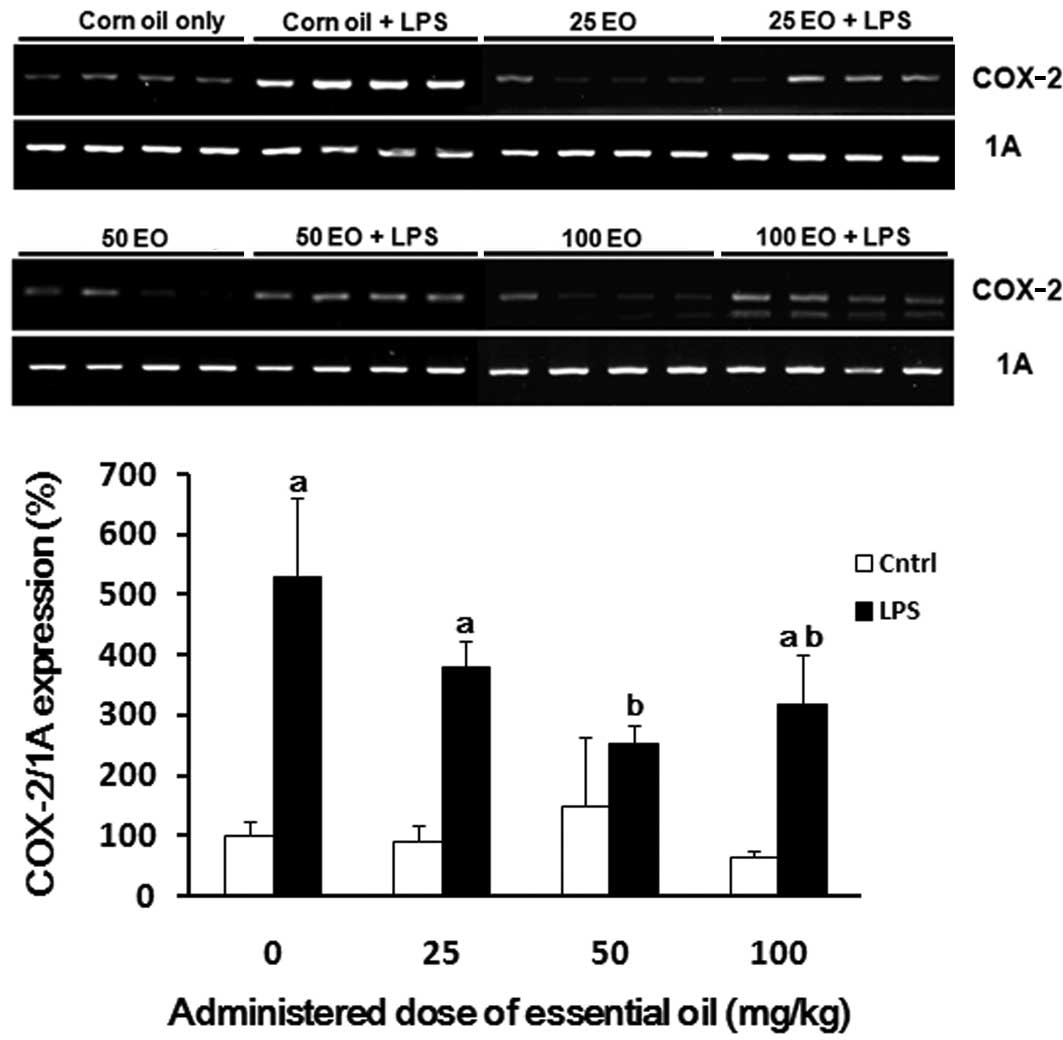
COX-2 mRNA expression was also assessed to determine
the mechanism via which the essential oil exerts it
anti-inflammatory effects (Fig.
4). The expression of COX-2 was increased by LPS treatment (up
to 5-fold) compared with the control group (Fig. 4). The induction of COX-2 expression
by LPS treatment was inhibited by pre-administration of the
essential oil; this inhibition was most marked at a dose of 50
mg/kg.
Discussion
Since ancient times, plants have been used for their
medicinal properties, which are partially attributed to essential
oils (17). Essential oils are
natural, complex and multi-component oils composed mainly of
terpenes with additional components (18). Essential oils are widely used to
prevent and treat human diseases, including cancer and
cardiovascular diseases. Furthermore, their bioactivity as
antibacterial, antiviral, antioxidant and antidiabetic agents has
previously been reported (18).
Recent studies have demonstrated the anti-inflammatory properties
of essential oils from several plants (19–21).
In the present study, we demonstrated that the essential oil from
C. obtusa has an anti-inflammatory effect on LPS-induced
inflammation in rats. The serum concentration of PGE2
was reduced when the rats were treated with the essential oil. In
addition, the synthesis of PGE2 from blood PMNCs showed
concomitant results with that of serum. These results suggest that
PGE2 is synthesized by blood monocytes following
stimulation by LPS, and that this inflammatory process is
ameliorated by C. obtusa essential oil.
PGE2 is produced in the body and
accumulates with LPS treatment through stimulation of the COX
pathway. The quantitative analysis of PGE2 represents
inflammatory activities in vivo and in vitro(22). In the present study, LPS-induced
PGE2 production was reduced in the serum and PMNCs of
rats by treatment with C. obtusa essential oil. Similar
results have been previously reported; Hyptis pectinata
essential oil has been shown to have anti-inflammatory effects by
inhibiting the production of PGE2 and nitric oxide
(23). Cryptomeria japonica
essential oil has been demonstrated to inhibit the LPS-induced
production of PGE2 and cytokines in macrophages,
including interleukin (IL)-1β and IL-6 (24); furthermore, the chemical
composition of Cryptomeria japonica essential oil was
analyzed and its major components were revealed to be elemol and
sabinene. Elemol and sabinene are also known to be terpenes of
C. obtusa essential oil (10,11).
In the present study, we investigated the effects of
C. obtusa essential oil on the regulation of TNFα mRNA
expression following LPS treatment. As expected, the expression of
TNFα was increased in the lung tissues of rats following LPS
treatment, which implied induction of the immune response by LPS.
Furthermore, the induced expression of TNFα was abrogated by
treatment with C. obtusa essential oil. To verify the
mechanism of action underlying the anti-inflammatory effects of
C. obtusa essential oil, we also examined the regulation of
COX-2 expression levels. Pathogen-associated immune responses are
antigenic, and are able to activate monocytes or macrophages to
synthesize various inflammatory cytokines, including IL-1, IL-6 and
TNF, which have been reported to be mediated via COX expression
(24–26). The expression of COX-2 was
increased following treatment with LPS, and this upregulation was
inhibited by C. obtusa essential oil in a dose-dependent
manner. These results suggest that the anti-inflammatory effects of
this essential oil occur via COX-2-mediated regulation of PGE2 and
TNFα signaling pathways.
The anti-inflammatory effects of essential oils
through the COX-2 signaling pathway have been previously
demonstrated; the essential oil from Houttuynia cordata has
been shown to possess anti-inflammatory properties through
inhibiting the LPS-induced production of PGE2(9). In this study, the anti-inflammatory
effects of the essential oil were mediated by the inhibition of
COX-2 gene expression, while COX-1, the non-inflammation-specific
isoform, was not involved. Katsuawa et al(27) also demonstrated that the essential
oil of lemongrass suppresses COX-2 promoter activity in human
macrophage-like U937 cells.
In conclusion, our results demonstrate that C.
obtusa essential oil exerts it anti-inflammatory effects by
regulating the production of PGE2 in the blood and the
gene expression of TNFα in rats. The anti-inflammatory effects of
C. obtusa essential oil are mediated by inhibiting the
expression of the inflammation-specific COX-2 enzyme. Our results
suggest that C. obtusa essential oil is a novel source of
inflammation-specific pharmacological drugs. However, further
studies are required to investigate the specific mechanisms of
action and potential side-effects of C. obtusa essential
oil.
Acknowledgements
This study was supported by a National Research
Foundation of Korea (NRF) grant funded by the Korea government
(MEST; no. 2010-0011433).
Abbreviations:
|
C. obtusa
|
Chamaecyparis obtusa
|
|
PGE2
|
prostaglandin E2
|
|
TNFα
|
transforming growth factor α
|
|
COX-2
|
cyclooxygenase-2
|
|
NSAIDS
|
nonsteroidal anti-inflammatory
drugs
|
|
LPS
|
endotoxin lipopolysaccharides
|
References
|
1
|
Simmons DL, Botting RM and Hla T:
Cyclooxygenase isozymes: the biology of prostaglandin synthesis and
inhibition. Pharmacol Rev. 56:387–437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith WL: Nutritionally essential fatty
acids and biologically indispensable cyclooxygenases. Trends
Biochem Sci. 33:27–37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koki A, Khan NK, Woerner BM, et al:
Cyclooxygenase-2 in human pathological disease. Adv Exp Med Biol.
507:177–184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zha S, Yegnasubramanian V, Nelson WG,
Isaacs WB and De Marzo AM: Cyclooxygenases in cancer: progress and
perspective. Cancer Lett. 215:1–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cuzick J, Otto F, Baron JA, et al: Aspirin
and non-steroidal anti-inflammatory drugs for cancer prevention: an
international consensus statement. Lancet Oncol. 10:501–507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thun MJ and Blackard B: Pharmacologic
effects of NSAIDs and implications for the risks and benefits of
long-term prophylactic use of aspirin to prevent cancer. Recent
Results Cancer Res. 181:215–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mukherjee D: Selective cyclooxygenase-2
(COX-2) inhibitors and potential risk of cardiovascular events.
Biochem Pharmacol. 63:817–821. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coruzzi G, Venturi N and Spaggiari S:
Gastrointestinal safety of novel nonsteroidal antiinflammatory
drugs: selective COX-2 inhibitors and beyond. Acta Biomed.
78:96–110. 2007.PubMed/NCBI
|
|
9
|
Li W, Zhou P, Zhang Y and He L:
Houttuynia cordata, a novel and selective COX-2 inhibitor
with anti-inflammatory activity. J Ethnopharmacol. 133:922–927.
2011. View Article : Google Scholar
|
|
10
|
Joo SS, Yoo YM, Ko SH, et al: Effects of
essential oil from Chamaecypris obtusa on the development of
atopic dermatitis-like skin lesions and the suppression of Th
cytokines. J Dermatol Sci. 60:122–125. 2010.PubMed/NCBI
|
|
11
|
Singh BK, Tripathi M, Chaudhari BP, Pandey
PK and Kakkar P: Natural terpenes prevent mitochondrial
dysfunction, oxidative stress and release of apoptotic proteins
during nimesulide-hepatotoxicity in rats. PLoS One. 7:e342002012.
View Article : Google Scholar
|
|
12
|
Lee GS, Byun HS, Kim MH, et al: The
beneficial effect of the sap of Acer mono in an animal with
low-calcium diet-induced osteoporosis-like symptoms. Br J Nutr.
100:1011–1018. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee GS, Hong EJ, Gwak KS, et al: The
essential oils of Chamaecyparis obtusa promote hair growth
through the induction of vascular endothelial growth factor gene.
Fitoterapia. 81:17–24. 2010.
|
|
14
|
Lee SC, Ju SA, Pack HN, et al: 4-1BB
(CD137) is required for rapid clearance of Listeria
monocytogenes infection. Infect Immun. 73:5144–5151. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hasegawa H, Suzuki K, Nakaji S and
Sugawara K: Analysis and assessment of the capacity of neutrophils
to produce reactive oxygen species in a 96-well microplate format
using lucigenin- and luminol-dependent chemiluminescence. J Immunol
Methods. 210:1–10. 1997. View Article : Google Scholar
|
|
16
|
Vo TT, An BS, Yang H, Jung EM, Hwang I and
Jeung EB: Calbindin-D9k as a sensitive molecular biomarker for
evaluating the synergistic impact of estrogenic chemicals on GH3
rat pituitary cells. Int J Mol Med. 30:1233–1240. 2012.PubMed/NCBI
|
|
17
|
Takayama C, de-Faria FM, de Almeida AC, et
al: Gastroprotective and ulcer healing effects of essential oil
from Hyptis spicigera Lam. (Lamiaceae). J Ethnopharmacol.
135:147–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edris AE: Pharmaceutical and therapeutic
potentials of essential oils and their individual volatile
constituents: a review. Phytother Res. 21:308–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Silva FV, Guimarães AG, Silva ER, et al:
Anti-inflammatory and anti-ulcer activities of carvacrol, a
monoterpene present in the essential oil of oregano. J Med Food.
15:984–991. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lima DK, Ballico LJ, Rocha Lapa F, et al:
Evaluation of the antinociceptive, anti-inflammatory and gastric
antiulcer activities of the essential oil from Piper
aleyreanum C.DC in rodents. J Ethnopharmacol. 142:274–282.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Veras HN, Araruna MK, Costa JG, et al:
Topical antiinflammatory activity of essential oil of Lippia
sidoides Cham: possible mechanism of action. Phytother Res.
27:179–185. 2012. View
Article : Google Scholar
|
|
22
|
Hennebert O, Pelissier MA, Le Mee S,
Wülfert E and Morfin R: Anti-inflammatory effects and changes in
prostaglandin patterns induced by 7beta-hydroxy-epiandrosterone in
rats with colitis. J Steroid Biochem Mol Biol. 110:255–262. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raymundo LJ, Guilhon CC, Alviano DS, et
al: Characterisation of the anti-inflammatory and antinociceptive
activities of the Hyptis pectinata (L.) Poit essential oil.
J Ethnopharmacol. 134:725–732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoon WJ, Kim SS, Oh TH, Lee NH and Hyun
CG: Cryptomeria japonica essential oil inhibits the growth
of drug-resistant skin pathogens and LPS-induced nitric oxide and
pro-inflammatory cytokine production. Pol J Microbiol. 58:61–68.
2009.
|
|
25
|
Chao LK, Hua KF, Hsu HY, et al:
Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from
monocytes/macrophages through suppression of intracellular
signaling. Food Chem Toxicol. 46:220–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takeda K, Kaisho T and Akira S: Toll-like
receptors. Annu Rev Immunol. 21:335–376. 2003. View Article : Google Scholar
|
|
27
|
Katsukawa M, Nakata R, Takizawa Y, Hori K,
Takahashi S and Inoue H: Citral, a component of lemongrass oil,
activates PPARalpha and gamma and suppresses COX-2 expression.
Biochim Biophys Acta. 1801:1214–1220. 2010. View Article : Google Scholar : PubMed/NCBI
|