Introduction
Gastric cancer is one of the most common type of
malignancies in China, of which the 5-year survival rate is ≤30%
(1,2). The most common treatments include
surgery and chemotherapy, however, these treatments are often
accompanied with unsatisfactory effects. Therefore, there is a
requirement for further study of its occurrence and the development
of mechanisms to guide the treatment.
In the last three decades, cancer has been
understood as a summary of altered genetic and epigenetic events.
The epigenetic pathway is, in contrast to genetic events, a
reversible alteration and is characterized by three primary
mechanisms: i) DNA hypermethylation leading to inactivation; ii)
DNA hypomethylation causing genomic instability and iii) histone
modifications affecting chromatin conformation (3). These processes, particularly aberrant
DNA methylation and histone modifications, are closely linked with
each other by a protein complex of transcript activators and
repressors, and they alter mRNA transcript expression of affected
genes (4). Characteristically, DNA
methylation does not change the genetic information. However, the
readability of the DNA is altered and results in the inactivation
of genes by subsequent mRNA transcript repression (3). In humans and other mammals, CpG
island methylation is an important physiological mechanism. The
inactivated X-chromosome of female silenced alleles of imprinted
genes or inserted viral genes and repeat elements are inactivated
through promoter methylation (5,6).
Moreover, with the progression of molecular biology
in cancer, increasing evidence has indicated that the occurrence of
gastric cancer results from multiple genetic alterations, including
activated oncogenes, inactivation of tumor suppressor genes, loss
of heterozygosity (LOH) and defects in DNA damage response and
repair mechanisms (3). Another
factor which plays a key role in the regulation of gene expression
is epigenetic changes in gene expression patterns due to mechanisms
other than mutations in the underlying DNA sequence, including DNA
methylation and histone modifications. According to a large number
of animal tests, trefoil factor 1 (TFF1) plays a regulatory
function in the mammals’ digestive system, namely in mucosal
protection and epithelial cell reconstruction, tumor suppression,
signal transduction and the regulation of apoptosis.
The trefoil peptides (TFF1, TFF2 and TFF3) are a
group of highly conserved small proteins, which are localized
within the mucous granules in mucus-secreting cells and are
expressed and secreted by epithelial cells which line mucous
membranes (7). TFF1 is expressed
predominantly by the gastric epithelia, in the upper portion of the
glandular pits and is ectopically expressed in a number of
adenocarcinomas, including breast cancer (7–9). In
the breast, TFF1 expression is highly expressed in estrogen
receptor-positive tumors and is inversely associated with
histological grade (9). In the
stomach, TFF1 is secreted as a component of the protective mucus
layer. TFF1 is synthesized and secreted by the mucus-secreting pit
cells of the corpus and antropyloric regions of the stomach
(7,10). TFF1 expression is markedly induced
following mucosal injury (11) and
is involved in stomach ontogenesis and maintenance of the integrity
of the mucosa (7,8). Molecular studies have shown frequent
loss of TFF1 expression in >2/3 of gastric carcinomas (GCs)
resulting from a mutation-independent mechanism (12–14).
The silencing of the TFF1 gene in GCs is due to LOH and methylation
of the TFF1 promoter region (12,15–18);
however, mutations are observed in ~5% of GCs (12,19).
Silencing of TFF1 may also be triggered by chromatin remodeling
associated with histone modifications, including H3K9 methylation
and H3 deacetylation at the TFF1 promoter, as observed in
N-methyl-N-nitrosourea-induced gastric carcinogenesis mouse model
(18). In addition,
transcriptional repression of TFF1 in gastric epithelial cells by
CCAAT/enhancer binding protein-β (20) and cofactor of BRCA1 has been
demonstrated (14). A previous
study showed that TFF1 is a candidate tumor suppressor gene which
inhibits cell growth (21). The
TFF1-knockout mouse model provided the first evidence supporting a
tumor suppressor role of TFF1 in gastric tumorigenesis,
demonstrating that it is essential for normal differentiation of
the antral and pyloric gastric mucosa (22). The spectrum of histological lesions
and the mechanisms and molecular pathways, which are mediated by
the loss of TFF1 in gastric tumorigenesis are not fully
understood.
To determine the correlation between TFF1 and
gastric cancer, the expression levels of TFF1 in GC tissue and cell
lines was examined. To clarify the potential effect of DNA
methylation in the promoter region in gastric carcinogenesis, the
methylation rate and mechanism in specimens and cell lines was
investigated. This study provides an experimental basis for gastric
cancer diagnosis and clinical treatment, and provides a significant
insight into epigenetic regulation in gastric cancer associated
with TFF1.
Materials and methods
Cell culture and 5-aza-2′-deoxycytidine
treatment
Human gastric cancer cell lines SGC-7901 and BGC-823
were cultured in Dulbecco’s modified Eagle’s medium supplemented
with 10% fetal bovine serum and 1% antibiotics
(penicillin-streptomycin solution) under identical conditions (37°C
in humidified 5% CO2/95% air). For drug treatment, cell
lines were treated with 5 μmol/l 5-aza-2′-deoxycytidine (Aza;
Sigma-Aldrich, St. Louis, MO, USA) for 3 days. Aza and medium were
changed every 24 h. Control cells were incubated with culture
medium.
Tissue and surgical specimens
GC, adjacent non-tumor tissue and distant normal
tissue specimens were obtained from 44 samples in the People’s
Liberation Army Air Force General Hospital and People’s Liberation
Army General Hospital (Beijing, China) under Institutional Review
Board-approved instructions. A further 15 normal tissue specimens
adjacent to benign gastric ulcers were obtained from patients in
the same two hospitals. The adjacent non-tumor tissues were removed
2 cm from the carcinoma border. Each non-tumor sample was divided
into two groups and the distal section was used following
confirmation that the proximal section was not cancerous. The
normal tissue distant from carcinoma and those adjacent to benign
gastric ulcer were removed >5 cm from the carcinoma border or
the ulcer. The samples were resected from surgical patients with
pathological diagnosis between October 2002 and December 2012,
prior to receiving any radiotherapy or chemotherapy. Following
resection, all specimens were snap frozen in liquid nitrogen and
stored at −70°C. All patient’s age, gender and pathological type
were recorded.
The use of human specimens in this research was
approved by the Ethics Committee of the People’s Liberation Army
Air Force General Hospital and People’s Liberation Army General
Hospital (Beijing, China) according to the Declaration of Helsinki.
All necessary consent was acquired from patients/patient’s families
involved in the study, including consent to participate in the
study and consent to publish.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
RNA was extracted from cells or patient tissue using
an RNA isolation reagent (TRIzol; Invitrogen Life Technologies,
Carlsbad, CA, USA). To prevent DNA contamination, total RNA was
treated with RNase-free DNase II (Invitrogen Life
Technologies).
The human glyceraldehyde-3-phosphate dehydrogenase
gene (GAPDH; forward primer, 5′-TCACCAGGGCTGCTT TTA-3′ and reverse
primer, 5′-TTCACACCC ATGACGAACA-3′) was used as an internal control
in PCR amplification. A two-step RT-PCR procedure was performed in
all experiments. First, total RNA samples (2 μg/reaction) were
reverse transcribed into cDNA by RT II reverse transcriptase
(Invitrogen Life Technologies). Then, the cDNAs were used as
templates in PCR with TFF1 gene-specific primers
(5′-TTTGGAGCAGAGAGGAGG-3′ and 5′-TTGAGTAGTCAAAGTCAGAGCAG-3′). The
primers for TP 53 were 5′-GGGTTAGTTTACAATCAGCCACATT-3′ and
5′-GGGCCTTGAAGTTAGAGAAAATTCA-3′. The amplification reactions were
performed using AmpliTaq Gold® DNA polymerase (Applied
Biosystems, Foster City, CA, USA). The PCR cycle was programmed as
follows: 2 min at 95°C and 30–32 cycles of 30 sec at 94°C, 30 sec
at 58–62°C and 30 sec at 72°C, with an extension for 10 min at
72°C. The PCR bands were visualized under UV light and images were
captured. Quantitative PCR was used to determine mRNA levels of
urea cycle genes for hepatocellular carcinoma (HCC) cell lines and
NOLC 1 mRNA levels for tissue on a StepOne Plus™ Real-Time PCR
system with SYBR-Green Master Mix (Applied Biosystems).
Immunohistochemical staining
Biopsy specimens were subjected to routine
immunohistochemical staining using a monoclonal antibody against
TFF1. Immunoreactivity, defined as the number of positive tumor
cells over total tumor cells, was scored independently by two
researchers. The number of TFF1-positive and -negative gastric
cancer cells was counted by light microscopy at a magnification of
×400, with only the cells exhibiting brown nucleoli on the section
considered TFF1 positive. For each slide, 7–10 microscopic fields
were randomly selected. Positive scores were then categorized into
weak staining (only one nucleolus was stained), moderate staining
(>1 nucleolus was stained) and strong staining (the nucleus and
nucleolus of the tumor cell staining). The average percentage of
TFF1-positive gastric cancer cells was calculated for each
group.
Western blot analysis
Lysates from the cultured cells were subjected to
routine western blot analysis as described previously (23). The antibodies used were monoclonal
anti-mouse antibodies against TFF1 and β-actin (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and polyclonal rabbit
antibodies against NF-κB and HIF-1α (Abcam, Cambridge, MA, USA).
The results shown are representative of two independent
experiments.
Bisulfite genomic sequencing
Genomic DNA was purified from cells with a kit
(Wizard Genomic DNA Purification kit; Promega Corporation, Madison,
WI, USA). DNA (2 μg) was bisulfite modified with the EZ DNA
Methylation Direct kit; Zymo Research, Irvine, CA, USA).
Sequence-specific primers to amplify the CpG-rich regions of
interest were designed using a computer program (MethPrimer;
http://www.urogene.org/methprimer). The
primers used for amplification were as follows: TFF1,
5′-TAGACGGAATGGGCTTCATG-3′ (forward) and 5′-GCAAACAGAGCCTGCCCTAT-3′
(reverse). The PCR products were amplified, purified and cloned
into a vector (pGEM-T Easy Vector; Promega Corporation). Clones
were selected using blue-white screening. Finally, the colonies
harboring the insert were sequenced in a 96-well plate using the
M13 reverse and/or forward primers.
The bisulfite-treated DNA was amplified using
primers, which specifically amplify the methylated or unmethylated
sequence of TFF1 promoter, respectively. The PCR was performed for
40 cycles, with an annealing temperature of 58°C for the methylated
reaction and 52°C for the unmethylated reaction. The human
methylated and unmethylated DNA was used as a control to verify the
specificity of the primers (Qiagen, Valencia, CA, USA).
DNA samples from each group were cleaved by
methylation-sensitive restriction enzyme or non-sensitive
restriction enzyme, and this was followed by PCR amplification with
the primer designed to be the DNA sequence outside where
methylation was to be detected. Methylation in the relevant
restriction enzyme site was confirmed if methylation was detected
in the PCR products from the samples which used
methylation-sensitive restriction enzyme. If no methylation was
detected, then there was no methylation in the restriction enzyme
site.
Plasmid and transfection
Cells were subcultured and transfected as previously
described (24). The cDNA encoding
TFF1, flanked by BamHI and SalI restriction sites,
was cloned into the mammalian expression vector pCDNA4 (Stratagene
Inc., La Jolla, CA, USA) to generate pCDNA-TFF1, which expresses an
N-terminal Myc-tagged TFF1 fusion protein. The promoter region of
TFF1 was cloned into the pGL3 plasmid. Subconfluent cells were
transiently transfected with pCDNA-TFF1 DNA (4 μg/dish) mixed with
Lipofectamine and PLUS reagent (Invitrogen Life Technologies),
according to the manufacturer’s instructions. Cells were harvested
~48 h following transfection (24).
RNA interference of TFF1
Stealth RNAi™ (Invitrogen Life Technologies)
oligonucleotides for TFF1 were designed to target TFF1 mRNA and
annealed as follows: siTFF1, 5′-AACAUGCAGUAA GGAUUCCACUUCC-3′
(sense) and 5′-GGAAGUGGAA UCCUUACUGCAUGUU-3′ (antisense); scrambled
siRNA (negative control siRNA duplexes, 12935-300; Invitrogen Life
Technologies). For transfection of siRNA, cells were plated into
6-well plates, grown until reaching 70–80% confluency and
transfected with 40 or 80 pmol of siRNA duplex using Lipofectamine
2000 (Invitrogen Life Technologies) following the manufacturer’s
instructions.
Luciferase assay
The cells were transfected with 0.6 μg of firefly
luciferase reporter plasmid and 0.05 μg of control plasmid
containing Renilla luciferase (pRL-TK; Promega Corporation).
A promoterless basic vector (pGL3; Promega Corporation) was used as
a negative control. To confirm the efficiency of transfection
(Lipofectin; Invitrogen Life Technologies), a luciferase expression
vector (pGL3-control; Promega Corporation) was used as a positive
control. Following 48 h, the cells were harvested for analysis.
Luciferase enzyme assays and colorimetric β-galactosidase assays
were performed according to the manufacturer’s instructions
(Promega Corporation). Luciferase activity was normalized to
β-galactosidase activity to assess the transfection efficiency.
When indicated, the firefly and Renilla luciferase
activities were measured using the Dual-Luciferase Reporter Assay
system (Promega Corporation), according to the manufacturer’s
instructions. The Renilla luciferase activity of pRL-TK was
used to normalize the firefly luciferase activity of the reporter
construct. Each transfection experiment was repeated three
times.
Cell growth and proliferation assay
Cell growth was determined by the colorimetric
tetrazolium derived sodium
3′-[1-(phenylamino-carbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)
benzenesulfonic acid hydrate (XTT) assay (Roche Diagnostics GmbH,
Mannheim, Germany) and DNA synthesis of cells was assessed by the
bromodeoxyuridine (BrdU) incorporation assay (Roche Diagnostics
GmbH). For the cell growth and proliferation assay, the cells of
each group at 48 h following treatment were re-seeded onto 96-well
plates at a density of 3×103 cells/well. Then, XTT and
incorporated BrdU were measured colorimetrically using a microtiter
plate reader (Bio-Rad, Hercules, CA, USA) at a wavelength of 450 nm
(25).
Determimation of apoptosis
Cells (3×105) were cultured for 48 h.
Apoptotic cells were identified using fluorescence-activated cell
sorting (FACS) Annexin V-FLUOS (BioLegend, San Diego, CA, USA)
following the manufacturer’s instructions. Following culturing,
cells were washed at 4°C for 30 min in PBS and stained with
Annexin-V staining solution (Annexin-V-FLUOS kit; Roche Diagnostics
GmbH) at 4°C for 3 h. Gels were washed 4 times in PBS at 4°C and
fixed at room temperature with 1% paraformaldehyde (Sigma-Aldrich)
in PBS for 15 min. For counterstaining, 7-AAD (2 μg/ml) was added
to the first washing step. The numbers of total,
Annexin-V-positive, 7-AAD-positive and double-positive cells were
determined respectively by FACS analysis. Apoptosis was verified by
detection of activated caspases (26).
Statistical analysis
Using GraphPad Prism software, a 2-tailed Student’s
t-test was used to compare the statistical difference between 2
groups and a one-way ANOVA Newman-Keuls Multiple Comparisons test
was used to compare the differences between 3 groups or more. The
correlation between 2 parameters, age and chronic inflammation
scores, was determined by Spearman’s correlation. P≤0.05 was
considered to indicate a statistically significant difference.
Results
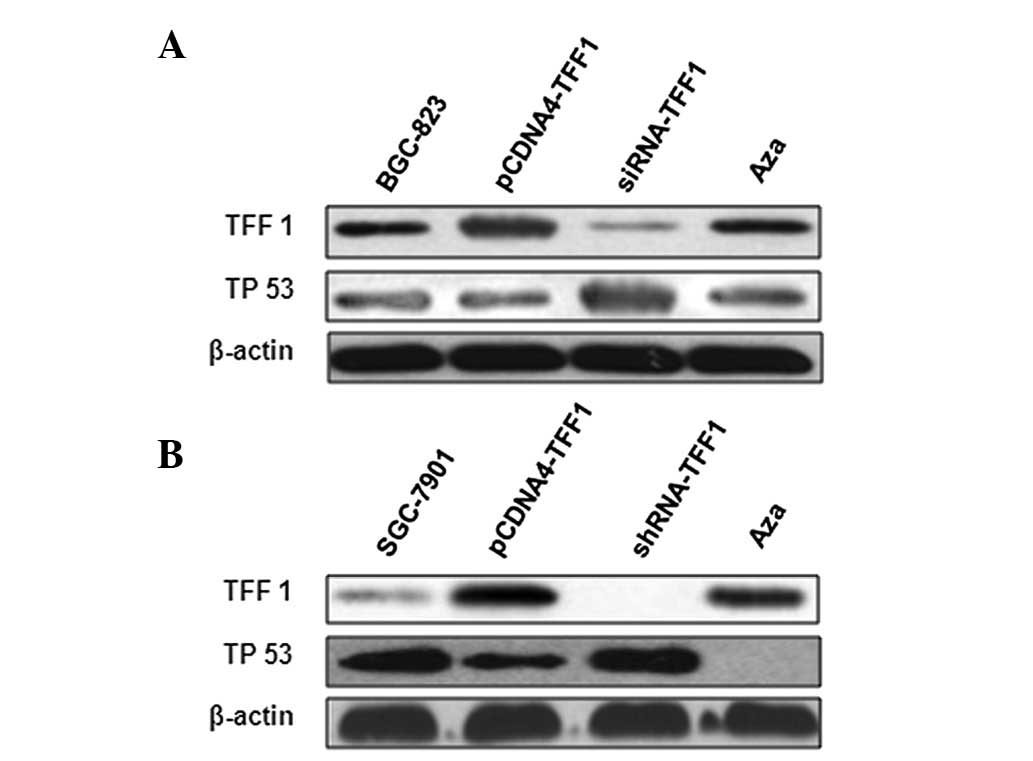
Low expression of TFF1 in gastric cancer
tissue is associated with TP 53
To determine the potential mechanisms by which TFF1
is regulated in GC, TFF1 expression levels at the transcription and
translation level in the tissue of patients was determined (N,
normal tissues adjacent to benign gastric ulcer; DN, distant normal
tissue of patients with GC; AN, adjacent non-tumor of patients with
GC; GC, GC tissue of patients with GC) using immunohistochemical
staining and RT-PCR. The results show that TFF1 was moderately
expressed in the mucosa of normal tissue adjacent to benign gastric
ulcer (Fig. 1A) and was expressed
at a low level in GC tissue (Fig.
1B). The expression levels of TFF1 mRNA was examined in 44
specimens of GC, adjacent non-tumor, distant normal tissues and 15
cases of normal tissue from patients with benign gastric ulcer,
using RT-PCR. The expression levels in GC specimens were
significantly lower compared with the other three specimens
(P<0.01). The results in adjacent non-tumor tissue were
significantly lower compared with the distant normal tissue and the
normal tissues adjacent to a benign gastric ulcer (P<0.05). By
contrast, the expression level of TFF1 between the adjacent
non-tumor and normal tissue adjacent to benign gastric ulcer showed
no significant difference (P>0.05; Fig. 1C and D). To determine the
association of mRNA transcription with protein expression, western
blot analysis was performed to examine the TFF1 protein expression
in patient tissues. As shown in Fig.
1E, a strong expression of TFF1 protein was detected in normal
tissue adjacent to benign gastric ulcer and weakly expressed in
distant normal tissue of patients with GC. TFF1 protein decreased
significantly in adjacent noncancerous specimens, particularly in
the tumor samples of patients.
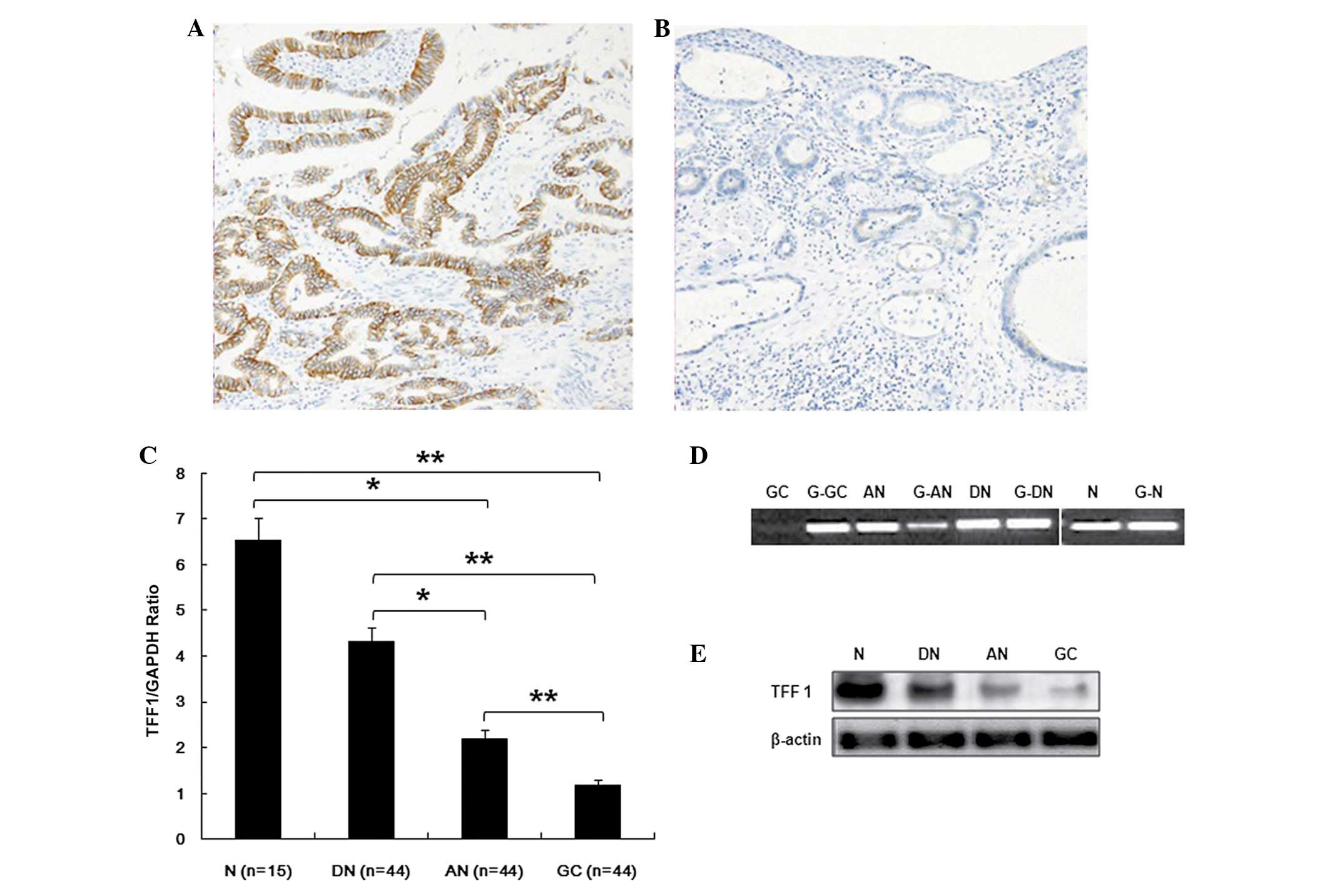 | Figure 1TFF1 mRNA and protein expression in
gastric cancer cell lines and patient specimens. (A) The protein
expression of TFF1 in normal tissue adjacent to N by IH staining.
(B) The protein expression of TFF1 in tumor tissues of patients
with GC by IH and DN, AN and GC. (C) TFF1 mRNA levels in tissues
were detected by RT-PCR in patients with gastric ulcer (n=15) and
patients with gastric carcinoma (n=44; N vs. AN, P=0.032; N vs. GC,
P=0.000; DN vs. AN, P=0.018; DN vs. GC, P=0.004; AN vs. GC,
P=0.000). (D) TFF1 mRNA levels in tissues were detected by
quantitative PCR in patients with gastric ulcer (n=15) and patients
with gastric carcinoma (n=44). (E) TFF1 protein levels in tissues
were detected by western blot analysis in patients with gastric
ulcer or GC. The data are presented as the mean ± SD from three
independent experiments *P<0.05,
**P<0.01. TFF1, trefoil factor 1; IH,
immunohistochemical; RT-PCR, reverse transcription-polymerase chain
reaction; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; N,
benign gastric ulcer group; DN, distant normal tissue of patients
with gastric carcinoma; AN, adjacent non-tumor of patients with
gastric carcinoma; GC, tumor tissues of patients with gastric
carcinoma. |
Fenoglio-Preisern et al(27) found that p53 alterations occur
early in the development of GC and are present in the
non-neoplastic mucosa and increase in frequency as GC development
progresses. p53 immunoreactivity is observed in 17–90.7% of
invasive GCs. p53 alterations occur more commonly in proximal
lesions compared with distal ones, suggesting that the molecular
events leading to the development of GC may be markedly different
in proximal vs. distal tumors. p53 mutations occur in 0–77% of GCs
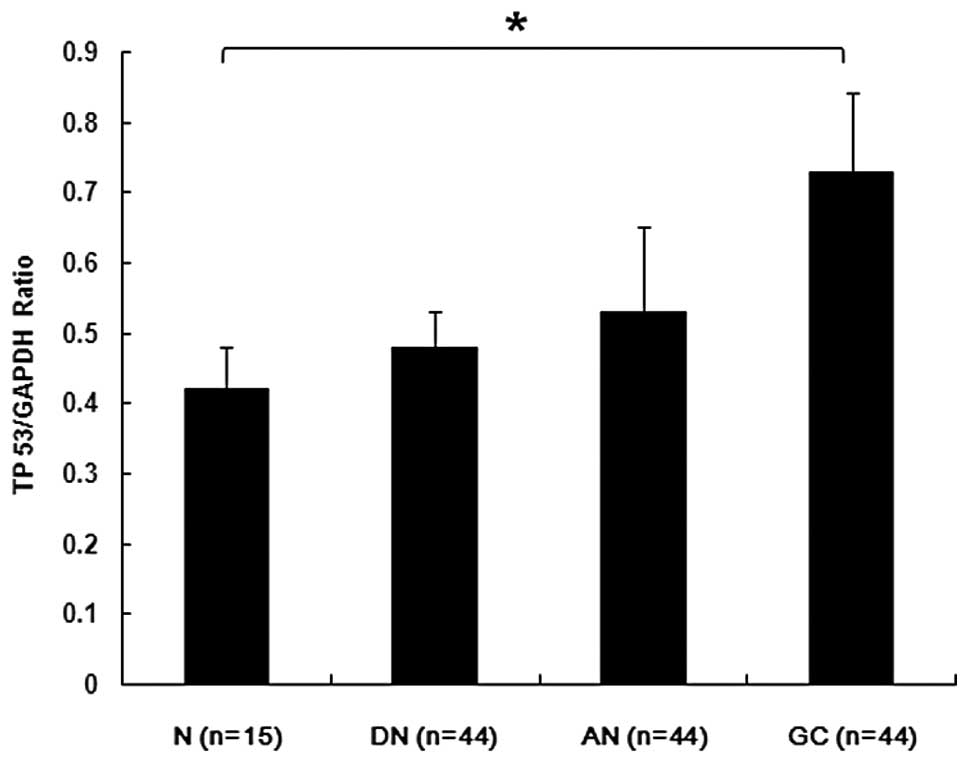
(27). TP 53 mRNA expression was
then examined in patient tissue. The TP 53 expression in tumor
tissue was higher compared with normal tissue adjacent to benign
gastric ulcer (Fig. 2).
TFF1 expression is regulated by DNA
methylation in GC tumor cells
Since numerous cancer cells exhibit aberrant
epigenetic regulation, it is possible that TFF1 expression is
regulated by epigenetic modification. To confirm that DNA
methylation regulates TFF1 expression, detailed methylation
analysis of the TFF1 gene sequence was performed using genomic DNA
extracted from cell lines and gastric tissue. To determine whether
TFF1 promoter methylation is relevant to TFF1 expression control in
gastric cancer cell lines and clinical specimens,
methylation-specific PCR was performed and compared with the
promoter methylation status in gastric cell lines SGC-7901 and in
tumor tissue with that in normal tissue adjacent to benign gastric
ulcers. Methylation of the CpG dinucleotides in the promoter region
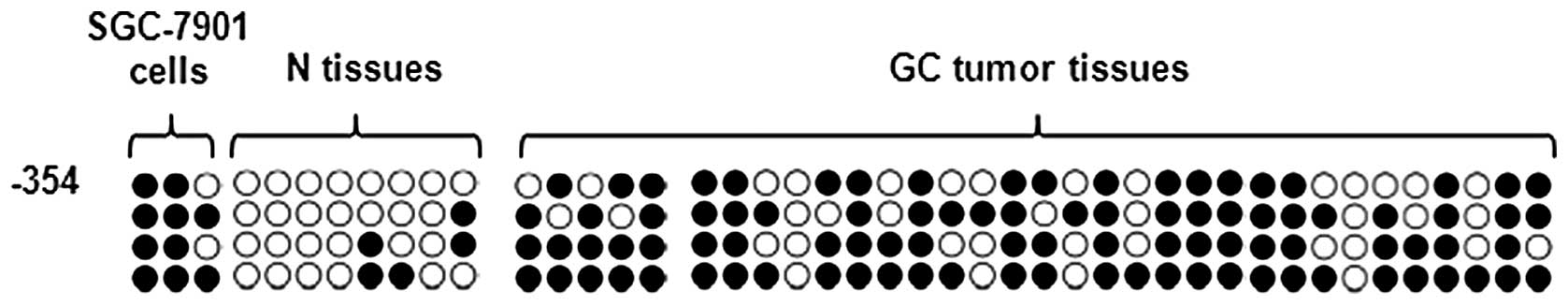
was detectable. As shown in Fig.
3, the results revealed that TFF1 were markedly methylated in
gastric cancer cell lines and tumor tissue (Fig. 3). The methylation status in the
promoter region appears to be correlated with TFF1 expression
levels in gastric cell lines or specimen tissue.
Effect of the CpG1 island on TFF1
promoter activity
To investigate the possible effect of methylation of
the promoter activity and to determine the functional significance,
a reporter gene construct was generated using a TFF1 promoter
sequence containing CpG island. The reporter construct (pTFF1),
together with pRL-TK Renilla luciferase expression vectorU,
were transiently transfected in BGC-823 and SGC-7901 cells,
followed by Aza treatment. The promoter activity was determined by
luciferase assay. Firefly and Renilla luciferase activities
were measured at the point indicated. Renilla luciferase
activity was used to normalize firefly luciferase activity of the
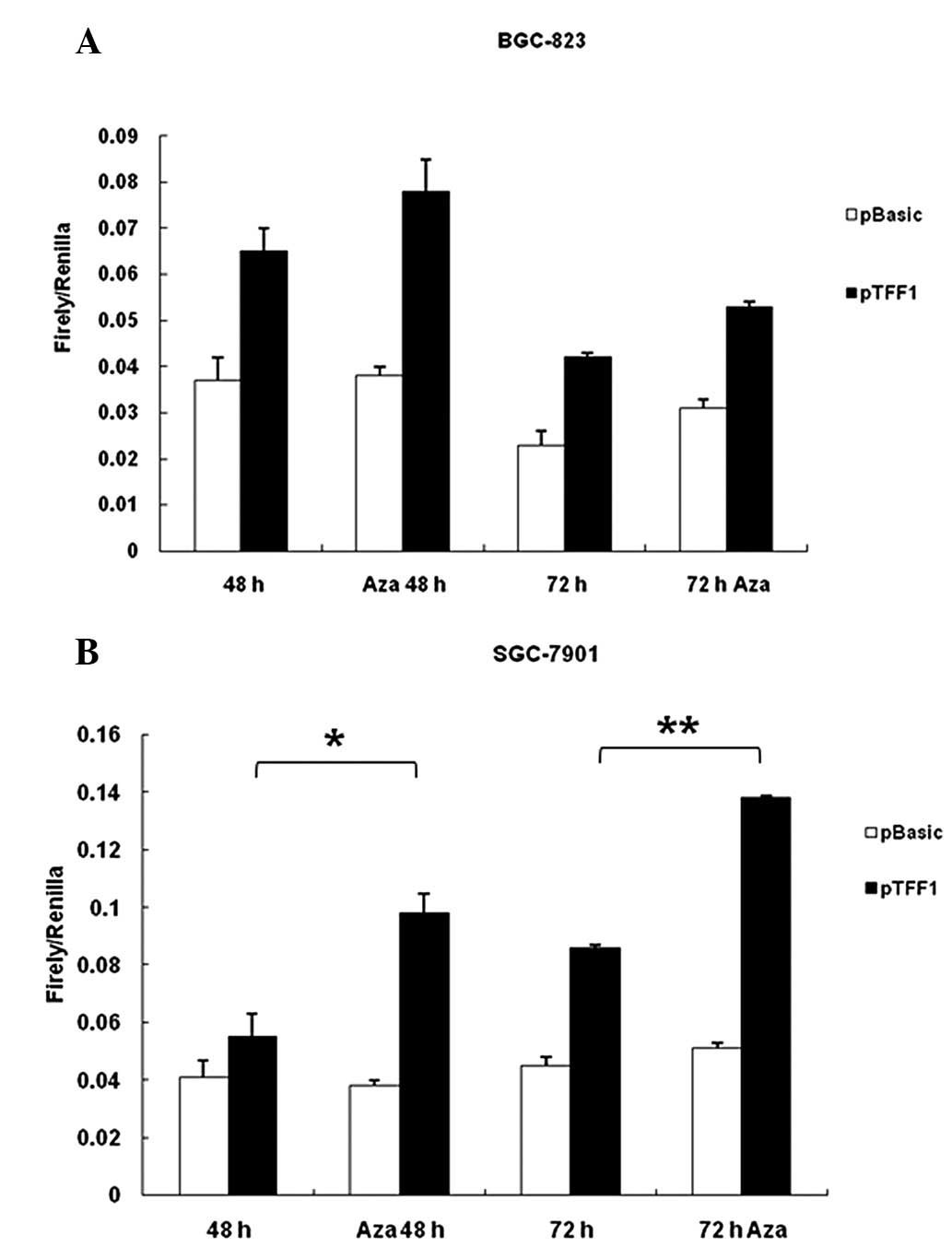
reporter constructs. As shown in Fig.
4, Aza treatment caused a significant increase in promoter
activity in the two cell lines, particularly in SGC-7901 cells
(Fig. 4B). Luciferase activity of
pGL3-basic, which has no promoter element, was not affected by Aza
treatment. The CpG islands in the plasmid were not methylated at
transfection (data not shown). Thus, the data suggest that the CpG
islands appear to be critical for TFF1 promoter activity.
Function of TFF1 in cell biology
It is unclear how TFF1 affects cell function. Cells
were synchronized at the G1/S boundary by double thymidine block
and underwent mitosis. Following 24 h, BrdU was added into the
medium at indicated time points to evaluate DNA synthesis. As shown
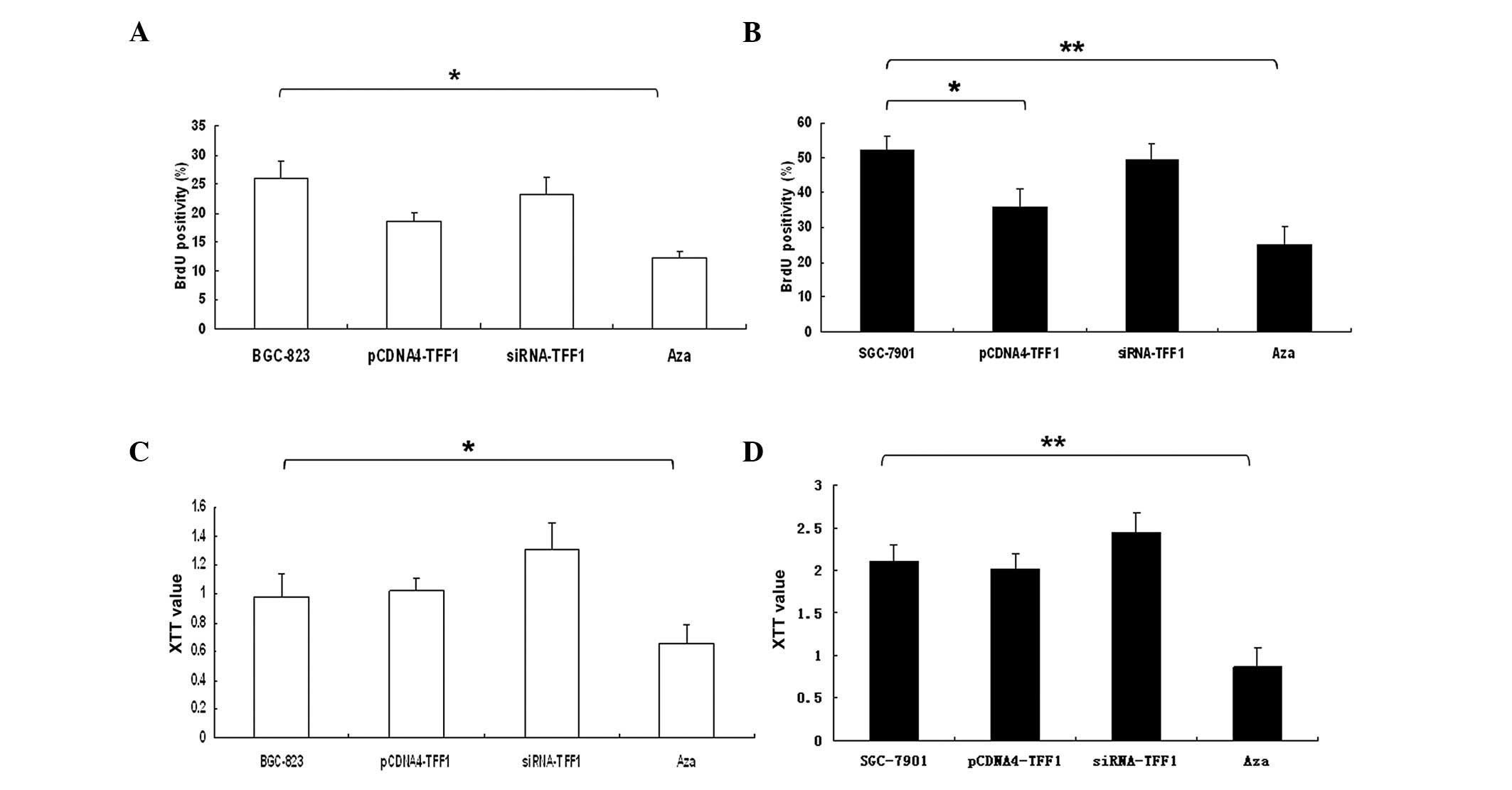
in Fig. 5A, incorporation of BrdU
into the control, accumulation of mitotic BGC-823 cells was
significantly inhibited by TFF1 siRNA (P<0.05). By contrast,
overexpression of TFF1 in SGC-7901 cells were significantly delayed
at 48 h (P<0.05), particularly in cells treated with Aza
(P<0.01) (Fig. 5B).
By XTT assays, TFF1 silencing of BGC-823 cells
resulted in a significant decreased of cell growth when compared
with control and others (P<0.05; Fig. 5C). However, Aza treatment in
SGC-7901 cells significantly inhibited cell growth compared with
the control group (P<0.01; Fig.
5D).
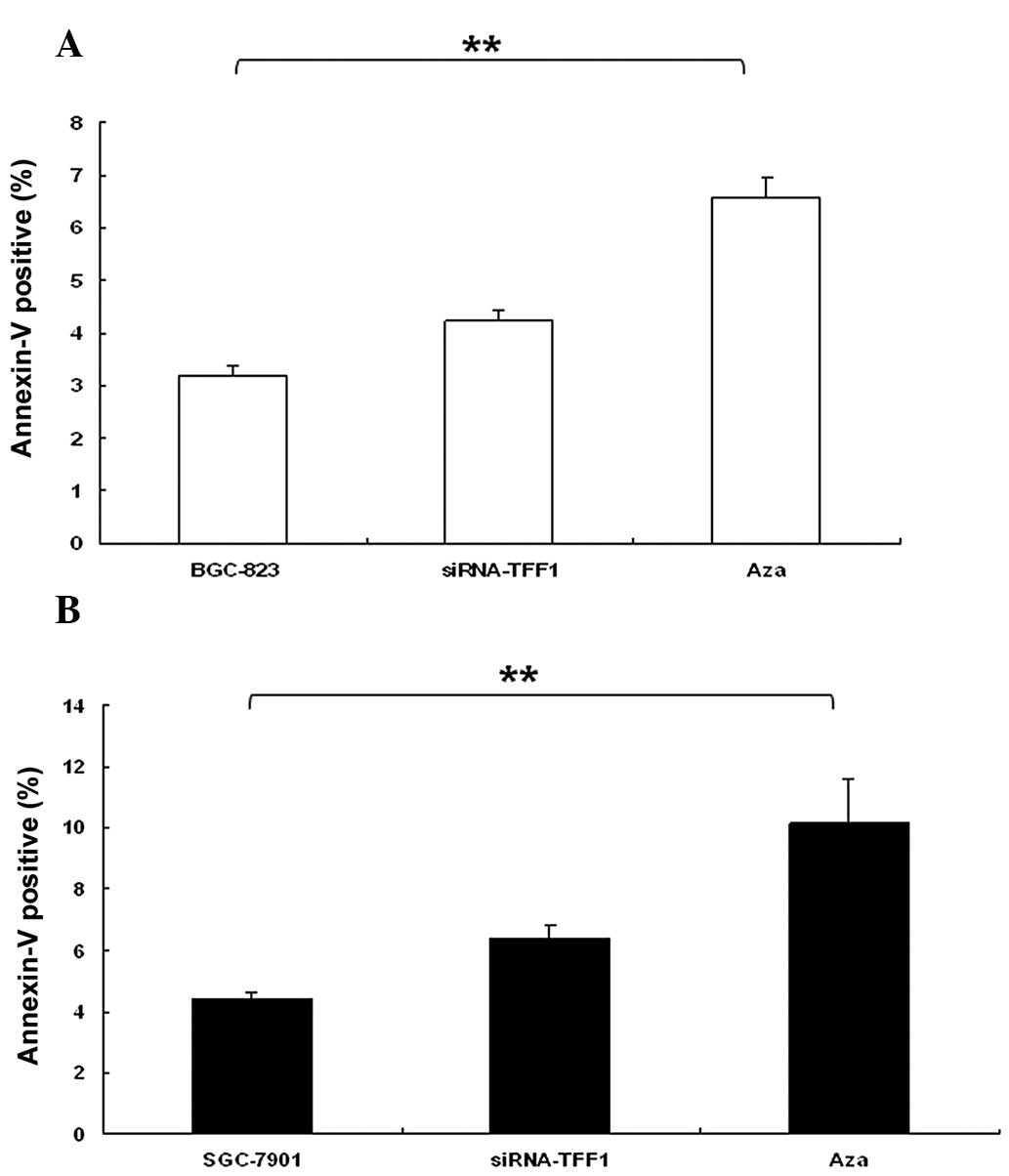
The effect of TFF1 expression on apoptosis
distribution was determined in BGC-823 and SGC-7901 cells by flow
cytometry. Downregulation of TFF1 in BGC-823 cells promoted
significant apoptosis (P<0.01; Fig.
6A). Furthermore, SGC-7901 cells treated with Aza induced an
increase in apoptosis (P<0.01; Fig.
6B). This maybe associated with low levels of TP 53,
particularly in SGC-7901 (Fig.
7B).
Discussion
It is well established that carcinogenesis undergoes
multistep progression, which includes gastric mucosa, chronic
gastritis, atrophy, intestinal metaplasia and dysplasia, with the
involvement of multiple genetic alterations (28). The molecular changes of DNA are
represented by the activation of oncogenes, inactivation of tumor
suppressor genes, LOH and defects in DNA damage response and repair
mechanisms. Another factor which plays a key role in the regulation
of gene expression is epigenetic changes in gene expression
patterns due to mechanisms other than mutation in the underlying
DNA sequence, including DNA methylation and histone modifications
(29).
The TFF1 gene has been localized to chromosome 21q,
the long arm of human chromosome 21 and has been identified with
three exons, two introns and two promoters. The small molecule
peptide TFF1 is primarily secreted by gastrointestinal mucosa cells
and has been confirmed to function in gastrointestinal mucosa
protection and repair (30). Under
normal conditions, the TFF1 gene is primarily expressed in mucosal
epithelial cells in the gastric body and antrum, while under
pathological conditions, its expression specificity disappears
(8) and TFF1 may be secreted in
any gastrointestinal mucosal injury sites (31). The molecular forms of TFF1
identified in normal human gastric mucosa are: TFF1 monomer, TFF1
dimer which is rather rare and the predominant TFF1 complex with a
molecular mass of ~25 kDa (32).
According to a large number of animal tests, trefoil peptide
conducts regulatory functions on mammals, namely mucosal protection
and epithelial cell reconstruction, tumor suppression, signal
transduction and regulation of apoptosis (28).
In a previous study (30), an altered DNA methylation pattern
was found to play a definitive role in the regulation of TFF1 gene
expression in human gastric cancers. In the current study, there is
evidence to support the hypothesis that DNA methylation is a key
mechanism of epigenetic regulation to suppress TFF1 expression in
gastric cancer cells and identify the precise methylation area in
the TFF1 gene. This study provides an important insight into
epigenetic regulation in GC.
Aberrant epigenetic states may predispose an
individual to genetic changes; however, genetic changes may also
initiate aberrant epigenetic events. Epigenetic and genetic
mechanisms may thus work together to silence key cellular genes and
destabilize the genome, leading to oncogenic transformation and
observed the complexity and heterogeneity in human cancers,
including GC (33–35). The development of GC results from a
multistep process beginning with the accumulation of genetic and
epigenetic alterations in regulatory genes (3). In general, cancer cells have global
hypomethylation; however, they exhibit hypermethylation in specific
genes. DNA hypermethylation in promoter regions is associated with
silencing of tumor suppressor genes due to direct or indirect
prevention to accessing transcription factors in the promoter
region (36). Previous studies
(3,37,38)
have demonstrated that CpG island hypermethylation, via silencing
of key cancer-related genes, plays a major causal role in cancer,
including GC.
The current study of TFF1 gene methylation provides
a novel insight into the epigenetic regulation in GC. In the study,
it is clear that TFF1 expression is suppressed in gastric cancer
cell lines and tumor tissue. Moreover, TFF1 expression in gastric
cancer cell lines may be restored by treatment with the
demethylating agent Aza, particularly in high differentiation
SGC-7901 associated with TP 53, but not associated with low
differentiation BGC-823. We demonstrate further that the
methylation status of the CpG-rich area in the promoter region is
correlated with TFF1 gene expression in gastric cancer cell lines.
The enhanced TFF1 promoter activity in a reporter assay in the
gastric cancer cell lines was tested. These observations indicate
that DNA methylation regulates TFF1 expression in gastric cancer
cells.
The majority of studies investigating the mechanisms
which regulate gene expression by CpG methylation focus on CpG
islands in the promoter. In the present study, the TFF1 promoter
region (−354) was found. Hypermethylation of CpG islands in
promoter sequences is associated with silencing of tumor suppressor
genes and tumor-related genes by subsequent downregulation of mRNA
transcription expression. Epigenetic silenced genes are involved in
important molecular pathways of carcinogenesis e.g., cell cycle
regulation, apoptosis, DNA repair or cell adhesion. The imbalance
between cell proliferation and death is considered to be an early
and significant event in the carcinogenic process, thus, it is
desirable to develop a new strategy to induce apoptosis and
proliferation inhibition in tumor cells. The results of the study
demonstrated that TFF1 inhibits the proliferation of gastric cancer
cell lines and promotes apoptosis. TFF1 is hypothesized to play a
role in suppressing GC tumorigenesis.
Another observation in this experiment was the high
methylation rate in GC. Therefore, it is hypothesized that
methylation may be involved in the downregulation of TFF1
expression in GC while the particular molecular mechanism remains
to be defined. Methylation is a salient feature of mammalian
genomes. It promotes gene diversity of humans, while the loss of
DNA methylation may result in gene mutation and rearrangement, and
contributes to genomic instability. In the human body, methylation
only occurs on cytosine residues, primarily those in CpG islands.
Under normal conditions, CpG islands are not methylated if they are
located upstream of house-keeping genes, which is expressed at
relatively constant levels. The high methylation levels of
house-keeping genes may inhibit their expression. In addition,
hypomethylation in the overall genome level and hypermethylation in
a number of particular gene sites, particularly promoter sequences,
is likely to break the normal methylation pattern and impact gene
expression regulation and cellular differentiation (39).
A number of studies observed potential connections
between hypermethylation in promoter sites and inactivation of
genes related to specific familial cancers, including RBI, CHL,
E-cad and P16. There are a number of genes in gastric cancer where
the promoter methylation rate is >50%: Cox22 (86.6%) (40), E-cadherin (75.9%), DAP-kinase
(70.3%), P15 (68.5%), P16 (66.7%) (41), 06-MGMT (61%) (42), RASSFIA (60%) (43), TMP-3 (57.4%) (44). Furthermore, the positive rate in
multigene methylation detection is significantly higher compared
with the detections at a single gene. In short, promoter
methylation in cancer-related genes is an early and frequent event
during carcinogenesis. Therefore, it holds a biological and
clinical significance in the early diagnosis for gastric cancer,
which is essential to cancer treatment and follow-up of surgical
patients. In addition, DNA methylation is beneficial for cancer
diagnosis since it is not only detected in resected tissues, but
also in various body fluids, including fluids in cancerous organs,
peripheral blood, saliva and sputum (44). To conclude, promoter methylation in
cancer-related genes is an extremely promising novel biomarker.
By contrast, DNA methylation is a reversible process
and gene expression may be restored with methylation inhibitors
(45). Thus, the normal growth
regulation pattern may be restored by demethylating genes prior to
mutation or other damages. It may also be a possible pathway for
cancer treatment in the future.
The marked downregulation or absence of TFF1 mRNA
and protein expression in GC tissue specimens and high expression
level in adjacent non-tumor tissue suggests that TFF1 may be
involved in the inhibition of gastric cancer. Adjacent non-tumor
tissues secrete additional TFF1 to suppress tumor growth. Moreover,
according to previous literature, the TFF1 expression level in the
gastric mucosa which borders gastric ulcers is higher compared with
the distant mucosa (46–49). The high expression level in mucosa
adjacent to GC or gastric ulcer suggests that TFF1 plays a
significant role in mucosal protection and epithelial cell
reconstruction. A potential mechanism by which this occurs is by
enhancing the replication of adjacent intact epithelial cells to
replace the damaged mucous membrane. In addition, there are two
possible causes for the low expression level or absence of TFF1 in
GC: i) potential genetic alterations, including gene mutation, LOH
and DNA methylation and ii) poorly differentiated glands and cells,
which are too damaged to secrete TFF1. In conclusion, TFF1 is a
potential suppressor of gastric cancer and may be involved in
inhibiting gastric carcinogenesis and development.
According to the results of these experiments, the
TFF1 promoter site was hypermethylated, the proportion being
40.91%. The location of these methylated sites was among three
CmCGG (cytosine-methylcytosine-guanine-guanine) sequences: −354,
−84 and −20 nt, particularly in −354. Although in the other ~60%
carcinoma samples, no methylation was detected, the TFF1 mRNA and
protein expression levels were significantly downregulated. There
are two possible causes for this phenomenon: i) methylation in
other sites which are outside the promoter region and ii) gene
mutation, LOH or other genetic alterations. DNA methylation blocks
the tumor suppressor role of the TFF1 gene since the secretion of
TFF1 mRNA and peptides were affected.
The marked downregulation of TFF1 secretion in GC
suggests that TFF1 may be an inhibitory factor of gastric cancer
and that the TFF1 gene may be a tumor suppressor gene. Also, the
high methylation rate in TFF1 of GC suggests that methylation in
the TFF1 DNA promoter region may attribute to the reduced or absent
TFF1 mRNA expression, hence carcinogenesis of gastric cancer. This
study provides a potential target point for the future treatment of
gastric cancer, however the molecular mechanisms for TFF1 in
carcinogenesis and progression of gastric cancer remains to be
studied further. There are a number of hypotheses which indicate
that DNA methylation in the promoter region is reversible. All
these studies are invaluable for further cancer research and
biologically targeted therapy for cancer treatment, particularly in
GC associated with TFF1.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 30600524, 81071990,
81172383 and 81201758).
References
|
1
|
Stadtländer CT and Waterbor JW: Molecular
epidemiology, pathogenesis and prevention of gastric cancer.
Carcinogenesis. 20:2195–2208. 1999.
|
|
2
|
Ren J, Chen Z, Juan SJ, Yong XY, Pan BR
and Fan DM: Detection of circulating gastric carcinoma-associated
antigen MG7-Ag in human sera using an established single
determinant immuno-polymerase chain reaction technique. Cancer.
88:280–285. 2000. View Article : Google Scholar
|
|
3
|
Tischoff I and Tannapfel A: DNA
methylation in hepatocellular carcinoma. World J Gastroenterol.
14:1741–1748. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baylin SB and Herman JG: DNA
hypermethylation in tumorigenesis: epigenetics joins genetics.
Trends Genet. 16:168–174. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Riggs AD and Pfeifer GP: X-chromosome
inactivation and cell memory. Trends Genet. 8:169–174. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Razin A and Cedar H: DNA methylation and
genomic imprinting. Cell. 77:473–476. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thim L and May FE: Structure of mammalian
trefoil factors and functional insights. Cell Mol Life Sci.
62:2956–2973. 2005.PubMed/NCBI
|
|
8
|
Ribieras S, Tomasetto C and Rio MC: The
pS2/TFF1 trefoil factor, from basic research to clinical
applications. Biochim Biophys Acta. 1378:F61–F77. 1998.PubMed/NCBI
|
|
9
|
Corte MD, Tamargo F, Alvarez A, Rodríguez
JC, Vázquez J, Sánchez R, Lamelas ML, González LO, Allende MT,
García-Muñiz JL, Fueyo A and Vizoso F: Cytosolic levels of TFF1/pS2
in breast cancer: their relationship with clinical-pathological
parameters and their prognostic significance. Breast Cancer Res
Treat. 96:63–72. 2006. View Article : Google Scholar
|
|
10
|
Rio MC, Bellocq JP, Daniel JY, Tomasetto
C, Lathe R, Chenard MP, Batzenschlager A and Chambon P: Breast
cancer-associated pS2 protein: synthesis and secretion by normal
stomach mucosa. Science. 241:705–708. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taupin D, Pedersen J, Familari M, Cook G,
Yeomans N and Giraud AS: Augmented intestinal trefoil factor (TFF3)
and loss of pS2 (TFF1) expression precedes metaplastic
differentiation of gastric epithelium. Lab Invest. 81:397–408.
2001. View Article : Google Scholar
|
|
12
|
Carvalho R, Kayademir T, Soares P, Canedo
P, Sousa S, Oliveira C, Leistenschneider P, Seruca R, Gött P, Blin
N, Carneiro F and Machado JC: Loss of heterozygosity and promoter
methylation, but not mutation, may underlie loss of TFF1 in gastric
carcinoma. Lab Invest. 82:1319–1326. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katoh M: Trefoil factors and human gastric
cancer (review). Int J Mol Med. 12:3–9. 2003.
|
|
14
|
McChesney PA, Aiyar SE, Lee OJ, Zaika A,
Moskaluk C, Li R and El-Rifai W: Cofactor of BRCA1: a novel
transcription factor regulator in upper gastrointestinal
adenocarcinomas. Cancer Res. 66:1346–1353. 2006. View Article : Google Scholar
|
|
15
|
Park WS, Oh RR, Park JY, Yoo NJ, Lee SH,
Shin MS, Kim SY, Kim YS, Lee JH, Kim HS, An WG and Lee JY: Mapping
of a new target region of allelic loss at 21q22 in primary gastric
cancers. Cancer Lett. 159:15–21. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ribieras S, Lefèbvre O, Tomasetto C and
Rio MC: Mouse Trefoil factor genes: genomic organization, sequences
and methylation analyses. Gene. 266:67–75. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujimoto J, Yasui W, Tahara H and Tahara
E, Kudo Y, Yokozaki H and Tahara E: DNA hypermethylation at the pS2
promoter region is associated with early stage of stomach
carcinogenesis. Cancer Lett. 149:125–134. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tomita H, Takaishi S, Menheniott TR, Yang
X, Shibata W, Jin G, Betz KS, Kawakami K, Minamoto T, Tomasetto C,
Rio MC, Lerkowit N, Varro A, Giraud AS and Wang TC: Inhibition of
gastric carcinogenesis by the hormone gastrin is mediated by
suppression of TFF1 epigenetic silencing. Gastroenterology.
140:879–891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park WS, Oh RR, Park JY, Lee JH, Shin MS,
Kim HS, Lee HK, Kim YS, Kim SY, Lee SH, Yoo NJ and Lee JY: Somatic
mutations of the trefoil factor family 1 gene in gastric cancer.
Gastroenterology. 119:691–698. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sankpal NV, Mayo MW and Powell SM:
Transcriptional repression of TFF1 in gastric epithelial cells by
CCAAT/enhancer binding protein-beta. Biochim Biophys Acta.
1728:1–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calnan DP, Westley BR, May FE, Floyd DN,
Marchbank T and Playford RJ: The trefoil peptide TFF1 inhibits the
growth of the human gastric adenocarcinoma cell line AGS. J Pathol.
188:312–317. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lefebvre O, Chenard MP, Masson R, Linares
J, Dierich A, LeMeur M, Wendling C, Tomasetto C, Chambon P and Rio
MC: Gastric mucosa abnormalities and tumorigenesis in mice lacking
the pS2 trefoil protein. Science. 274:259–262. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng Z, Chen J, Wei H, Gao P, Shi J, Zhang
J and Zhao F: The risk factor of gallbladder cancer: hyperplasia of
mucous epithelium caused by gallstones associates with
p16/CyclinD1/CDK4 pathway. Exp Mol Pathol. 91:569–577. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Liu H, Chen K, Xiao J, He K, Zhang
J and Xiang G: SIRT1 promotes tumorigenesis of hepatocellular
carcinoma through PI3K/PTEN/AKT signaling. Oncol Rep. 28:311–318.
2012.PubMed/NCBI
|
|
25
|
Li L, Zhang J, Yang Y, Wang Q, Gao L, Yang
Y, Chang T, Zhang X, Xiang G, Cao Y, Shi Z, Zhao M and Gao G:
Single-wall carbon nanohorns inhibited activation of microglia
induced by lipopolysaccharide through blocking of Sirt3. Nanoscale
Res Lett. 8:1002013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, Bergeron L, Cryns V, Pasternack MS,
Zhu H, Shi L, Greenberg A and Yuan J: Activation of caspase-2 in
apoptosis. J Biol Chem. 272:21010–21017. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fenoglio-Preisern CM, Wang J, Stemmermann
GN and Noffsinger A: TP53 and gastric carcinoma: a review. Hum
Mutat. 21:258–270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tahara E: Genetic pathways of two types of
gastric cancer. IARC Sci Pub. 157:327–349. 2004.PubMed/NCBI
|
|
29
|
Ballestar E and Wolffe AP:
Methyl-CpG-binding proteins. Targeting specific gene repression.
Eur J Biochem. 268:1–6. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Babyatsky MW, deBeaumont M, Thim L and
Podolsky DK: Oral trefoil peptides protect against ethanol and
indomethacin-induced gastric injury in rats. Gastroenterology.
110:489–497. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pera M, Heppell J, Poulsom R, Teixeira FV
and Williams J: Ulcer associated cell lineage glands expressing
trefoil peptide genes are induced by chronic ulceration in ileal
pouch mucosa. Gut. 48:792–796. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Newton JL, Allen A, Westley BR and May FE:
The human trefoil peptide, TFF1, is present in different molecular
forms that are intimately associated with mucus in normal stomach.
Gut. 46:312–320. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Herath NI, Leggett BA and MacDonald GA:
Review of genetic and epigenetic alterations in
hepatocarcinogenesis. J Gastroenterol Hepatol. 21:15–21. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li HP, Leu YW and Chang YS: Epigenetic
changes in virus-associated human cancers. Cell Res. 15:262–271.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Herceg Z: Epigenetics and cancer: towards
an evaluation of the impact of environmental and dietary factors.
Mutagenesis. 22:91–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adrien LR, Schlecht NF, Kawachi N, Smith
RV, Brandwein-Gensler M, Massimi A, Chen S, Prystowsky MB, Childs G
and Belbin TJ: Classification of DNA methylation patterns in tumor
cell genomes using a CpG island microarray. Cytogenet Genome Res.
114:16–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lund AH and van Lohuizen M: Epigenetics
and cancer. Genes Dev. 18:2315–2335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sparmann A and van Lohuizen M: Polycomb
silencers control cell fate, development and cancer. Nat Rev
Cancer. 6:846–856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baylin SB: Mechanisms underlying
epigenetically mediated gene silencing in cancer. Semin Cancer
Biol. 12:331–337. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hur K, Song SH, Lee HS, Ho Kim W, Bang YJ
and Yang HK: Aberrant methylation of the specific CpG island
portion regulates cyclooxygenase-2 gene expression in human gastric
carcinomas. Biochem Biophys Res Commun. 310:844–851. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee TL, Leung WK, Chan MW, Ng EK, Tong JH,
Lo KW, Chung SC, Sung JJ and To KF: Detection of gene promoter
hypermethylation in the tumor and serum of patients with gastric
carcinoma. Clin Cancer Res. 8:1761–1766. 2002.PubMed/NCBI
|
|
42
|
Carvalho B, Pinto M, Cirnes L, Oliveira C,
Machado JC, Suriano G, Hamelin R, Carneiro F and Seruca R:
Concurrent hypermethylation of gene promoters is associated with a
MSH-H phenotype and diploidy in gastric carcinomas. Eur J Cancer.
39:1222–1227. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Byun DS, Lee MG, Chae KS, Ryu BG and Chi
SG: Frequent epigenetic inactivation of RASSF1A by aberrant
promoter hypermethylation in human gastric adenocarcinoma. Cancer
Res. 61:7034–7038. 2001.PubMed/NCBI
|
|
44
|
Sozzi G, Conte D, Leon M, Ciricione R, Roz
L, Ratcliffe C, Roz E, Cirenei N, Bellomi M, Pelosi G, Pierotti MA
and Pastorino U: Quantification of free circulating DNA as a
diagnostic marker in lung cancer. J Clin Oncol. 21:3902–3908. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Toyota M and Issa JP: The role of DNA
hypermethylation in human neoplasisa. Electrophoresis. 21:329–333.
2000. View Article : Google Scholar
|
|
46
|
Poulsen SS, Thulesen J, Christensen L,
Nexo E and Thim L: Metabolism of oral trefoil factor 2 (TFF2) and
the effect of oral and parenteral TFF2 on gastric and duodenal
ulcer healing in the rat. Gut. 45:516–522. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wong WM, Poulsom R and Wright NA: Trefoil
peptides. Gut. 44:890–895. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Longman RJ, Douthwaite J, Sylvester PA,
Poulsom R, Corfield AP, Thomas MG and Wright NA: Coordinated
localisation of mucins and trefoil peptides in the ulcer associated
cell lineage and the gastrointestinal mucosa. Gut. 47:792–800.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dignass A, Lynch-Devaney K, Kindon H, Thim
L and Podolsky DK: Trefoil peptides promote epithelial migration
through a transforming growth factor beta-independent pathway. J
Clin Invest. 94:376–383. 1994. View Article : Google Scholar : PubMed/NCBI
|