Introduction
Sepsis is a major cause of multiple organ
dysfunction syndromes (MODS) in intensive care units (1,2). It
is associated with the development of acute multiorgan dysfunction,
morbidity and mortality and great increases in treatment
expenditure. Sepsis is usually initiated by microbial agents or
their products, such as lipopolysaccharide (LPS), an outer membrane
component of gram-negative bacteria. Sepsis induces a rapid release
of inflammatory mediators, including tumor necrosis factor α
(TNF-α), γ interferon, interleukin-1β (IL-lβ) and interleukin-6
(IL-6) (3,4), which leads to systemic inflammatory
response syndrome (SIRS), MODS and mortality. When SIRS results in
MODS and organ failure, the mortality becomes higher and may be
>50% (4–6).
Local anesthetics have been shown to modulate
inflammatory cascades (7) and
provide protection from ischemic reperfusion injury in the heart
(8), lung (9,10)
and liver (11). Local anesthetics
are capable of exerting anti-inflammatory effects on various cell
types, including monocytes, macrophages and neutrophils (7). Lidocaine has been used as a
traditional local anesthetic in medicine for >100 years. In
addition to its anesthetic properties, lidocaine has been shown to
attenuate inflammatory responses in vivo and in vitro
and to possess anti-inflammatory and -infective effects. Lidocaine
may also significantly improve the survival of mice and rats
suffering from endotoxic shock (12) and protect them against liver and
renal injury resulting from cecal ligation and puncture-induced
septic peritonitis (13). Although
lidocaine is crucial for immune function and inflammation, the
mechanisms involved in its action are poorly understood.
Toll-like receptors (TLRs) recognize distinct
microbial components that initiate the innate and adaptive immune
responses. TLR activation culminates in the expression of
appropriate proinflammatory and immunomodulatory factors to meet
pathogenic challenges. Toll-like receptor 4 (TLR4), the first TLR
found in mammals, is important for regulating the immune response
and inflammatory reaction (14,15).
Stimulation of TLR4 by pathogens activates signal transduction
pathways that lead to the induction of a range of antimicrobial
genes and inflammatory cytokines (16).
In the present study, the mechanisms of whether the
protective effects of lidocaine against LPS-induced renal and
hepatic dysfunction in sepsis are associated with the activities of
the TLR4 signaling pathway and nuclear factor κB (NF-κB) were
explored in vivo. Establishment of a rat model of sepsis
that produced sepsis characterized by an initial hyperinflammatory
response through lidocaine treatment was attempted in order to
assess the relevance between the anti-inflammatory effects of
lidocaine-based drugs and their mechanism of action. The results
showed that lidocaine modifies the inflammatory response induced by
LPS and affects the outcome from sepsis, which may be via
inhibition of the TLR4 signaling pathway and inflammatory
response.
Materials and methods
Materials
Lidocaine (10%) was donated by Shanghai Chaohui
Pharma Ltd. (Shanghai, China). TLR4 and NF-κB antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). qPCR kits were purchased from Takara Bio, Inc. (Otsu, Japan)
and IL-6 ELISA kit was purchased from Mai Biotechnology Institute
(Hangzhou, China).
Animal care and treatment
Male Sprague-Dawley (SD) rats (SPF grade; 220–250 g)
were provided by the Shanghai Jiaotong University Medical School
(SHSMU; Shanghai, China). The procedures for experiments and animal
care were approved by the Institutional Animal Care and Use
Committee of SHSMU and conformed to the Guide for the Care and Use
of Laboratory Animals by the National Institutes of Health (NIH
Publication no. 80–23). Animals were maintained under standard
laboratory conditions at 22–30°C and normal photoperiod (12-h
dark/light). Adult male SD rats (n=30) were randomly divided into
the following three groups: control, sepsis model and 10% lidocaine
(5 mg/kg). The sepsis models were established by injection of LPS
(5 mg/kg) into the intraperitoneal cavity of rats. The same volume
of saline was injected intraperitoneally (i.p.) into rats of the
control group instead of LPS. The lidocaine group of rats were
treated with 10% lidocaine through tail vein injection, once every
2 h continuously for 24 h following LPS (the administration times
were calculated according to the half-time of lidocaine). The rats
were then anesthetized with pentobarbital (30 mg/kg; i.p.). All the
rats were handled at 24 h as follows: i) the right eyeball of each
model rat was removed to collect a 3 ml blood sample for
biochemical tests; and ii) rats were sacrificed, the abdominal
cavity was rapidly opened and the appropriate liver and kidney
tissues extracted for subsequent examinations.
Blood tests
Venous blood (3 ml) was collected from animals in
all the groups. Serum was prepared and stored in aliquots at −70°C
prior to analysis. Serum concentrations of aspartate
aminotransferase (AST), alanine aminotransferase (ALT), creatinine
(Cr) and blood urea nitrogen (BUN) were determined using a Hitachi
Automatic Biochemical Analyzer (Hitachi High-Technologies
Corporation (Tokyo, Japan). according to the manufacturer’s
instructions.
Hematoxylin and eosin (H&E)
staining
Tissue blocks were collected randomly from the
liver, lungs and kidneys, fixed with 4% paraformaldehyde for ~12 h
and embedded with wax. Coronal sections (4 μm) were dewaxed,
stained with H&E and examined under light microscopy for
histological changes. Digital images were captured.
qPCR detection of TLR4 mRNA
expression
Total RNA from the liver and kidney samples was
isolated with TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer’s instructions. Reverse transcription
was carried out using a RevertAid First Strand cDNA Synthesis kit
(Qiagen, Hilden, Germany) and the resultant single strand cDNA was
stored at −20°C for later use in the PCR reactions. qPCR was
performed by application of the SYBR®
ExScript® RT-PCR kit (Takara Bio, Inc., Otsu, Japan) and
using a two-step PCR reaction under the following conditions: 95°C
for 10 sec, 95°C for 5 sec and 40 cycles at 60°C for 30 sec.
β-actin was used as the housekeeping gene, according to Gene Bank
in TLR4 and β-actin (NM_019178 and NM_001101.3). The primers used
were: TLR4 forward, 5′-AGCCATTGCTGCCAA CATCA-3′ and reverse,
5-ATGCAGGGGTTCTGG-3′ (148 bp); and β-actin forward,
5′-CCCATCTATGAGGGTT ACGC-3′ and reverse,
5′-TTTAATGTCACGCACGATTTC-3′ (150 bp). TLR4 mRNA levels were
calculated based on the 2−ΔΔCt method.
Flow cytometry
Suitable liver and kidney samples were transferred
into flat plates with Hank’s solution and ground into single cells
with a homogenizer. The single cell suspension was filtered with
200 holes grit and cells were centrifuged twice with Hank’s
solution at 1,000 rpm for 10 min. The supernatant solution was
discarded and 0.5 ml distilled water was added for 20 sec, rapidly
followed by the addition of 1 ml sodium chloride solution (1.7%)
and mixed. Hank’s solution (8 ml) was added and cells were
centrifuged at 1,000 rpm for 10 min. The cellular sediment was
resuspended in Hank’s solution. The cell survival rate was detected
by trypan blue stain and the cell number was adjusted to
2×107/ml with Hank’s solution. TLR4 antibody (0.5 μg)
was added into the 100 μl cell suspension, incubated in a dark room
at 4°C for 30 min and then PBS (pH 7.2–7.4) was added to a total
volume to 0.5 ml. The cell suspension was mixed gently and then the
cells were detected by flow cytometry (Becton-Dickinson, Franklin
Lakes, NJ, USA).
Western blotting
Liver and kidney sample lysates were prepared by
sonication using a RIPA buffer solution with protease inhibitor
(Roche Diagnostics, Indianapolis, IN, USA). Extracts were
centrifuged at 12,000 × g and the supernatant was retained. Protein
concentrations were determined using the BCA Protein Assay kit
(Pierce Biotechnology Inc., Rockford, IL, USA). Prior to loading,
2.5% β-mercaptoethanol and 0.0125% bromophenol blue were added and
lysates were boiled for 5 min. The lysed protein was separated by
12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and
transferred onto PVDF membranes (Millipore, Billerica, MA, USA) by
wet transfer at 200 mA and 4°C for 2 h. The membrane was blocked
with 5% (w/v) non-fat milk in Tris-buffered saline/0.05% Tween
(v/v) for 2 h and incubated with the following primary antibodies
at 4°C overnight: anti-NF-κB, 1:1,000; or anti-β-actin, 1:1,000 (as
control). The membranes were incubated with the proper radish
peroxidase-conjugated secondary antibody at room temperature for 1
h. Immunoblots were visualized using an enhanced chemiluminescence
detection kit (Pierce Biotechnology, Inc.).
IL-6 measurement
Liver and kidney tissues were prepared and organized
in a UP-200H ultrasounic homogenizer (Hielscher Ultrasonics GmbH,
Teltow, Germany). The homogenized liquid was centrifuged at 4,000
rpm for 15 min. The rat IL-6 ELISA kit (Mai Biotechnology
Institute) was used for detection according to the manufacturer’s
instructions.
Statistical analysis
Data are expressed as mean ± SEM. Comparisons of
data between groups were made using a two-sample t-test, assuming
equal variances. P<0.05 was considered to indicate a
statistically significant difference. All data were analyzed using
SPSS 10.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Lidocaine reduces mortality and protects
renal and hepatic injury in septic rats
To determine whether lidocaine reduces mortality and
protects renal and hepatic injury in septic rats, mortality was
first assessed in all the groups. By the end of the experiment, all
10 rats had survived in the control group. However, the 5th and 1st
rat in the LPS-induced sepsis and lidocaine groups, respectively,
had died. Compared with the sepsis group, the mortality rate was
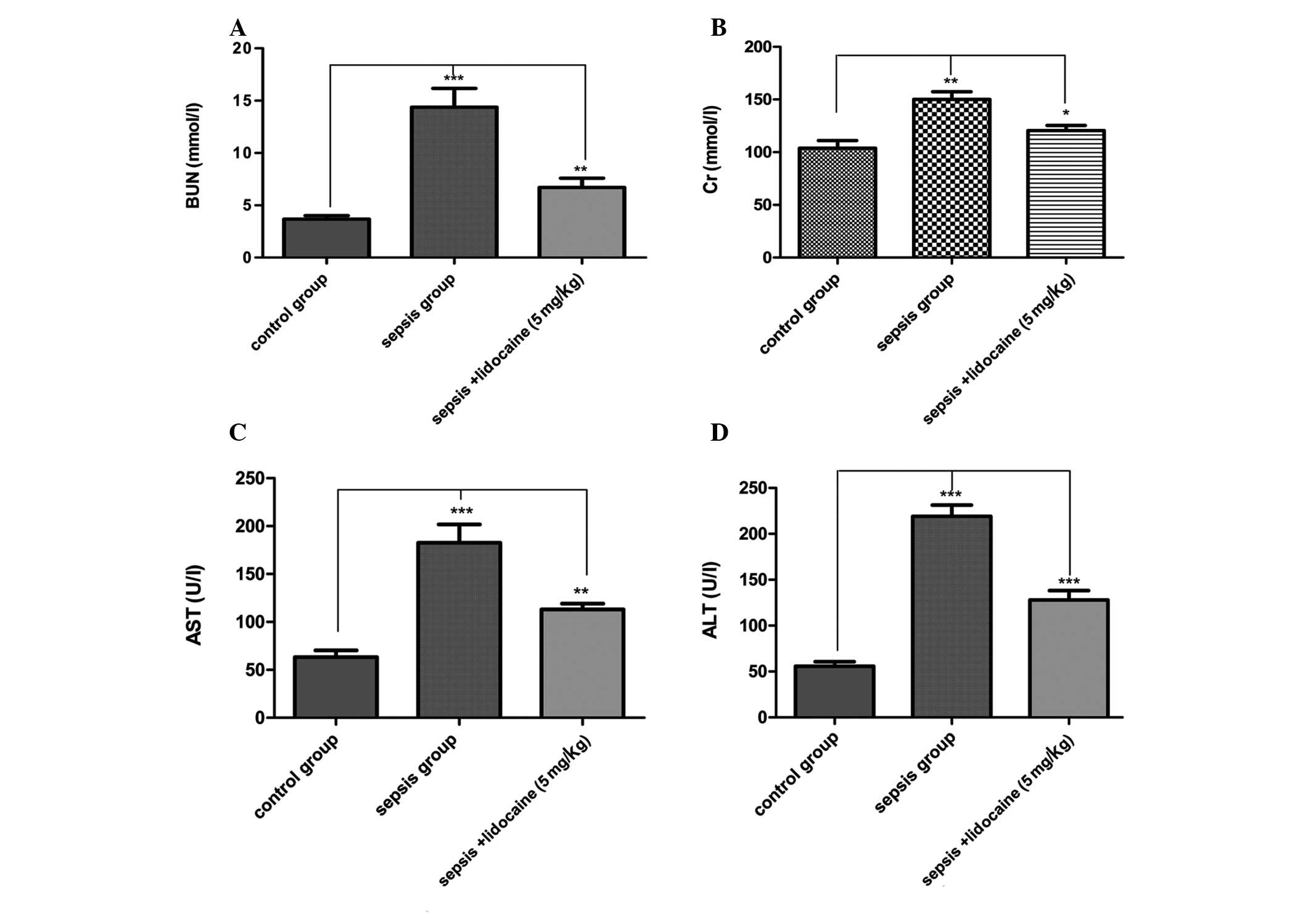
significantly lower in the lidocaine group. To evaluate changes in
the cellular integrity and functionality of the organs in the
various groups, blood tests were performed for renal and hepatic
function following sepsis by measuring plasma AST, ALT, Cr and BUN
concentrations. For all the parameters tested, the values were
significantly elevated in the LPS-induced sepsis group compared
with those of the control group of animals. However, lidocaine
treatment evidently blocked this elevation in the LPS-induced
sepsis group of animals (Fig.
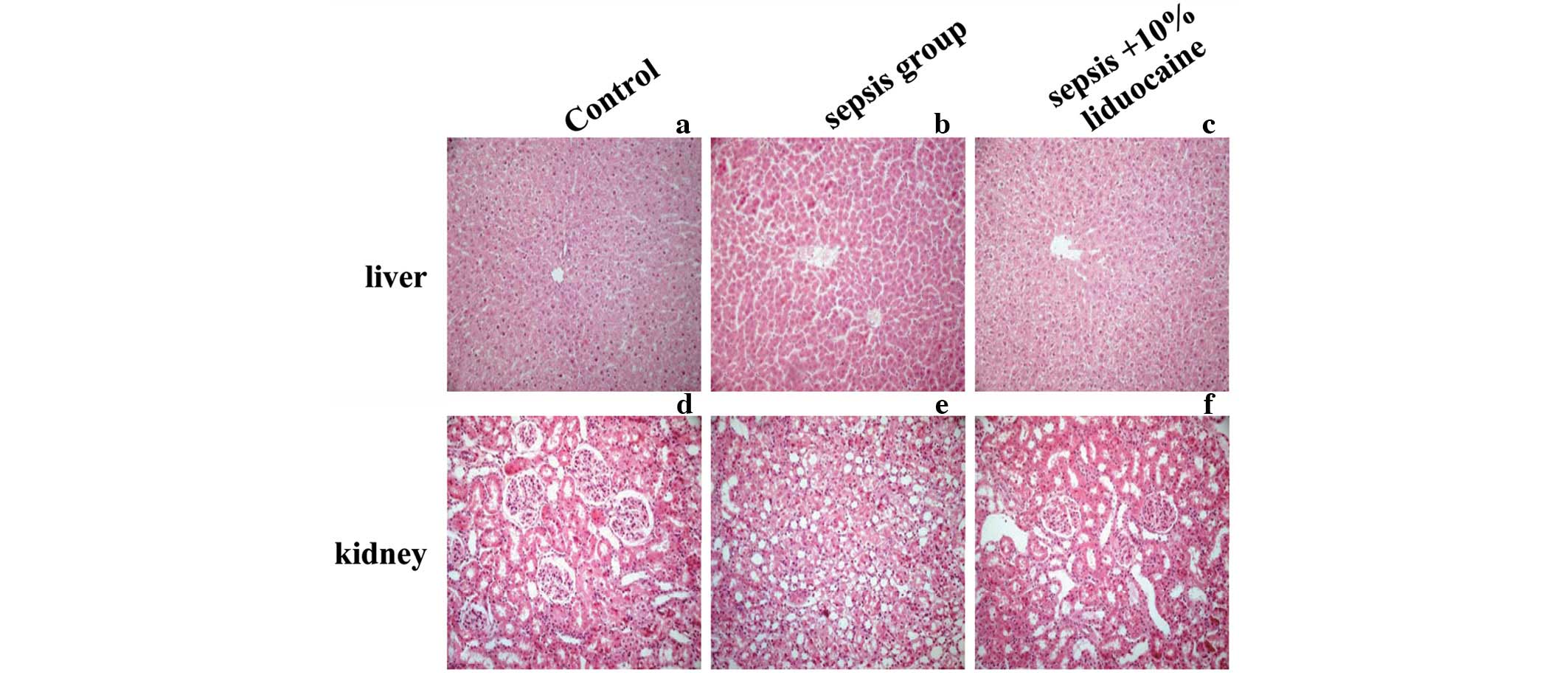
1A–D). To validate LPS-induced organ damage, the histological
changes were examined in the renal and hepatic tissue of the
various groups. No apparent degeneration or necrosis was observed
in the control groups. However, injury to the liver and kidneys was
serious in the sepsis groups. In the kidneys, nephron atrophy was
observed with a large number of proximal tubular epithelial cells
with edema in the renal cortex (Fig.
2A and B). In the liver, narrowed hepatic central veins,
sinusoidal dilatation, congestion, hepatocyte karyopyknosis and
necrotic lesions with signs of hepatocyte atrophy were observed in
the sepsis groups (Fig. 2D and E).
No significant improvements of histopathology were identified with
the application of lidocaine. Renal and hepatic cells lined up
sequentially, cell membrane integrity remained and reduced necrosis
or other pathological changes were detected (Fig. 2C and F). These results indicated
that lidocaine reduces mortality and ameliorates renal and hepatic
injury in septic rats induced by LPS.
Lidocaine decreases renal and hepatic
TLR4 expression in septic rats
Although lidocaine reduces mortality and protects
renal and hepatic injury in septic rats induced by LPS, the
mechanisms of whether the protective effects of lidocaine in sepsis
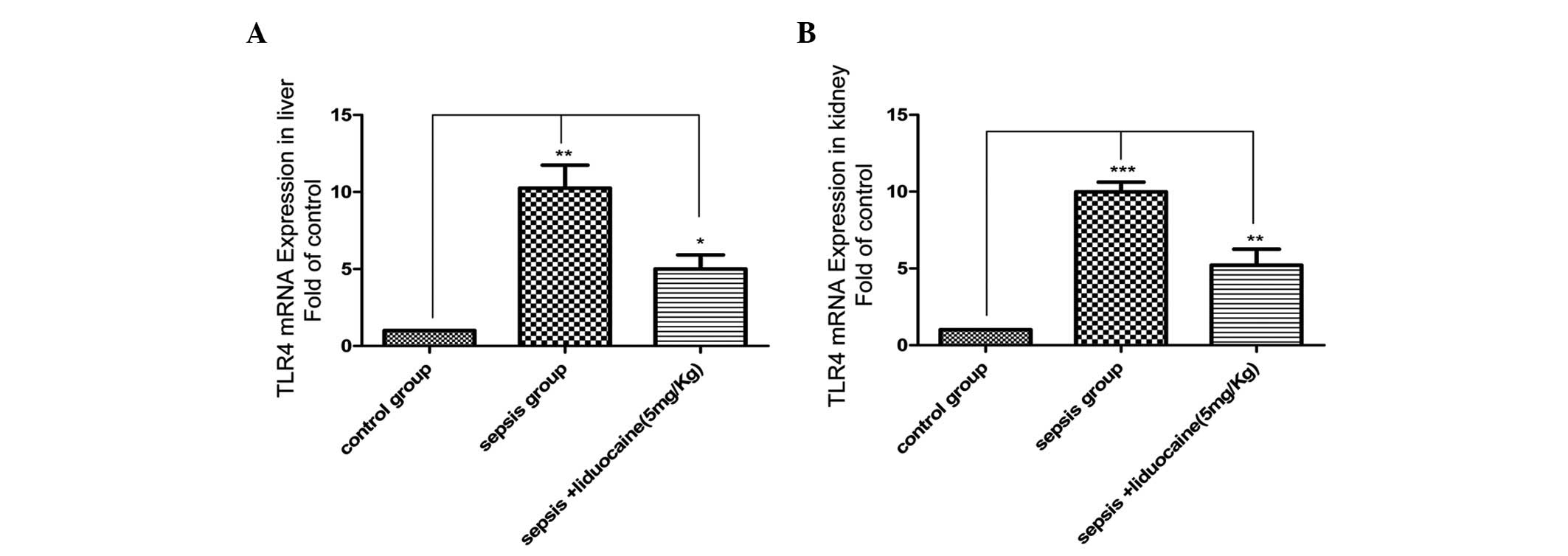
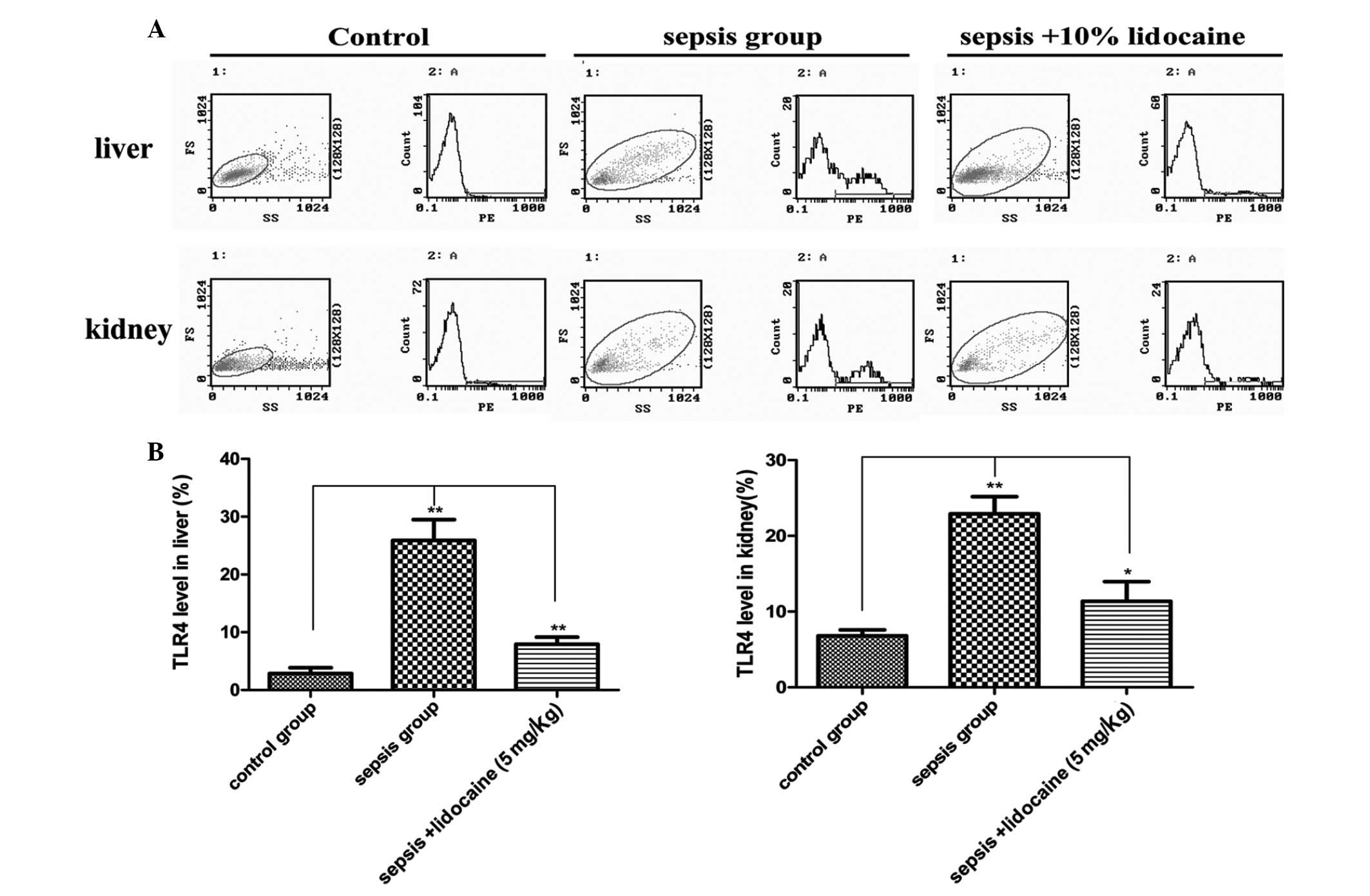
are associated with the TLR4 signaling pathway were explored. In
the liver of animals, the expression of TLR4 mRNA in the
LPS-induced sepsis group was found to be significantly increased
when compared with that of the control group, but this increase was
markedly eliminated with lidocaine treatment (Fig. 3A). The changes of TLR4 mRNA
expression were also found in the kidneys (Fig. 3B), further confirming the
determination of the protein levels of TLR4 in the various
treatment groups (Fig. 4A and B).
These results demonstrated that lidocaine protects against renal
and hepatic dysfunction in septic rats via the downregulation of
TLR4, indicating that lidocaine may act on the TLR4 signaling
pathway.
Lidocaine inhibits NF-κB expression in
sepsis
The signal transduction pathway of TLR4 mediated by
myeloid differentiation factor 88 (MyD88)-dependent pathways
activating NF-κB is clearly understood. This in turn activates a
wide variety of genes responsible for the synthesis of pro- and
anti-inflammatory cytokines (17,18).
To confirm whether NF-κB, an important signaling molecule
associated with the TLR4 signaling pathway, is involved in the
protection of lidocaine in sepsis, NF-κB levels in the liver and
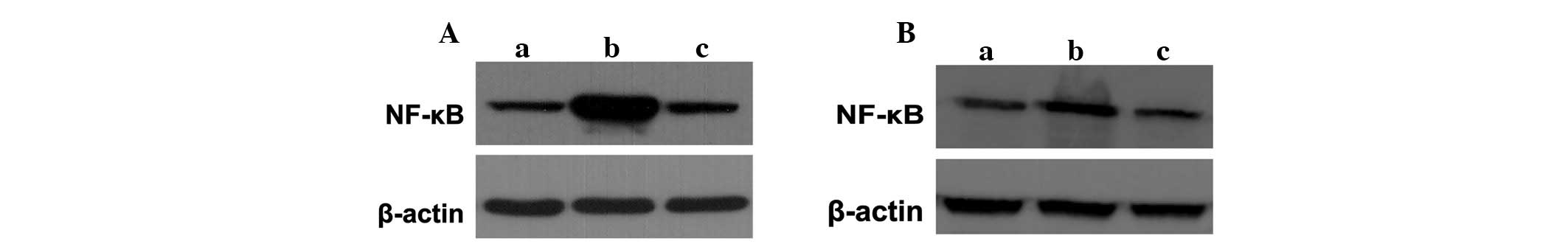
kidneys of animals were detected in the various groups. Western
blotting demonstrated that the expression of the NF-κB protein
increased markedly in the sepsis groups compared with that of the
control group, which was inhibited significantly with lidocaine
treatment (Fig. 5A and B). These
results showed that lidocaine inhibited NF-κB activation and that
it is involved in the protection of lidocaine in sepsis.
Lidocaine significantly attenuates
proinflammatory IL-6 levels in renal and hepatic tissues in
LPS-induced sepsis
TLR4 and NF-κB are considered important for the
protection of lidocaine in sepsis, however, there has been no
direct confirmation that lidocaine decreases the release of
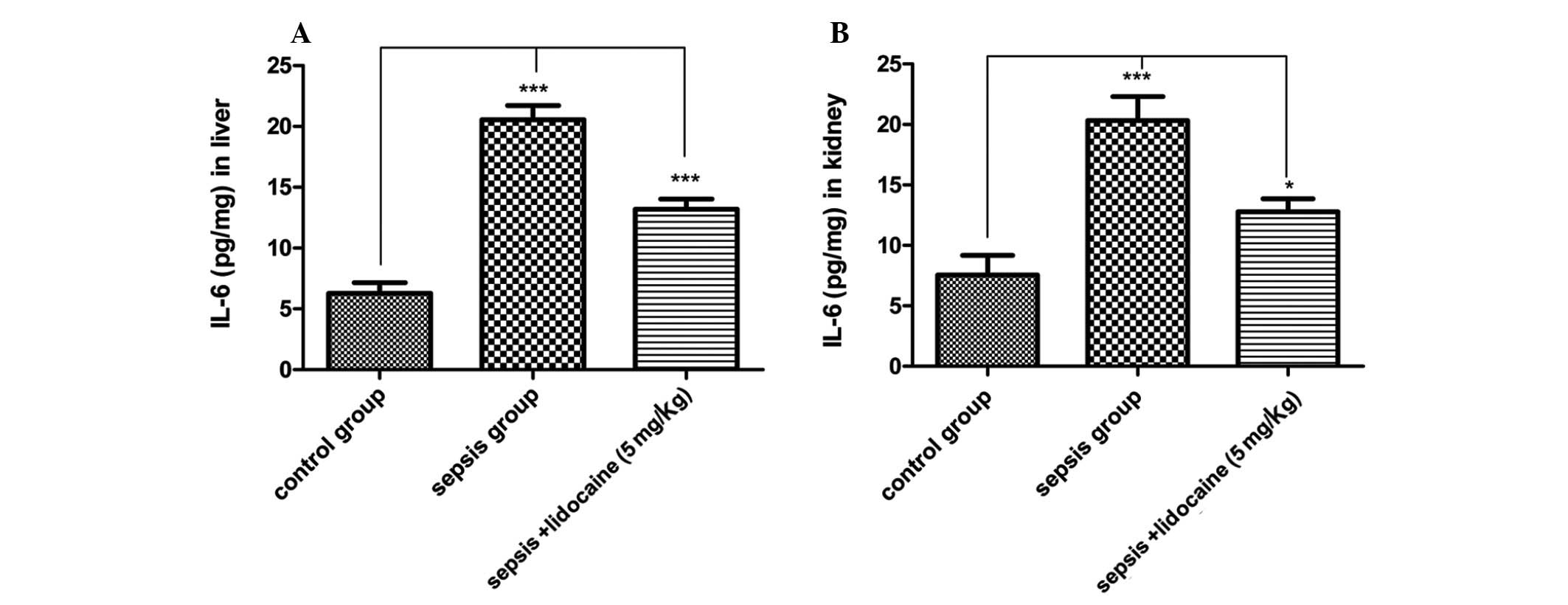
pro-inflammatory cytokines in sepsis. Therefore, the levels of IL-6
protein were detected in the liver and kidneys of animals in the
various groups. As predicted for the TLR4 signaling pathway, the
levels of IL-6 in the sepsis group were significantly increased
compared with those of the control group, whereas, this increase
was notably eliminated with lidocaine treatment in the liver
(Fig. 6A) and kidneys (Fig. 6B) of animals. These results
demonstrated that lidocaine significantly attenuates
proinflammatory IL-6 levels in the liver and kidneys in LPS-induced
sepsis via inhibition of the TLR4 signaling pathway.
Discussion
The results of the current study have demonstrated
that there is a complete TLR4-NF-κB proinflammatory cytokine
signaling pathway involved in the pathological process of renal and
hepatic injury in sepsis. Lidocaine provides significant protection
from acute renal and hepatic dysfunctions and attenuates the
hyperinflammatory response associated with rat sepsis induced by
LPS via the inhibition of the TLR4-NF-κB proinflammatory cytokine
signaling pathway.
The most important pathogenic mechanism underlying
sepsis is immune deregulation (19). TLRs are pathogen-associated
molecular pattern recognition molecules that are crucial for innate
immunity as the first defense system against microbial infection
(20). TLR4, the first TLR found
in mammals, is presented mainly by polymorphonuclear leukocytes,
monocytes, macrophages and dendritic cells (21,22),
as well as various other cell types, including epithelial and
endothelial cells (23–25). Different TLRs recognize various
pathogen-associated molecular patterns, with TLR4 mediating the
response to LPS from gram-negative bacteria (26–28).
The signal transduction pathway of TLR4 is mainly mediated by
MyD88-dependent pathways to activate NF-κB (29). NF-κB is one of the most important
regulators of proinflammatory gene expression. During inflammation,
NF-κB regulates the transcription of proinflammatory genes and
enzymes under signals received by TLRs (30–32).
The stimulation of TLR4 by LPS induces the release of critical
proinflammatory cytokines that are necessary to activate potent
immune responses (33–36).
Based on the experimental results of the present
study, we hypothesized that TLR4/NF-κB may be involved in the
inhibition process of lidocaine in sepsis. To address this
possibility, LPS was introduced to establish the septic model. The
amount of renal and hepatic cell surface TLR4 protein increased the
sensitivity of cells to LPS. Lidocaine almost completely blocked
LPS-triggered NF-κB activation in renal and hepatic tissues in
sepsis. This demonstrated the functional involvement of TLR4 in the
signal transduction pathway of lidocaine in LPS-induced sepsis.
Thus, it appeared that the TLR4-dependent signaling pathway is
involved in lidocaine-induced inhibitory effects. TLR antagonists
have previously been found to block components of the immune
response that are necessary to ward off subsequent infection and to
overcome the septic state (37).
Moreover, TLR4-deficient mice were hyporesponsive to LPS (38,39).
Previously, Johnson et al have shown that the ability of
heparin sulfate and pancreatic elastase to induce SIRS is greatly
reduced in TLR4-mutant mice, indicating that SIRS may be induced by
signaling through TLR4. These results from transgenic mice
highlighted further support for a contribution of specific TLRs to
the pathogenesis of SIRS (40).
Synthesis of cytokines, including TNF-α, IL-1β and
IL-6, are mediated by NF-κB and the current study found that
lidocaine inhibited LPS-induced IL-6 production and downregulated
TLR4 and NF-κB. Thus, we hypothesized that the NF-κB signaling
pathway is involved in the lidocaine-mediated signaling pathway.
Stimulus-induced NF-κB activity is the central mediator of
inflammatory responses. LPS-stimulated NF-κB transcription activity
was investigated in all groups. It was found that NF-κB
transcription activity was increased significantly following
treatment with LPS in sepsis, which was inhibited by lidocaine. We
hypothesized that the lidocaine-induced anti-inflammatory response
is involved in signaling mediated by the NF-κB signaling
pathway.
It is well known that mortality in sepsis is
markedly affected by the development of organ injury and
dysfunction (41). There exists
ample evidence of the potent anti-inflammatory effects of lidocaine
(13,42). In a previous rabbit model of lung
injury, Takao et al demonstrated that lidocaine injection
decreased various chemotactic factors, which resulted in reduced
neutrophil infiltration (43). In
a separate study, lidocaine and bupivacaine inhibited the release
of inflammatory mediators leukotriene B4 and interleukin-1 from
human neutrophils and monocytes (44). In concordance, the present study
showed that lidocaine injection not only decreased mortality in
LPS-induced sepsis, but also significantly reduced the magnitude of
renal and hepatic injury. Lidocaine has the ability to decrease Cr,
BUN, ALT and AST, indicating that the protection conferred by
lidocaine is global and not restricted to a particular organ. IL-6
is a prototypic example of a proinflammatory cytokine that is
important for propagating a host of secondary inflammatory
cascades. The current study has demonstrated that lidocaine
injection resulted in a marked decrease in circulating
concentrations of IL-6 in septic rats.
Overall, investigating TLR4/NF-κB-regulated
proinflammatory cytokine and enzyme expression is a good strategy
for novel anti-inflammatory drug development. Lidocaine may
decrease renal and hepatic tissue TLR4 expression and provide
global protection from organ injury and dysfunction resulting from
sepsis. The exploration of the protective function of lidocaine to
sepsis and its mechanism has great significance in the field of
emergency medicine.
References
|
1
|
Wang H and Ma S: The cytokine storm and
factors determining the sequence and severity of organ dysfunction
in multiple organ dysfunction syndrome. Am J Emerg Med. 26:711–715.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dewar DC and Balogh ZJ: The epidemiology
of multiple-organ failure: a definition controversy. Acta
Anaesthesiol Scand. 55:248–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dinarello CA: Proinflammatory and
anti-inflammatory cytokines as mediators in the pathogenesis of
septic shock. Chest. 112:S321–S329. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen J: The immunopathogenesis of sepsis.
Nature. 420:885–891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brun-Buisson C: The epidemiology of the
systemic inflammatory response. Intensive Care Med. 26(Suppl 1):
S64–S74. 2000. View Article : Google Scholar
|
|
6
|
Angus DC and Wax RS: Epidemiology of
sepsis: an update. Crit Care Med. 29:S109–S116. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hollmann MW and Durieux ME: Local
anesthetics and the inflammatory response: a new therapeutic
indication? Anesthesiology. 93:858–875. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strohm C, Barancik T, Bruhl ML, Kilian SA
and Schaper W: Inhibition of the ER-kinase cascade by PD98059 and
UO126 counteracts ischemic preconditioning in pig myocardium. J
Cardiovasc Pharmacol. 36:218–229. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Das KC and Misra HP: Prevention of
reperfusion lung injury by lidocaine in isolated rat lung
ventilated with higher oxygen levels. J Postgrad Med. 49:17–20.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmid RA, Yamashita M, ando K, Tanaka Y,
Cooper JD and Patterson GA: Lidocaine reduces reperfusion injury
and neutrophil migration in canine lung allografts. Ann Thorac
Surg. 61:949–955. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomori H, Shiraishi M, Koga H, et al:
Protective effects of lidocaine in hepatic ischemia/reperfusion
injury in vitro. Transplant Proc. 30:3740–3742. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fuentes JM, Talamini MA, Fulton WB, Hanly
EJ, Aurora AR and De Maio A: General anesthesia delays the
inflammatory response and increases survival for mice with
endotoxic shock. Clin Vaccine Immunol. 13:281–288. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gallos G, Jones DR, Nasr SH, Emala CW and
Lee HT: Local anesthetics reduce mortality and protect against
renal and hepatic dysfunction in murine septic peritonitis.
Anesthesiology. 101:902–911. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawai T and Akira S: TLR signaling. Cell
Death Differ. 13:816–825. 2006. View Article : Google Scholar
|
|
15
|
Akira S: TLR signaling. Curr Top Microbiol
Immunol. 311:1–16. 2006.
|
|
16
|
Compton T, Kurt-Jones EA, Boehme KW, et
al: Human cytomegalovirus activates inflammatory cytokine responses
via CD14 and toll-like receptor 2. J Virol. 77:4588–4596. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanimura N, Saitoh S, Matsumoto F,
Akashi-Takamura S and Miyake K: Roles for LPS-dependent interaction
and relocation of TLR4 and TRAM in TRIF-signaling. Biochem Biophys
Res Commun. 368:94–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: update on
toll-like receptors. Nat Immunol. 11:373–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rittirsch D, Flierl MA and Ward PA:
Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 8:776–787.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akira S and Hemmi H: Recognition of
pathogen-associated molecular patterns by TLR family. Immunol Lett.
85:85–95. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Muzio M, Bosisio D, Polentarutti N, et al:
Differential expression and regulation of toll-like receptors (TLR)
in human leukocytes: selective expression of TLR3 in dendritic
cells. J Immunol. 164:5998–6004. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O’Neill LAJ and Bowie AG: The family of
five: TIR- domain-containing adaptors in toll-like receptor
signalling. Nat Rev Immunol. 7:353–364. 2007.
|
|
23
|
Zhang FX, Kirschning CJ, Mancinelli R, et
al: Bacterial lipopolysaccharide activates nuclear factor-kappaB
through interleukin-1 signaling mediators in cultured human dermal
endothelial cells and mononuclear phagocytes. J Biol Chem.
274:7611–7614. 1999. View Article : Google Scholar
|
|
24
|
Cario E, Rosenberg IM, Brandwein SL, Beck
PL, Reinecker HC and Podolsky DK: Lipopolysaccharide activates
distinct signaling pathways in intestinal epithelial cell lines
expressing toll-like receptors. J Immunol. 164:966–972. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Faure E, Equils O, Sieling PA, et al:
Bacterial lipopolysaccharide activates NF-kappaB through toll-like
receptor 4 (TLR-4) in cultured human dermal endothelial cells.
Differential expression of TLR-4 and TLR-2 in endothelial cells. J
Biol Chem. 275:11058–11063. 2000. View Article : Google Scholar
|
|
26
|
Underhill DM, Ozinsky A, Smith KD and
Aderem A: Toll-like receptor-2 mediates mycobacteria-induced
proinflammatory signaling in macrophages. Proc Natl Acad Sci USA.
96:14459–14463. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ozinsky A, Underhill DM, Fontenot JD, et
al: The repertoire for pattern recognition of pathogens by the
innate immune system is defined by cooperation between toll-like
receptors. Proc Natl Acad Sci USA. 97:13766–13771. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Poltorak A, He X, Smirnova I, et al:
Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations
in Tlr4 gene. Science. 282:2085–2088. 1998. View Article : Google Scholar
|
|
29
|
Ahn KS, Sethi G and Aggarwal BB: Nuclear
factor-kappa B: from clone to clinic. Curr Mol Med. 7:619–637.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar
|
|
31
|
Hoffmann A, Natoli G and Ghosh G:
Transcriptional regulation via the NF-kappaB signaling module.
Oncogene. 25:6706–6716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hoffmann A, Levchenko A, Scott ML and
Baltimore D: The IkappaB-NF-kappaB signaling module: temporal
control and selective gene activation. Science. 298:1241–1245.
2002. View Article : Google Scholar
|
|
33
|
Couture LA, Piao W, Ru LW, Vogel SN and
Toshchakov VY: Targeting toll-like receptor (TLR) signaling by
toll/interleukin-1 receptor (TIR) domain-containing adapter
protein/MyD88 adapter-like (TIRAP/Mal)-derived decoy peptides. J
Biol Chem. 287:24641–24648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fitzgerald KA, Palsson-McDermott EM, Bowie
AG, et al: Mal (MyD88-adapter-like) is required for toll-like
receptor-4 signal transduction. Nature. 413:78–83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gray P, Dunne A, Brikos C, Jefferies CA,
Doyle SL and O’Neill LA: MyD88 adapter-like (Mal) is phosphorylated
by Bruton’s tyrosine kinase during TLR2 and TLR4 signal
transduction. J Biol Chem. 281:10489–10495. 2006.PubMed/NCBI
|
|
36
|
Verstak B, Nagpal K, Bottomley SP,
Golenbock DT, Hertzog PJ and Mansell A: MyD88 adapter-like
(Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and
TLR4-mediated NF-kappaB proinflammatory responses. J Biol Chem.
284:24192–24203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brown KL, Cosseau C, Gardy JL and Hancock
RE: Complexities of targeting innate immunity to treat infection.
Trends Immunol. 28:260–266. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hoshino K, Takeuchi O, Kawai T, et al:
Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are
hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps
gene product. J Immunol. 162:3749–3752. 1999.PubMed/NCBI
|
|
39
|
Arbour NC, Lorenz E, Schutte BC, et al:
TLR4 mutations are associated with endotoxin hyporesponsiveness in
humans. Nat Genet. 25:187–191. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Johnson GB, Brunn GJ and Platt JL: Cutting
edge: an endogenous pathway to systemic inflammatory response
syndrome (SIRS)-like reactions through Toll-like receptor 4. J
Immunol. 172:20–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Angus DC, Linde-Zwirble WT, Lidicker J,
Clermont G, Carcillo J and Pinsky MR: Epidemiology of severe sepsis
in the United States: analysis of incidence, outcome and associated
costs of care. Crit Care Med. 29:1303–1310. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li CY, Tsai CS, Hsu PC, Chueh SH, Wong CS
and Ho ST: Lidocaine attenuates monocyte chemoattractant protein-1
production and chemotaxis in human monocytes: possible mechanisms
for its effect on inflammation. Anesth Analg. 97:1312–1316.
2003.PubMed/NCBI
|
|
43
|
Takao Y, Mikawa K, Nishina K, Maekawa N
and Obara H: Lidocaine attenuates hyperoxic lung injury in rabbits.
Acta Anaesthesiol Scand. 40:318–325. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sinclair R, Eriksson AS, Gretzer C,
Cassuto J and Thomsen P: Inhibitory effects of amide local
anaesthetics on stimulus-induced human leukocyte metabolic
activation, LTB4 release and IL-1 secretion in vitro. Acta
Anaesthesiol Scand. 37:159–165. 1993. View Article : Google Scholar
|