Introduction
Hemangiomas are the most common type of benign
hepatic tumor. In adults, the major histological type is a
cavernous hemangioma. By contrast, infantile hemangioma (IH), also
referred to as infantile hemangioendothelioma, is a vascular liver
tumor arising in infancy or early childhood. The majority of cases
are diagnosed by the age of six months. IH in adults is extremely
rare, and only a few cases have been reported in the English
literature thus far. The present review reports the case of a
vascular tumor, morphologically resembling IH, that presented in a
47-year-old female, and a review of the literature.
Case report
A 47-year-old female visited Koga hospital for a
medical check. An abdominal ultrasound revealed five tumors in the
right lobe of the liver. Laboratory studies, including liver
function tests and tumor markers, were within the normal range.
Serological markers for hepatitis B or C viral infections were
undetectable.
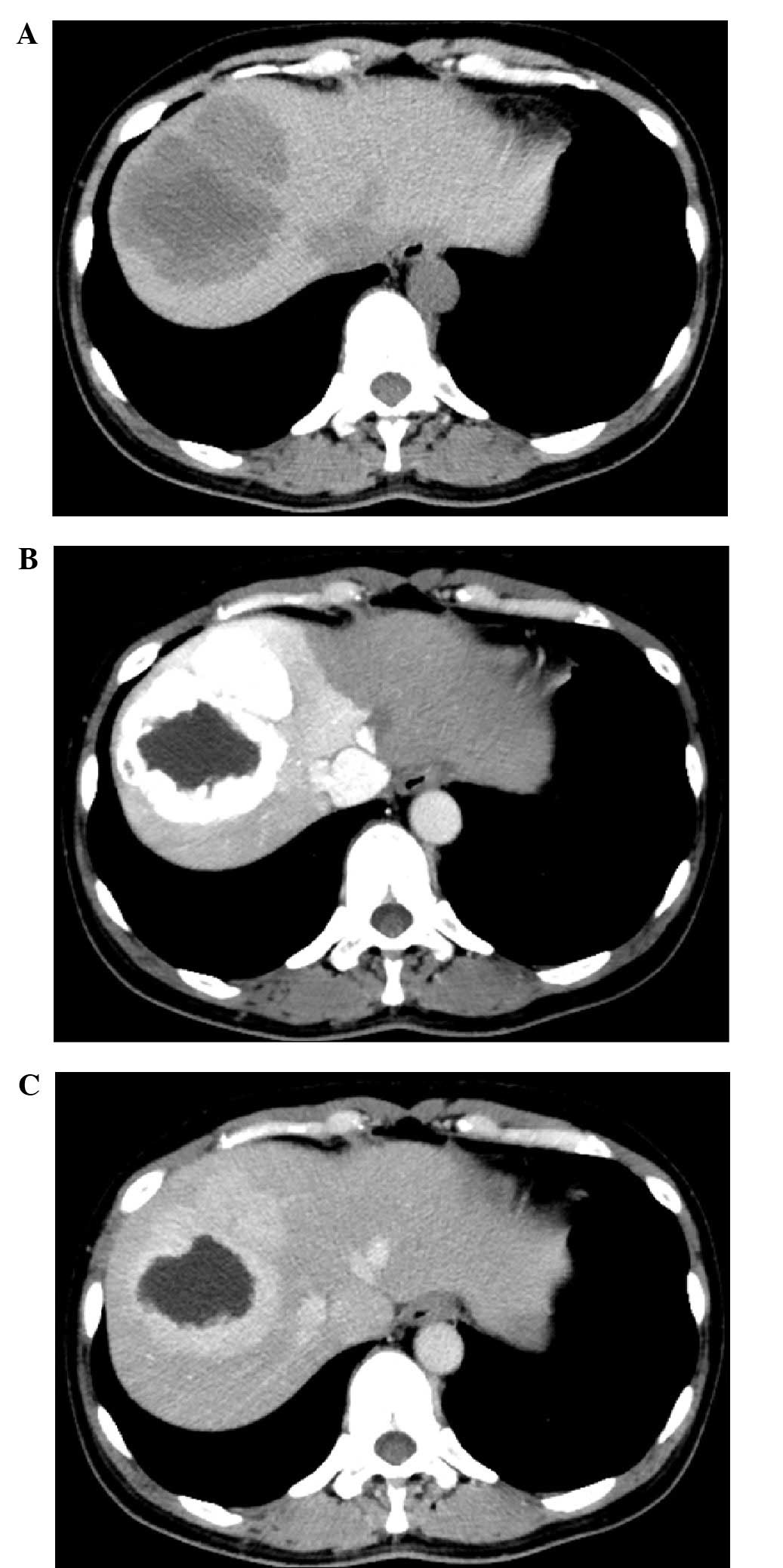
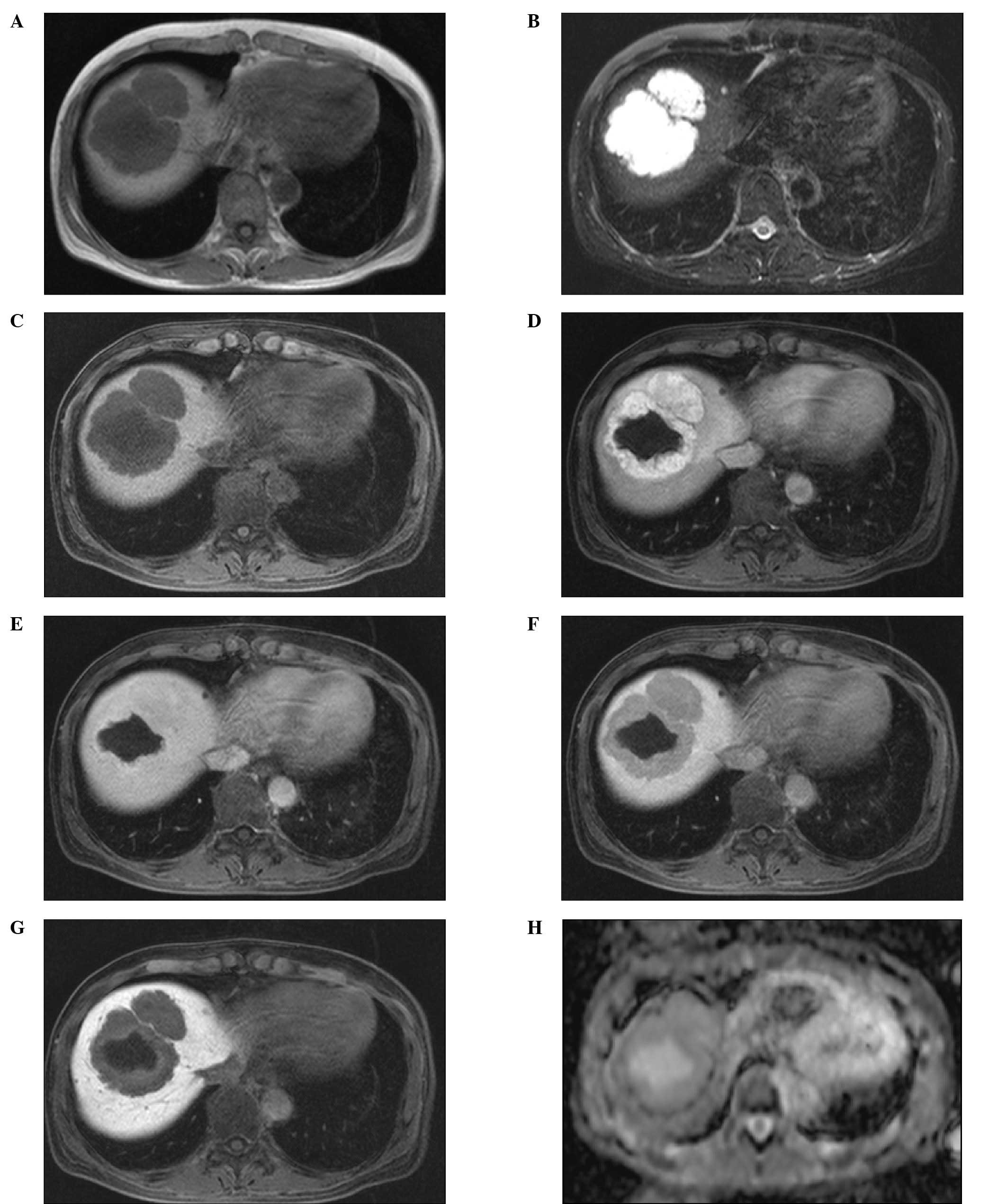
Abdominal computed tomography (CT) and gadolinium
ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA)
enhanced magnetic resonance imaging (MRI) findings, which are shown
in Figs. 1 and 2, respectively. A plain CT image revealed
five hypoattenuating lesions in the right lobe of the liver. The
arterial phase of the dynamic CT revealed marked enhancement of the
tumors. The largest tumor had no enhanced area within the tumor
considered to be necrosis or cystic/mucinous degeneration. The
tumors showed hyperattenuation compared with the surrounding liver
parenchyma in the delayed phase (Fig.
1). Tumors appeared as hypointense masses on the T1-weighted
images and as hyperintensities on the fat-suppression T2-weighted
images. Following the administration of Gd-EOB-DTPA, the tumors
appeared to be well-enhanced in the arterial phase (30 sec). In the
portal phase (80 sec), the tumors demonstrated isointensity
compared with the surrounding liver parenchyma, and hypointensity
in the equilibrium (4 min) and hepatobiliary (20 min) phases. In
the CT, the largest tumor had no enhanced area within the tumor.
The apparent diffusion coefficient (ADC) values ranged from 2.0 to
2.4×10−3 mm2/sec (Fig. 2).
Clinically, these tumors were considered to be
hemangiomas. As the tumors were enlarged during follow-up, a liver
biopsy was performed, and the tumors were diagnosed histologically
as hemangiomas.
The patient was followed up without therapy for ~4
years, but the tumors increased in size. As a result, the patient
underwent a lobectomy of the right liver.
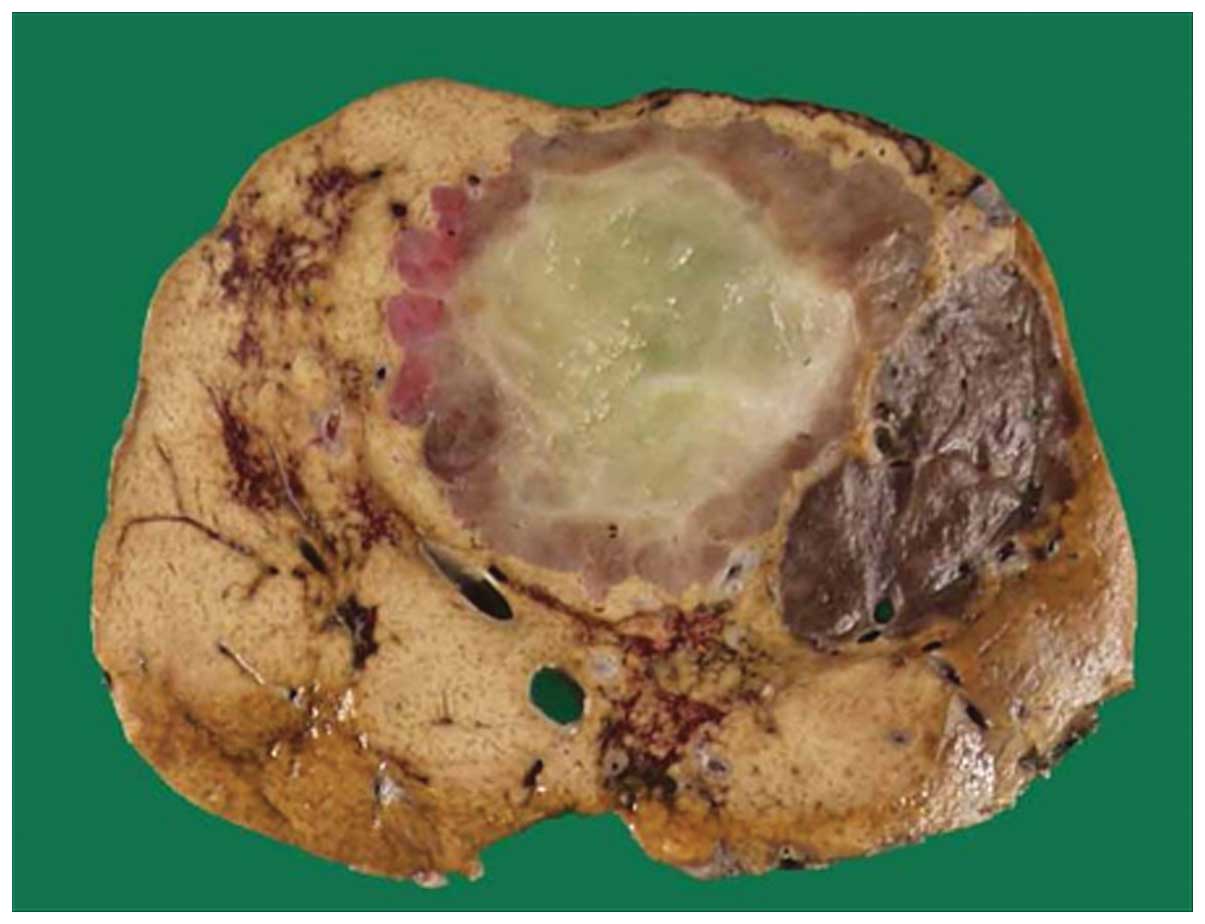
Grossly, the tumors were well defined and brown to
tan, and the largest tumor measured 66×57 mm in diameter with a
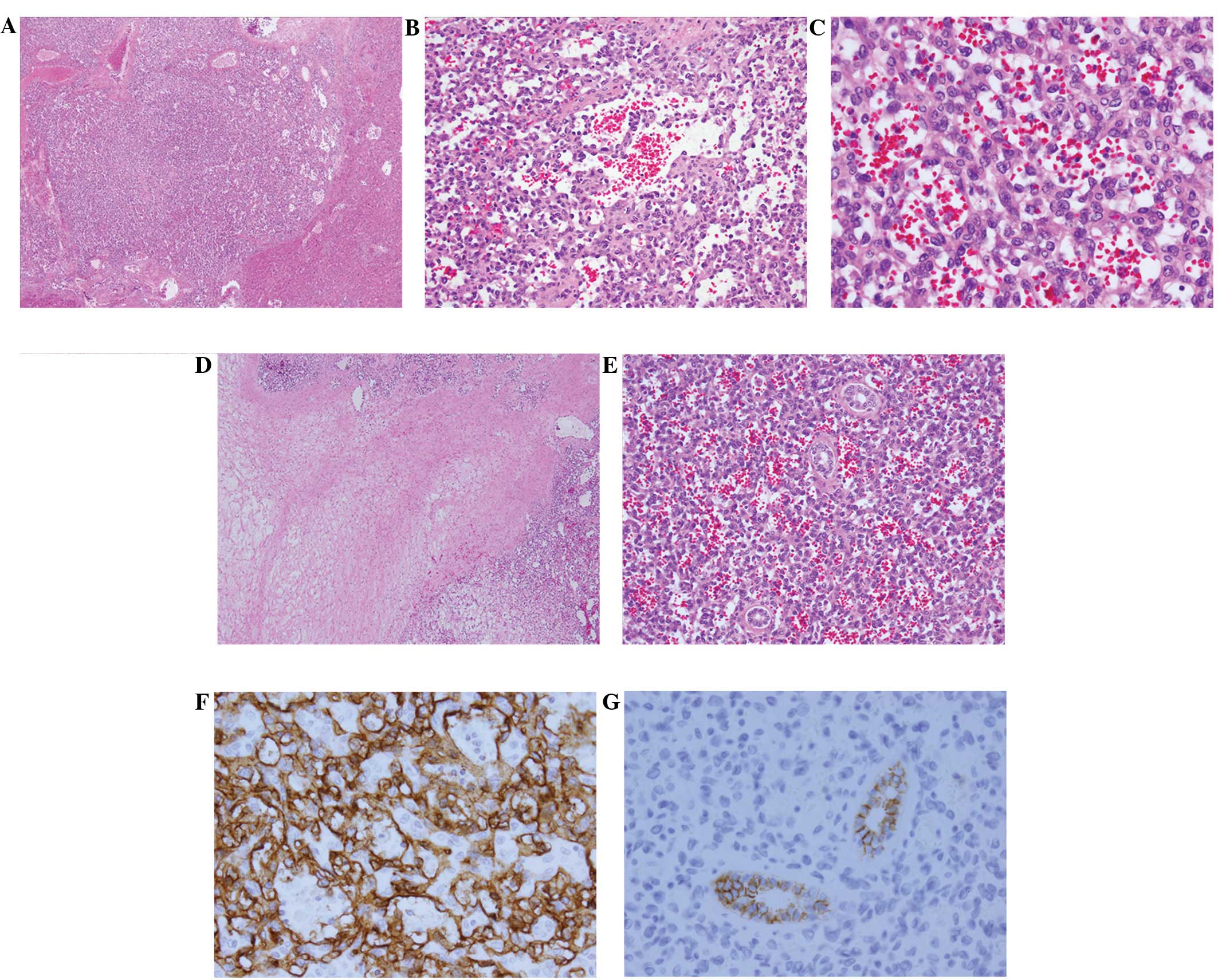
yellowish area considered to be myxoid degeneration (Fig. 3). Microscopically, the tumors
consisted of numerous capillary-like vessels lined with endothelial
cells without atypia or mitoses. A myxomatous component was noted
in the center of the largest tumor. Immunohistochemically, the
endothelial cells were positive for CD34 and vimentin, and negative
for Glut-1, HMB45 and CAM5.2. The small bile ducts trapped within
the tumor were positive for CD56 (Fig.
4).
Clinically, no apparent recurrence was noted in the
subsequent 12 months.
Discussion
IH is one of the most common benign vascular liver
tumors in infants and children. Most IHs are diagnosed before six
months of age, and enlarge in the first 8–18 months (1,2).
Following this period, the occurrence of IH is exceedingly rare.
There are only two adult case reports of IH, occurring in a
54-year-old female and an 18-year-old adolescent (1,3). The
majority of patients present with abdominal distention and certain
patients present with congestive heart failure or consumption
coagulopathy (1,4). Selby et al reported that the
most prognostically important symptoms are congestive heart failure
and jaundice (1). Up to two-thirds
of symptomatic patients die as a result of the tumor. If patients
harboring IH do not have any complications, the majority of tumors
spontaneously regress with age (5,6). In
the present case, the patient had no symptoms or complications
although the tumors gradually increased in size.
MRI findings of IH have been previously reported.
Although MRI findings reveal various intensities due to
hemorrhaging, infarction or degeneration, the tumor shows
heterogeneous hypointensity relative to the surrounding liver
parenchyma on T1-weighted images and heterogeneous hyperintensity
on T2-weighted images. These findings are similar to those of
cavernous hemangioma (7,8–11).
In the present case, the tumors demonstrated similar signal
intensities to those identified in the previous reports.
To the best of our knowledge, there have been no
previous reports that present Gd-EOB-DTPA-enhanced MRI image
findings of IH. However, there is a report on the enhancement
pattern in a dynamic study with Gd-EOB-DTPA of typical and
high-flow hemangiomas (12). In
typical cases, tumors demonstrate peripheral nodular enhancement
and a progressive fill-in pattern in the arterial phase, although
high-flow type hemangiomas exhibit uniform enhancement in the
arterial phase. Tamada et al reported that 59% of
hemangiomas were isointense during the portal phase and 69% were
isointense to slightly hypointense during the equilibrium phase. In
the hepatobiliary phase (20 min), all the tumors demonstrated
hypointensity relative to the surrounding liver parenchyma and
there were no differences in these enhancement patterns between
typical hemangiomas and high-flow hemangiomas (12). However, particularly with large
lesions, tumors contain unenhanced areas due to infarctions or
hemorrhaging (8,9). The present case was considered to be
a high-flow-type hemangioma and the largest tumor had a
non-enhancement area that was identified microscopically as a
myxoid change.
Although no previous reports have demonstrated the
DWI or ADC value of IH, certain reports have described that of
hemangioma in adult cases. Vossen et al reported that there
was a statistically significant difference in the ADC values
between hemangiomas and other hypervascular liver lesions (focal
nodular hyperplasia, hepatocellular carcinoma and hypervascular
liver metastases) (13). Taouli
et al also reported that the mean ADC value of benign
tumors, including hemangiomas, (2.45×10−3±0.96
mm2/sec) was significantly higher than that of malignant
lesions (1.08×10−3±0.50 mm2/sec) (14). In the present case, the ADC values
of IH ranged from 2.0 to 2.4×10−3 mm2/sec and
were similar to those of previous studies on hemangiomas.
Pathological studies on IH revealed that the tumor
margin is macroscopically well demarcated in 65% of IH cases, and
the cut surface is red-brown to light tan or white depending on the
degree of vascularity, hemorrhaging or degeneration (1,5,15).
Multiple occurrence is observed in 45% of IH cases (1,5).
Those findings are also consistent with this case. Microscopically,
IH tumors are composed of numerous capillary-like small vessels
lined by plump endothelial cells, usually arranged in a single
layer without mitoses (3,4,15–18).
The periphery of these lesions may also exhibit a certain degree of
cavernous changes (18).
Exclusively in IHs, small bile ducts are trapped and scattered
within the tumor. Extramedullary hematopoiesis is also often
observed (4,15,17,18).
Involutional changes, such as infarction, necrosis, hemorrhaging,
scarring, myxoid changes and calcifications are frequently present
(4,17,18).
Immunohistochemically, the tumor cells express CD31, CD34 and
factor VIII-related antigen (1,4,5,15,18).
The majority of these findings were observed in the present
case.
Angiosarcomas, composed of a vascular channel and
typically occurring in adults, should be considered as a different
diagnosis. However, angiosarcomas are usually composed of an
irregular vascular channel lined by variably atypical endothelial
cells and often exhibit multilayering and high mitotic activity
(4). There were no atypical cells
or mitoses in the present case. Angiosarcoma is usually a highly
aggressive tumor and its prognosis is extremely poor. The present
case had a clinically favorable outcome. By contrast, small bile
ducts were trapped within the tumor. This finding is rare in a
cavernous hemangioma, which is a representative benign vascular
tumor occurring in adults. Collectively, these findings suggest
that this case may be IH occurring in adults. IH-like tumors occur
in various organs, however, they usually demonstrate a positive
reaction immunohistochemically for Glut-1 (19). The present case lacked a positive
reaction for Glut-1 in spite of exhibiting typical morphological
findings of IH. Although these discrepancies remain unclear, the
lack of Glut-1 expression may be a characteristic immunophenotype
in adult onset IH. However, little is known about the Glut-1 status
in adult onset IH owing to its rarity.
There are therapeutic methods available. The
first-line therapy involves drugs such as steroids or interferon-α.
Arterial embolization, surgical resection or liver transplantation
are selected if medication is ineffective (4,6,7).
In conclusion, we presented a case of IH of the
liver in an adult. IH is one of the most common benign liver tumors
in infants and children. Although IH is a biologically benign
tumor, its mortality rate is relatively high in patients with
complications. Thus, radiologists and pathologists should
understand the characteristics of IH in order to administer
appropriate treatment.
References
|
1
|
Selby DM, Stocker JT, Waclawiw MA,
Hitchcock CL and Ishak KG: Infantile hemangioendothelioma of the
liver. Hepatology. 20:39–45. 1994.PubMed/NCBI
|
|
2
|
Woltering MC, Robben S and Egeler RM:
Hepatic hemangioendothelioma of infancy: treatment with interferon
alpha. J Pediatr Gastroenterol Nutr. 24:348–351. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diment J, Yurim O and Pappo O: Infantile
hemangioendothelioma of the liver in an adult. Arch Pathol Lab Med.
125:931–932. 2001.PubMed/NCBI
|
|
4
|
Bosman FT, Carineiro F, Hruban RH and
Theise ND: WHO Classification of Tumors of the Digestive System.
4th edition. World Health Organisation; Geneva: pp. 242–243.
2010
|
|
5
|
Burt AD, Portmann BC and Ferrell LD:
MacSween’s Pathology of the liver. 6th edition. Elsevier Limited;
Philadelphia, PA: pp. 796–797. 2012
|
|
6
|
Kuntz E and Kuntz HD: Hepatology Textbook
and Atlas. Third edition. Springer; Medizin Verlag, Heidelberg: pp.
781–782. 2008
|
|
7
|
Kassarjian A, Zurakowski D, Dubois J,
Paltiel HJ, Fishman SJ and Burrows PE: Infantile hepatic
hemangiomas: clinical and imaging findings and their correlation
with therapy. AJR Am J Roentgenol. 182:785–795. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mortele KJ, Mergo PJ, Urrutia M and Ros
PR: Dynamic gadolinium-enhanced MR findings in infantile hepatic
hemangioendothelioma. J Comput Assist Tomogr. 22:714–717. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keslar PJ, Buck JL and Selby DM: From the
archives of the AFIP. Infantile hemangioendothelioma of the liver
revisited. Radiographics. 13:657–670. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng ST, Chan T, Ching AS, Sun CH, Guo HY,
Fan M, Meng QF and Li ZP: CT and MR imaging characteristics of
infantile hepatic hemangioendothelioma. Eur J Radiol. 76:e24–e29.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mortelé KJ, Vanzieleghem B, Mortelé B,
Benoit Y and Ros PR: Solitary hepatic infantile
hemangioendothelioma: dynamic gadolinium-enhanced MR imaging
findings. Eur Radiol. 12:862–865. 2002.PubMed/NCBI
|
|
12
|
Tamada T, Ito K, Yamamoto A, Sone T, Kanki
A, Tanaka F and Higashi H: Hepatic hemangiomas: evaluation of
enhancement patterns at dynamic MRI with gadoxetate disodium. AJR
Am J Roentgenol. 196:824–830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vossen JA, Buijs M, Liapi E, Eng J,
Bluemke DA and Kamel IR: Receiver operating characteristic analysis
of diffusion-weighted magnetic resonance imaging in differentiating
hepatic hemangioma from other hypervascular liver lesions. J Comput
Assist Tomogr. 32:750–756. 2008. View Article : Google Scholar
|
|
14
|
Taouli B, Vilgrain V, Dumont E, Daire JL,
Fan B and Menu Y: Evaluation of liver diffusion isotropy and
characterization of focal hepatic lesions with two single-shot
echo-planar MR imaging sequences: prospective study in 66 patients.
Radiology. 226:71–78. 2003. View Article : Google Scholar
|
|
15
|
Ishak KG, Goodman ZD and Stocker TJ: Atlas
of Tumor Pathology. Tumors of the Liver and Intrahepatic Bile
Ducts. Armed Forces Institute of Pathology; Washington, D.C:
2001
|
|
16
|
Dachman AH, Lichtenstein JE, Friedman AC
and Hartman DS: Infantile hemangioendothelioma of the liver: a
radiologicpathologic-clinical correlation. AJR Am J Roentgenol.
140:1091–1096. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okuda K and Ishak KG: Neoplasms of the
Liver. Springer-Verlag; Tokyo: 1987, View Article : Google Scholar
|
|
18
|
Kanel GC and Korula J: Atlas of Liver
Pathology. 3rd edition. Elsevier Saunders; Philadelphia: pp.
260–261. 2011
|
|
19
|
Drut RM and Drut R: Extracutaneous
infantile haemangioma is also Glut1 positive. J Clin Pathol.
57:1197–1200. 2004. View Article : Google Scholar : PubMed/NCBI
|